We previously reported (1) in PNAS that zinc depletion in Mycobacterium smegmatis leads to reprogramming of ribosomes in which five ribosomal proteins (r-proteins) with a conserved zinc-finger CXXC motif (C+) are replaced by their paralogs lacking the motif (C−). Notably, the reprogrammed ribosomes enter into a hibernating state by binding to a mycobacterial protein Y (MPY) encoded by MSMEG_1878 (1). Binding of MPY locks the ribosome in an inactive, unrotated state that is drug tolerant. MPY is expressed constitutively but is specifically recruited to the ribosome by a zinc-responsive factor [MPY recruitment factor (MRF)] encoded within the c- ribosomal-gene operon.
The data presented by Tobiasson et al. (2) are not in conflict with our findings; rather, they strengthen our conclusion that assembly of the C− ribosome is not sufficient for MPY recruitment (figure 3B in ref. 1). Tobiasson et al. show that C− ribosomes can be assembled in the absence of MPY. We suggest that the growth conditions used by Tobiasson et al. fail to activate MRF to recruit MPY. Consistent with this, we previously showed that MRF-dependent recruitment of MPY requires zinc depletion, even in cells in which MRF and C− r-proteins are constitutively expressed (figure 3B in ref. 1). We believe that additional factors and signals modulate MPY recruitment, presumably at a zinc level lower than that inducing the c- operon. However, the requirement of MRF unequivocally supports that MPY-dependent hibernation in mycobacteria is responsive to environmental zinc.
The studies (refs. 3 and 4) cited by Tobiasson et al. (2) provide insufficient evidence to support zinc-independent recruitment of MPY to ribosomes. In one study, Trauner et al. (3) used mass spectrometry to detect peptides corresponding to MPY and MSMEG_3935 (a related protein with the signature βαβββα motif) in ribosomes from stationary-phase M. smegmatis cells. However, these authors acknowledged that the abundance of peptides corresponding to these proteins was too low to conclude that their presence in stationary-phase ribosomes was statistically different from that in exponential phase (3). Moreover, zinc levels in the cultures were not defined and, thus, further prevent a direct comparison with our data (3). In another study, Mishra et al. (4) reported that stationary-phase ribosomes of M. smegmatis, cultured in LB, Tween 80, and 0.2% glycerol (i.e., without zinc depletion), were occupied by MPY. However, density referred to as MSMEG_1878 in their structure was not of sufficient resolution to allow ab initio modeling that could distinguish it from MSMEG_3935 (4).
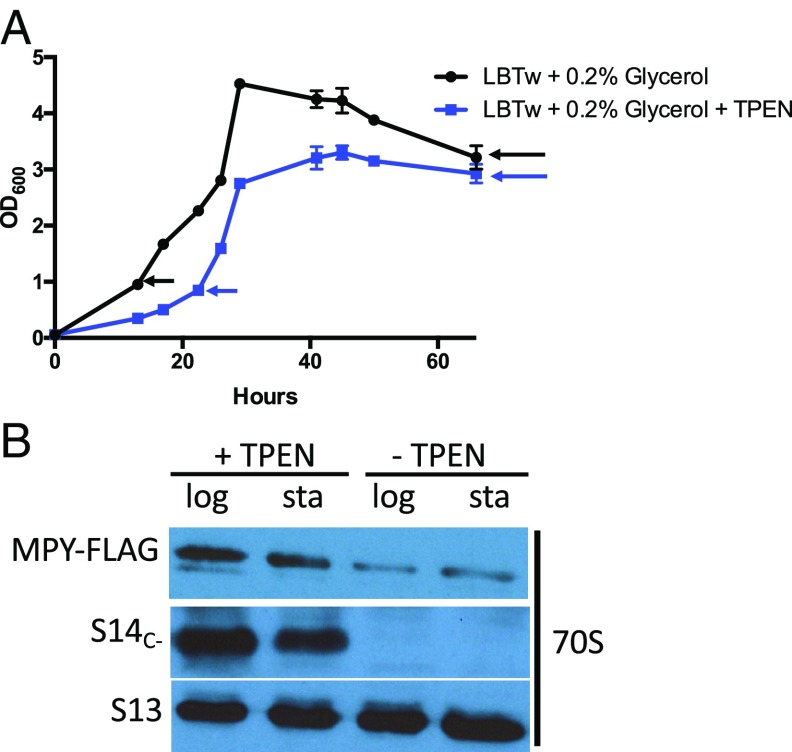
To further address the points raised by Tobiasson et al. (2) in the context of these other studies (3, 4), we directly tested the effect of zinc depletion on MPY binding to ribosomes in an LB medium containing Tween 80 and 0.2% glycerol. Depletion of zinc increases association of MPY with the ribosome, regardless of the growth phase of cultures (Fig. 1). Moreover, the levels of MPY-bound ribosomes did not change between logarithmic and stationary phases in zinc-rich medium (Fig. 1). Thus, zinc depletion appears to be a specific signal for induction of MPY-dependent ribosome hibernation that requires a dedicated role of MRF in the hibernation process (1).
Fig. 1.
Growth phase-independent binding of MPY to C− ribosomes in zinc-depleted cultures. (A) Growth curve of a recombinant M. smegmatis carrying FLAG-tagged MPY in LB + 0.05% Tween 80 (LBTw) + 0.2% glycerol, and in the same medium but with 20 µM N,N,N′,N′-tetrakis(2-pyridylmethyl)-ethylenediamine (TPEN), a zinc-specific chelator. The genotype of this strain is described in ref. 1. This medium has sufficient zinc to prevent expression of C− ribosomes by M. smegmatis in stationary phase (4). The arrows indicate the growth points at which 70S ribosomes from the cultures were purified for the analysis described in B. (B) The 70S ribosomes purified from logarithmic (log)- and stationary (sta)-phase cells from zinc-rich and -depleted cultures were analyzed for MPY levels using the protocols described in ref. 1. While antibodies against S14C- differentiate C− ribosomes from their C+ counterparts, those against S13 were used as loading controls (1). Increased MPY binding to the ribosome in the zinc-depleted medium is consistent with reduced growth rate of cells observed in A.
Footnotes
The authors declare no conflict of interest.
References
- 1.Li Y, et al. Zinc depletion induces ribosome hibernation in mycobacteria. Proc Natl Acad Sci USA. 2018;115:8191–8196. doi: 10.1073/pnas.1804555115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tobiasson V, Dow A, Prisic S, Amunts A. Zinc depletion does not necessarily induce ribosome hibernation in mycobacteria. Proc Natl Acad Sci USA. 2019;116:2395–2397. doi: 10.1073/pnas.1817490116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trauner A, Lougheed KE, Bennett MH, Hingley-Wilson SM, Williams HD. The dormancy regulator DosR controls ribosome stability in hypoxic mycobacteria. J Biol Chem. 2012;287:24053–24063. doi: 10.1074/jbc.M112.364851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mishra S, Ahmed T, Tyagi A, Shi J, Bhushan S. Structures of Mycobacterium smegmatis 70S ribosomes in complex with HPF, tmRNA, and P-tRNA. Sci Rep. 2018;8:13587. doi: 10.1038/s41598-018-31850-3. [DOI] [PMC free article] [PubMed] [Google Scholar]