Abstract
Background
Liver vitamin A (VA) concentration is the gold standard for VA status, but is not routinely accessible. Adipose tissue VA concentrations, as retinol and retinyl esters, may be correlated to liver VA. α-VA (as α-retinol) is a cleavage product of α-carotene that traces postprandial VA distribution to tissues but cannot recirculate from hepatic stores, providing insight into tissue VA sources.
Objective
We performed a secondary analysis of VA and α-VA in Mongolian gerbil liver and adipose to determine the suitability of adipose tissue VA as a biomarker of VA status.
Methods
Gerbils (n = 186) consumed feeds containing 0–15.9 μg (0–55.6 nmol) retinol activity equivalents/g as preformed VA and/or provitamin A carotenoids for 36–62 d in 3 studies. Body fat percentage was determined in the final study by MRI. Serum and liver retinol, α-retinol, and retinyl esters were extracted and analyzed by HPLC or ultra-performance LC (UPLC). Epididymal and retroperitoneal adipose tissue retinol and α-retinol were determined by UPLC after homogenization, saponification, and extraction. Linear regression models with appropriate data transformations identified determinants of adipose VA concentrations.
Results
Liver VA did not predict serum retinol concentrations. After logarithmic transformation of adipose and liver values, liver VA positively predicted both adipose depots’ VA concentrations (P < 0.001, R2 > 0.7). Adding serum retinol or body fat percentage did not significantly increase the adjusted R2. In liver, α-VA concentration explained much of the variation of VA (P < 0.001, R2 > 0.7), but far less in epididymal and retroperitoneal adipose (P = 0.004 and 0.012, respectively, R2 < 0.4).
Conclusions
Adipose VA is correlated with liver VA and is a potential biomarker of VA status. It is not fully explained by chylomicron deposition and is negatively affected by serum retinol. Adipose biopsy validation is needed for human applications.
Keywords: biomarkers, adipose tissue, chylomicron remnants, retinol-binding protein, vitamin A
Introduction
Vitamin A (VA) is an essential nutrient obtained from preformed retinyl esters in animal products or supplements and from provitamin A carotenoids (e.g., α-carotene, β-carotene, β-cryptoxanthin) in plant sources or supplements. VA is best known for its essentiality in vision as 11-cis-retinal, but is also vital for growth, immunity, and cell signaling through the binding of the oxidation product retinoic acid to nuclear receptors (i.e., retinoic acid and retinoid X receptors) (1). Both deficient and elevated concentrations of VA can be harmful (1), so it is necessary to monitor populations at risk of these conditions.
Liver VA concentration is considered the “gold standard” for VA status assessment because >80% of total body VA stores are in hepatic stellate cells in VA-adequate adults (1). Because liver biopsies are not routinely accessible, public health researchers use other biomarkers of VA status to determine the prevalence of VA deficiency, hypervitaminosis, or toxicity (1). Serum retinol is the most commonly used biomarker: the WHO defines a prevalence of >20% of a population with serum retinol concentrations <0.7 μmol/L as representing a serious risk of deficiency (2); however, no concentration has been identified that reflects elevated VA status. Serum retinol suffers from insensitivity (3) and is affected by infection and inflammation (4). Other indicators, such as the modified-relative-dose-response test, qualitatively determine VA deficiency or sufficiency (1). Retinol isotope dilution measures total body VA stores from deficiency through toxicity in groups of individuals using sophisticated mass spectrometric techniques (1). However, an inexpensive biomarker is needed that accurately predicts VA status over a wide range of values that can be broadly applied to allow researchers to determine deficiency and hypervitaminosis A at the population level. Adipose tissue VA concentration, potentially taken from a needle biopsy of subcutaneous fat, could be one such biomarker.
Humans and rodents maintain adipose VA concentrations between 1 and 24 nmol/g tissue (5–8), much lower than liver VA concentrations (100–700 nmol/g in optimal VA status) (1). Estimates of the relative proportion of total VA in adipose to that in liver vary significantly. For example, adipose VA stores were estimated to be 15–20% of those in liver in rats when assuming adipose and liver comprise 15% and 4% of body mass, respectively (9). In Mongolian gerbils (Meriones unguiculatus), adipose VA stores are <5% of those in liver using the same assumptions (7). In rats, VA concentrations in liver and several adipose depots varied positively according to VA intake (6, 10). Similarly, an increase in VA concentration occurred in liver and abdominal subcutaneous adipose tissue of ferrets supplemented with β-carotene compared with controls (11). Gerbils fed varying amounts of β-cryptoxanthin that did not have differences in liver VA had no difference in adipose tissue VA (7). These correlations suggest that changes in VA status may be reflected in adipose. Correlation of liver and adipose VA may be due to increased dietary VA delivery to tissues by chylomicra after a meal, rather than resulting from redistribution of retinol secreted from hepatic stores on retinol-binding protein (RBP4) (6). This theory has been supported by studies showing no difference in VA concentrations in extrahepatic tissues (other than the eye) in mice lacking RBP4 compared with wild-type mice (12). In addition, humans with RBP4 mutations do not have overt symptoms of VA deficiency when consuming a VA-adequate diet (13).
α-Retinol is a retinol isomer found in liver and tissues of animals and humans consuming α-carotene (14) that may provide insight into the relative importance of different VA sources without disrupting protein function. Unlike β-carotene, which produces 2 retinal molecules when cleaved, α-carotene yields 1 retinal and 1 α-retinal, which are reduced to their respective alcohol forms, esterified, and packaged into chylomicra. In gerbils fed α-carotene, hepatic α-retinol and retinol were present in equal amounts, indicating that the initial steps of α-retinol metabolism quantitatively trace retinol metabolism (15). However, once in the liver, α-retinol cannot bind RBP4 for secretion into plasma (16), making it an effective tracer of chylomicron distribution, but not hepatic recirculation. VA-sufficient diets deposit adequate VA into tissues through chylomicra (12), thus not disrupting the hepatic 1:1 ratio of retinol:α-retinol.
The study presented herein was a secondary analysis of 3 studies in gerbils with a range of dietary VA concentrations from deficient through excessive as preformed retinyl esters and provitamin A carotenoids. VA concentrations in liver, epididymal (EPI), and retroperitoneal (RP) adipose were analyzed to evaluate adipose VA concentration as a biomarker of VA status against liver VA concentration. In the third study, body fat was measured to determine the effect of increased fat mass on adipose VA concentration, and α-VA was analyzed to provide insight into the source of adipose VA.
Methods
Animals and feeds
Approval for the 3 studies from which tissues and data were taken was obtained from the University of Wisconsin-Madison College of Agricultural and Life Sciences Animal Care and Use Committee. The primary outcomes and analyses of these studies will be reported elsewhere or have been partially published (17). The feeds and study protocols are summarized in Table 1. Briefly, male Mongolian gerbils (n = 40–80) were fed a VA-deficient feed for 14–21 d, then divided into groups (n = 5–10/group) and given preformed VA and/or provitamin A carotenoids as biofortified (orange) maize and/or orange carrots (Study 1, n = 80), dried cassava leaves (Study 2, n = 40), or high-carotene orange carrots (Study 3, n = 66) for 36–62 d. Study 1 included a low concentration of VA fortificant as retinyl palmitate [0.2–0.4 μg retinol activity equivalents (RAE)/g feed, equivalent to approximately half the estimated daily requirement of gerbils from prior studies (18, 19)]; Study 2 included a retinyl acetate supplement as a positive control; and Study 3 included retinyl palmitate VA fortificant at 1 or 2 times the same estimated daily requirement for gerbils as followed in Study 1 (0.4–0.5 and 1.0 μg RAE/g feed). Control feeds contained white maize and/or white carrots. In Study 3, body fat percentage was determined in live gerbils <30 min before death by whole body MRI (EchoMRI), which was calibrated against a known oil-based phantom. Full matched sets of EPI and RP adipose depots, serum, and liver were not completely available for all gerbils in all 3 studies, therefore sample sizes are noted for each analysis.
TABLE 1.
Characteristics of 3 studies combining dietary provitamin A carotenoids and preformed vitamin A in male Mongolian gerbils
| Study 1 | Study 2 | Study 3 | |
|---|---|---|---|
| Characteristic | |||
| Number of gerbils | 80 | 40 | 66 |
| Baseline age of gerbils, wk | 4 | 4.5 | 4–5 |
| Endline age of gerbils, wk | 14.9 | 12.6 | 15–16 |
| Vitamin A depletion, d | 14 | 21 | 14 |
| Treatment time, d | 62 | 36 | 62 |
| Baseline body weight (mean ± SD), g | 27.9 ± 2.5 | 39.4 ± 2.0 | 36.7 ± 3.7 |
| Endline body weight (mean ± SD), g | 74.3 ± 6.6 | 63.2 ± 4.2 | 71.2 ± 3.8 |
| Vitamin A components in feed | |||
| Preformed vitamin A | Fortificant in feed1 | Oil supplement2 | Fortificant in feed1 |
| Provitamin A carotenoids | Orange carrot, orange maize | Dried cassava leaves | Orange carrot |
| Preformed vitamin A intakes, μg retinol activity equivalents/d3 | |||
| Control group | 0 | 0 | 0 |
| VA+ group | 1.1–2.3 | 7.1 | 2.2–2.4 |
| VA++ group | — | — | 5.3 |
| Provitamin A intakes, µg β-carotene equivalents/d4 | |||
| Control group | 0.1 | 0 | 0.1–0.2 |
| Orange carrot, orange maize | 111–112 | — | — |
| Orange carrot, white maize | 72.3–79.4 | — | 116–127 |
| White carrot, orange maize | 33.4–39.5 | — | — |
| Dried cassava leaves | — | 6.6–6.7 | — |
Provided as retinyl palmitate [“Dry Vitamin A Palmitate” (250,000 IU VA/g; DSM Nutritional Products Ltd.)].
Provided as retinyl acetate (Sigma-Aldrich).
1 μg retinol activity equivalent = 1.15 μg retinyl acetate or 1.83 μg retinyl palmitate.
1 μg β-carotene equivalent = 1 μg β-carotene or 2 μg β-cryptoxanthin or α-carotene.
Sample collection and preparation
Gerbils were anesthetized by isoflurane and killed by exsanguination through cardiac puncture. Blood, EPI and RP fat pads, and whole liver were collected. Blood was allowed to clot at room temperature for 20 min and then serum was separated by centrifugation at 2800 × g for 15 min at 4°C. All tissues were weighed and stored at −80°C until analysis.
Serum, liver, and adipose tissue VA and α-VA analysis
Serum and liver retinol were determined by HPLC (Studies 1 and 3) and ultra-performance LC (UPLC; Waters Acquity H-class) (Study 2) as previously described (20, 21). In the first 2 studies, total liver VA concentration was determined by summing the area of the retinol peak and all identifiable retinyl ester peaks. In the third study, both total liver VA and α-VA concentrations were determined from a saponified fraction.
For adipose VA concentrations in all 3 studies, ∼200 mg of tissue was weighed into a 13 × 100 mm glass culture tube (Fisher Scientific); 100 μL 8′-β-apo-carotenal (∼15 ng, as internal standard; Sigma-Aldrich), 2 mL dichloromethane, and 1 mL methanol were added for a modified Folch total lipid extraction (22, 23). The tissue was mechanically homogenized (T10 ULTRA-TURRAX, IKA), mixed with a vortex for 30 s, and centrifuged at 1400 × g at room temperature for 10 min to remove solids. The organic layer was washed by the addition of 0.9% NaCl(aq), mixed for 30 s, and centrifuged as above for 5 min. Also at room temperature, the extracted lipid was dried under N2 and saponified by resuspension in 1 mL 5% ethanolic KOH (wt:vol) and 0.1% butylated hydroxytoluene (wt:vol) for 10 min at 60°C (24). One milliliter of distilled H2O was added to quench the reaction and then the solution was extracted twice with 3 mL hexanes. The pooled organic layers were dried under N2 and resuspended in 100 μL 75:25 methanol:dichloroethane (vol:vol). Two microliters were injected onto the UPLC equipped with a guard column, a C18 1.8-μm, 2.1 × 150 mm column (Waters Acquity HSS), and a heater set to 30°C (Waters). Solvent A was 70:25:5 acetonitrile:water:isopropanol (vol:vol:vol) and solvent B was 75:25 methanol:isopropanol (vol:vol). Each sample was run for 29 min according to a previously described gradient (25). Retinol and α-retinol were quantified at 325 and 311 nm, respectively, by external standardization to HPLC-purified, UV-visible spectrophotometer-authenticated standards [saponified retinyl acetate (Sigma-Aldrich) and α-retinyl acetate synthesized using α-ionone in a published procedure for retinyl acetate (26)], after correction for recovery of internal standard.
Statistical analysis
Preliminary analysis of the data was performed using Spearman's rank-sum coefficient, after which single and multiple linear regressions were performed using the “LM” procedure in the R statistical package (27). Two extreme outliers for EPI adipose VA concentration (1 in each of Studies 2 and 3, Figure 1A) were identified by Grubb's test for outliers (P < 0.05 within each study) and those animals were retroactively excluded from all statistical analyses. These values had excessive influence on regression coefficients [e.g., Cook's distance > (4 × mean Cook's distance) in the full model described in Supplemental Table 1], resulting in conclusions of strong significance that became nonsignificant when those points were removed. Liver and adipose VA concentration were transformed by base-10 logarithm (log10) to achieve normality of errors and homoscedasticity in the general linear model.
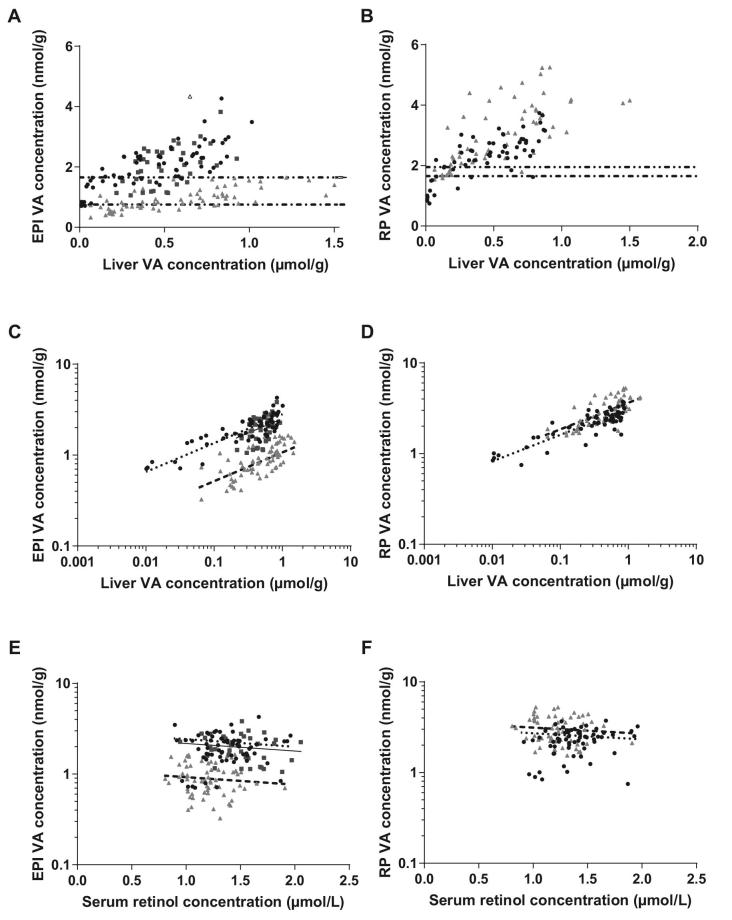
FIGURE 1.
Tissue VA (as retinol + retinyl esters), determined by HPLC (liver and serum) or UPLC (adipose tissue) in Studies 1 (black circles), 2 (dark grey squares), and 3 (light grey triangles). RP tissue was only available in Studies 1 and 3. (A, B)  Example cutoff for deficiency for Studies 1 and 2.
Example cutoff for deficiency for Studies 1 and 2.  Example cutoff for deficiency for Study 3. (A) Liver and EPI retinol concentrations. The data point with an open symbol was an outlier and excluded from further analysis. A further outlier at (0.33, 6.50) was off-axis and also excluded. (B) Liver and RP retinol concentrations. (C–F) ANCOVA including liver, serum, and either EPI (C, E) or RP (D, F) tissue VA was performed after base-10 logarithmic transformation of liver and adipose VA concentrations in Studies 1 (
Example cutoff for deficiency for Study 3. (A) Liver and EPI retinol concentrations. The data point with an open symbol was an outlier and excluded from further analysis. A further outlier at (0.33, 6.50) was off-axis and also excluded. (B) Liver and RP retinol concentrations. (C–F) ANCOVA including liver, serum, and either EPI (C, E) or RP (D, F) tissue VA was performed after base-10 logarithmic transformation of liver and adipose VA concentrations in Studies 1 (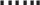 ), 2 (—), and 3 (– – –), with study as a covariate. (C, D) Liver VA concentration explained 28% (D, EPI) and 57% (E, RP) of the variation in adipose VA concentration (P < 0.001). (E, F) Serum retinol concentration explained a negligible fraction of the variation in adipose VA concentration. EPI, epididymal adipose; RP, retroperitoneal adipose; UPLC, ultra-performance LC; VA, vitamin A.
), 2 (—), and 3 (– – –), with study as a covariate. (C, D) Liver VA concentration explained 28% (D, EPI) and 57% (E, RP) of the variation in adipose VA concentration (P < 0.001). (E, F) Serum retinol concentration explained a negligible fraction of the variation in adipose VA concentration. EPI, epididymal adipose; RP, retroperitoneal adipose; UPLC, ultra-performance LC; VA, vitamin A.
Two separate ANCOVAs were performed to explain EPI or RP adipose VA concentration using both independent variables available for all 3 studies (liver VA and serum retinol concentration) as well as the study (1, 2, or 3) as a covariate. For each analysis, we compared a model using different slopes for each study with a model using the same slope for each study. In both the EPI and RP analyses, the different-slopes model did not explain more variance than the simpler same-slopes model according to the additional sum of squares test. Models for EPI and RP were constructed in Study 3 to determine if any variability of EPI and RP adipose VA concentrations was due to body fat percentage. The model included log10-liver VA and serum retinol as independent variables and tested the significance of inclusion or exclusion of body fat percentage using the aforementioned additional sum of squares test.
ANCOVA was used to determine if EPI and RP adipose VA were correlated within studies, and if that correlation varied between studies, using RP adipose VA concentration as the dependent variable and EPI adipose VA concentration as the independent variable, including study as a covariate. In this case, both the slopes were clearly different, so no further reduction of the model was applied. VA and α-VA concentrations in liver and both adipose tissues were analyzed using ANCOVA to explain VA concentrations from all sources, with α-VA from dietary deposition as an independent variable and treatment group as a covariate. The covariate was necessary because each of the 3 groups had different fortificant concentrations (i.e., 0, 0.5, or 1 μg RAE/g feed) and therefore different ratios of sources of VA to α-VA available in their feed. As above, the different-slopes and same-slopes models were compared and the same-slopes model was used. The regression in liver was repeated with and without including the gerbils that were missing RP tissues to be comparable with the EPI and RP regressions, respectively.
Data are reported as mean ± SD. Significance was defined as P ≤ 0.01 rather than P ≤ 0.05 to account for multiple testing throughout analyses; however, the importance of an independent variable was primarily examined by the magnitude of its contribution to variation in the dependent variable (measured as adjusted R2); P values for these changes were determined from the additional sum of squares test for completeness. To plot multiple regression lines, the independent variable that was not plotted was held constant at its mean effect. Sensitivity and specificity for a biomarker were calculated as the percentage of VA-deficient gerbils correctly identified as such and the percentage of VA-sufficient gerbils correctly identified as such, respectively. Example cutoffs for population health studies were determined by calculating sensitivity and specificity for cutoff values every 0.05 nmol/g from 0 to 5, then maximizing Youden's index (J = sensitivity + specificity – 1) (28).
Results
Serum retinol is not correlated with liver VA
Liver VA and serum retinol concentrations were not correlated by Spearman's rank-sum test in any study (Supplemental Figure 1). No gerbil had a serum retinol concentration <0.7 μmol/L, the current WHO-recommended cutoff to define VA deficiency in humans (2), despite 12 gerbils with liver stores defined as VA deficient (<0.1 μmol/g) (1).
Adipose VA concentration correlates with liver VA concentrations
Study 1, which spanned extremely deficient to high-normal liver VA stores (Figure 1A, B), revealed that a logarithmic data transformation was appropriate for both liver and adipose VA concentrations. The final models explaining EPI and RP adipose VA concentration were both significant (P < 0.001, Supplemental Table 1, Figure 1C–F). The slopes of the regressions were not different between any study and intercepts did not significantly vary between Studies 1 and 2 in EPI adipose depots; Study 3 had a significantly lower intercept than Studies 1 and 2 (EPI) and a higher intercept than Study 1 (RP) (P < 0.001). In EPI and RP adipose, log10-transformed liver VA concentration, serum retinol concentration, and study accounted for 78% and 70% of total variance (measured as adjusted R2), respectively, 28% and 57% of which was due to liver VA concentration, respectively. Conversely, removing serum from the model did not change the adjusted R2 (<0.01 difference in either model, P = 0.02 and 0.08, respectively). EPI and RP adipose VA concentrations were correlated (adjusted R2 = 0.64, P < 0.001; Figure 2, Supplemental Table 2); however, the relation was different between studies (P < 0.001).
FIGURE 2.
EPI and RP VA concentrations are correlated in gerbils. Adipose retinol concentrations were determined by UPLC and plotted against each other for Studies 1 (black circles) and 3 (light grey triangles). ANCOVA with study as a covariate revealed that retinol concentrations were not the same between depots, the relations were different between studies, and they were significantly related overall (adjusted R2 = 0.64, P < 0.001). EPI, epididymal adipose; RP, retroperitoneal adipose; UPLC, ultra-performance LC; VA, vitamin A.
Adipose VA concentrations were evaluated as a potential qualitative biomarker of deficient liver VA stores (i.e., <0.1 μmol/g) by calculating example cutoff values to predict deficiency or sufficiency (Figure 1A, B). In Studies 1 and 2, a reference value of 1.65 nmol VA/g adipose tissue maximized Youden's index in both EPI (J = 0.76) and RP (J = 0.85), resulting in a sensitivity of 100% (12 of 12 gerbils) in EPI and 91% (10 of 11 gerbils) in RP, and a specificity of 76% (70 of 92 gerbils) in EPI and 94% (48 of 51 gerbils) in RP. Study 3 was examined separately because the gerbils had significantly different adipose VA concentrations over the range of liver values, as revealed by the different intercept coefficients in the ANCOVA models (Supplemental Table 1). Only 2 gerbils in Study 3 had deficient liver VA stores, and a reference value of 0.75 nmol/g EPI or 1.95 nmol/g RP adipose yielded 100% sensitivity (2 of 2 gerbils) and a specificity of 63% (40 of 52 gerbils) in EPI or 86% (49 of 52 gerbils) in RP to predict values <0.1 μmol/g liver.
Body fat percentage does not predict adipose VA concentrations
In Study 3, the body fat percentage (12% ± 4.3%) determined by MRI ranged from 3% to 24%. The estimated contribution of adipose retinol to total body VA stores, which was determined by multiplying the VA concentration by the MRI-predicted total fat mass, was minimal (Table 2). The addition of body fat percentage to the models predicting adipose VA (in Study 3 alone, assuming the inclusion of log10-liver VA and serum retinol concentration; Supplemental Table 3) in EPI and RP adipose did not affect the model, resulting in negligible changes in adjusted R2 from 49% to 51% (P = 0.047) and 58% to 60% (P = 0.061) upon inclusion, respectively.
TABLE 2.
Distribution of vitamin A in male Mongolian gerbils in Study 3, which included carrots as a provitamin A source and retinyl palmitate as a fortificant1
| Liver | Adipose | Serum | Total body | |
|---|---|---|---|---|
| Total vitamin A concentration2 | 0.585 ± 0.353 | 0.975 ± 0.544 | 1.21 ± 0.21 | — |
| Mass/volume of tissue3 | 2.61 ± 0.565 | 8.13 ± 3.29 | 1.72 ± 0.134 | 55.9 ± 4.1 |
| Total vitamin A in tissue, μmol | 1.55 ± 1.09 | 0.0077 ± 0.0052 | 0.0021 ± 0.0004 | 1.56 ± 1.105 |
| Total body vitamin A stores, % | 99.4 | 0.5 | 0.1 | 100 |
Values are means ± SDs, n = 66.
Values are in μmol/g (for liver), nmol/g (for adipose), and μmol/L (for serum).
Values are in g except for serum (mL).
Calculated assuming 7.7 mL blood/100 g body weight (29) and a serum fraction of blood of ∼0.4.
Calculated as the sum of total vitamin A in these 3 tissues, which are predicted to contain >90% of all vitamin A in the body (1).
α-Retinol concentrations
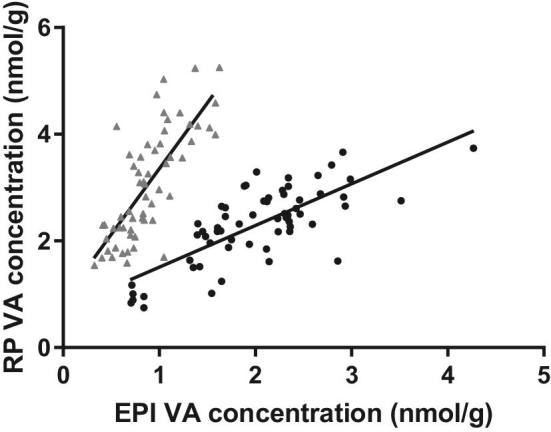
In Study 1, adipose α-VA concentrations ranged from 0 to 0.77 nmol/g tissue, but liver α-VA concentrations were not measured in that study. α-VA concentrations were not quantifiable in Study 2 because carrot was not fed (Table 1). In Study 3, VA concentrations were more strongly correlated to α-VA concentrations in liver than in adipose (Supplemental Tables 4 and 5): variation in liver α-VA (including or excluding samples missing from the RP set) accounted for 62% or 72% of the variation in retinol, respectively (P < 0.001; Figure 3A), whereas α-VA only accounted for 16% and 25% of the variation in EPI and RP adipose VA concentrations, respectively (P < 0.001, Figure 3B, C).
FIGURE 3.
The fraction of the variation in tissue VA concentration from all sources explained by chylomicron deposition in ANCOVA, measured as α-VA concentration, both determined by HPLC (liver) or UPLC (adipose). Dietary treatment was added as a covariate to control for different quantities of preformed VA [0 (●,  ); 0.5 (■, —); 1 (▲, – – –) μg/g feed] in each group. (A) α-VA concentrations explained >60% (P < 0.001) of the variation in VA concentration in liver. Data points with open symbols were from gerbils which were included in the EPI analysis but lacked RP samples. (B, C) α-VA concentration explained 15% (P = 0.012; B, epididymal) and 25% (P = 0.004; C, retroperitoneal) of the variation in retinol concentration in adipose. EPI, epididymal adipose; RP, retroperitoneal adipose; UPLC, ultra-performance LC; VA, vitamin A.
); 0.5 (■, —); 1 (▲, – – –) μg/g feed] in each group. (A) α-VA concentrations explained >60% (P < 0.001) of the variation in VA concentration in liver. Data points with open symbols were from gerbils which were included in the EPI analysis but lacked RP samples. (B, C) α-VA concentration explained 15% (P = 0.012; B, epididymal) and 25% (P = 0.004; C, retroperitoneal) of the variation in retinol concentration in adipose. EPI, epididymal adipose; RP, retroperitoneal adipose; UPLC, ultra-performance LC; VA, vitamin A.
Discussion
Liver and adipose VA concentrations maintained a nonlinear correlation in all 3 studies in 2 different adipose depots, hence adipose VA concentrations represented a potential biomarker of liver VA and therefore VA status. Body fat percentage and serum retinol concentrations were not significant predictors of adipose VA concentration. Putative reference ranges for VA deficiency using adipose concentrations gave 63–94% specificity and 86–100% sensitivity to diagnose VA deficiency in the studies from which they were generated, which was not possible with serum retinol concentrations. The adipose depots examined were collected owing to their size and ease of collection in gerbils. Concentrations varied between depots and between studies, with RP having ∼4 times the VA concentration of EPI in Study 3, but similar concentrations were observed in both depots in Study 1. In rats, adipose VA was similarly correlated with VA status, but was not different between depots (6). For translational applications in humans, targeted studies comparing subcutaneous fat concentrations with isotope dilution–estimated or biopsy-determined liver VA status in more advanced models (e.g., rhesus monkeys, swine) or humans should be conducted, because of the relative complexity of the human diet and tissues compared with gerbils consuming an unchanging diet with few (≤3) VA sources. Furthermore, the presence of 2 outliers for EPI adipose VA concentration indicates the potential for this relation to be disrupted among individuals. For this reason, adipose VA concentration may be a technique more useful in population surveys than for individual VA status assessment; in the former, the influence of rare outliers would be minimized.
Interestingly, the slope of the liver-adipose VA regression lines did not vary between studies. The logarithmic relation indicates that there is a slowing of VA storage in adipose tissue as liver VA increases, perhaps indicating a maximum capacity or nonlinear induction of degradation. As a potential biomarker of VA status, especially for excessive VA stores, this is an important consideration.
Study 3’s intercept differed from those of the first 2 studies; adipose retinol was lower than the other 2 studies in EPI and higher than Study 1 in RP. This difference could be attributable to heterogeneity between gerbils in the first 2 studies and those in the third. To our knowledge, the only impactful difference was that Study 3 gerbils were older and more variable in age than those in the first 2 studies. Age influences VA-modulating parameters such as body weight and immune function (1), and the impact of age on adipose VA metabolism could be an interesting avenue of research given the information already known on the interaction of adipose retinoid metabolism and disruptions in whole body energy metabolism, including insulin resistance and hepatic steatosis (30, 31). Unfortunately, individual gerbil age was not available in Study 3 because gerbils were shipped together and therefore age could not be controlled for as a predictor of stores. Other differences between studies could be due to a longer time at the supplier where perhaps they were consuming a different feed or being exposed to various stressors that uniformly modulated their adipose stores, somehow differently in either depot. The studies were also completed at different times, which could mean that some genetic event or crossbreeding had occurred at the supplier between studies, as the status of the gerbil genome is less well-monitored than for other rodent species (e.g., C57BL/6J mice). Regardless of the source, this difference between studies indicates that the use of adipose as a biomarker likely requires standardization of reference ranges to each population or age group for application; however, regardless of differences between studies or adipose depots, the correlation of adipose and liver VA concentrations was maintained.
Blaner et al. (6) proposed that the changes in adipose and liver VA concentrations without corresponding changes in serum retinol indicated that dietary deposition was responsible for tissue concentrations because this variation was noted when varying the dietary amount of VA. Since then, studies from the same group have shown that chylomicron delivery was sufficient to maintain mean VA concentrations in tissues other than the eye in mice lacking RBP4 (12) or its transmembrane receptor Stimulated by Retinoic Acid 6 (STRA6) (32). These data suggest that RBP4 delivery of VA to tissues other than the eye in dietary VA sufficiency is dispensable. In the absence of a method to differentiate dietary and recirculated hepatic VA within individual animals, these conclusions are also supported by our study, given that the differing liver values between groups of gerbils were achieved by variations in dietary VA.
Upon analyzing α-VA in α-carotene–consuming gerbils in Study 3, however, chylomicron-delivered α-VA accounted for variation in liver VA stores more strongly than in adipose VA stores. α-VA and VA are initially derived from the diet at some ratio, which could be measured in chylomicra in a targeted study. This ratio varies according to the amount of additional VA fortificant added to the feeds of 3 different groups (0, 1, and 2 μg RAE/g feed), so this concentration was used as a covariate in analysis of ratios of VA to α-VA in tissues. Although this ratio could theoretically change by discrimination for or against α-VA by physical phenomena (e.g., packing into lipoproteins) or enzyme rates (e.g., differential esterification or hydrolysis), evidence suggests that it is delivered to the liver at the expected ratio (15). In this case, we propose that this discrimination is due to RBP4 delivery of retinol (but not α-retinol, which cannot bind RBP4) to the tissue of interest according to VA status. Less likely, but still possible, is that α-VA could be added by differential cleavage of α-carotene and the generation of α-retinol by adipose tissue β-carotene-15, 15′-oxygenase in situ; however, no α-carotene was detected by photo-diode array analysis in the adipose tissue at 450 nm during UPLC analysis.
Liver VA stores contain >99% of the VA in the tissues examined in these gerbils (Table 2); therefore, it is not surprising that the feed (rather than some other storage organ) is the major source of VA in the liver, which is apparent from dietary-derived α-VA explaining >60% of the variation in liver VA. Conversely, although a ratio of α-VA to VA is deposited in both adipose depots examined, the VA concentration appears to be changed by a variable amount in each animal, weakening the correlation such that α-VA explains <25% of the VA concentrations in adipose tissue. This could suggest that RBP4 delivery of hepatic VA stores to peripheral tissues is important to maintain a particular adipose VA concentration.
In our 3 studies and other reports (1), serum retinol concentration did not positively correlate with dietary intake or liver VA and did not predict adipose VA. The mechanism behind the relation between liver and adipose VA concentrations without strongly correlated changes in serum retinol concentrations could lie in a change in flux of VA out of liver to serum and then into tissue despite a long-term, near-constant concentration in serum. This flux may be controlled by another metabolite (e.g., retinoic acid, retinyl- or retinoyl-β-glucuronide adducts) affecting either or both of liver release and adipose uptake, or by secreting liver VA in short pulses that acutely increase adipose VA uptake but are not reflected in fasting serum retinol measurements made at the time of death. Retinoyl-β-glucuronide decreased serum retinol concentrations in rats after oral administration, demonstrating potential regulatory action (33). Further research must explore these potential effects to determine how the correlation between adipose and liver VA is maintained.
Biomarkers of VA status that are minimally invasive are necessary for population assessment (1), especially with the widespread use of VA supplements and fortificants. Although stable-isotope methods are an indirect method to assess VA status in research settings, they are cost-prohibitive and technically challenging to perform routinely on large groups of subjects for population monitoring. Thus, a method to predict VA status during a single visit, rather than multiple blood draws over several weeks and sophisticated isotope MS, would improve the ability of health agencies to assess the impact of VA interventions.
This study showed that adipose VA concentrations were correlated to liver VA concentrations in gerbils, but there was a potential plateau at higher concentrations. This plateau, as well as clinically relevant concentrations corresponding to deficiency, likely vary according to factors such as species, dietary VA source, age, and genetic background. For example, human adipose VA concentrations have been reported to be similar to, or ≤3 times higher than, adipose VA concentrations in these gerbils (8, 34, 35), but still lower than those in rats (9). Within the results reported here, studies with different ages and feeds yielded different mean adipose VA concentrations despite following the same logarithmic correlation. Finally, we recognize that historical measurements of human adipose and liver VA concentrations have not yielded a clear correlation between the 2 (5, 36); however, these evaluations preceded modern chromatographic techniques, such as HPLC or UPLC, likely lacking the accuracy to detect small changes within a narrow range of adipose VA concentrations. Thus, human studies using sensitive analytical techniques are needed to determine the correlation of adipose and liver VA concentrations within and between populations to determine the utility of adipose retinol concentration as a biomarker of VA status.
Supplementary Material
Acknowledgments
We thank Peter Crump, University of Wisconsin-Madison College of Agricultural and Life Sciences Statistical Consulting Service, for statistical analysis consultation. The authors’ contributions were as follows—SAT: designed the research and had primary responsibility for the final content; MS, LM, LTZ, CRD, and JS: conducted the research; JS: analyzed the data; JS and SAT: wrote the paper; PWS: provided the high-carotene mass carrots; and all authors: read and approved the final manuscript.
Notes
Supported by a Vilas Associate Professor research award (to SAT), an endowment entitled “Friday Chair for Vegetable Processing Research” (to SAT), USDA Hatch WIS01528 (to SAT), a postdoctoral fellowship from the National Council for Scientific and Technological Development in Brazil (to LM), a Fulbright Scholarship (to LTZ), and USDA award 2016-51181-25400 (to PWS).
Author disclosures: JS, MS, LM, LTZ, CRD, PWS, and SAT, no conflicts of interest.
Supplemental Tables 1–5 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used:
- EPI
epididymal
- RAE
retinol activity equivalent
- RBP4
retinol-binding protein
- RP
retroperitoneal
- UPLC
ultra-performance LC
- VA
vitamin A
References
- 1. Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, Lietz G, Schulze K, Raiten DJ. Biomarkers of Nutrition for Development (BOND)—vitamin A review. J Nutr 2016;146:1816S–48S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations [Internet] Geneva, Switzerland: WHO; 2011; [cited 19 Feb, 2018]. Available from: http://www.who.int/vmnis/indicators/retinol.pdf. [Google Scholar]
- 3. Suri DJ, Tanumihardjo JP, Gannon BM, Pinkaew S, Kaliwile C, Chileshe J, Tanumihardjo SA. Serum retinol concentrations demonstrate high specificity after correcting for inflammation but questionable sensitivity compared with liver stores calculated from isotope dilution in determining vitamin A deficiency in Thai and Zambian children. Am J Clin Nutr 2015;102:1259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thurnham DI, McCabe GP, Northrop-Clewes CA, Nestel P. Effects of subclinical infection on plasma retinol concentrations and assessment of prevalence of vitamin A deficiency: meta-analysis. Lancet 2003;362:2052–8. [DOI] [PubMed] [Google Scholar]
- 5. Dagadu JM. Distribution of carotene and vitamin A in liver, pancreas and body fat of Ghanaians. Br J Nutr 1967;21:453–6. [DOI] [PubMed] [Google Scholar]
- 6. Blaner WS, Obunike JC, Kurlandsky SB, al-Haideri M, Piantedosi R, Deckelbaum RJ, Goldberg IJ. Lipoprotein lipase hydrolysis of retinyl ester. Possible implications for retinoid uptake by cells. J Biol Chem 1994;269:16559–65. [PubMed] [Google Scholar]
- 7. La Frano MR, Zhu C, Burri BJ. Assessment of tissue distribution and concentration of β-cryptoxanthin in response to varying amounts of dietary β-cryptoxanthin in the Mongolian gerbil. Br J Nutr 2013;111:968–78. [DOI] [PubMed] [Google Scholar]
- 8. Rautalahti M, Albanes D, Hyvönen L, Piironen V, Heinonen M. Effect of sampling site on retinol, carotenoid, tocopherol, and tocotrienol concentration of adipose tissue of human breast with cancer. Ann Nutr Metab 1990;34:37–41. [DOI] [PubMed] [Google Scholar]
- 9. Tsutsumi C, Okuno M, Tannous L, Piantedosi R, Allan M, Goodman DS, Blaner WS. Retinoids and retinoid-binding protein expression in rat adipocytes. J Biol Chem 1992;267:1805–10. [PubMed] [Google Scholar]
- 10. Escaron AL, Green MH, Tanumihardjo SA. Plasma turnover of 3,4-didehydroretinol (vitamin A2) increases in vitamin A-deficient rats fed low versus high dietary fat. J Lipid Res 2009;50:694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ribaya-Mercado JD, Fox JG, Rosenblad WD, Blanco MC, Russell RM. β-Carotene, retinol and retinyl ester concentrations in serum and selected tissues of ferrets fed β-carotene. J Nutr 1992;122:1898–903. [DOI] [PubMed] [Google Scholar]
- 12. Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. Impaired retinal function and vitamin A availability in mice lacking retinol binding protein. EMBO J 1999;18:4633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Biesalski HK, Frank J, Beck SC, Heinrich F, Illek B, Reifen R, Gollnick H, Seeliger MW, Wissinger B, Zrenner E. Biochemical but not clinical vitamin A deficiency results from mutations in the gene for retinol binding protein. Am J Clin Nutr 1999;69:931–6. [DOI] [PubMed] [Google Scholar]
- 14. Riabroy N, Dever JT, Tanumihardjo SA. α-Retinol and 3,4-didehydroretinol support growth in rats when fed at equimolar amounts and α-retinol is not toxic after repeated administration of large doses. Br J Nutr 2013;111:1373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tanumihardjo SA, Howe JA. Twice the amount of α-carotene isolated from carrots is as effective as β-carotene in maintaining the vitamin A status of Mongolian gerbils. J Nutr 2005;135:2622–6. [DOI] [PubMed] [Google Scholar]
- 16. Dever JT, Surles RL, Davis CR, Tanumihardjo SA. α-Retinol is distributed through serum retinol-binding protein-independent mechanisms in the lactating sow-nursing piglet dyad. J Nutr 2011;141:42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gannon BM, Pungarcher I, Mourao L, Davis CR, Simon P, Pixley KV, Tanumihardjo SA. 13C natural abundance of serum retinol is a novel biomarker for evaluating provitamin A carotenoid-biofortified maize consumption in male Mongolian gerbils. J Nutr 2016;146:1290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee CM, Lederman JD, Hofmann NE, Erdman JJW. The Mongolian gerbil (Merionesunguiculatus) is an appropriate animal model for evaluation of the conversion of β-carotene to vitamin A. J Nutr 1998;128:280–6. [DOI] [PubMed] [Google Scholar]
- 19. Bresnahan KA, Davis CR, Tanumihardjo SA. Relative vitamin A values of 9-cis- and 13-cis-β-carotene do not differ when fed at physiological levels during vitamin A depletion in Mongolian gerbils (Merionesunguiculatus). Br J Nutr 2014;112:162–9. [DOI] [PubMed] [Google Scholar]
- 20. Surles RL, Mills JP, Valentine AR, Tanumihardjo SA. One-time graded doses of vitamin A to weanling piglets enhance hepatic retinol but do not always prevent vitamin A deficiency. Am J Clin Nutr 2007;86:1045–53. [DOI] [PubMed] [Google Scholar]
- 21. Gannon BM, Valentine AR, Davis CR, Howe JA, Tanumihardjo SA. Duration of retinol isotope dilution studies with compartmental modeling affects model complexity, kinetic parameters, and calculated vitamin A stores in US women. J Nutr 2018;148:1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 1957;226:497–509. [PubMed] [Google Scholar]
- 23. Cequier-Sánchez E, Rodríguez C, Ravelo ÁG, Zárate R. Dichloromethane as a solvent for lipid extraction and assessment of lipid classes and fatty acids from samples of different natures. J Agric Food Chem 2008;56:4297–303. [DOI] [PubMed] [Google Scholar]
- 24. Parker RS. Carotenoid and tocopherol composition of human adipose tissue. Am J Clin Nutr 1988;47:33–6. [DOI] [PubMed] [Google Scholar]
- 25. Mondloch S, Tanumihardjo SA, Davis CR, van Jaarsveld PJ. Vervets (Chlorocebusaethiops) consuming oil palm-derived carotenoids have higher hepatic vitamin A concentrations than controls. J Am Assoc Lab Anim Sci 2018;57:456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tanumihardjo SA. Synthesis of 10,11,14,15-13C4-and 14,15-13C2-retinyl acetate. J Labelled Comp Radiopharm 2001;44:365–72. [Google Scholar]
- 27. R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2005. Available from: http://www.R-project.org. [Google Scholar]
- 28. Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–5. [DOI] [PubMed] [Google Scholar]
- 29. Heatley JJ, Harris MC. Hamsters and gerbils. In: Tully TN, editor. Manual of exotic pet practice. St. Louis, MO: Saunders Elsevier; 2009. p. 406–32. [Google Scholar]
- 30. Norseen J, Hosooka T, Hammarstedt A, Yore MM, Kant S, Aryal P, Kiernan UA, Phillips DA, Maruyama H, Kraus BJ, et al. Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Mol Cell Biol 2012;32:2010–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee S-A, Yuen JJ, Jiang H, Kahn BB, Blaner WS. Adipocyte-specific overexpression of retinol-binding protein 4 causes hepatic steatosis in mice. Hepatology 2016;64:1534–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berry DC, Jacobs H, Marwarha G, Gely-Pernot A, O'Byrne SM, DeSantis D, Klopfenstein M, Feret B, Dennefeld C, Blaner WS, et al. The STRA6 receptor is essential for retinol-binding protein-induced insulin resistance but not for maintaining vitamin A homeostasis in tissues other than the eye. J Biol Chem 2013;288:24528–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Romans DA, Barua AB, Olson JA. Pharmacokinetics of all-trans retinoyl beta-glucuronide in rats following intraperitoneal and oral administration. Int J Vitam Nutr Res 2003;73:251–7. [DOI] [PubMed] [Google Scholar]
- 34. Virtanen SM, van't Veer P, Kok F, Kardinaal AF, Aro A. Predictors of adipose tissue carotenoid and retinol levels in nine countries. The EURAMIC study. Am J Epidemiol 1996;144:968–79. [DOI] [PubMed] [Google Scholar]
- 35. Lunetta JM, Zulim RA, Dueker SR, Lin Y, Flaig V, Schneider PD, Wolfe BM, Clifford AJ. Method for the simultaneous determination of retinol and β-carotene concentrations in human tissues and plasma. Anal Biochem 2002;304:100–9. [DOI] [PubMed] [Google Scholar]
- 36. Scott J, Raica JN, Lowry L, Sauberlich HE. Vitamin A concentration in human tissues collected from five areas in the United States. Am J Clin Nutr 1972;25:291–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.