Abstract
Introduction
Phosphodiesterase type 5 inhibitors (PDE5-Is) have an excellent efficacy and tolerability profile and remain the first-line choice for the treatment of erectile dysfunction (ED). However, ED is still an underdiagnosed and undertreated condition, and many men prematurely discontinue therapy with conventional dosage formulations despite successful intercourse.
Aim
To review the unmet needs and expectations of patients with ED and describe the latest pharmaceutical innovations in the field of PDE5-I formulations designed to address these needs, with particular reference to a new orodispersible film (ODF) formulation of the PDE5-I, sildenafil.
Methods
Online literature search in PubMed and the Cochrane Library.
Main Outcome Measure
To identify English-language publications relevant to the aims of the present review.
Results
Improved recognition and management of ED would enable the early diagnosis of underlying and comorbid conditions that contribute to ED, leading to improved patient health and health-related quality of life. To ensure successful outcomes and patient and partner satisfaction, the complex and personal issues that influence the patient’s needs and expectations regarding treatment for ED must be considered along with their personal experiences and preferences. Innovative drug delivery systems, including orally disintegrating formulations, have been developed as alternatives to conventional dosage forms with the aim of improving patient convenience and acceptability and enhancing compliance. These alternative formulations include the sildenafil ODF, which is designed to improve acceptance and compliance over conventional solid dosage forms and extend the treatment options for men with ED by offering a convenient and discrete dosage form of a drug with proven efficacy.
Conclusion
The sildenafil ODF is an example of an innovative dosage formulation for ED that can be used interchangeably with the conventional film-coated formulation to better address the needs and expectations of men with ED.
Jannini EA, Droupy S. Needs and Expectations of Patients with Erectile Dysfunction: An Update on Pharmacological Innovations in Phosphodiesterase Type 5 Inhibition with Focus on Sildenafil. Sex Med 2019;7:1–10.
Key Words: Compliance, Erectile Dysfunction, Orodispersible Dosage Formulations, Fast Dissolving Oral Films, Orodispersible Tablets, PDE5 Inhibitors
Introduction
A range of treatment options, both non-invasive and invasive, is available for the management of erectile dysfunction (ED). Treatment approaches include psychosexological strategies, intraurethral or intracavernosal alprostadil self-injections, vacuum-assisted erection devices, low-intensity extracorporeal shock wave treatment, and penile implants.1, 2, 3 However, oral pharmacological management with phosphodiesterase 5 inhibitors (PDE5-Is) remains the first-line treatment choice for ED because of the excellent efficacy and safety profile of PDE5-Is.1, 3, 4
Four PDE5-Is—sildenafil, tadalafil, vardenafil, and avanafil—are approved and marketed for ED in Europe and the United States (Table 1). There is substantial evidence for the efficacy and tolerability of these PDE5-Is for the treatment of ED related to organic and non-organic etiologies, and they have essentially comparable efficacy, with a better profile of safety and tolerability for avanafil.1, 3, 5, 6, 7 Sildenafil, the first-in-class selective PDE5-I, is a potent and selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific PDE5, an enzyme that promotes degradation of cGMP. Since its launch for the treatment of erectile dysfunction in 1998, a particularly strong evidence base for the efficacy and safety of sildenafil in the treatment of ED has been established, comprising the highest number of studies and published scientific papers in this drug class. For example, more than twice as many clinical studies assessing the use of sildenafil in men with ED are indexed in the MEDLINE database than for the next-most-studied PDE5-I, tadalafil. In a review of clinical trial data, it has been shown that ∼80% of men taking sildenafil in the dosage range of 25–100 mg report improvements in erections, compared with 25% of men taking a placebo, a highly statistically significant finding.8 The proven efficacy of sildenafil in ED is irrespective of age, baseline severity of the condition, or etiology of ED.1 Headache and flushing, both transient and of mild severity, are the most commonly reported side effects.1
Table 1.
Comparison of marketed PDE5-Is
| Property | Sildenafil | Vardenafil | Tadalafil | Avanafil |
|---|---|---|---|---|
| Year of first market authorization | 1998 | 2003 | 2003 | 2013 |
| Generation | First | First | First | Second |
| Absorption and elimination | Relatively rapid; medium half-life | Relatively rapid; medium half-life | Relatively slow; long half-life | Rapid; relatively long half-life |
| Formulations available | Film-coated tablet, ODT, ODF | Film-coated tablet, ODT | Film-coated tablet | Film-coated tablet |
| Marketed dosages | ||||
| Conventional tablets | 25, 50, 100 mg | 5, 10, 20 mg | 2.5, 5, 10, 20 mg | 50, 100, 200 mg |
| ODF | 25, 50, 75, 100 mg | 10 mg | ||
| Common adverse reactions | Headache, flushing, dyspepsia, nasal congestion, naso-pharyngitis, visual abnormalities | Headache, flushing, dyspepsia, nasal congestion, naso-pharyngitis, visual abnormalities | Headache, flushing, dyspepsia, nasal congestion, back pain, naso-pharyngitis, visual abnormalities | Headache, flushing, dyspepsia, nasal congestion, naso-pharyngitis |
ODF = orodispersible film; ODT = orodispersible tablet; PDE5-Is = phosphodiesterase type 5 inhibitors.
Although the efficacy of PDE5-Is has been thoroughly demonstrated, the available evidence shows that a high percentage of patients discontinue this pharmacological therapy prematurely because they are not completely satisfied with the prescribed therapy despite successful intercourse.1, 5, 9, 10, 11, 12, 13 Data show that ≤50% of men stop their treatment with first-generation PDE5-Is (sildenafil, tadalafil, vardenafil) in traditional formulations within a year, although data are limited for the second-generation PDE5-I, avanafil, which was designed to overcome some of the limitations of first-generation agents.5, 6, 13
Patient satisfaction with ED treatment is a complex and personal issue that contributes to underdiagnosis and undertreatment.9, 14 It is essential that the patient’s needs and expectations regarding treatment for ED, together with their personal experiences and preferences, are taken into consideration to ensure successful outcomes and satisfaction regarding the pharmacological therapy.1, 3
Innovative drug delivery systems, including orally disintegrating formulations, have been developed as an alternative to conventional marketed dosage formulations to improve patient convenience and acceptability and enhance compliance.15, 16, 17, 18 Sildenafil orodispersible film (ODF) is one of the novel formulations that have been recently made available. Sildenafil ODF disintegrates in the mouth without the need for water and could offer several advantages over conventional film-coated tablet formulations for patients with ED.19, 20, 21, 22, 23
This review discusses the unmet needs and expectations of patients with ED and describes the latest pharmaceutical innovations in the field of PDE5-I formulations designed to address these needs, with a focus on the sildenafil ODF.
Search Strategy
An online PubMed and the Cochrane Library literature search was conducted to identify English language publications from inception to February 2018 using combinations of the terms [“phosphodiesterase type 5 inhibitor,” “phosphodiesterase 5 inhibitor,” “PDE5 inhibitor,” “sildenafil,” “vardenafil,” “tadalafil,” “avanafil,” “erectile dysfunction,” “unmet needs,” “patient expectations,” “patient satisfaction,” “compliance,” “drug delivery,” “innovative dosage formulations,” “novel drug delivery,” “buccal delivery,” “orally dispersible,” “orodispersible,” “orally disintegrating,” “ODT,” “ODF”]. Other relevant articles were identified by manually reviewing the reference lists of selected articles. A supplementary search to identify recently published articles was conducted at the time of writing the manuscript.
Rationale for cGMP Inhibition
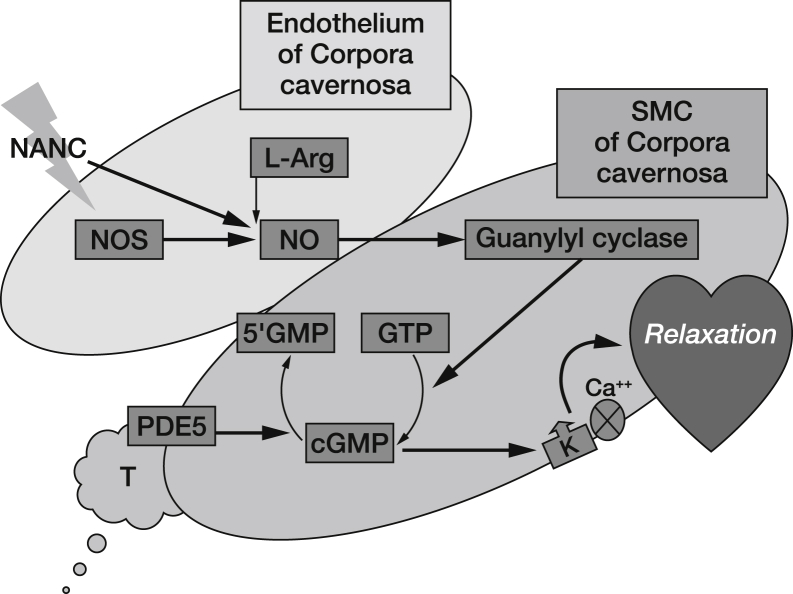
The nitric oxide (NO)–cGMP signaling pathway is a key physiological mediator of smooth muscle function through activation of intracellular protein kinases and modulation of intracellular calcium.24, 25 cGMP is a cyclic nucleotide derived from guanosine triphosphate that, along with other molecules such as cyclic adenosine monophosphate, regulates the function of the smooth muscle cells that encircle blood vessels and sinusoidal spaces. NO has an established role in the physiology of erections; release of NO stimulates the production of cGMP and lowers intracellular calcium levels, triggering the relaxation of arterial and trabecular smooth muscle.24 The resulting arterial dilatation and venous constriction increase the accumulation of blood in the corpus cavernosum, facilitating penile erection.24, 25
The mammalian cyclic nucleotide phosphodiesterases are a superfamily of enzymes that consist of 11 subfamilies (PDE1–PDE11) so far characterized. The predominant PDE present in the corpora cavernosa is the cGMP-specific PDE5; it has been hypothesized that degradation of cGMP by process of hydrolyzation allows penile smooth muscle cells to remain in the contracted state for extended periods of time.26, 27, 28 In ED, when this process is impeded by peripheral vascular diseases, diabetes, dyslipidemia, or other contributors to endothelial dysfunction, PDE5-Is such as sildenafil prevent the catalytic degradation of cGMP by PDE5, potentiating increases in pro-erectile cGMP and exerting a relaxant effect on the corpora cavernosa.24, 25 A representation of how PDE5 inhibition increases levels of cGMP in the penile cell, potentiating penile erection, is shown in Figure 1.
Figure 1.
PDE5 inhibition increases levels of cGMP in the penile cell, potentiating penile erection. GTP = guanosine triphosphate; NANC = nonadrenergic noncholinergic nerve fibers; NO = nitric oxide; NOS = nitric oxide synthase; SMC = smooth muscle cells.
Of interest, advances in the understanding of cGMP have resulted in successful targeting of the NO-cGMP pathway in other disease states related to vascular and bronchial smooth muscle, including pulmonary arterial hypertension and lower urinary tract symptoms (reviewed in Das et al,29 Francis et al,25 Hutchings et al,30 Rybalkin et al,31 and Uthayathas et al32). Furthermore, PDE5-Is may have potential cardioprotective and anticancer properties.29, 30
Which PDE5-I?
Data from head-to-head clinical trials of PDE5-Is are lacking, and data from available comparative studies may be of limited value owing to design flaws, including nonequivalent dose comparisons, inadequate treatment duration, variable inclusion and exclusion criteria, and biased dosing instructions.33 The process of penile erection is a complex interplay of tissular, vascular, neurohormonal, and biopsychosocial factors which interweave endocrine, cardiovascular, and neurologic conditions; diseases of the urinary tract; problems with drugs and alcohol; social stressors; and psychosocial and partner interactions. Therefore, criteria to effectively compare the effectiveness of agents used in ED should, according to expert opinion, consider both objective and subjective parameters.33
To date, no specific randomized clinical trials comparing sildenafil, tadalafil, vardenafil, and avanafil have been published. The multicenter ENDOTRIAL Study was designed with an approach reflecting the use of sildenafil 50-mg, sildenafil 100-mg, tadalafil 20-mg, and vardenafil 20-mg treatment regimens in everyday real-life settings.34 The primary outcome measure was an improvement in the erectile function domains of the abridged International Index of Erectile Function (IIEF5+1) from baseline to endpoint (week 8 of treatment). Secondary objectives included analysis of penile flow parameters, specifically peak-systolic velocities (PSVs), end-diastolic velocities, and resistive index (RI), and the percentage of men with normal penile hemodynamic parameters after each treatment.
In the 4 treatment groups, there was a statistically significant improvement in IIEF5+1 scores from baseline to endpoint; analysis of regression coefficients confirmed that the treatments were equivalent according to IIEF and penile flow parameters.34 However, there was a dose-dependent amelioration in penile flow parameters with sildenafil that was not seen with tadalafil or vardenafil. In men taking sildenafil 50 mg, PSV improved from baseline by 8.43 cm per second (P = .010), and PSV and RI improved by 7.0 cm per second (P = .027) and 0.075 (P = .034), respectively, in men taking sildenafil 100 mg. A similar proportion of men in each group had normal IIEF5+1 after treatment. Although the study had a number of limitations, the findings suggest that sildenafil, but not tadalafil or vardenafil, routinely used in an on-demand regimen, may improve penile vascular performance in a manner that offers a clinical advantage to the patient. Certainly, the findings justify further evaluation in a higher number of patients and for a longer duration of treatment. Of the 3 main randomized controlled placebo trials published on erectile rehabilitation using PDE5-Is after nerve-sparing radical prostatectomy, the study from Padma-Nathan et al35 using sildenafil is the only one positively showing an improvement of spontaneous erections versus placebo after 9 months of sildenafil treatment and a washout period. The REACTT36 and REINVENT37 studies used the same methodology but did not show any ability of tadalafil or vardenafil to improve spontaneous erections after nerve-sparing radical prostatectomy. Furthermore, early penile rehabilitation with sildenafil immediately after urethral catheter removal has been shown to significantly improved full erectile function recovery over 12 months following nerve-sparing radical prostatectomy, compared with delayed rehabilitation with the same regimen starting 3 months after surgery.38 These data support the fact that sildenafil routinely used may improve penile performance in a rehabilitation objective.
Unmet Needs and Expectations
Despite the widespread public awareness of ED and the availability of effective treatment with PDE5-Is, a high proportion of men with ED delay, or avoid, discussing their condition with their doctor, with the result that ED is still underdiagnosed and undertreated.39 This was confirmed by recent data from a large National Health and Wellness Survey (NHWS) conducted in France, Germany, Italy, Spain, and the United Kingdom that showed the extent of ED underdiagnosis and undertreatment across Europe.9 52% of men with ED, regardless of age group, had not discussed their condition with a physician, and <50% of men surveyed had a close enough alliance with their physician that would support the open dialogue essential to ensuring patient satisfaction and compliance with therapeutic decisions. Among those men who had consulted their physician about ED, treatment rates remained low, with the physician seldom recommending a therapy.9 Given the accepted negative effect of ED on health-related quality of life and work productivity, these findings suggest deficiencies in the quality of the patient/physician relationship that are a barrier to the reducing the psychological and social burden of ED.
Men with ED show a strong preference toward pharmacological management with oral PDE5-Is compared with other methods, although there is no clearly established and robust evidence for a preference between different PDE5-Is.40, 41, 42, 43, 44 However, the high percentage of patients who prematurely discontinue pharmacological therapy for ED, even during successful treatment, is of concern.5, 10, 11, 12 Moreover, some studies have also shown that a significant percentage of men are unsatisfied with their actual therapy and alternate between several available drugs.11 The reasons for lack of satisfaction with or discontinuation of PDE5-Is are diverse and include perceived non-effectiveness, psychological factors (eg, fear, anxiety, masculinity issues, negative emotions), relationship issues, recovery of erectile function, cost, reluctance for chronic medication-dependent intercourse, and concerns about side effects or long-term safety.11, 12
A better understanding of how an ED treatment meets the rational and realistic expectations of the patient and their sexual partner is essential to ensure an optimal treatment strategy that satisfies the needs and demands on modern ED management toward restoring a more satisfying sexual relationship. Therefore, it is important to identify factors that improve patient acceptance, adherence, and satisfaction with therapy.
A large observational study of 1,567 men with ED wishing to initiate or change ED treatment with tadalafil found that expectations important to the patients were efficient and sustained maintenance of erection until completion of intercourse (>92% of patients), confidence and satisfaction of the partner and naturalness of intercourse (>84%), and rapid and sustained effect of the drug (>75%).45 Higher effectiveness, a supportive partner and a good relationship, and good drug tolerance increased treatment satisfaction, which was associated with treatment continuation.45
Thus, when the basic functional aspects of achieving and maintaining an erection are reached, additional factors, such as spontaneity and naturalness of sexual relationship and pleasing and acceptance of the treatment by the partner become more important factors that contribute to overall improvement of sexual satisfaction and adherence to ED treatment.46 In support of this, data from the NHWS survey of men with ED conducted in Europe provides evidence that “on-demand” use of ED treatment better fits with the needs and preferences of men and their partners and reflects a reluctance for chronic medicalization to solve the problem of ED.9 Furthermore, men with ED value a treatment with a relatively rapid onset of action and a duration of effect that diminishes naturally over a few hours, corresponding as much as possible to the normal physiology and naturalness of the sexual act, together with a convenient and discreet modality of use.46 Conversely, the majority of men consider a duration of action of more than 12 hours to be too long.46
The ideal therapy aims to be a treatment that responds as much as possible to the normal psychology and naturalness of the relationship. Furthermore, such considerations provide an insight into the key pharmacokinetic properties together with the discreet mode of intake expected of an ideal oral therapy for ED. In fact, Jannini et al9 demonstrated that when only filmcoated PDE5-I were available, the stigma of a well-recognizable pill for ED was considered a major issue against the use of these drugs.9 This suggested the need for a discreet version of the effective PDE5-Is.
Pharmacological Approaches to Meeting Unmet Needs
Although a number of novel PDE5 and other molecules under development may hold promise for the treatment of ED,2, 47 in the medium term, new formulations designed to overcome some of the limitations of existing treatments by offering improved discretion and flexibility are likely to contribute most effectively to reducing the above-mentioned stigma that is often still associated with ED, and encourage acceptance of and continuation with therapy.
The ultimate benefits of improved recognition and management of ED would be enabling the early diagnosis of underlying and comorbid conditions that contribute to ED, leading to improved patient health and health-related quality of life (HR-QoL) outcomes by changing lifestyle.48
Although no single ED therapy can meet all requirements for efficacy and patient satisfaction, PDE5-Is remain the first-line treatment of choice for ED,1, 3 and formulations tailored to fit the clinical characteristics of the patient with ED and chosen in consultation to meet the needs and expectations of men and their partners will play a key role in the effective future management of ED.
Overview of Latest Developments in PDE5-Is
Novel approaches for the treatment of ED are currently being investigated, including stem cell therapy, molecules targeting alternative vasoactive pathways, trophic factors, gene therapy, and new technologies such as penile vibratory stimulation, external penile support devices, internal pudendal artery stenting, extracorporeal low-intensity shockwave therapy, endovascular revascularization, and tissue engineering. However, PDE5 remains the most understood molecular target in the effective pharmacological management of ED.1, 2, 47 Accordingly, innovative dosage formulations of PDE5-Is are being developed that are designed to optimize efficacy, safety and patient acceptability, convenience, and compliance.
Orodispersible Dosage Systems
ODFs and orodispersible tablets (ODTs) are innovative drug delivery systems that are formulated to disperse and disintegrate rapidly when placed in the mouth, without the need for water.49, 50, 51 They offer a discreet and convenient mode of administration, without risk of choking or difficulty in swallowing that may limit compliance with conventional tablets or capsules in some patients, and are of particular relevance in children or special patient populations such as the elderly with comorbid conditions (eg, renal impairment or congestive heart failure), and patients with dysphasia. Furthermore, their convenience, together with superior dosing accuracy and rapid onset of action, have resulted in strong patient preference for orodispersible dosage formulations across a wide range of patient groups, with research indicating that the majority of patients prefer orally disintegrating dosage forms over conventional solid oral dosage forms.52, 53, 54
A detailed description of the manufacturing technologies used in the production of orodispersible dosage formulations is beyond the scope of this article. However, the technologies have been comprehensively described by Bala et al,20 Goel et al,15 Hoffmann et al,49 Karki et al,22 and Irfan et al.50
ODT formulations, compounded with an appropriate disintegrating agent and using highly water-soluble excipients, are designed to disintegrate rapidly in the mouth following entry of water into a porous tablet matrix.15, 20, 50 The performance of ODTs is dependent on manufacturing processes that maximize the porous structure of the tablet matrix, incorporating a disintegrating agent, and the use of highly water-soluble excipients to allow quick ingress of water, facilitating rapid oral disintegration.15 The various manufacturing technologies used in the production of ODTs fall under the broad classifications of lyophilized systems and compressed tablet–based systems. A balance between fast disintegration and fragility must be established to minimize problems of packaging, storage, handling, and administration. In brief, lyophilization or freeze drying, a technology commonly used in the manufacture of ODTs, involves molding tablet-shaped units of a drug in suspension or solution together with other structural excipients, followed by freezing and lyophilization in the pack or mold.15, 20 The very high porosity of the resulting ODTs allows rapid water or saliva penetration and rapid disintegration. Compressed tablet–based systems use standard tableting technology by direct compression of the active pharmaceutical ingredient and excipients designed to achieve the required disintegration performance and packaging requirements.
In more recent years, ODFs have been developed with the aim to improve patients’ compliance and acceptability over conventional solid dosing forms. The manufacturing of thin film ODFs has evolved from the technology for producing transdermal patches, a process that is less expensive than lyophilization, resulting in a stable, thin and flexible dosage form, with high mechanical strength, that can be manufactured in a range of sizes and shapes that are easily transported and stored.15, 20, 55
ODFs share the accurate dosage and ease of administration of conventional tablet formulations with the ease of swallowing and rapid bioavailability due to the reduced hepatic first-pass effect of liquid dosing forms.50, 51 They offer a convenient and accurate dosage form that rapidly disintegrates and dissolves in the oral cavity.20, 22, 49, 50, 51 ODFs have a number of advantages over ODTs, including lack of friability, no risk of suffocation or choking during administration, and the ability to carry individual strips without requiring the secondary container.22, 50 The film is hydrated by saliva without the need for water when placed on the tongue or in the oral cavity, rapidly disintegrating to release the medication for oromucosal and/or systemic absorption.
Table 2 summarizes the classification of the technologies used in orodispersible dosage formulations. Table 3 presents key characteristics of conventional solid dosage forms and orodispersible formulations.
Table 2.
Classification of fast dissolving technologies for the production of orodispersible dosage formulations
| Property | ODT |
ODF | |
|---|---|---|---|
| Lyophilized system | Compressed tablet–based system | ||
| Composition | Solution or suspension of drug with excipients | Active pharmaceutical ingredient with super-disintegrants | Hydrophilic polymers with drug and other excipients |
| Technology used | Lyophilization | Direct compression | Solvent casting, hot melt extrusion |
| Characteristics | High porosity that allows rapid water or saliva penetration and disintegration | Different levels of hardness and friability that results in varying disintegration and packaging needs | Large surface area leads to rapid disintegration |
| Packaging | Blister pack | High-density polyethylene bottles | Blister cards with multi-units |
ODF = orodispersible film; ODT = orodispersible tablet.
Table adapted with permission from Wolters Kluwer, from Bala et al.20
Table 3.
Key characteristics of orodispersible dosage formulations
| ODF | ODT |
|---|---|
| Hydrated by saliva without the need for water. | Some do not need to be taken with water. |
| No choking risk compared with conventional solid dosage forms. | Reduced risk of choking. |
| Convenient and easy administration. | Instruction not to chew or swallow must be given. |
| Dose accuracy compared with liquid dosage forms. | Dose accuracy compared with liquid dosage forms. |
| Convenience of single-dose sachet or multi-unit film packaging. | Multi-dose blister packs or bottles. |
| Large surface area that allows rapid disintegration and dissolution in the buccal cavity. | Take longer to disintegrate than thin film preparations. |
| Thin, flexible stable; can be manufactured in a range of shapes and sizes. | May be fragile and brittle. |
| Easily transported and stored. | More stringent storage and transportation requirements compared with ODFs. |
| Reduces hepatic first-pass effect when the active substance absorption occurs mainly through the oral mucosae. | Hepatic first-pass effect may still be a consideration. |
| Patient preference compared with conventional solid dosage forms. | Patient preference compared with conventional solid dosage forms. |
| Improved compliance in special patient populations. | Improved compliance in special patient populations. |
| Require moisture-protecting packaging. | Issues of fragility and friability during manufacture, storage, handling, and administration. |
| Some technical challenges in achieving dose uniformity. | More complicated and expensive manufacturing processes compared with ODFs. |
| Taste masking may be necessary to ensure patient acceptability and compliance. | Taste masking may be necessary to ensure patient acceptability and compliance. |
| High doses cannot be incorporated. | More flexible dose loading capacity. |
ODF = orodispersible film; ODT = orodispersible tablet.
Orodispersible PDE5-Is
A number of drugs have been successfully formulated as ODTs, including ODT formulations of the PDE5-Is vardenafil and sildenafil that disintegrate in the mouth without the need for water.
A rapidly disintegrating ODT formulation of vardenafil 10 mg has been developed and is marketed in Europe, the United States, and other countries. The efficacy and safety of the vardenafil 10-mg ODT formulation administered on demand were established in the POTENT I and POTENT II randomized, placebo-controlled trials.17, 18, 56 In both studies, vardenafil ODT therapy was statistically significantly superior to placebo for all primary and secondary measures of erectile function, regardless of age, baseline severity of ED, or underlying comorbid condition.18 Treatment-emergent adverse events were mostly mild to moderate in severity and were comparable in incidence and type with those associated with the film-coated tablet formulation.
In general, the ODT formulation of vardenafil (in the unique, fixed dosage of 10 mg, which does not correspond to the available dosages of the same drug in the traditional form) had a similar pharmacokinetic profile to the marketed vardenafil film-coated tablet.57 However, the ODT has significantly greater bioavailability and is not interchangeable with the conventional film-coated formulation. This effect was considered related to significant drug absorption via the oral mucosa with vardenafil ODT.57
An ODT formulation of sildenafil citrate, designed to offer an alternative dosage form to the marketed film-coated tablet, has been developed. A randomized, open-label, 3-treatment, 3-period, single-dose crossover bioequivalence study in healthy Asian male subjects found that the ODT formulation administered without water was bioequivalent to the marketed sildenafil film-coated tablet, suggesting minimal buccal absorption from the ODT formulation in the short time it remains in the mouth.58 The results of the food-effect study showed that the sildenafil ODT should be taken on an empty stomach.
ODF formulations of PDE5-Is designed to dissolve rapidly in the mouth are expected to have a number of advantages over conventional dosage forms, enhancing sexual health and supporting a sense of psychological well-being in patients and their partners. A novel ODF of sildenafil (IBSA Institut Biochimique SA, Pambio-Noranco, Switzerland) is approved in Europe and is available as 25, 50, 75, or 100 mg of sildenafil (as citrate), which allows for precisely tailored therapy for the individual patient. Notably, the new dose of 75 mg, intermediate between the most popular (50 mg) and the most powerful (100 mg), is likely to raise high clinical interest, being available for the first time.
Each sildenafil 100-mg ODF contains 140.4 mg of sildenafil citrate, equivalent to 100 mg of sildenafil, in the form of a rectangular, flexible, opaque light azure-blue film 40 × 45 mm. Other ingredients comprise the pharmaceutical excipients maltodextrin, glycerol, polysorbate 20, propylene glycol monocaprylate, polyvinyl acetate dispersion, flavors, sucralose, titanium dioxide, and indigotine.23
The pharmacokinetics of the sildenafil ODF formulation are similar to that of the conventional film-coated tablet and meet the criterion of assumed bioequivalence; the orodispersible formulation could be used interchangeably with the marketed conventional film-coated tablet.23, 59 In the recent sildenafil ODF bioequivalence study, the mean plasma concentration-time profiles up to 24 hours for the sildenafil 100-mg ODF and its metabolite, N-desmethyl-sildenafil, after single dose administration were nearly superimposable with those of the film-coated tablet.23 The test for bioequivalence was fully satisfied for both sildenafil and its metabolite in terms of the rate and extent of bioavailability (peak plasma concentration [Cmax] area under the curve [AUC] from administration to last observed concentration time [AUC0-t] and AUC extrapolated to infinity [AUC0-∞]). There were no new safety concerns with the ODF formulation; adverse events, which were of mild to moderate severity, occurred at similar rates for the ODF and the reference film-coated tablet formulations.
In the first clinical trial to assess the efficacy of the new sildenafil ODF formulation, sildenafil 75-mg ODF was compared with the marketed sildenafil 100-mg film-coated tablet.60 Both formulations were safe and effective, and no additional side effects were identified for the ODF formulation, thus suggesting that oral film can be used interchangeably with the conventional oral forms.
Discussion
Erectile dysfunction remains an underdiagnosed and undertreated condition that imparts a high physical, psychosocial, and relationship burden with associated impairment of HR-QoL and work productivity and activity.9, 48, 61, 62, 63, 64 Conversely, interventions that ameliorate sexual function have the potential to improve patient health and HR-QoL.64, 65 Furthermore, there is increasing evidence that ED, and sexual dysfunction in general, may be an efficient gender-dependent predictor of overall systemic health and an early marker of chronic non-communicable diseases (NCDs).66 This is due to the strong biological basis for the developmental origins of health and chronic disease that suggests the value of identifying appropriate biomarkers to better understand chronic disease processes and to better evaluate and monitor risk factors for interventions aimed at preventing or minimizing chronic disease.66 This opens up the possibility of a new systems medicine approach to understanding and managing, at the same time with shared strategies on lifestyles, both NCDs and sexual dysfunction. This can be obtained by incorporating an innovative, integrative, interdisciplinary approach to the management of NCDs that acknowledges the complex association between ED and chronic disease. In the future, sexual dysfunction, as a powerful marker of systemic health, may be recognized as an essential component in an effective and integrated approach to the diagnosis, treatment, and prevention of the current dramatic epidemics of NCDs.66
Having clearly established that the reduction of the risk factors related to lifestyle is to be considered the first aim for doctors managing ED,48 PDE5-Is have an established place as first-line pharmacological therapy for the majority of patients with ED because of their efficacy and favorable safety profile. However, despite the potency of the available drugs, ED is a unique condition that can be challenging to manage effectively. Poor compliance and low satisfaction levels with conventional dosage forms of PDE5-Is are recognized issues that need to be addressed. Although erectile response and potential side effects are important considerations, how well the treatment meets the needs and expectations of the patient and enhances the dynamics of the relationship are essential to the long-term success of therapy.45
A desire to restore the spontaneity and naturalness of the couple relationship and a reluctance for chronic medicalization are among unmet needs identified for reestablishing a more satisfying sexual relationship. To find an optimal treatment choice tailored to individual needs, it is crucial that the wishes, expectations, and fears of the patient and the sexual partner are considered. As such, the development of delivery platforms that are not only safe and effective but also attractive to the patient is essential. Speed of onset and duration of pharmacological effect are undoubtedly important (although the former much more than the latter) but not in themselves sufficient to ensure that an ED treatment is well accepted; moreover, the success of therapy depends on how well the cure meets the expectations and psychological well-being of the patient and partner. There is, in fact, considerable variance in the needs and expectations of patients. Involving the patient and, preferably, his sexual partner in the decision-making process and providing relevant information about the therapy options and their expected benefits, possible adverse effects, or complications is essential in deciding on the most appropriate treatment approach for ED. To this end, physicians should learn the patient’s psychosocial profile and work with the patient to ensure that his special needs and expectations are acknowledged and met. Follow-up evaluation to review the success of the intervention, identify any side effects, discuss the patient’s satisfaction with treatment, and revisit their expectations is essential to ensure the optimal long-term treatment strategy.
The risk/benefit profile of sildenafil has been established over >20 years of clinical experience. It has a proven role as an effective and readily manageable treatment option for ED in men, regardless of comorbidities such as hypertension, depression, congestive heart failure, stable coronary artery disease, diabetes mellitus, traumatic spinal cord injury, multiple sclerosis, obstructive sleep apnea, renal dysfunction, prostate cancer, Parkinson’s disease, and traumatic stress disorders.67
Orodispersible dosage forms, in particular ODFs, have been developed to obtain a high level of acceptance and compliance across a range of patient populations and medications. Strong patient preference for orodispersible dosage formulations over conventional solid dosage forms further supports recent developments in PDE5-Is for the treatment of ED. The development of orodispersible formulations of PDE5-Is extends the treatment options for men with ED by offering convenient dosage forms that may better meet the needs and expectations of men and their partners. An individually tailored treatment plan formulated jointly by the physician and patient and taking into consideration the concerns, expectations, and preferences of the patient, ideally with the involvement of the patient’s partner, is key to identifying factors that improve patient acceptance, adherence, and satisfaction with therapy.
Conclusion
Sildenafil ODF, recently marketed as the first sildenafil ODF in Europe, is an example of an innovative dosage form for the treatment of ED that can be used interchangeably with the conventional film-coated formulation to better address the needs and expectations of men with ED.
Statement of authorship
Category 1
-
(a)Conception and Design
- Emmanuele A. Jannini; Stéphane Droupy
-
(b)Acquisition of Data
- Emmanuele A. Jannini; Stéphane Droupy
-
(c)Analysis and Interpretation of Data
- Emmanuele A. Jannini; Stéphane Droupy
Category 2
-
(a)Drafting the Article
- Emmanuele A. Jannini; Stéphane Droupy
-
(b)Revising It for Intellectual Content
- Emmanuele A. Jannini; Stéphane Droupy
Category 3
-
(a)Final Approval of the Completed Article
- Emmanuele A. Jannini; Stéphane Droupy
Acknowledgments
We thank Ray Hill, an independent medical writer acting on behalf of Springer Healthcare Communications, who provided medical writing support funded by IBSA Institut Biochimique SA, Switzerland.
Footnotes
Conflict of interest: E.A.J. is, or has recently been, a paid consultant and/or paid speaker for Bayer, IBSA, Lundbeck, Menarini, Otsuka, Pfizer, and Shionogi. S.D. has served as a consultant and has received speaker honoraria in the field of erectile dysfunction for Bayer Healthcare, Eli Lilly, Pfizer, Majorelle, Genevrier, Novelator, and Boston Scientific.
Funding: Medical writing support for this article was funded by IBSA Institut Biochimique SA, Switzerland.
References
- 1.Hatzimouratidis K., Eardley I., Giuliano F. EAU Guidelines on male sexual dysfunction: Erectile dysfunction and premature ejaculation. https://uroweb.org/guideline/male-sexual-dysfunction/ Updated February 19, 2018. Available at: Accessed July 19, 2018. [DOI] [PubMed]
- 2.Le B.V., Burnett A.L. The future of erectile dysfunction therapy II: Novel pharmacotherapy and innovative technology. In: Lipshultz L.I., Pastuszak A.W., Goldstein A.T., Giraldi A., Perelman M.A., editors. Management of Sexual Dysfunction in Men and Women. Springer; New York: 2016. pp. 109–121. [Google Scholar]
- 3.Burnett A.L., Nehra A., Breau R.H. Erectile dysfunction: AUA guideline. J Urol. 2018 doi: 10.1016/j.juro.2018.05.004. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Hatzimouratidis K., Salonia A., Adaikan G. Pharmacotherapy for erectile dysfunction: Recommendations from the Fourth International Consultation for Sexual Medicine (ICSM 2015) J Sex Med. 2016;13:465–488. doi: 10.1016/j.jsxm.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Corona G., Rastrelli G., Burri A. First-generation phosphodiesterase type 5 inhibitors dropout: A comprehensive review and meta-analysis. Andrology. 2016;4:1002–1009. doi: 10.1111/andr.12255. [DOI] [PubMed] [Google Scholar]
- 6.Limoncin E., Gravina G.L., Corona G. Erectile function recovery in men treated with phosphodiesterase type 5 inhibitor administration after bilateral nerve-sparing radical prostatectomy: A systematic review of placebo-controlled randomized trials with trial sequential analysis. Andrology. 2017;5:863–872. doi: 10.1111/andr.12403. [DOI] [PubMed] [Google Scholar]
- 7.Jannini E.A., McCabe M.P., Salonia A. Organic vs. psychogenic? The Manichean diagnosis in sexual medicine. J Sex Med. 2010;7:1726–1733. doi: 10.1111/j.1743-6109.2010.01824.x. [DOI] [PubMed] [Google Scholar]
- 8.Bruzziches R., Francomano D., Gareri P. An update on pharmacological treatment of erectile dysfunction with phosphodiesterase type 5 inhibitors. Expert Opin Pharmacother. 2013;14:1333–1344. doi: 10.1517/14656566.2013.799665. [DOI] [PubMed] [Google Scholar]
- 9.Jannini E.A., Sternbach N., Limoncin E. Health-related characteristics and unmet needs of men with erectile dysfunction: A survey in five European countries. J Sex Med. 2014;11:40–50. doi: 10.1111/jsm.12344. [DOI] [PubMed] [Google Scholar]
- 10.Sato Y., Tanda H., Kato S. How long do patients with erectile dysfunction continue to use sildenafil citrate? Dropout rate from treatment course as outcome in real life. Int J Urol. 2007;14:339–342. doi: 10.1111/j.1442-2042.2007.01608.x. [DOI] [PubMed] [Google Scholar]
- 11.Carvalheira A.A., Pereira N.M., Maroco J. Dropout in the treatment of erectile dysfunction with PDE5: A study on predictors and a qualitative analysis of reasons for discontinuation. J Sex Med. 2012;9:2361–2369. doi: 10.1111/j.1743-6109.2012.02787.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim S.C., Lee Y.S., Seo K.K. Reasons and predictive factors for discontinuation of PDE-5 inhibitors despite successful intercourse in erectile dysfunction patients. Int J Impot Res. 2014;26:87–93. doi: 10.1038/ijir.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corona G., Maggi M., Jannini E.A. EDEUS, a real-life study on the users of phosphodiesterase type 5 inhibitors: Prevalence, perceptions, and health care-seeking behavior among European men with a focus on 2nd-generation avanafil. Sex Med. 2018;6:15–23. doi: 10.1016/j.esxm.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frost M., Wraae K., Gudex C. Chronic diseases in elderly men: Underreporting and underdiagnosis. Age Ageing. 2012;41:177–183. doi: 10.1093/ageing/afr153. [DOI] [PubMed] [Google Scholar]
- 15.Goel H., Rai P., Rana V. Orally disintegrating systems: Innovations in formulation and technology. Recent Pat Drug Deliv Formul. 2008;2:258–274. doi: 10.2174/187221108786241660. [DOI] [PubMed] [Google Scholar]
- 16.Debruyne F.M., Gittelman M., Sperling H. Time to onset of action of vardenafil: A retrospective analysis of the pivotal trials for the orodispersible and film-coated tablet formulations. J Sex Med. 2011;8:2912–2923. doi: 10.1111/j.1743-6109.2011.02462.x. [DOI] [PubMed] [Google Scholar]
- 17.Gittelman M., McMahon C.G., Rodriguez-Rivera J.A. The POTENT II randomised trial: Efficacy and safety of an orodispersible vardenafil formulation for the treatment of erectile dysfunction. Int J Clin Pract. 2010;64:594–603. doi: 10.1111/j.1742-1241.2010.02358.x. [DOI] [PubMed] [Google Scholar]
- 18.Sperling H., Gittelman M., Norenberg C. Efficacy and safety of an orodispersible vardenafil formulation for the treatment of erectile dysfunction in elderly men and those with underlying conditions: An integrated analysis of two pivotal trials. J Sex Med. 2011;8:261–271. doi: 10.1111/j.1743-6109.2010.02005.x. [DOI] [PubMed] [Google Scholar]
- 19.Scaglione F., Donde S., Hassan T.A. Phosphodiesterase type 5 inhibitors for the treatment of erectile dysfunction: Pharmacology and clinical impact of the sildenafil citrate orodispersible tablet formulation. Clin Ther. 2017;39:370–377. doi: 10.1016/j.clinthera.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Bala R., Pawar P., Khanna S. Orally dissolving strips: A new approach to oral drug delivery system. Int J Pharm Investig. 2013;3:67–76. doi: 10.4103/2230-973X.114897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Efremov E.A., Kasatonova E.V., Melnik Y.I. [PDE-5 inhibitors: patients’ preferences] Urologiia. 2017;3:120–126. [PubMed] [Google Scholar]
- 22.Karki S., Kim H., Na S.-J. Thin films as an emerging platform for drug delivery. Asian J Pharm Sci. 2016;11:559–574. [Google Scholar]
- 23.Radicioni M., Castiglioni C., Giori A. Bioequivalence study of a new sildenafil 100 mg orodispersible film compared to the conventional film-coated 100 mg tablet administered to healthy male volunteers. Drug Des Devel Ther. 2017;11:1183–1192. doi: 10.2147/DDDT.S124034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corbin J.D. Mechanisms of action of PDE5 inhibition in erectile dysfunction. Int J Impot Res. 2004;16(Suppl. 1):S4–S7. doi: 10.1038/sj.ijir.3901205. [DOI] [PubMed] [Google Scholar]
- 25.Francis S.H., Busch J.L., Corbin J.D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010;62:525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dolci S., Belmonte A., Santone R. Subcellular localization and regulation of type-1C and type-5 phosphodiesterases. Biochem Biophys Res Commun. 2006;341:837–846. doi: 10.1016/j.bbrc.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 27.Carosa E., Rossi S., Giansante N. The ontogenetic expression pattern of type 5 phosphodiesterase correlates with androgen receptor expression in rat corpora cavernosa. J Sex Med. 2009;6:388–396. doi: 10.1111/j.1743-6109.2008.01091.x. [DOI] [PubMed] [Google Scholar]
- 28.Carosa E., Castri A., Forcella C. Platelet-derived growth factor regulation of type-5 phosphodiesterase in human and rat penile smooth muscle cells. J Sex Med. 2014;11:1675–1684. doi: 10.1111/jsm.12568. [DOI] [PubMed] [Google Scholar]
- 29.Das A., Durrant D., Salloum F.N. PDE5 inhibitors as therapeutics for heart disease, diabetes and cancer. Pharmacol Ther. 2015;147:12–21. doi: 10.1016/j.pharmthera.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutchings D.C., Anderson S.G., Caldwell J.L. Phosphodiesterase-5 inhibitors and the heart: Compound cardioprotection? Heart. 2018;104:1244–1250. doi: 10.1136/heartjnl-2017-312865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rybalkin S.D., Yan C., Bornfeldt K.E. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ Res. 2003;93:280–291. doi: 10.1161/01.RES.0000087541.15600.2B. [DOI] [PubMed] [Google Scholar]
- 32.Uthayathas S., Karuppagounder S.S., Thrash B.M. Versatile effects of sildenafil: Recent pharmacological applications. Pharmacol Rep. 2007;59:150–163. [PubMed] [Google Scholar]
- 33.Jannini E.A., DeRogatis L.R., Chung E. How to evaluate the efficacy of the phosphodiesterase type 5 inhibitors. J Sex Med. 2012;9:26–33. doi: 10.1111/j.1743-6109.2011.02611.x. [DOI] [PubMed] [Google Scholar]
- 34.Jannini E.A., Isidori A.M., Gravina G.L. The ENDOTRIAL study: A spontaneous, open-label, randomized, multicenter, crossover study on the efficacy of sildenafil, tadalafil, and vardenafil in the treatment of erectile dysfunction. J Sex Med. 2009;6:2547–2560. doi: 10.1111/j.1743-6109.2009.01375.x. [DOI] [PubMed] [Google Scholar]
- 35.Padma-Nathan H., McCullough A.R., Levine L.A. Randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil citrate for the prevention of erectile dysfunction after bilateral nerve-sparing radical prostatectomy. Int J Impot Res. 2008;20:479–486. doi: 10.1038/ijir.2008.33. [DOI] [PubMed] [Google Scholar]
- 36.Montorsi F., Brock G., Stolzenburg J.U. Effects of tadalafil treatment on erectile function recovery following bilateral nerve-sparing radical prostatectomy: A randomised placebo-controlled study (REACTT) Eur Urol. 2014;65:587–596. doi: 10.1016/j.eururo.2013.09.051. [DOI] [PubMed] [Google Scholar]
- 37.Montorsi F., Brock G., Lee J. Effect of nightly versus on-demand vardenafil on recovery of erectile function in men following bilateral nerve-sparing radical prostatectomy. Eur Urol. 2008;54:924–931. doi: 10.1016/j.eururo.2008.06.083. [DOI] [PubMed] [Google Scholar]
- 38.Jo J.K., Jeong S.J., Oh J.J. Effect of starting penile rehabilitation with sildenafil immediately after robot-assisted laparoscopic radical prostatectomy on erectile function recovery: A prospective randomized trial. J Urol. 2018;199:1600–1606. doi: 10.1016/j.juro.2017.12.060. [DOI] [PubMed] [Google Scholar]
- 39.Aversa A., Isidori A.M., Gianfrilli D. Are subjects with erectile dysfunction aware of their condition? Results from a retrospective study based on an Italian free-call information service. J Endocrinol Invest. 2004;27:548–556. doi: 10.1007/BF03347477. [DOI] [PubMed] [Google Scholar]
- 40.Hackett G.I. Patient preferences in treatment of erectile dysfunction: The continuing importance of patient education. Clin Cornerstone. 2005;7:57–65. doi: 10.1016/s1098-3597(05)80049-3. [DOI] [PubMed] [Google Scholar]
- 41.Mirone V., Fusco F., Rossi A. Tadalafil and vardenafil vs sildenafil: A review of patient-preference studies. BJU Int. 2009;103:1212–1217. doi: 10.1111/j.1464-410X.2008.08267.x. [DOI] [PubMed] [Google Scholar]
- 42.Raheem A.A., Kell P. Patient preference and satisfaction in erectile dysfunction therapy: A comparison of the three phosphodiesterase-5 inhibitors sildenafil, vardenafil and tadalafil. Patient Prefer Adherence. 2009;3:99–104. doi: 10.2147/ppa.s3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith W.B., 2nd, McCaslin I.R., Gokce A. PDE5 inhibitors: Considerations for preference and long-term adherence. Int J Clin Pract. 2013;67:768–780. doi: 10.1111/ijcp.12074. [DOI] [PubMed] [Google Scholar]
- 44.Doggrell S. Do vardenafil and tadalafil have advantages over sildenafil in the treatment of erectile dysfunction? Int J Impot Res. 2007;19:281–295. doi: 10.1038/sj.ijir.3901525. [DOI] [PubMed] [Google Scholar]
- 45.Perimenis P., Roumeguere T., Heidler H. Evaluation of patient expectations and treatment satisfaction after 1-year tadalafil therapy for erectile dysfunction: The DETECT study. J Sex Med. 2009;6:257–267. doi: 10.1111/j.1743-6109.2008.01027.x. [DOI] [PubMed] [Google Scholar]
- 46.Burri A., Porst H. Results from an online survey investigating ED patients’ insights and treatment expectations. Int J Impot Res. 2015;27:191–196. doi: 10.1038/ijir.2015.14. [DOI] [PubMed] [Google Scholar]
- 47.Smith-Harrison L., Starke N.R., Smith R.P. Drugs in preclinical to phase II clinical development for the treatment of erectile dysfunction. Expert Opin Investig Drugs. 2017;26:669–675. doi: 10.1080/13543784.2017.1324570. [DOI] [PubMed] [Google Scholar]
- 48.Mollaioli D., Ciocca G., Limoncin L. Lifestyle and sexuality in men and women: The gender perspective in sexual medicine. Reprod Biol Endocrinol. 2018 doi: 10.1186/s12958-019-0557-9. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffmann E.M., Breitenbach A., Breitkreutz J. Advances in orodispersible films for drug delivery. Expert Opin Drug Deliv. 2011;8:299–316. doi: 10.1517/17425247.2011.553217. [DOI] [PubMed] [Google Scholar]
- 50.Irfan M., Rabel S., Bukhtar Q. Orally disintegrating films: A modern expansion in drug delivery system. Saudi Pharm J. 2016;24:537–546. doi: 10.1016/j.jsps.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kathpalia H., Gupte A. An introduction to fast dissolving oral thin film drug delivery systems: A review. Curr Drug Deliv. 2013;10:667–684. doi: 10.2174/156720181006131125150249. [DOI] [PubMed] [Google Scholar]
- 52.Vondrak B., Barnhart S. Dissolvable films for flexible product format. Pharm Technol. 2008;32:s20. [Google Scholar]
- 53.Carnaby-Mann G., Crary M. Pill swallowing by adults with dysphagia. Arch Otolaryngol Head Neck Surg. 2005;131:970–975. doi: 10.1001/archotol.131.11.970. [DOI] [PubMed] [Google Scholar]
- 54.Dowson A.J., Charlesworth B.R. Patients with migraine prefer zolmitriptan orally disintegrating tablet to sumatriptan conventional oral tablet. Int J Clin Pract. 2003;57:573–576. [PubMed] [Google Scholar]
- 55.Thakur N., Bansal M., Sharma N. Overview “a novel approach of fast dissolving films and their patents. Adv Biol Res (Rennes) 2013;7:50–58. [Google Scholar]
- 56.Sperling H., Debruyne F., Boermans A. The POTENT I randomized trial: efficacy and safety of an orodispersible vardenafil formulation for the treatment of erectile dysfunction. J Sex Med. 2010;7:1497–1507. doi: 10.1111/j.1743-6109.2010.01806.x. [DOI] [PubMed] [Google Scholar]
- 57.Heinig R., Weimann B., Dietrich H. Pharmacokinetics of a new orodispersible tablet formulation of vardenafil: Results of three clinical trials. Clin Drug Investig. 2011;31:27–41. doi: 10.2165/11584950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 58.Damle B., Duczynski G., Jeffers B.W. Pharmacokinetics of a novel orodispersible tablet of sildenafil in healthy subjects. Clin Ther. 2014;36:236–244. doi: 10.1016/j.clinthera.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 59.Roh H., Son H., Lee D. Pharmacokinetic comparison of an orally disintegrating film formulation with a film-coated tablet formulation of sildenafil in healthy Korean subjects: a randomized, open-label, single-dose, 2-period crossover study. Clin Ther. 2013;35:205–214. doi: 10.1016/j.clinthera.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 60.Cocci A., Capece M., Cito G. Effectiveness and safety of oro-dispersible sildenafil in a new film formulation for the treatment of erectile dysfunction: Comparison between sildenafil 100-mg film-coated tablet and 75-mg oro-dispersible film. J Sex Med. 2017;14:1606–1611. doi: 10.1016/j.jsxm.2017.10.066. [DOI] [PubMed] [Google Scholar]
- 61.Fisher W.A., Eardley I., McCabe M. Erectile dysfunction (ED) is a shared sexual concern of couples I: Couple conceptions of ED. J Sex Med. 2009;6:2746–2760. doi: 10.1111/j.1743-6109.2009.01457.x. [DOI] [PubMed] [Google Scholar]
- 62.Litwin M.S., Nied R.J., Dhanani N. Health-related quality of life in men with erectile dysfunction. J Gen Intern Med. 1998;13:159–166. doi: 10.1046/j.1525-1497.1998.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salonia A., Castagna G., Sacca A. Is erectile dysfunction a reliable proxy of general male health status? The case for the International Index of Erectile Function-Erectile Function domain. J Sex Med. 2012;9:2708–2715. doi: 10.1111/j.1743-6109.2012.02869.x. [DOI] [PubMed] [Google Scholar]
- 64.Sanchez-Cruz J.J., Cabrera-Leon A., Martin-Morales A. Male erectile dysfunction and health-related quality of life. Eur Urol. 2003;44:245–253. doi: 10.1016/s0302-2838(03)00215-x. [DOI] [PubMed] [Google Scholar]
- 65.Cappelleri J.C., Althof S.E., Siegel R.L. Development and validation of the Self-Esteem And Relationship (SEAR) questionnaire in erectile dysfunction. Int J Impot Res. 2004;16:30–38. doi: 10.1038/sj.ijir.3901095. [DOI] [PubMed] [Google Scholar]
- 66.Jannini E.A. SM = SM: The interface of systems medicine and sexual medicine for facing non-communicable diseases in a gender-dependent manner. Sex Med Rev. 2017;5:349–364. doi: 10.1016/j.sxmr.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 67.Leoni L.A., Leite G.S., Wichi R.B. Sildenafil: Two decades of benefits or risks? Aging Male. 2013;16:85–91. doi: 10.3109/13685538.2013.801952. [DOI] [PubMed] [Google Scholar]