Abstract
The objective of this study was to evaluate the effect of sodium butyrate (SB) infusion on rumen papillae growth and volatile fatty acid (VFA) uptake and metabolism in neonatal lambs. Seven pairs of newborn twin lambs were used. Within each pair, lambs were assigned to receive an oral infusion of SB at 0.36 g/kg body weight (BW) (SB, n = 7) or the same volume of saline (Con, n = 7). Treatments were administered from 10 to 49 d of age, when all lambs were slaughtered. Results showed that the average daily feed intake (ADFI) of starter, average daily gain (ADG), BW of lambs at ages of 5 and 6 wk in SB group were greater (P < 0.05) than those in Con group. Infusion of SB increased (P < 0.05) the concentrations of acetate, butyrate, and total VFA in the rumen fluid and elevated (P < 0.05) the levels of β-hydroxybutyrate acid (BHBA), insulin-like growth factor-1 (IGF-1), and insulin in plasma. Infusion of SB promoted rumen papillae growth, depicted by higher emptied rumen weight, larger rumen papillae length, width, and surface area, and greater thickness of stratum corneum and total epithelium. Sodium butyrate infusion upregulated (P < 0.05) mRNA expression of cyclin A2, cyclin D1, and cyclin-dependent kinases 6 (CDK6), and downregulated (P < 0.05) mRNA expression of caspase-3 and Bcl-2-associated X protein (Bax) in the rumen epithelia. Moreover, SB infusion also upregulated (P < 0.05) mRNA expression of insulin-like growth factor-1 receptor (IGF-1R), and insulin-like growth factor-binding protein 5 (IGFBP-5), and downregulated (P < 0.05) mRNA expression of insulin-like growth factor-binding protein 3 (IGFBP-3) in the rumen epithelia. Sodium butyrate infusion also enhanced (P < 0.05) gene expressions of monocarboxylate transporter isoform 1 (MCT1), downregulated in adenoma (DRA), 3-hydroxy-3-methylglutaryl-CoA synthase isoform 2 (HMGCS2), and 3-hydroxy-3-methylglutaryl-CoA lyase (HMGCL), while depressed (P < 0.05) mRNA expression of sodium/proton exchanger isoform 2 (NHE2) in the rumen epithelia. Our results suggest that the SB infusion can improve animal performance, promote the ruminal papillae growth, and enhance expression of genes related to ruminal epithelial VFA uptake and metabolism in preweaning twin lambs. These findings provide a better understanding of the molecular mechanism of SB promoting rumen epithelial development and function in preweaning lambs.
Keywords: cell apoptosis, cell cycle, lamb, rumen papillae growth, sodium butyrate, volatile fatty acid uptake and metabolism
INTRODUCTION
In young ruminants, the rumen is not fully developed and is unprepared for the intake and digestion of solid food (Gorka et al., 2018). Many previous studies showed that promoting the rumen development in early life will help facilitate early weaning, reduce weaning stress, and allow for optimal performance (Ferreira and Bittar, 2011; Sun et al., 2018). Therefore, accelerating the rumen development and maturation during early life through nutritional strategies may have great potential for long-term animal performance and health in ruminants.
Numerous studies have demonstrated that exogenous sodium butyrate (SB) could improve animal performance and rumen papillae growth at a morphology level in calves (Górka et al., 2018) and lambs (Cavini et al., 2015). However, the molecular mechanism by which supplementation with SB facilitates ruminal papillae growth in lambs is not well understood. Reports showed that exogenous butyrate facilitated rumen epithelial growth by enhancing cell cycle in goats (Malhi et al., 2013) and reducing apoptosis in calves (Mentschel et al., 2001). In addition, morphological development may accompany the molecular adaptation of nutrients absorption and metabolism in the rumen epithelium of young ruminants. However, the effect of SB supplementation on ruminal VFA absorption and metabolism is also largely unexplored in sheep lambs.
In order to address the knowledge gaps, this study will adopt the model of natural ewe’s milk-feeding and twin lambs, which diminishes potential differences among experimental animals due to the genetic similarities between twins, to: 1) evaluate the effect of the infusion of SB on the ruminal development including morphological changes and mRNA expression of genes related to cell proliferation and apoptosis; and 2) to investigate the changes in expression of genes involved in VFA absorption and metabolism of rumen epithelium after the infusion of SB.
MATERIALS AND METHODS
The experimental design and process for this study was followed according to the Animal Care and Use Guidelines of the Animal Care Committee, Nanjing Agricultural University, Nanjing, China.
Experimental Design and Animal Management
This animal experiment was conducted using Hu sheep at a commercial farm in Jiangsu Province of China from November to December 2016. Seven pairs of newborn twin Hu lambs, born in continuous 3 d, were chosen. At 10 d of age, 1 lamb from each pair was randomly assigned to receive an oral infusion of SB (Cayman Chemical, Ann Arbor, MI) at 1.8 mL/kg body weight (BW) (0.36 g/kg BW) (SB, n = 7), while the other lamb was given the same volume infusion of saline (Con, n = 7). Treatments were administered from 10 to 49 d of age at 0800 h, according to the previous study on the goats (Malhi et al., 2013). The SB powder was dissolved in physiological saline at a concentration of 0.2 g/mL. The infusion of all lambs was finished within 20 min, and the amount of infusion was adjusted weekly according to BW. The lambs were separated from their mothers (0500 to 1100, 1230 to 1530, and 1700 to 2000 h) every day and fed in individual pens during the infusion period. Meanwhile, the lambs sucked ewe’s milk at fixed times (3 times daily: 1100 to 1230, 1530 to 1700, and 2000 to 0500 h the next day). Fresh starter (Table 1) was delivered 3 times daily (0530, 1230, and 1730 h) to ensure that all lambs had starter available at all times, and alfalfa (18.09% crude protein, 26.06% crude fiber) and oat grass (10.05% crude protein, 28.71% crude fiber) were provided ad libitum during the experimental period. All lambs had free access to water. No lambs could contact the ewes’ concentrated feed. The starter intake of lambs was recorded per day at the beginning of 14 d of age. The BW of lambs was measured at 7 d of age and subsequently measured every 7 d before the morning feeding of starter until sacrifice. The starter diet was designed to meet the nutrient requirements according to the NRC (2007). At 49 d of age, the lambs were sacrificed 2 to 3 h after the final SB infusion with 2, 2, and 3 pairs of lambs sacrificed at the first, second, and third slaughter day, respectively, depending on the different birth days. During the slaughter day, when sampling of a pair was completed, experimental procedures began in the next pair of lambs. Hence, all lambs were sacrificed within 1 h within each day.
Table 1.
Ingredient and nutrient composition of the starter diet
| Item | Value |
|---|---|
| Ingredient composition (dry matter basis) | |
| Corn starch, % | 50.69 |
| Soybean meal, % | 27.75 |
| Corn gluten meal, % | 14.86 |
| Soybean oil, % | 1.61 |
| Limestone meal, % | 0.76 |
| NaCl, % | 0.58 |
| Calcium hydrogen phosphate, % | 1.75 |
| Premix1, % | 2.00 |
| Nutrient composition (dry matter basis)2 | |
| Dry matter, % | 90.14 |
| Metabolic energy, MJ/kg | 15.47 |
| Crude protein, % | 17.53 |
| Crude fat, % | 3.16 |
| Crude fiber, % | 2.50 |
| Neutral detergent fiber, % | 5.68 |
| Acid detergent fiber, % | 3.75 |
| Ash, % | 2.07 |
| Starch, % | 53.61 |
| Calcium, % | 0.97 |
| Phosphate, % | 0.62 |
1Per kg premix contains: Fe 3.0 g, Zn 5.0 g, Cu 0.5 g, Mn 3.0 g, Co 0.1 g, I 50 mg, Se 40 mg, vitamin A 500,000 IU, vitamin D 50,000 IU, and vitamin E 2,000 IU.
2Dry matter, crude protein, crude fat, ash, calcium, and phosphate were analyzed according to the Association of Official Analytical Chemists (AOAC, 2000), acid detergent fiber, and neutral detergent fiber were analyzed according to the procedures of Van Soest et al. (1991), starch content was analyzed using Total Starch Kit, whereas metabolic energy was calculated according to the NRC (2007).
Chemical Analysis
Samples of starter were mixed thoroughly and analyzed for dry matter (DM) (method 930.15), crude protein (method 984.13), crude fat (method 920.02), crude fiber (method 978.10), ash (method 942.05), and minerals (method 985.01) content according to the Association of Official Analytical Chemists (AOAC, 2000). Acid detergent fiber (ADF) and neutral detergent fiber (NDF) were analyzed according to the procedures of Van Soest et al. (1991) using an ANKOM Fiber Analyzer (ANKOM Technology Corporation, Fairport, NY). Starch content was analyzed using Total Starch Kit (Megazyme, Bray, Ireland). All analyses are reported on a dry matter (DM) basis.
Sample Collection
Before they were sacrificed, blood was sampled from the jugular vein and then taken into Na-heparin tubes, which contained 40 KIU/mL of blood. Blood samples were centrifuged at 1,000 × g at 4 °C for 10 min to obtain the plasma and then stored at −20 °C until analysis for β-hydroxybutyrate acid (BHBA), insulin-like growth factor-1 (IGF-1), glucose and insulin concentrations. After the blood harvest, the lambs were immediately stunned by captive bolt, sacrificed by exsanguinations in the slaughterhouse, and then eviscerated to obtain the rumen. The rumen including contents were weighed, and then the rumen content was removed and washed with ice-cold saline, the emptied rumen was reweighed. A representative sample of at least 200 mL rumen content was gathered, and the pH value was determined. The rumen content was filtered through 4 layers of cheesecloth. Each 5 mL of ruminal fluid was preserved by adding 1 mL of 25% (wt/vol) metaphosphoric acid and then stored at −20 °C until later analysis of VFA concentration. A representative segment of rumen ventral sac tissues was sampled and washed clean with ice-cold saline. Images of the rumen tissues were taken with a camera (Canon 750d; Canon, Tokyo, Japan) and then 1 portion was cut into pieces that were less than 0.5 cm2, and immediately frozen in liquid nitrogen for RNA extraction. Another portion rumen tissues (1 cm2) was immediately fixed in 4% paraformaldehyde (PFA) (Sigma, St. Louis, MO) for morphological analysis.
Rumen and Blood Plasma Parameters Determination
The ruminal pH value was determined using a portable pH meter (HI 9024C; HANNA Instruments, Woonsocket, RI). Concentration of VFA was analyzed using gas chromatography (GC-14B, Shimadzu, Japan) (Qin, 1982). The BHBA concentration in plasma was measured with the BHBA test kit (Jiancheng Bioengineering Institute, Nanjing, China). The plasma glucose concentration was analyzed using the glucose oxidase-peroxidase method (Rongsheng Biological Pharmaceutical, Shanghai, China) as described previously (Penner et al., 2009). The plasma insulin concentration was analyzed using a sheep insulin ELISA kit (AngleGene BioTechnology Co. Ltd, Nanjing, China). The plasma IGF-1 concentration was determined using sheep IGF-1 ELISA kit (Abbkine Scientific Co., Ltd, California) based on the method described by Sugino et al. (2004). All sample concentrations were measured in triplicate and the intra-assay coefficients of variations were <6%.
Determination of Rumen Papillae Morphology
Five samples of rumen epithelium from the ventral sac of each lamb were prepared for light microscopy histomorphometric analysis, based on the methods previously described by Odongo et al. (2006). The papillary samples were PFA-fixed, embedded in paraffin, cut into 6 μm thickness, and stained with hematoxylin and eosin. The standard sectioning procedure was performed as described previously (Holle and Birtles, 1990). The microscopist was blinded to treatment conditions during the histomorphometric analysis. The length and width of the papillae were measured using Image Pro Plus software (Media Cybernetics, Bethesda, MD). The density of papillae (1 × 1 cm) was determined using a standing magnifying glass with 2.5× magnification (MG3B-1A, Shanghai, China). The surface area of papillae/cm2 was calculated as length × width × density of the papillae (number of papillae/cm2) × 2, whereas the 2-fold multiplying factor is to account for the front and back sides of papillae. Each stratum was measured under a 40× objective lens, and 5 different areas of each rumen papillae were captured for a total of 25 replicates per measurement per animal. Image Pro Plus software (Media Cybernetics, Bethesda, MD) was used to measure the thickness of the stratum corneum, stratum granulosum, stratum spinosum, and stratum basale according to the predefined criteria previously described by Malhi et al. (2013).
Total RNA Extract and cDNA Synthesis
Total RNA was extracted from homogenized rumen epithelium tissue using RNAiso Plus (Takara Bio, Otsu, Japan) as described by the manufacturer’s instructions. The concentration and purity of extracted RNA were monitored using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Madison, WI). All samples had an absorption ratio of 260/280 nm between 1.80 and 2.10, which indicates high RNA purity. The rRNA integrity was assessed using 1.4% agarose-formaldehyde gel. Synthesis of cDNA was performed using 1 μg of total RNA per 20-μL sample reaction and the Prime Script RT reagent kit with gDNA Eraser (Takara Bio, Otsu, Japan) according to the manufacturer’s instructions. The reaction conditions were as follows: 2 min at 42 °C, 15 min at 37 °C, and 5 s at 85 °C. Negative control reactions in the absence of reverse transcriptase were performed on each sample to verify the absence of genomic DNA contamination of the RNA samples.
Primer Design and Quantitative Real-Time PCR
The primers for cyclin A2, cysteinyl aspartate specific proteinase 8 (caspase-8), putative anion transporter isoform 1 (PAT1), downregulated in adenoma (DRA), β-hydroxybutyrate dehydrogenase-1 (BDH1), β-Hydroxybutyrate dehydrogenase-2 (BDH2), insulin-like growth factor-1 receptor (IGF-1R), sodium/proton exchanger isoform 1 (NHE1), sodium/proton exchanger isoform 2 (NHE2), sodium/proton exchanger isoform 3 (NHE3), monocarboxylate transporter isoform 1 (MCT1), monocarboxylate transporter isoform 4 (MCT4), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as described in the published literature (Supplementary Table S1). The primers for the others were designed by Primer Premier 6 computer program (Premier Biosoft International, Palo Alto, CA; www.premierbiosoft.com) and were identified using the BLAST (Basic Local Alignment Search Tool) computer program (National Center for Biotechnology Information, Bethesda, MD; https://blast.ncbi.nlm.nih.gov/Blast.cgi). All primers used in this study were commercially synthesized by Invitrogen Life Technologies (Shanghai, China). The relative mRNA expression of target genes and GAPDH were evaluated using the ABI 7500 Real-Time PCR Instrument (Applied Biosystems, Foster City, CA) and analyzed using QuantStudio version 1.4 software (Thermo Fisher Scientific, Shanghai, China). The quantitative real-time PCRs were performed in a 20 μL reaction mixture and contained 2 μL cDNA, 0.8 μL primer (the final primer concentration was 0.4 μM in the reaction), and SYBR Green PCR Master Mix (Takara Bio, Otsu, Japan) as a fluorescent dye. The amplification conditions were as follows: 30 s at 95 °C followed by 40 cycles composed of 5 s at 95 °C, 34 s at 60 °C, 15 s at 95 °C, 60 s at 60 °C, and 15 s at 95 °C. A reverse-transcription-negative blank of each sample and a no-template blank served as negative controls. Melt curve analysis showed no primer dimer formation in the assays. The PCR amplification efficiencies of all the primers ranged between 92.5% and 106.0% and linearity of standard curves was acceptable (R2 > 0.994). Final PCR products were sequenced to verify their identity (Invitrogen Biological Technologies, Shanghai, China) and all amplicons were verified as 100% homologous to their target sequence. The primers, amplicon sizes, and amplification efficiencies of all genes are presented in Supplementary Table S1. All samples were performed in triplicate. Relative quantification of target gene expression was normalized to GAPDH. The relative mRNA expression was analyzed according to the 2−ΔΔCT method (He et al., 2014).
Statistical Analysis
The results are presented as means ± standard error of the means (SEM). The data of BW were analyzed by repeated measures analysis of variance (ANOVA) of SPSS software (version 25, SPSS Inc., Chicago, IL). The paired sample t-test was applied to the analysis of statistical significance of BW at specific time and other measurements using SPSS version 25 software (SPSS Inc., Chicago, IL). Results with P < 0.05 were considered significant differences and 0.05 < P < 0.10 was defined tendency to be different between the Con group and the SB group.
RESULTS
Animal Performance
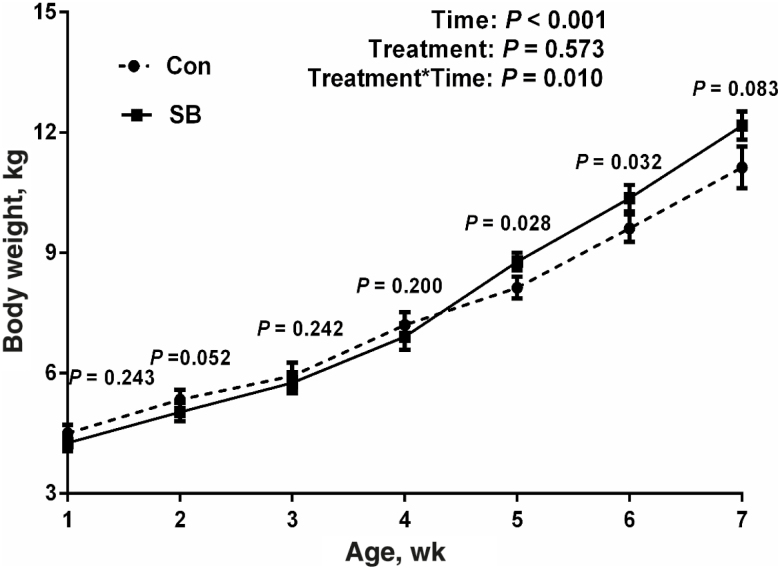
At 7 d of age, there was no significant difference (P = 0.243) in the body weight between the lambs in the Con group (4.50 ± 0.21 kg) and those in the SB group (4.30 ± 0.20 kg). Sodium butyrate infusion increased the average daily feed intake (ADFI) of starter (103.39 ± 3.47 vs. 118.29 ± 2.08 g/d, P = 0.010) and average daily gain (ADG) (165.31 ± 13.41 vs. 204.08 ± 8.66 g/d, P = 0.022) in the lambs at the ages of 2 to 7 wk. As shown in Fig. 1, results showed that time (P < 0.001) and interaction between treatment and time (P = 0.010) significantly affected the BW of lambs at the ages of 1 to 7 wk. The BW of the lambs in the SB group was greater than that of lambs in the Con group at the ages of 5 wk (P = 0.028), 6 wk (P = 0.032). In addition, infusion of SB also tended to increase the BW of lambs at the ages of 7 wk (P = 0.083).
Figure 1.
The effects of oral infusion of sodium butyrate at 0.36 g/kg body weight (BW) once daily for 39 d on BW of the lambs at different ages. The BW of lambs was measured at 7 d of age and subsequently measured every 7 d before the morning feeding of starter until sacrifice. The values are means ± SEM, n = 7.
Rumen and Blood Plasma Parameters
As shown in Table 2, an oral infusion with SB increased emptied rumen weight (P = 0.043), total VFA (P = 0.020), acetate (P = 0.048), butyrate (P = 0.002), and butyrate proportion (P = 0.001). Moreover, the rumen weight (P = 0.074) and emptied rumen weight/live BW (P = 0.071) also had an increased trend in the lambs, but the rumen weight/live BW (P = 0.119), rumen pH (P = 0.344), propionate (P = 0.237), acetate proportion (P = 0.249), propionate proportion (P = 0.722), and acetate/propionate (P = 0.707) showed no significant change between the 2 groups. Meanwhile, SB supplementation increased the concentration of BHBA (P = 0.009), IGF-1 (P = 0.010), and insulin (P = 0.003) in the blood plasma, whereas there was no significant difference in the plasma glucose concentration (P = 0.524) of preweaning lambs.
Table 2.
The effects of oral infusion of sodium butyrate at 0.36 g/kg body weight once daily for 39 d on rumen parameters and blood parameters in preweaning lambs1
| Item | Con | SB | P-value |
|---|---|---|---|
| Ruminal parameters | |||
| Rumen weight, kg | 1.25 ± 0.13 | 1.59 ± 0.08 | 0.074 |
| Emptied rumen weight, kg | 0.21 ± 0.02 | 0.25 ± 0.01 | 0.043 |
| Rumen weight/live body weight, % | 11.05 ± 0.91 | 13.03 ± 0.36 | 0.119 |
| Emptied rumen weight/live body weight, % | 1.85 ± 0.08 | 2.02 ± 0.05 | 0.071 |
| pH | 5.81 ± 0.11 | 5.64 ± 0.20 | 0.344 |
| Total VFA2, mM | 102.57 ± 1.29 | 110.31 ± 2.04 | 0.020 |
| Acetate, mM | 55.70 ± 1.20 | 58.04 ± 0.61 | 0.048 |
| Propionate, mM | 34.69 ± 1.16 | 36.96 ± 1.67 | 0.237 |
| Butyrate, mM | 9.38 ± 0.34 | 12.65 ± 0.42 | 0.002 |
| Acetate, mol/100 mol of VFA | 54.31 ± 1.02 | 52.71 ± 0.98 | 0.249 |
| Propionate, mol/100 mol of VFA | 33.82 ± 1.07 | 33.43 ± 0.97 | 0.722 |
| Butyrate, mol/100 mol of VFA | 9.09 ± 0.26 | 12.40 ± 0.42 | 0.001 |
| Acetate: Propionate | 1.62 ± 0.08 | 1.59 ± 0.07 | 0.707 |
| Blood parameters | |||
| Plasma BHBA3, mmol/Liter | 0.23 ± 0.04 | 0.36 ± 0.02 | 0.009 |
| Plasma IGF-14, ng/mL | 265.73 ± 8.39 | 320.49 ± 8.85 | 0.010 |
| Plasma glucose, mmol/Liter | 4.15 ± 0.12 | 4.30 ± 0.15 | 0.524 |
| Plasma insulin, μIU/mL | 24.07 ± 1.00 | 27.27 ± 1.12 | 0.003 |
1The values shown are means ± SEM; Con (n = 7), SB (n = 7); rumen and blood samples were collected 2 to 3 h after the final SB infusion at the ages of 49 d in lambs. P < 0.05 indicated that mean values were significantly difference between the control group (Con) and the sodium butyrate group (SB).
2VFA = volatile fatty acids.
3BHBA = beta-hydroxybutyrate.
4IGF-1 = insulin-like growth factor-1.
Morphology of Rumen Papillae
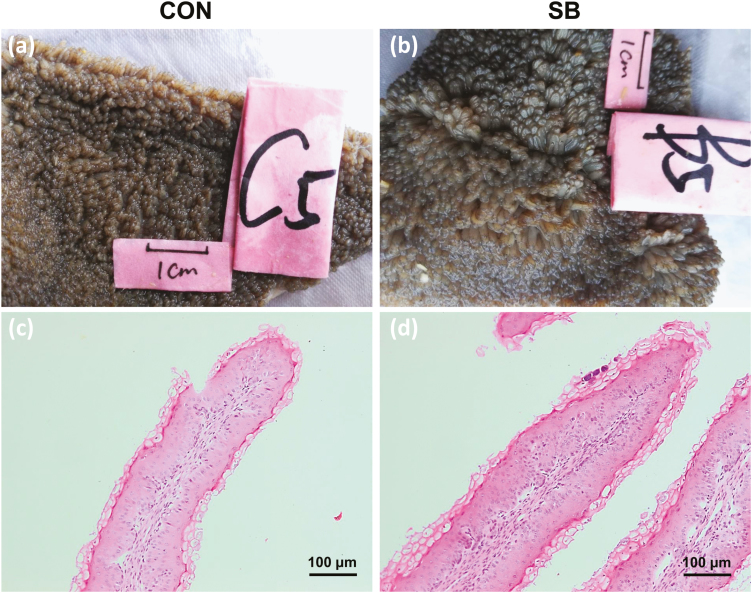
As shown in Fig. 2 and Table 3, the ruminal papillae length (P = 0.017), width (P = 0.004), and surface area (P = 0.002) were increased in response to the infusion of SB. However, the density (P = 0.291) of the rumen papillae showed no significant change between the Con group and the SB group. The thicknesses of total epithelium (P = 0.048) and stratum corneum (P = 0.017) in the rumen epithelium were greater in the SB group than those in the Con group. In addition, the thickness of stratum granulosum (P = 0.054) in the SB group also had an increased trend in the rumen epithelium, whereas the thickness of stratum spinosum + stratum basale (P = 0.574) showed no difference in the rumen epithelium between the 2 groups.
Figure 2.
The effects of oral infusion of sodium butyrate at 0.36 g/kg body weight once daily for 39 d on ruminal papillae morphology in preweaning lambs. Rumen tissues were collected 2 to 3 h after the final SB infusion at the ages of 49 d in lambs. Representative rumen epithelial visual graph (a, b) and micrograph (c, d) of the lambs between the control group (Con) and the sodium butyrate group (SB). Visual images of the rumen tissues were taken with a camera. Micrographs of rumen papillae were captured under a 10× objective lens of microscope.
Table 3.
The effects of oral infusion of sodium butyrate at 0.36 g/kg body weight once daily for 39 d on papillae morphology and the thickness of different stratum of rumen papillae in preweaning lambs1
| Item | Con | SB | P-value |
|---|---|---|---|
| Papillae morphology | |||
| Length, mm | 2.76 ± 0.07 | 3.33 ± 0.20 | 0.017 |
| Width, mm | 1.13 ± 0.05 | 1.31 ± 0.04 | 0.004 |
| Density, n/cm2 | 144 ± 1.38 | 139 ± 3.41 | 0.291 |
| Surface area2, mm2/cm2 | 898.27 ± 48.56 | 1,205.13 ± 45.42 | 0.002 |
| Thickness of different stratum3 | |||
| Total epithelium, μm | 116.56 ± 4.16 | 129.03 ± 4.87 | 0.048 |
| Stratum corneum, μm | 21.54 ± 0.69 | 26.68 ± 1.44 | 0.017 |
| Stratum granulosum, μm | 19.41 ± 0.66 | 22.97 ± 0.93 | 0.054 |
| Stratum spinosum and basale, μm | 75.61 ± 3.63 | 79.37 ± 3.06 | 0.574 |
1The values shown are means ± SEM; Con (n = 7), SB (n = 7); samples of rumen tissue were collected 2 to 3 h after the final SB infusion at the ages of 49 d in lambs.
2The surface area of papillae/cm2 was calculated as length × width × density of the papillae (number of papillae/cm2) × 2, whereas the 2-fold multiplying factor is to account for the front and back sides of papillae.
3Each stratum was measured under a 40× objective lens, and 5 different areas of each rumen papillae were captured for a total of 25 replicates per measurement per animal. P < 0.05 indicated that mean values were significantly difference between the control group (Con) and the sodium butyrate group (SB).
mRNA Expression Analyses
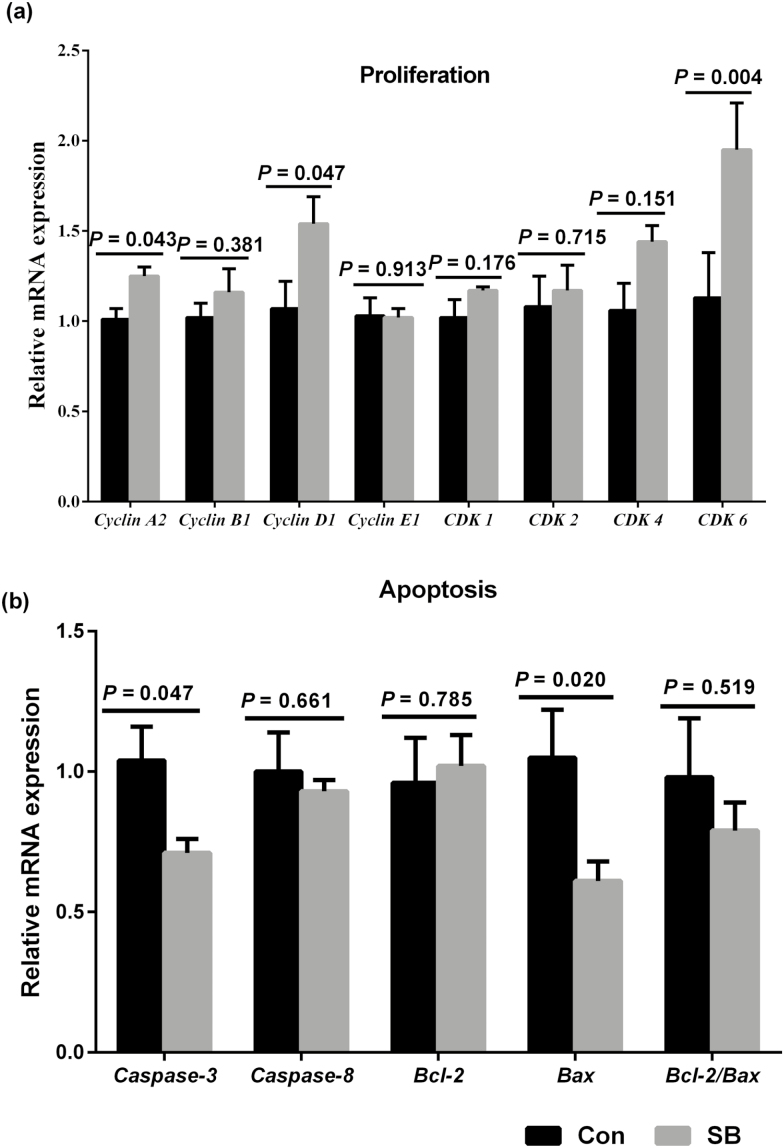
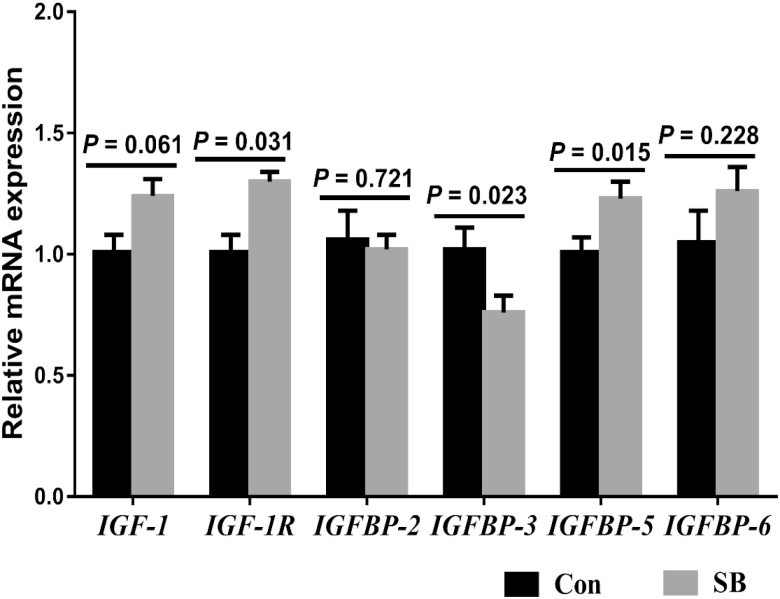
In cell proliferation-related genes (Fig. 3a), the SB treatment upregulated the cyclin A2 (P = 0.043), cyclin D1 (P = 0.047), and CDK6 (P = 0.004) expression, whereas there was no significant difference in the mRNA expression of cyclin B1 (P = 0.381), cyclin E1 (P = 0.913), CDK1 (P = 0.176), CDK2 (P = 0.715), and CDK4 (P = 0.151). Meanwhile, in cell apoptosis-related genes (Fig. 3b), we found that SB supplementation decreased the caspase-3 (P = 0.047) and Bax (P = 0.020) expression, while there was no significant difference in the expression of caspase-8 (P = 0.661), Bcl-2 (P = 0.785), and Bcl-2/Bax (P = 0.519). The SB treatment increased the mRNA expression of IGF-1R (P = 0.031) and IGFBP-5 (P = 0.015), decreased the mRNA expression of IGFBP-3 (P = 0.023), and tended to increase IGF-1 mRNA expression (P = 0. 061) in the rumen epithelium (Fig. 4). However, there was no significant difference in the mRNA expression of IGFBP-2 (P = 0.721) and IGFBP-6 (P = 0.228).
Figure 3.
The effects of oral infusion of sodium butyrate at 0.36 g/kg body weight once daily for 39 d on messenger RNA (mRNA) expression of genes involved in rumen epithelial proliferation (a) and apoptosis (b) in preweaning lambs. Rumen tissues were collected 2 to 3 h after the final SB infusion at the ages of 49 d in lambs. Quantitative RT-PCR results were expressed as relative mRNA expression (fold of Con group, normalized to GAPDH) and the data were analyzed by the 2−ΔΔCT method. The values are means ± SEM, n = 7. CDK1 = cyclin-dependent kinase 1; CDK2 = cyclin-dependent kinase 2; CDK4 = cyclin-dependent kinase 4; CDK6 = cyclin-dependent kinase 6; caspase-3 = cysteinyl aspartate specific proteinase 3; caspase-8 = cysteinyl aspartate specific proteinase 8; Bax = Bcl-2-associated X protein; Bcl-2 = B-cell lymphoma 2.
Figure 4.
The effects of oral infusion of sodium butyrate at 0.36 g/kg body weight once daily for 39 d on rumen epithelial IGF-1 and IGF-binding protein-related genes messenger RNA (mRNA) expression in preweaning lambs. Rumen tissues were collected 2 to 3 h after the final SB infusion at the ages of 49 d in lambs. Quantitative RT-PCR results were expressed as relative mRNA expression (fold of Con group, normalized to GAPDH) and the data were analyzed by the 2−ΔΔCT method. The values are means ± SEM, n = 7. IGF-1 = insulin-like growth factor-1; IGF-1R = insulin-like growth factor-1 receptor; IFGBP-2 = insulin-like growth factor-binding protein 2; IFGBP-3 = insulin-like growth factor-binding protein 3; IFGBP-5 = insulin-like growth factor-binding protein 5; IFGBP-6 = insulin-like growth factor-binding protein 6.
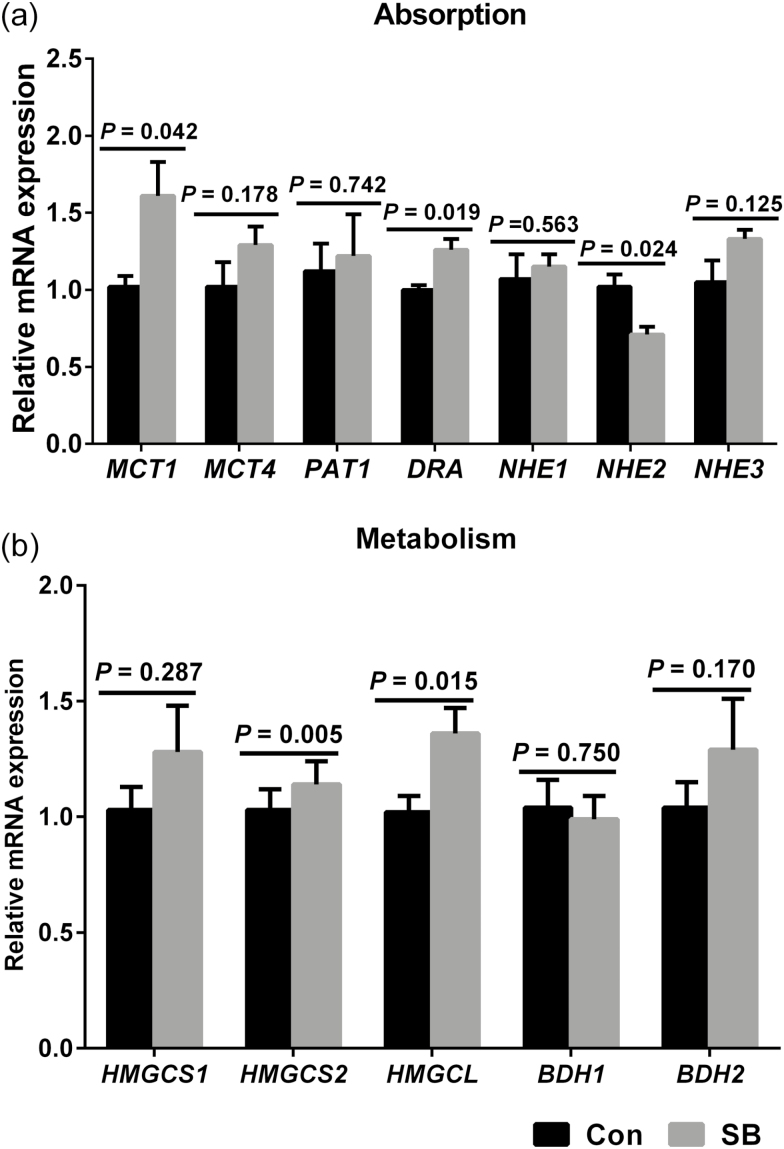
As shown in Fig. 5, SB treatment increased the MCT1 (P = 0.042), DRA (P = 0.019), HMGCS2 (P = 0.005), and HMGCL (P = 0.015) expressions of genes involved in VFA absorption and metabolism in the rumen epithelium. However, NHE2 expression (P = 0.024) was decreased in the SB treatment group. No significant difference in the mRNA expression of MCT4 (P = 0.178), PAT1 (P = 0.742), NHE1 (P = 0.563), NHE3 (P = 0.125), HMGCS1 (P = 0.287), BDH1 (P = 0.750), and BDH2 (P = 0.170) were observed in the present study.
Figure 5.
The effects of oral infusion of sodium butyrate at 0.36 g/kg body weight once daily for 39 d on messenger RNA (mRNA) expression of genes involved in ruminal epithelial VFA absorption (a) and metabolism (b) in preweaning lambs. Rumen tissues were collected 2 to 3 h after the final SB infusion at the ages of 49 d in lambs. Quantitative RT-PCR results are expressed as relative mRNA expression (fold of Con group, normalized to GAPDH), and the data were analyzed by the 2−ΔΔCT method. The values are means ± SEM, n = 7. MCT1 = monocarboxylate transporter isoform 1; MCT4 = monocarboxylate transporter isoform 4; PAT1 = putative anion transporter isoform 1; DRA = downregulated in adenoma; NHE1 = sodium/proton exchanger isoform 1; NHE2 = sodium/proton exchanger isoform 2; NHE3 = sodium/proton exchanger isoform 3; HMGCS1 = 3-hydroxy-3-methylglutaryl-CoA synthase isoform 1; HMGCS2 = 3-hydroxy-3-methylglutaryl-CoA synthase isoform 2; HMGCL = 3-hydroxy-3-methylglutaryl-CoA lyase; BDH1 = β-hydroxybutyrate dehydrogenase-1; BDH2 = β-hydroxybutyrate dehydrogenase-2.
DISCUSSION
In this study, the model of natural ewe’s milk-feeding strategy was adopted to monitor the practical lamb production better. In this feeding strategy, the milk intake of each lamb could not be detected and only the feed intake of starter and BW of each lamb were determined. The results suggested that an infusion of SB increased the ADFI of starter, ADG, and BW in preweaning lambs, which is consistent with the finding of Serbester et al. (2014) and Gorka et al. (2009), who reported that adding SB in the milk replacer and starter increased starter intake, BW, and feed conversion efficiency in preweaning calves. In this study, the elevated BW and ADG may be due to the SB infusion and increased starter intake, which could provide more energy for animal growth in preweaning lambs.
Volatile fatty acids are an important source of energy for ruminants, especially butyrate, and it is considered as the main stimulator of rumen development (Gorka et al., 2009; Górka et al., 2011). The results of this study showed that SB infusion elevated the concentration of total VFA, acetate, and butyrate in the rumen, which are consistent with the reports mentioned earlier in young calves (Laarman et al., 2012; Khan et al., 2016). The increased VFA in the rumen may be due to an increased feed intake of starter with infusion of SB. In addition, this elevated concentration of butyrate was also partly derived from the conversion of exogenous SB.
We found that supplementation with SB positively affected the emptied rumen weight in preweaning lambs. Moolchand et al. (2013) showed that ruminal infusion of butyrate increased the full weight of rumen as percent of total stomach weight in goats. Another study also shows that an increase in the proportion of ruminal butyrate is associated with an increase in ruminal mass (Yang et al., 2012). This positive change of the rumen weight may be attributed to an increase in papillae length, papillae width, and rumen wall thickness during SB infusion. Lesmeister et al. (2004) reported that the rumen papillae length and width are the most important indicators for assessing rumen development, followed by the density of the rumen papillae and the thickness of the rumen wall. In this experiment, infusion of SB facilitated the growth of rumen papillae, such as greater papillae length, width, and thus, surface area. Similarly, Malhi et al. (2013) reported that intraruminal infusion of SB at 0.3 g/kg of body weight every day has a positive effect on papillae size, density, and surface area in goats. These results indicated that the administration of exogenous butyrate is marked with growth in terms of papillae size and density, which results in an enlarged surface area of the rumen papillae. Further histomorphometric analysis reveals that the infusion of SB positively affected the thickness of total epithelium, stratum corneum, and stratum granulosum of rumen epithelium in preweaning lambs. Malhi et al. (2013) also found that intraruminal infusion of butyrate for 28 d resulted in a larger number of cells and total thickness of the ruminal epithelium for approximately 4-mo-old goats. Similar histological changes were also observed in the ruminal epithelium of cattle that had received butyrate treatment (Mentschel et al., 2001). On the one hand, accelerating development and maturation of the rumen papillae may facilitate absorption and digestion of feed components, which could provide nutrients for the physiological requirements of the young ruminants. A larger rumen may also consume a greater proportion of the animal’s gross energy for maintenance.
Based on the previous results and discussion, we conclude that exogenous infusion of SB was beneficial to modeling the rumen epithelial morphology for preweaning lambs. However, the mechanism responsible for the development of rumen papillae with a butyrate infusion needs to be elucidated. Thus, we studied the molecular mechanism of SB action for the promotion of rumen papillary growth and development. The development of rumen epithelium is closely related to changes in the cell cycle. A small family of cyclins and CDKs are relevant to cell cycle progression in mammalian cells (Sherr, 1993; Vermeulen et al., 2003). This study shows that supplementation with SB significantly upregulates the expression of cyclin A2, cyclin D1, and CDK6 in the rumen epithelium. Cyclin A2 functions in the S phase, G2, and early mitosis (Lee et al., 2017). Cyclin A2 is involved in the initiation and completion of DNA replication during the S phase (Bendris et al., 2011). From G1 into the S phase, cyclin A2, which replaces cyclin E1, functions with CDK2. The cyclin A2-CDK2 complex driven chromosome duplication by the phosphorylation is important for DNA replication (Kanakkanthara et al., 2016). The formation of CDK6 with cyclin D1 complexes could activate cell cycle in the early G1 phase (Mathew et al., 2010). Malhi et al. (2013) found that exogenous butyrate treatment could promote cell cycle progression by increasing the cellular fraction from G0/G1 phase to S phase transition and increasing mRNA expression of cyclin D1 and CDK4 in the ruminal epithelial cells of goats. Any changes in the duration of 1 or more phases of the cell cycle will affect cell cycle progression and cell growth. These changes may result in an increase in rumen papillae size and surface area, which further enhances the absorptive ability of the rumen epithelium.
In addition to cell proliferation, apoptosis is also an important part in the development of the rumen epithelium. To date, Bcl-2 and Caspases are 2 important families that control cell apoptosis progression. In this study, we found that the infusion of SB downregulated gene expression of caspase-3 in rumen epithelium. The caspase-3 was activated by butyrate and led to cellular substrates cleavage in Caco-2 cells (Schwab et al., 2006), which is irreplaceable in cell apoptosis. Two primary pathways were identified in activating caspase-3, one was the receptor-mediated pathway and the another was the intrinsic pathway (Cory and Adams, 2002). In the 2 evolutionarily conserved signal transduction pathways of apoptosis, the Bcl-2/Bax ratio is the molecular switch that initiates apoptosis. Korsmeyer et al. (1993) reported that the intrinsic pathway is mainly regulated by the Bcl-2 family and that Bcl-2/Bax is an important index for evaluating cell apoptosis. In this study, the expression of Bax was downregulated in the rumen epithelium, while Bcl-2/Bax had no significant change between the 2 groups. These results indicate that the infusion of SB promotes the development of rumen papillae related to the receptor-mediated pathway of apoptosis rather than the intrinsic pathway. We inferred that the infusion of SB beneficial to the development of ruminal epithelium, which relates to an accelerated cell cycle and inhibited apoptosis in preweaning lambs.
Recent studies indicate that the activity of the endocrine system was also affected by butyrate. The concentration of IGF-1 in plasma was increased in response to infusion of mixed VFA containing 10% butyrate in sheep rumen (Zhao and Sun, 2010). In this study, infusion of SB increased the concentration of IGF-1 in plasma and IGF-1R expression in the rumen epithelium, which are consistent with the reports mentioned earlier in calves (Gorka et al., 2009; Guilloteau et al., 2010). Previous studies indicated that IGF-1 can promote cyclin expression, accelerate cell proliferation and differentiation (Hayashi et al., 2004; Hayashi et al., 2005), and promote the development of rumen papillae. IGF-1 induces cellular response by regulating IGF-binding proteins (IGFBPs) (Firth and Baxter, 2002). IGFBP-5, which is known to potentiate IGF-1 effects, may encourage proliferation in the rumen epithelium when it was upregulated (Firth and Baxter, 2002), whereas IGFBP-3 modulates IGF-1 cellular events in an opposing fashion (Baxter, 2001). In this study, infusion of SB increased mRNA expression of IGFBP-5 and decreased mRNA expression of IGFBP-3 in the rumen epithelium, which may contribute to rumen epithelial cell proliferation. However, it has not been concluded whether variations in mRNA expression of IGF-1 and IGF-binding proteins in the rumen epithelia are the factors that contribute to the rumen epithelial proliferation and development during SB infusion in lambs. Indeed, a recent study revealed a mismatch in gene and protein expression in response to different grain level (Hollmann et al., 2013), indicating that the changes in the expression of genes related to IGF-1 and IGF-binding proteins might not result in increased expression of the corresponding proteins. Thus, future studies are needed to determine whether SB infusion affect the actual activity and protein expression of IGF-1 and IGF-binding proteins in the rumen epithelia of lambs.
Infusion of SB also increased the concentration of BHBA in plasma herein, which is consistent with the findings by Herrick et al. (2017). The increased BHBA in the plasma may be due to increased feed intake of starter with infusion of SB, which leads to increased rumen VFA production, is could also be partly derived from the conversion of exogenous SB. In this study, infusion of SB increased the concentrate of insulin in plasma, which is consistent with the findings of Nazari et al. (2012), who report that the concentration of insulin is significantly increased in blood in response to adding coated calcium butyrate to milk replacer in milk-fed calves. However, Herrick et al. (2018) found that infusion of butyrate at 2 g/kg of BW did not significantly affect the concentrate of plasma insulin in lactating dairy cows. It appears that insulin response to butyrate may depend upon several factors, such as the amount of butyrate, the physiological state of the animals, and the duration of the butyrate administration.
The main manifestation of rumen epithelial metabolism is the ketogenic effect, which converts VFA into ketone bodies to provide energy for the body (Allen, 2014). Thus, we also determined the related genes expression involved in VFA uptake and metabolism. The membrane-bound transporters and pH values homeostasis were primarily regulated by MCT and NHE family (Graham et al., 2007; Kuzinski et al., 2012). The MCT1, localized on the basolateral side of the rumen epithelium, is beneficial because it transports VFA and their metabolites from the epithelium into the blood (Müller et al., 2002). The increased butyrate concentration in rumen stimulates the activities of MCT1 and MCT4 in delivering carboxylic acids in the rumen epithelial cells in calves (Laarman et al., 2012) and in goats (Yan et al., 2014). In the present study, the infusion of SB upregulated the mRNA expression of MCT1 in rumen epithelium. The positive relationship between the activities of MCT1 and the intracellular concentrations of butyrate and ketone bodies reveals that MCT1 regulate butyrate and its metabolites in the ruminal epithelium (Dengler et al., 2015). Similarly, other reports also suggest that co-treatment of low pH and VFA has an effect on the expression of MCT1 (Yan et al., 2014). NHE family was used by ruminal epithelial cells to regulate intracellular pH. Graham et al. (2007) found that NHE1, NHE2, and NHE3 are present in the rumen epithelial cells, and that NHE2 is located at the apical side of epithelial cells, where Na+ is imported to cells and H+ is exported to extracellular spaces. In the current study, the infusion of SB only downregulated the NHE2 expression in the rumen epithelium. This lower NHE2 expression may indicate decreased proton recycling into the lumen and a greater net proton uptake by the rumen epithelium, which may explain why rumen pH was not different despite higher VFA concentration for SB treatment in the preweaning lambs. These candidate transporters include DRA and PAT1 on the apical membrane, where they import dissociated VFA and export bicarbonate from epithelial cells to neutralize acid in the rumen (Schlau et al., 2012). Infusion of SB also upregulated the mRNA expression of DRA, which may have a positive effect on VFA uptake in the rumen epithelia of preweaning lambs. The potential increase in DRA-mediated bicarbonate-dependent transport infusion of SB is also beneficial for modulating ruminal pH. The upregulation of MCT1, DRA and downregulation of NHE2 may increase the ability of VFA uptake in the rumen. These results may explain the reason why the infusion of SB leads to an increase in VFA concentration within rumen in preweaning lambs.
The increase in concentration of butyrate in calf rumen stimulates changes in the expressions of genes and proteins involved in the ketogenesis pathway (Niwinska et al., 2017). HMG-CoA can be used by HMG-CoA lyase (HMGCL) to compound the ketone bodies, such as acetoacetate and BHBA. In the current study, the expression of HMGCL and HMGCS2 was higher in the SB group than those in the Con group. The mRNA expression of HMGCS2 was upregulated in the rumen epithelium of calves in response to transition from milk replacer to hay or grain feeding (Connor et al., 2013). Kato et al. (2016) report that the HMGCS2 expression is significantly higher in the rumen epithelium of calves in postweaning than those in preweaning, which supports the existence that there is a positive relationship between ketogenesis and an increase in rumen fermentation induced by the intake of more solid feed. It is possible that BHBA production increases when providing more substrate resulting from an increase in starter feed intake, and thus this process activates the activity of enzymes controlling ketogenesis in preweaning lambs. Ketogenesis is the primary pathway of VFA metabolism in the rumen epithelium cells (Penner et al., 2011). In addition to ketogenic effects, cholesterol biosynthesis is an alternative pathway for VFA metabolism in the rumen epithelium cells (Steele et al., 2011). However, we did not detect a significant difference in the expression of genes (HMGCS1) associated with VFA metabolism via cholesterol pathways between Con and SB lambs. These results indicated that the infusion of SB increased the metabolic activity of VFA via the ketogenesis pathway in the rumen epithelium, which controls ketone metabolism and provides an important source of energy for the body.
Overall, the differences in rumen development and rumen epithelial VFA uptake and metabolism reported herein may have resulted from the greater energy intake induced by SB infusion. In fact, our recent study found that starter supplementation promoted rumen epithelial cell proliferation and VFA metabolism in lambs (Sun et al., 2018). Moreover, differences in water, milk, and hay intake induced by SB may also affect the development and growth of rumen in lambs. Infusion of SB may impact appetite, whereas increased feed intake is known to promote rumen development and function (Niwinska et al., 2017).
CONCLUSIONS
In summary, our results indicated that the infusion of SB for 39 d at 0.36 g/kg BW during early life can improve animal performance, promote the rumen papillae growth related to cell proliferation accelerated and cell apoptosis inhibited, and enhance expression of genes involved in rumen epithelial VFA uptake and metabolism in preweaning lambs. To our knowledge, we report for the first time the effects of in vivo SB infusion on mRNA expression of genes-related cell proliferation, apoptotic, and VFA absorption and metabolism in the rumen epithelium of preweaning lambs. These findings provide a better understanding of the molecular mechanism of SB promoting rumen epithelial development and function in preweaning lambs.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (no. 31501980), National Key Research and Development Plan (2018YFD0501900), and Fundamental Research Funds for the Central Universities (KJQN201610 and KYDS201808). There are no conflicts of interest to declare.
LITERATURE CITED
- Allen M. S. 2014. Drives and limits to feed intake in ruminants. Anim. Prod. Sci. 54:1513–1524. doi: 10.1071/AN14478 [DOI] [Google Scholar]
- Baxter M. C. 2001. Signaling pathways involved in anti-proliferative effects of IGFBP-3: a review. Mol. Pathol. 54:145–148. doi: 10.1136/mp.54.3.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendris N., B. Lemmers J. M. Blanchard, and Arsic N.. 2011. Cyclin A2 mutagenesis analysis: a new insight into CDK activation and cellular localization requirements. PLoS One 6:e22879. doi: 10.1371/journal.pone.0022879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavini S., Iraira S., Siurana A., Foskolos A., Ferret A., and Calsamiglia S.. 2015. Effect of sodium butyrate administered in the concentrate on rumen development and productive performance of lambs in intensive production system during the suckling and the fattening periods. Small Ruminant Res. 123:212–217. doi: 10.1016/j.smallrumres.2014.11.009 [DOI] [Google Scholar]
- Connor E. E., R. L. Baldwin C. J. Li R. W. Li, and Chung H.. 2013. Gene expression in bovine rumen epithelium during weaning identifies molecular regulators of rumen development and growth. Funct. Integr. Genomics 13:133–142. doi: 10.1007/s10142-012-0308-x [DOI] [PubMed] [Google Scholar]
- Cory S. and Adams J. M.. 2002. The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2:647–656. doi: 10.1038/nrc883 [DOI] [PubMed] [Google Scholar]
- Dengler F., R. Rackwitz F. Benesch H. Pfannkuche, and Gäbel G.. 2015. Both butyrate incubation and hypoxia upregulate genes involved in the ruminal transport of SCFA and their metabolites. J. Anim. Physiol. Anim. Nutr. (Berl). 99:379–390. doi: 10.1111/jpn.12201 [DOI] [PubMed] [Google Scholar]
- Ferreira L. S. and Bittar C. M.. 2011. Performance and plasma metabolites of dairy calves fed starter containing sodium butyrate, calcium propionate or sodium monensin. Animal 5:239–245. doi: 10.1017/S1751731110001965 [DOI] [PubMed] [Google Scholar]
- Firth S. M. and Baxter R. C.. 2002. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 23:824–854. doi: 10.1210/er.2001-0033 [DOI] [PubMed] [Google Scholar]
- Górka P., Z. M. Kowalski P. Pietrzak A. Kotunia W. Jagusiak J. J. Holst P. Guilloteau, and Zabielski R.. 2011. Effect of method of delivery of sodium butyrate on rumen development in newborn calves. J. Dairy Sci. 94:5578–5588. doi: 10.3168/jds.2011-4166 [DOI] [PubMed] [Google Scholar]
- Gorka P., Z. M. Kowalski P. Pietrzak A. Kotunia R. Kiljanczyk J. Flaga J. J. Holst P. Guilloteau, and Zabielski R.. 2009. Effect of sodium butyrate supplementation in milk replacer and starter diet on rumen development in calves. J. Physiol. Pharmacol. 60(Suppl. 3):47–53. [PubMed] [Google Scholar]
- Górka P., Z. M. Kowalski R. Zabielski, and Guilloteau P.. 2018. Invited review: use of butyrate to promote gastrointestinal tract development in calves. J. Dairy Sci. 101:4785–4800. doi: 10.3168/jds.2017-14086 [DOI] [PubMed] [Google Scholar]
- Graham C., I. Gatherar I. Haslam M. Glanville, and Simmons N. L.. 2007. Expression and localization of monocarboxylate transporters and sodium/proton exchangers in bovine rumen epithelium. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292:R997–R1007. doi: 10.1152/ajpregu.00343.2006 [DOI] [PubMed] [Google Scholar]
- Guilloteau P., G. Savary Y. Jaguelin-Peyrault V. Romé L. Le Normand, and Zabielski R.. 2010. Dietary sodium butyrate supplementation increases digestibility and pancreatic secretion in young milk-fed calves. J. Dairy Sci. 93:5842–5850. doi: 10.3168/jds.2009-2751 [DOI] [PubMed] [Google Scholar]
- Hayashi K., K. D. Carpenter, and Spencer T. E.. 2004. Neonatal estrogen exposure disrupts uterine development in the postnatal sheep. Endocrinology 145:3247–3257. doi: 10.1210/en.2004-0178 [DOI] [PubMed] [Google Scholar]
- Hayashi K., K. D. Carpenter T. H. Welsh R. C. Jr Burghardt L. J. Spicer, and Spencer T. E.. 2005. The IGF system in the neonatal ovine uterus. Reproduction 129:337–347. doi: 10.1530/rep.1.00342 [DOI] [PubMed] [Google Scholar]
- He Z. X., Z. H. Sun W. Z. Yang K. A. Beauchemin S. X. Tang C. S. Zhou X. F. Han M. Wang J. H. Kang, and Tan Z. L.. 2014. Effects of maternal protein or energy restriction during late gestation on immune status and responses to lipopolysaccharide challenge in postnatal young goats. J. Anim. Sci. 92:4856–4864. doi: 10.2527/jas.2014-7904 [DOI] [PubMed] [Google Scholar]
- Herrick K. J., A. R. Hippen K. F. Kalscheur D. J. Schingoethe D. P. Casper S. C. Moreland, and van Eys J. E.. 2017. Single-dose infusion of sodium butyrate, but not lactose, increases plasma β-hydroxybutyrate and insulin in lactating dairy cows. J. Dairy Sci. 100:757–768. doi: 10.3168/jds.2016-11634 [DOI] [PubMed] [Google Scholar]
- Herrick K. J., A. R. Hippen K. F. Kalscheur D. J. Schingoethe S. D. Ranathunga J. L. Anderson S. C. Moreland, and van Eys J. E.. 2018. Infusion of butyrate affects plasma glucose, butyrate, and β-hydroxybutyrate but not plasma insulin in lactating dairy cows. J. Dairy Sci. 101:3524–3536. doi: 10.3168/jds.2017-13842 [DOI] [PubMed] [Google Scholar]
- Holle S. A. and Birtles M. J.. 1990. An immunocytochemical method for studying patterns of cell proliferation in the wool follicle. N. Z. Vet. J. 38:89–93. doi: 10.1080/00480169.1990.35625 [DOI] [PubMed] [Google Scholar]
- Hollmann M., I. Miller K. Hummel S. Sabitzer B. U. Metzler-Zebeli E. Razzazi-Fazeli, and Zebeli Q.. 2013. Downregulation of cellular protective factors of rumen epithelium in goats fed high energy diet. PLoS One 8:e81602. doi: 10.1371/journal.pone.0081602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakkanthara A., K. B., Jeganathan J. F., Limzerwala D. J., Baker M., Hamada H. J., Nam W. H., van Deursen N., Hamada R. M., Naylor N. A., Becker, et al. 2016. Cyclin A2 is an RNA binding protein that controls Mre11 mRNA translation. Science 353:1549–1552. doi: 10.1126/science.aaf7463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato D., Y. Suzuki S. Haga K. So E. Yamauchi M. Nakano H. Ishizaki K. Choi K. Katoh, and Roh S. G.. 2016. Utilization of digital differential display to identify differentially expressed genes related to rumen development. Anim. Sci. J. 87:584–590. doi: 10.1111/asj.12448 [DOI] [PubMed] [Google Scholar]
- Khan M. A., A. Bach D. M. Weary, and von Keyserlingk M. A. G.. 2016. Invited review: transitioning from milk to solid feed in dairy heifers. J. Dairy Sci. 99:885–902. doi: 10.3168/jds.2015-9975 [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J., J. R. Shutter D. J. Veis D. E. Merry, and Oltvai Z. N.. 1993. Bcl-2/Bax: a rheostat that regulates an anti-oxidant pathway and cell death. Semin. Cancer Biol. 4:327–332. [PubMed] [Google Scholar]
- Kuzinski J., R. Zitnan E. Albrecht T. Viergutz, and Schweigel-Röntgen M.. 2012. Modulation of vH+-ATPase is part of the functional adaptation of sheep rumen epithelium to high-energy diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 303:R909–R920. doi: 10.1152/ajpregu.00597.2011 [DOI] [PubMed] [Google Scholar]
- Laarman A. H., Ruiz-Sanchez A. L., Sugino T., Guan L. L., and Oba M.. 2012. Effects of feeding a calf starter on molecular adaptations in the ruminal epithelium and liver of Holstein dairy calves. J. Dairy Sci. 95:2585–2594. doi: 10.3168/jds.2011-4788 [DOI] [PubMed] [Google Scholar]
- Lee H. J., M. P., Jedrychowski A., Vinayagam N., Wu N., Shyh-Chang Y., Hu C., Min-Wen J. K., Moore J. M., Asara C. A., Lyssiotis, et al. 2017. Proteomic and metabolomic characterization of a mammalian cellular transition from quiescence to proliferation. Cell Rep. 20:721–736. doi: 10.1016/j.celrep.2017.06.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesmeister K. E., P. R. Tozer, and Heinrichs A. J.. 2004. Development and analysis of a rumen tissue sampling procedure. J. Dairy Sci. 87:1336–1344. doi: 10.3168/jds.S0022-0302(04)73283-X [DOI] [PubMed] [Google Scholar]
- Malhi M., H. Gui L. Yao J. R. Aschenbach G. Gäbel, and Shen Z.. 2013. Increased papillae growth and enhanced short-chain fatty acid absorption in the rumen of goats are associated with transient increases in cyclin D1 expression after ruminal butyrate infusion. J. Dairy Sci. 96:7603–7616. doi: 10.3168/jds.2013-6700 [DOI] [PubMed] [Google Scholar]
- Mathew O. P., K. Ranganna, and Yatsu F. M.. 2010. Butyrate, an HDAC inhibitor, stimulates interplay between different posttranslational modifications of histone H3 and differently alters G1-specific cell cycle proteins in vascular smooth muscle cells. Biomed. Pharmacother. 64:733–740. doi: 10.1016/j.biopha.2010.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentschel J., R. Leiser C. Mülling C. Pfarrer, and Claus R.. 2001. Butyric acid stimulates rumen mucosa development in the calf mainly by a reduction of apoptosis. Arch. Tierernahr. 55:85–102. doi: 10.1080/17450390109386185 [DOI] [PubMed] [Google Scholar]
- Moolchand M., Wang J., Gui H., and Shen Z.. 2013. Ruminal butyrate infusion increased papillae size and digesta weight but did not change liquid flow rate in the rumen of goats. J. Anim. Plant Sci. 23:2013–1516. [Google Scholar]
- Müller F., K. Huber H. Pfannkuche J. R. Aschenbach G. Breves, and Gäbel G.. 2002. Transport of ketone bodies and lactate in the sheep ruminal epithelium by monocarboxylate transporter 1. Am. J. Physiol. Gastrointest. Liver Physiol. 283:G1139–G1146. doi: 10.1152/ajpgi.00268.2001 [DOI] [PubMed] [Google Scholar]
- Nazari M., Karkoodi K., and Alizadeh A.. 2012. Performance and physiological responses of milk-fed calves to coated calcium butyrate supplementation. S. Afr. J. Anim. Sci. 42:296–303. doi: 10.4314/sajas.v42i3.12 [DOI] [Google Scholar]
- Niwińska B., E. Hanczakowska M. B. Arciszewski, and Klebaniuk R.. 2017. Review: exogenous butyrate: implications for the functional development of ruminal epithelium and calf performance. Animal 11:1522–1530. doi: 10.1017/S1751731117000167 [DOI] [PubMed] [Google Scholar]
- Odongo N. E., O. Alzahal M. I. Lindinger T. F. Duffield E. V. Valdes S. P. Terrell, and McBride B. W.. 2006. Effects of mild heat stress and grain challenge on acid-base balance and rumen tissue histology in lambs. J. Anim. Sci. 84:447–455. [DOI] [PubMed] [Google Scholar]
- Penner G. B., M. A. Steele J. R. Aschenbach, and McBride B. W.. 2011. Ruminant nutrition symposium: molecular adaptation of ruminal epithelia to highly fermentable diets. J. Anim. Sci. 89:1108–1119. doi: 10.2527/jas.2010-3378 [DOI] [PubMed] [Google Scholar]
- Penner G. B., Taniguchi M., Guan L. L., Beauchemin K. A., and Oba M.. 2009. Effect of dietary forage to concentrate ratio on volatile fatty acid absorption and the expression of genes related to volatile fatty acid absorption and metabolism in ruminal tissue. J. Dairy Sci. 92:2767–2781. doi: 10.3168/jds.2008-1716 [DOI] [PubMed] [Google Scholar]
- Qin W. L. 1982. Determination of rumen volatile fatty acids by means of gas chromatography. J. Nanjing Agric. U. China 4:110–116. [Google Scholar]
- Schlau N., L. L. Guan, and Oba M.. 2012. The relationship between rumen acidosis resistance and expression of genes involved in regulation of intracellular ph and butyrate metabolism of ruminal epithelial cells in steers. J. Dairy Sci. 95:5866–5875. doi: 10.3168/jds.2011-5167 [DOI] [PubMed] [Google Scholar]
- Schwab M., V. Reynders S. Ulrich N. Zahn J. Stein, and Schröder O.. 2006. PPARgamma is a key target of butyrate-induced caspase-3 activation in the colorectal cancer cell line Caco-2. Apoptosis 11:1801–1811. doi: 10.1007/s10495-006-9788-2 [DOI] [PubMed] [Google Scholar]
- Serbester U., Çakmakçi C., Göncü S., and Görgülü M.. 2014. Effect of feeding starter containing butyrate salt on pre- and post-weaning performance of early or normally weaned calves. Revue Med. Vet. 165:44–48. [Google Scholar]
- Sherr C. J. 1993. Mammalian G1 cyclins. Cell 73:1059–1065. [DOI] [PubMed] [Google Scholar]
- Steele M. A., G. Vandervoort O. Alzahal S. E. Hook J. C. Matthews, and McBride B. W.. 2011. Rumen epithelial adaptation to high-grain diets involves the coordinated regulation of genes involved in cholesterol homeostasis. Physiol. Genomics 43:308–316. doi: 10.1152/physiolgenomics.00117.2010 [DOI] [PubMed] [Google Scholar]
- Sugino T., Y. Hasegawa Y. Kurose M. Kojima K. Kangawa, and Terashima Y.. 2004. Effects of ghrelin on food intake and neuroendocrine function in sheep. Anim. Reprod. Sci. 82:183–194. doi: 10.1016/j.anireprosci.2004.05.001 [DOI] [PubMed] [Google Scholar]
- Sun D. M., Mao S. Y., Zhu W. Y., and Liu J. H.. 2018. Effect of starter diet supplementation on rumen epithelial morphology and expression of genes involved in cell proliferation and metabolism in pre-weaned lambs. Animal 26:1–10. doi: 10.1017/S1751731118000290 [DOI] [PubMed] [Google Scholar]
- Van Soest P. J., J. B. Robertson, and Lewis B. A.. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]
- Vermeulen K., D. R. Van Bockstaele, and Berneman Z. N.. 2003. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 36:131–149. doi: 10.1046/j.1365-2184.2003.00266.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., B. Zhang, and Shen Z.. 2014. Dietary modulation of the expression of genes involved in short-chain fatty acid absorption in the rumen epithelium is related to short-chain fatty acid concentration and pH in the rumen of goats. J. Dairy Sci. 97:5668–5675. doi: 10.3168/jds.2013-7807 [DOI] [PubMed] [Google Scholar]
- Yang W., Shen Z., and Martens H.. 2012. An energy-rich diet enhances expression of Na(+)/H(+) exchanger isoform 1 and 3 messenger RNA in rumen epithelium of goat. J. Anim. Sci. 90:307–317. doi: 10.2527/jas.2011-3854 [DOI] [PubMed] [Google Scholar]
- Zhao G. Y., and Sun Y. B.. 2010. Effects of volatile fatty acids on IGF-I, IGFBP-3, GH, insulin and glucagon in plasma, and IGF-I and IGFBP-3 in different tissues of growing sheep nourished by total intragastric infusions. Asian Australas J. Anim. Sci. 23:366–371. doi: 10.5713/ajas.2010.90355 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.