Abstract
Introduction
Now that HIV infection has become a chronic disease, optimizing health status is an important goal of care for HIV-infected patients. Testosterone insufficiency (TI) can compromise health status, but little is known about the prevalence of TI and possible related factors in HIV-infected women.
Aim
To investigate the prevalence of TI among HIV-infected women attending our HIV outpatient clinic, and to study the relationship between TI and sexual function, fatigue, health status, and depression.
Methods
56 HIV-infected women aged ≥18 years who attended the HIV outpatient clinic of the Amsterdam University Medical Center, The Netherlands, were included. Blood samples were taken for endocrinologic testing and patients filled out 6 validated questionnaires measuring sexual function, fatigue, health, and depression.
Main Outcome Measure
TI, the Female Sexual Function Index, the Female Sexual Distress Scale-Revised, the Multidimensional Fatigue Inventory, the Medical Outcomes Studies Short Form 36-item health survey, and the Beck Depression Inventory were assessed.
Results
A relatively high prevalence of TI, 37%, was found. Plasma viral load and CD4 cell count did not differ between women with or without TI. Clinical fatigue, physical fatigue, and impaired cognitive function were significantly more prevalent in women with TI. Women with TI also tended to report decreased sexual desire, reduced physical activity, increased mental fatigue, reduced physical function, increased health distress, and clinical depression.
Conclusion
We recommend that in all HIV-positive women with complaints typical for TI, testosterone is measured, and that in women with TI, testosterone replacement be considered as a treatment option. However, given that complaints are also prevalent in HIV-positive women without TI, the approach to women with these complaints should include sexual and psychological evaluation.
Laan ETM, Prins JM, van Lunsen RHW, et al. Testosterone Insufficiency in Human Immunodeficiency Virus–Infected Women: A Cross-Sectional Study. Sex Med 2019;7:72–79.
Key Words: HIV-Infected Women, Testosterone Insufficiency, Sexual Dysfunction, Fatigue, Health Status
Introduction
Considering that HIV infection has become a chronic disease in resource-rich settings, optimizing the health status of people living with HIV is becoming an increasingly important goal of care for these patients.
Testosterone insufficiency (TI) is one of the conditions that influence health status of people with HIV. In men, health problems related to TI are reduced sexual arousability, fatigue, weight and muscle mass loss, depressed mood, anemia, and loss of energy.1, 2, 3, 4, 5, 6, 7 Several studies have shown that TI is also present in women with HIV.8, 9, 10, 11, 12, 13, 14 A U.S. study found a prevalence of TI of 48% in HIV-positive women not taking combination antiretroviral therapy (cART).12 2 studies in HIV-positive women with weight loss and wasting reported a prevalence of 49% and 66%, respectively.13, 14 Little is known about the prevalence and possible related factors of TI among unselected HIV-infected women attending a general HIV outpatient clinic.
The causes of TI in HIV-infected patients are diverse. In HIV-positive men, TI was found to be related to HIV infection itself, with changes in fat metabolism related to the use of cART.1, 2 Another cause of TI is the presence of increased levels of SHBG, which can result from age, pregnancy, hormonal contraception, and hyperthyroidism.13 Increased SHBG levels may lead to lower levels of bioavailable testosterone (T) despite normal total T levels.1 In HIV-positive women, duration of HIV infection, cART use, lower CD4 cell values, wasting, and weight loss were found to be associated with low levels of bioavailable T.14, 15
The most frequently reported clinical symptoms of decreased bioavailable T in women are sexual problems, persistent unexplained fatigue, and depression.13, 16 Many women with HIV report these complaints as well. The question is to what extent these complaints are related to TI. In this cross-sectional study among HIV-infected women attending the HIV outpatient clinic of the Amsterdam University Medical Center (AUMC), we investigated the prevalence of TI, and studied the relationship between TI and sexual function, fatigue, health status, and depression.
Methods
Study Design
The study was a cross-sectional cohort study, enrolling all eligible and consenting HIV-infected women who visited the HIV outpatient clinic of the AUMC between February 2008 and February 2009.
Patients
Eligible were HIV-infected women aged ≥18 years who were willing to complete 6 questionnaires related to sexual function, fatigue, health status, mental health, cognitive functioning, and depression and who were willing to provide an extra blood sample. Exclusion criteria were the use of androgen therapy and pregnancy and lactation during the 6 months before study start, hypo- or hyperthyroidism, transgender hormonal or surgical therapies in the past, and ovariectomy or radiation of the ovaries. HIV nurse-counselors and physicians approached all eligible patients during routine visits and provided verbal and written information about the study. The study was approved by the hospital Medical Ethics Committee, and all patients provided written informed consent.
Study Procedures
Patients who were willing to participate first signed the informed consent form, after which the questionnaires were completed. Eligibility, partnership status, comorbidity, and medication use were assessed in a brief clinical interview. Blood for endocrinologic testing was collected at the same time as the standard blood tests, which are usually drawn at the end of each visit. The maximum window between completion of the questionnaires and the collection of the blood sample was 2 weeks. We measured plasma levels of total T, SHBG, luteinizing hormone (LH), follicle-stimulating hormone (FSH), prolactin, 17β-estradiol, albumin, and TSH. Plasma samples were analyzed at the AUMC laboratory using in-house standardized radioimmunoassay with extraction (RIAaumc) to measure total extracted T and SHBG. For another study conducted in our hospital using RIAaumc, plasma samples were re-analyzed in another laboratory using liquid chromatography/mass spectrometry (LC-MS/MS).17, 18 Total T values using LC-MS/MS were found to be highly equivalent to the RIAaumc values (r = 0.928, unpublished data). Albumin was measured using a bromocresol assay. The lower limits of quantification were as follows: total T, 0.3 nmol/L; SHBG, 5 nmol/L; albumin, 35 g/L. Because a method for directly measuring free T was not yet available when the study was performed, free T was calculated based on total T, SHBG, and albumin concentration.19, 20 The free T index (FTI) was calculated using the following formula: FTI = 100 × T/SHBG, where T is total T. Following recommendations where TI is defined as T levels within the lowest quartile16 and recommendations with respect to estimating T levels using radioimmunoassay,21 in this study TI was defined as total T ≤0.3 if SHBG levels were within the normal range (<100 nmol/L), and as total T × FTI ≤0.5 in patients with elevated SHBG levels (>100 nmol/L).
Measures
Sexual problems were assessed using the Female Sexual Functioning Index (FSFI) and the Female Sexual Distress Scale-Revised (FSDS-R). The FSFI measures sexual dysfunction in women based on Diagnostic and Statistical Manual for Mental Disorders-IV-TR criteria.22 The questionnaire consists of 6 subscales: Desire (2 items, range 1–5), Arousal (4 items, range 0–5), Lubrication (4 items, range 0–5), Orgasm (3 items, range 0–5), Satisfaction (3 items, range 0–5), and Pain (3 items, range 0–5).23 Low scores represent worse sexual functioning. Total score ranges from 2 to 36. An FSFI total score of 26.55 was found to be the optimal cut-off score for differentiating women with and without sexual dysfunction. The FSFI has a good internal consistency (α = .80) and is able to reliably distinguish between different clinical groups and healthy controls. The psychometric properties of the Dutch translation are excellent.24 For women who are not sexually active, even though they may have a sexual partner, low FSFI total scores may be related to sexual inactivity rather than being indicative of sexual dysfunction.25 Therefore, for analyses with this measure, only scores of sexually active women were used.
The FSDS-R measures the extent to which women experience sexual distress.26 The questionnaire consists of 13 items with 5 answering categories ranging from 0 = never to 4 = always. Total score ranges from 0 to 52, with higher scores representing more sexual distress. The questionnaire has good internal consistency and discriminant validity. The “minimized error” optimum cutoff score on the FSDS-R was found to be ≥11, which effectively discriminated between women with hypoactive sexual desire disorder and women without sexual problems. The psychometric properties of the Dutch translation of the FSDS are excellent as well.24 In accordance with other authors, we defined sexual dysfunction as a FSFI total score <26.55 and a FSDS-R total score ≥11.23, 26
Fatigue was measured with the Multidimensional Fatigue Inventory (MFI). The current version contains 20 statements that cover different aspects of fatigue, organized into 5 scales: General fatigue, Physical fatigue, Reduced activity, Reduced motivation, and Mental fatigue. Each scale contains 4 items. The scales are balanced to reduce the influence of response tendencies as much as possible; each scale contains 2 items indicative of fatigue and 2 items contraindicative of fatigue. Indicative items (eg, “I tire easily”) are formulated in such a way that a high score suggests a high degree of fatigue. In case of contraindicative items (eg, “I feel fit”), a high total score indicates a low degree of fatigue.27 Following Schwarz et al and Hinz et al,28, 29 we used a cut-off of ≥53 to indicate clinically significant fatigue.
We assessed Health-Related Quality of Life (HRQL) using the Medical Outcomes Studies Short Form 36-item health survey (SF-36).30 The SF-36 is composed of 36 questions and standardized response choices, organized into 8 multi-item scales: ability to perform usual and vigorous activities (Physical function), ability to participate in social and occupational activities (Social function, Physical role function, and Emotional role function), moods (Mental health), amount of energy and pain (Vitality and pain), and current health (General health perception). All raw scale scores were linearly converted to a 0–100 scale, with higher scores indicating higher levels of functioning or well-being. The 8 dimensions of the SF-36 score were converted to standard scores on the basis of the scores of an age- and sex-matched representative reference sample of the Dutch population. Standard scores were calculated by dividing the difference between the patients’ SF-36 score and the mean score of the matched reference population by the SDs of the reference population. A standard score thus indicates how many SDs the observed SF-36 score falls below or above the score of the reference population. Consequently, scores of the reference population are set at 0. Because it is similar to the effect-size calculation, a mean standard score of 0.20 is considered to indicate a small deviation from the reference population31 and mean standard scores of 0.50 and 0.80 are considered to indicate moderate and large deviations from the reference population, respectively.32 Because 2 highly relevant health domains for HIV-infected people, health distress and cognitive functioning, are not covered by the SF-36, 2 additional scales of the Medical Outcomes Study HIV Health Survey (MOS-HIV) were used to measure HRQL (Health distress and Cognitive function). Subscale scores of the MOS-HIV range from 0 to 100, with higher scores indicating better health status.33
Depression was measured using the Beck Depression Inventory (BDI-II). The range for the BDI-II total score is 0–63, with higher scores indicating more depressive symptoms. We defined depression as BDI-II total score ≥20.34, 35
Statistical Analyses
For confirmation of a normal distribution, we used the Kolmogorov-Smirnov test and the Levene test for the equality of error variances. Differences between women with and without TI in baseline variables, plasma hormone levels, and the questionnaire scores were analyzed using χ2 or t-tests, or non-parametric tests (Mann-Whitney U tests) where appropriate. Associations between T and indices of sexual function were calculated using Pearson or Spearman correlations where appropriate. The level of significance was set at 5%. With a power of .80 and α set at 0.05, 64 subjects are required to detect differences with a medium effect size of 0.3. Because of the small sample size, P values between .05 and <.1 are reported as well. All statistical analyses were conducted using SPSS 24.0 software (SPSS Inc, Chicago, IL, USA).
Results
Participants
Of the 80 women who were invited to participate, 56 agreed (participation rate 70%). The reasons for declining participation were being too busy, not being interested in the subject, and relationship problems. 6 patients were excluded because questionnaire data were incomplete and 1 was excluded because the blood sample was missing and questionnaires were incomplete. Mean age of the remaining 49 participants was 39 years (range 24–70). 42 women were in a sexual relationship. Most of the women were using antiretroviral treatment (78%). Women had been diagnosed with HIV on average 8.75 years ago. There was no significant difference among the groups in duration of the HIV infection.
Prevalence of TI
Of the 49 women, 18 (37%) were found to have a TI as defined in the study procedures. Women with TI were no more likely to use cART than women without TI, and plasma viral load and CD4 cell counts were also comparable (Table 1). T levels have a diurnal variation and peak in the morning, hence, a morning specimen is preferable. Because we wanted to minimize the burden for participants, we took blood samples during regular outpatient visits. Therefore, for 3 women (7%), samples were taken after 11 AM. An analysis using samples taken before 11 AM only yielded a comparable prevalence of TI (33%). Average total T in women with TI was 0.48 nmol/L (mean) and 0.295 nmol/L (median), and that of women without TI was 1.23 nmol/L (mean) and 1.00 nmol/L (median). No other differences in baseline characteristics were found.
Table 1.
Baseline characteristics of women with and without TI
| Patient characteristics | Women with TI N =18 |
Women without TI N =31 |
P value |
|---|---|---|---|
| Age, y | 42 (11) | 38 (10) | 0.12 |
| Premenopausal, N (%) | 15 (83) | 26 (84) | 0.96 |
| Duration of HIV infection, y | 9.8 (4.8) | 8.2 (5.4) | 0.30 |
| Hormonal contraception, N (%) | 3 (17) | 2 (6) | 0.34 |
| Total T, nmol/L, mean Total T, nmol/L, median |
0.48 (0.27) 0.295 |
1.2 (0.79) 1.00 |
0.001 |
| cART use, N (%) | 16 (89) | 22 (72) | 0.18 |
| CD4 cell count, 10E6/L | 653 (376) | 572 (272) | 0.52 |
| Plasma viral load undetectable, N (%) | 16 (88) | 22 (71) | 0.15 |
| SHBG, nmol/L | 96.3 (75.2) | 75.9 (43.0) | 0.50 |
| Prolactin, μg/L | 12.0 (12.1) | 12.8 (9.5) | 0.51 |
cART = combination anti-retroviral therapy; T = testosterone; TI = testosterone insufficiency.
Differences Between Women With and Without TI
Sexual Dysfunction
Of all women, 41% had a combined FSFI total score <26.55 and FSDS-R score ≥11, indicative of distress-related sexual dysfunction. In the TI group, prevalence of distress-related sexual dysfunction was 44%, and in the non-TI group, the prevalence of distress-related sexual dysfunction was 38% (P > .70). FSFI total scores in women with and without TI were not significantly different (P > .30). However, women with TI tended to have lower sexual desire scores than the women without TI (t [40] = 1.825, P = .08; Table 2).
Table 2.
Sexual functioning and sexual distress in women with and without TI
| Sexual functioning | Women with TI N = 16 |
Women without TI N = 26 |
P | CI 95% |
|
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Desire | 2.88 (1.49) | 3.72 (1.39) | 0.08 | –1.88 | –0.04 |
| Arousal | 3.56 (2.37) | 4.02 (1.71) | 0.47 | –2.14 | 0.59 |
| Lubrication | 3.73 (2.65) | 4.50 (1.78) | 0.27 | –2.63 | 0.34 |
| Orgasm | 3.25 (2.60) | 3.88 (1.96) | 0.41 | –2.47 | 0.57 |
| Satisfaction | 3.80 (1.89) | 4.26 (1.39) | 0.37 | –1.78 | 0.41 |
| Pain | 3.68 (2.70) | 4.08 (2.25) | 0.62 | –2.36 | 0.88 |
| FSFI total score | 20.90 (12.70) | 24.37 (8.59) | 0.35 | –12.37 | 1.86 |
| FSDS-R total score | 14.75 (12.94) | 11.85 (9.31) | 0.40 | –4.48 | 9.58 |
| Sexual dysfunction, N (%)∗ | 7 (44%) | 10 (38%) | 0.74 | ||
FSFI = Female Sexual Function Index; FSDS-R = Female Sexual Distress Scale-Revised; TI = testosterone insufficiency.
FSFI <26.55 and FSDS-R >11.
Fatigue
The prevalence of clinically significant fatigue (MFI total score ≥53) in all women was 41%. Women in the TI group were significantly more clinically fatigued (61%) compared with the non-TI group (29%; χ2 [1] = 4.851, P = .03). Women with TI had a significantly higher level of physical fatigue than the women without TI (t [47] = 2.400, P = .02), and they had marginally higher levels of reduced activity and mental fatigue (.05 ≥ P ≤ .1; Table 3).
Table 3.
Fatigue, health status, and depression in women with and without TI
| Women with TI (N = 18) |
Women without TI (N = 31) |
P value | CI 95% |
||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Fatigue | |||||
| MFI subscales | |||||
| General fatigue | 13.22 (4.66) | 11.32 (5.15) | 0.17 | –1.10 | 4.87 |
| Physical fatigue | 12.11 (5.13) | 8.55 (4.94) | 0.02 | 0.58 | 6.55 |
| Reduced activity | 10.83 (4.82) | 8.58 (3.81) | 0.097 | –0.25 | 4.76 |
| Reduced motivation | 10.22 (4.51) | 8.16 (3.63) | 0.14 | –0.31 | 4.43 |
| Mental fatigue | 11.78 (5.09) | 9.19 (4.90) | 0.08 | –0.37 | 5.55 |
| MFI total score | 58.17 (20.33) | 45.81 (17.99) | 0.04 | 1.11 | 23.61 |
| Clinically significant fatigue (N, %)∗ | 11 (61%) | 9 (29%) | 0.03 | ||
| Health status | |||||
| SF-36 subscales | |||||
| Physical function | 75.83 (24.09) | 84.51 (21.34) | 0.06 | –22.02 | 4.66 |
| Social function | 65.97 (30.26) | 75.81 (24.78) | 0.27 | –25.86 | 6.20 |
| Physical role function | 44.44 (47.40) | 62.90 (41.27) | 0.20 | –44.44 | 7.52 |
| Emotional role function | 51.85 (46.05) | 58.06 (43.86) | 0.64 | –32.83 | 20.41 |
| Mental health | 66.35 (22.81) | 68.64 (18.91) | 0.71 | –14.65 | 10.07 |
| Vitality | 50.59 (25.43) | 60.84 (21.65) | 0.13 | –23.89 | 4.10 |
| Bodily pain | 71.72 (23.9) | 73.83 (25.17) | 0.76 | –16.85 | 12.62 |
| General health perception | 48.11 (23.10) | 59.13 (25.23) | 0.14 | –25.60 | 3.57 |
| MOS–HIV subscales | |||||
| Health distress | 57.11 (23.95) | 67.74 (19.15) | 0.09 | –23.16 | 1.90 |
| Cognitive function | 58.89 (22.66) | 71.29 (17.27) | 0.04 | –23.96 | –.84 |
| Depression | |||||
| BDI-II | |||||
| Total score BDI-II | 18.11 (16.65) | 11.81 (8.97) | 0.15 | –2.48 | 15.09 |
| Clinical depression, N (%)† | 7 (39%) | 5 (16%) | 0.07 | ||
BDI = Beck Depression Inventory; MFI = Multidimensional Fatigue Inventory; MOS-HIV = Medical Outcomes Study HIV Health Survey; SF-36 = Studies Short Form 36-item health survey; TI = testosterone insufficiency.
MFI total score ≥53.
BDI-II total score ≥20.
Health Status
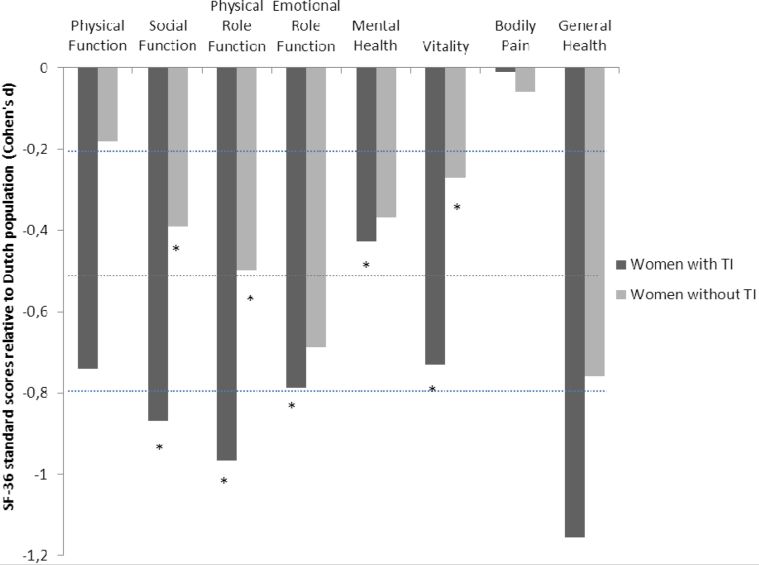
Women with TI tended to have worse physical function relative to women without TI (t [40] = 1.825, P = .06; Table 3). The other scales of the SF-36 showed no significant differences between the 2 groups. However, relative to an age-matched Dutch population, women with HIV had lower health scores (Figure 1). Women with TI had scores indicating worse health on 5 of 8 scales (Social function, Physical role function, Emotional role function, Mental health, and Vitality), whereas women without TI had scores indicating worse health on 3 of the scales (Social function, Physical role function, and Vitality).
Figure 1.
Short form 36-item health survey health standard scores for women with and without testosterone insufficiency. Standard scores <0 indicate that health is worse than that of an age-matched Dutch reference population. Dotted lines at –0.2, –0.5, and at –0.8 indicate a small, moderate, or large deviation from the reference population, respectively. Scores marked with an asterisk (*) indicate significant differences (P < .05) relative to the reference population.
Cognitive function as measured by MOS-HIV was significantly worse in the TI group relative to the women without TI (t [47] = –2.158, P = .04; Table 3). Health distress tended to be higher in the TI group (t [47] = –1.707, P = .09).
Depression
Of the entire group, 25% had BDI-II total scores >20, indicative of clinical depression. There were no significant mean differences between women with and without TI for the BDI-II total score (t [23] = 1.486, P = .15). However, more women in the TI group (39%) than in the non-TI group (16%) tended to be clinically depressed (χ2 [1] = 3.190, P = .07; Table 3).
Associations Between T and Sexual Function Indices
To further explore the association between T and sexual function indices, Pearson correlation coefficients were calculated between plasma T levels and the FSFI domain and total scores, and the FSDS-R total score. Correlations between plasma T and the FSFI and FSDS-R total scores were r = –0.14, P > .30 and r = 0.19, P > .20, respectively. Plasma T was only significantly correlated with the FSFI desire domain score (r = 0.30, P < .05).
Discussion
In this study, we investigated the prevalence of TI and its consequences in women living with HIV attending a general HIV outpatient clinic. The prevalence of TI in the investigated group was 37%, which seems somewhat lower than the prevalence of TI in HIV-positive women in other studies.12, 13, 14 This lower prevalence may be related to the present sample being healthier than in other studies, which included women with complications such as wasting and weight loss. Of course, the differences may also be related to differences in how TI was defined. Women with TI of the present study had a plasma viral load and CD4 cell counts comparable to those of women without TI, yet they were more likely to report reduced sexual desire, fatigue, impaired health, and depression than women without TI. These findings cannot be attributed to high rates of hormonal contraception use. Hormonal contraception is known to elevate SHBG levels and to decrease bioavailable T,36 but among our patients rates of hormonal contraception use were low.
In the Dutch population, the prevalence of sexual dysfunction among women is 27%.37 In this study, 41% of the women with HIV had a sexual dysfunction, 44% of those with TI, and 38% of those without TI. These results indicate that distress-related sexual dysfunction is a major problem in all HIV-infected women. However, HIV-infected women with TI may be at a greater risk of developing sexual desire complaints than HIV-infected women without TI. This conclusion is corroborated by women with TI reporting lower levels of sexual desire than the women without TI, as well as sexual desire levels being significantly related to plasma T levels.
The prevalence of clinically significant fatigue was 41% in the total group, and significantly higher (61%) in the TI group in comparison with the non-TI group (29%). Fatigue impairs physical functioning and compromises health status.38 This may contribute to the reported physical and mental fatigue and reduced activity in the women with TI. Compared with an age-matched Dutch population, the women with HIV perceived worse health status. Women with TI reported increased health distress and significantly worse cognitive function. Their decreased physical function might be related to the finding that muscle strength decreases when TI is present in HIV-infected women.12
Finally, the prevalence of depression in the entire investigated group (25%) was higher than the rate of 6.2% in unselected Dutch women between ages 18 and 65 years.39 In addition, the prevalence of clinical depression was significantly higher in the TI group.
These high rates of sexual dysfunction, fatigue, perceived lower health, and depression among HIV-positive women are in line with earlier findings. For instance, in a study in European HIV-positive women, 79% of whom were taking cART, <60% had FSFI scores within the dysfunctional range.40 Because partnership status was not assessed in that study, it is unknown whether low FSFI scores were related to sexual inactivity or to sexual dysfunction. A large U.S. study found that HIV-positive women had more sexual problems than HIV-negative women.41
Fatigue was very common in HIV patients before the introduction of cART, and it is still highly prevalent today despite viral suppression and good immune function.42 Of 297 HIV-positive Canadian women, 54% were depressed.43 The depressed sample had significantly poorer general health. We now show that these complaints, particularly fatigue and clinical depression, are even more prevalent in women with TI. It is not very likely that this can be indirectly explained by stage of HIV infection or severity of immune suppression, because women in the TI group had, on average, a comparable viral load and CD4 cell counts.
With respect to age, our sample was representative of the total population of HIV-infected women in the AUMC. The mean CD4 cell count of the women was high, indicating an absence of severe immune dysfunctions and related infectious complications. Therefore, the results cannot be extrapolated to women with more advanced diseases or to women in different settings, such as those in resource-poor countries.
A limitation of the present study is the limited power; in this explorative study, only a small number of patients were included. Hence, it is important to replicate this study using a larger sample size. In addition, measurement of total T using the gold standard represented by LC-MS/MS was not yet available when we started the study.44 Finally, the design of the study is cross sectional, and therefore no causal interferences can be made.
A strength of the study is the use of a stringent definition of sexual dysfunction (sexual symptoms that are distressing). Because partnership status was assessed, it is certain that the prevalence found in this study represents actual sexual dysfunction and not sexual inactivity.
Conclusion
This study showed that many HIV-positive women who present in a general HIV outpatient clinic have complaints of sexual dysfunction, fatigue, lower perceived physical or mental health, and depression, which can be aggravated by TI. These findings suggest that it is important to actively question women with HIV about their sexual, mental, and physical health, and to measure T levels in women with HIV who report problems in these domains. We recommend that in women with TI, T replacement be considered as a treatment option, because earlier studies suggested that T replacement may improve these complaints.10, 45 However, given that these complaints are also prevalent in HIV-positive women without TI, all HIV-positive women should be offered integrated and tailor-made multidisciplinary care addressing their sexual and mental health complaints.
Statement of authorship
Category 1
-
(a)Conception and Design
- Ellen Laan, Jan Prins, Rik van Lunsen, Pythia Nieuwkerk, Marian Nievaard-Boon
-
(b)Acquisition of Data
- Marian Nievaard-Boon
-
(c)Analysis and Interpretation of Data
- Ellen Laan, Pythia Nieuwkerk, Marian Nievaard-Boon
Category 2
-
(a)Drafting the Article
- Ellen Laan, Marian Nievaard-Boon
-
(b)Revising It for Intellectual Content
- Ellen Laan, Jan Prins, Rik van Lunsen, Marian Nievaard-Boon
Category 3
-
(a)Final Approval of the Completed Article
- Ellen Laan, Jan Prins, Rik van Lunsen, Pythia Nieuwkerk, Marian Nievaard-Boon
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
Funding: None.
References
- 1.Crum N.F., Furtek K.J., Olson P.E. A review of hypogonadism and erectile dysfunction among HIV-infected men during the pre- and post-HAART eras: Diagnosis, pathogenesis, and management. AIDS Patient Care STDs. 2005;19:655–671. doi: 10.1089/apc.2005.19.655. [DOI] [PubMed] [Google Scholar]
- 2.Wunder D.M., Fu C.A., Bersinger N.A. Androgen and gonadotropin patterns differ in HIV-1-infected men who develop lipoatrophy during antiretroviral therapy: A case–control study. HIV Med. 2008;9:427–432. doi: 10.1111/j.1468-1293.2008.00578.x. [DOI] [PubMed] [Google Scholar]
- 3.Rhoden E.L., Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med. 2004;29(350):482–492. doi: 10.1056/NEJMra022251. [DOI] [PubMed] [Google Scholar]
- 4.Rochira V., Guaraldi G. Hypogonadism in the HIV-infected man. Endocrinol Metab Clin North Am. 2014;43:709–730. doi: 10.1016/j.ecl.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Wong N., Levy M., Stephenson I. Hypogonadism in the HIV-infected man. Curr Treat Options Infect Dis. 2017;9:104–116. doi: 10.1007/s40506-017-0110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salmimies P., Kockott G., Pirke K.M. Effects of testosterone replacement on sexual behavior in hypogonadal men. Arch Sex Behav. 1982;11:345–353. doi: 10.1007/BF01541595. [DOI] [PubMed] [Google Scholar]
- 7.Grinspoon S., Corcoran C., Stanley T. Effects of hypogonadism and testosterone administration on depression indices in HIV-infected men. J Clin Endocrinol Metab. 2000;85:60–65. doi: 10.1210/jcem.85.1.6224. [DOI] [PubMed] [Google Scholar]
- 8.Dolan Looby S., Wilkie S., Aliabadi N. Effects of testosterone administration in human immunodeficiency virus-infected women with low weight: A randomized placebo-controlled study. Arch Intern Med. 2004;164:897–904. doi: 10.1001/archinte.164.8.897. [DOI] [PubMed] [Google Scholar]
- 9.Grinspoon S., Corcoran C., Stanley T. Mechanisms of androgen deficiency in human immunodeficiency virus-infected women with the wasting syndrome. J Clin Endocrinol Metab. 2001;86:4120–4126. doi: 10.1210/jcem.86.9.7843. [DOI] [PubMed] [Google Scholar]
- 10.Miller K., Corcoran C., Armstrong C. Transdermal testosterone administration in women with acquired immunodeficiency syndrome wasting: A pilot study. J Clin Endocrinol Metab. 1998;83:2717–2725. doi: 10.1210/jcem.83.8.5051. [DOI] [PubMed] [Google Scholar]
- 11.Grinspoon S., Corcoran C., Miller K. Body composition and endocrine function in women with acquired immunodeficiency syndrome wasting. J Clin Endocrinol Metab. 1997;82:1332–1337. doi: 10.1210/jcem.82.5.3907. [DOI] [PubMed] [Google Scholar]
- 12.Goggin K., Engelson E.S., Rabkin J.G. The relationship of mood, endocrine, and sexual disorders in human immunodeficiency virus positive (HIV+) women: An exploratory study. Psychosom Med. 1998;60:11–16. doi: 10.1097/00006842-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Bolour S., Braunstein G. Testosterone therapy in women: A review. Int J Impot Res. 2005;17:399–408. doi: 10.1038/sj.ijir.3901334. [DOI] [PubMed] [Google Scholar]
- 14.Huang J.S., Wilkie S.J., Dolan S. Reduced testosterone levels in human immunodeficiency virus-infected women with weight loss and low weight. Clin Infect Dis. 2003;36:499–506. doi: 10.1086/367642. [DOI] [PubMed] [Google Scholar]
- 15.Mylonakis E., Koutkia P., Grinspoon S. Diagnosis and treatment of androgen deficiency in human immunodeficiency virus-infected men and women. Clin Infect Dis. 2001;33:857–864. doi: 10.1086/322695. [DOI] [PubMed] [Google Scholar]
- 16.Bachman G., Bancroft J., Braunstein G. Female androgen insufficiency: The Princeton consensus statement on definition, classification, and assessment. Fertil Steril. 2002;77:660–665. doi: 10.1016/s0015-0282(02)02969-2. [DOI] [PubMed] [Google Scholar]
- 17.Coelingh Bennink H.J.T., Zimmerman Y., Laan E. Maintaining physiological testosterone levels by adding dehydroepiandrosterone to combined oral contraceptives: I. Endocrine effects. Contraception. 2017;96:322–329. doi: 10.1016/j.contraception.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Lunsen R.H.W., van Zimmerman Y., Coelingh Bennink H.J.T. Maintaining physiological testosterone levels by adding dehydroepiandrosterone to combined oral contraceptives: II. Effects on sexual function. Contraception. 2018;98:56–62. doi: 10.1016/j.contraception.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 19.Sodergard R., Backstrom T., Shanbhag V. Calculation of free and bound fractions of testosterone and estradiol-17 beta to human plasma proteins at body temperature. J Steroid Biochem. 1982;16:801–810. doi: 10.1016/0022-4731(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 20.Vermeulen A., Verdonck L., Kaufman J.M. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 21.Ly L.P., Handelsman D.J. Empirical estimation of free testosterone from testosterone and sex hormone-binding globulin immunoassays. Eur J Endocrinol. 2005;152:471–478. doi: 10.1530/eje.1.01844. [DOI] [PubMed] [Google Scholar]
- 22.American Psychiatric Association . American Psychiatric Association; Washington, DC: 2000. Diagnostic and statistical manual of mental disorders. 4th edition, text rev. [Google Scholar]
- 23.Wiegel M., Meston C., Rosen R. The Female Sexual Function Index (FSFI): Cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31:1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- 24.Kuile ter M.M., Brauer M., Laan E. The Female Sexual Function Index (FSFI) and the Female Sexual Distress Scale (FSDS): Psychometric properties within a Dutch population. J Sex Marital Ther. 2006;32:289–304. doi: 10.1080/00926230600666261. [DOI] [PubMed] [Google Scholar]
- 25.Meyer-Bahlburg H.F., Dolezal C. The Female Sexual Function Index: A methodological critique and suggestions for improvement. J Sex Marital Ther. 2007;33:217–224. doi: 10.1080/00926230701267852. [DOI] [PubMed] [Google Scholar]
- 26.Derogatis L., Clayton A., Lewis-D’Agostino D. Validation of the female sexual distress scale—Revised for assessing distress in women with hypoactive sexual desire disorder. J Sex Med. 2008;5:357–364. doi: 10.1111/j.1743-6109.2007.00672.x. [DOI] [PubMed] [Google Scholar]
- 27.Smets E.M.A., Garssen B., Bonke B. The Multidimensional Fatigue Inventory (MFI): Psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 28.Schwarz R., Krauss O., Hinz A. Fatigue in the general population. Onkol. 2003;26:140–144. doi: 10.1159/000069834. [DOI] [PubMed] [Google Scholar]
- 29.Hinz A., Fleischer M., Brähler E. Fatigue in patients with sarcoidosis, compared with the general population. Gen Hosp Psychiat. 2011;33:462–468. doi: 10.1016/j.genhosppsych.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Aaronson N.K., Muller M., Cohen P.D. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol. 1998;51:1055–1068. doi: 10.1016/s0895-4356(98)00097-3. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J. Erlbaum; Mahwah, NJ: 1988. Statistical power analysis for the behavioural sciences. [Google Scholar]
- 32.Lettinga K.D., Verbon A., Nieuwkerk P.T. Health-related quality of life and posttraumatic stress disorder among survivors of an outbreak of Legionnaires disease. Clin Infect Dis. 2002;35:11–17. doi: 10.1086/340738. [DOI] [PubMed] [Google Scholar]
- 33.Revicki D.A., Sorensen S., Wu A.W. Reliability and validity of physical and mental health summary scores from the Medical Outcomes Study HIV Health Survey. Med Care. 1998;36(2):126–137. doi: 10.1097/00005650-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Beck A.T., Steer A., Brown G.K. Psychological Corporation; San Antonio, TX: 1996. Beck Depression Index-II. [Google Scholar]
- 35.Does van der A.W.J. The Psychological Corporation/Swets Test Publishers; Lisse, The Netherlands: 2002. Handleiding bij de Nederlandse bewerking van de BDI–II (Manual of the Dutch version of the BDI-II) [Google Scholar]
- 36.Zimmerman Y., Eijkemans M.J.C., Coelingh Bennink H.J.T. The effect of combined oral contraception on testosterone levels in healthy women: A systematic review and meta-analysis. Hum Reprod Update. 2014;20:76–105. doi: 10.1093/humupd/dmt038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kedde H. Seksuele disfuncties in Nederland: Prevalentie en samenhangende factoren. Tijdschr Seksuol. 2012;36:98–108. [Google Scholar]
- 38.Rose L., Pugh L.C., Lears K. The fatigue experience: Persons with HIV infection. J Advanced Nurs. 1998;28:295–304. doi: 10.1046/j.1365-2648.1998.00789.x. [DOI] [PubMed] [Google Scholar]
- 39.Graaf de R., Have ten M., Dorsselaer van S. Design of the general population study NEMESIS-2: Netherlands Mental Health Survey and Incidence Study-2. Tijdschr Psychiatr. 2012;54:17–26. [PubMed] [Google Scholar]
- 40.Florence E., Schrooten W., Dreezen C. Prevalence and factors associated with sexual dysfunction among HIV-positive women in Europe. AIDS Care. 2004;16:550–557. doi: 10.1080/09540120410001716333. [DOI] [PubMed] [Google Scholar]
- 41.Wilson T.E., Girardin J.L., Schwartz R. HIV infection and women’s sexual functioning. J Acquir Immune Defic Syndr. 2010;54:360–367. doi: 10.1097/QAI.0b013e3181d01b14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Payne B.A.I., Hateley C.L., Ong E.L.C. HIV-associated fatigue in the era of highly active antiretroviral therapy: Novel biological mechanisms? HIV Med. 2013;14:247–251. doi: 10.1111/j.1468-1293.2012.01050.x. [DOI] [PubMed] [Google Scholar]
- 43.Williams P., Narciso L., Browne G. The prevalence, correlates, and costs of depression in people living with HIV/AIDS in Ontario: Implications for service directions. AIDS Educ Prev. 2005;17:119–130. doi: 10.1521/aeap.17.3.119.62903. [DOI] [PubMed] [Google Scholar]
- 44.Rosner W., Auchus R.J., Azziz R. Position statement: Utility, limitations, and pitfalls in measuring testosterone: An Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92:405–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- 45.Dolan Looby S.E., Collins M., Lee H. Effects of long-term testosterone administration in HIV-infected women: A randomized, placebo-controlled trial. AIDS. 2009;23:951–959. doi: 10.1097/QAD.0b013e3283299145. [DOI] [PMC free article] [PubMed] [Google Scholar]