Significance
Elucidating the mechanisms by which homeostasis is established and maintained in the oral mucosa is important for human health. In this study we investigated the ontogeny and function of gingival γδT cells. The majority of these cells originate from embryonic precursors that maintain themselves locally independent of circulating γδT cells arising from adult precursors. The oral microbiota regulates the development of γδT cells, particularly those of embryonic origin. γδT cells represent the major sources of IL-17–producing cells in the gingiva, and they play a critical role in down-modulating gingival steady-state immunity that subsequently shapes oral microbial diversity. This suggests that oral homeostasis requires mutual interactions between γδT cells and the microbiota.
Keywords: oral mucosa, γδT cells, Vγ6, microbiota, gingiva
Abstract
γδT cells are a major component of epithelial tissues and play a role in tissue homeostasis and host defense. γδT cells also reside in the gingiva, an oral tissue covered with specialized epithelium that continuously monitors the challenging dental biofilm. Whereas most research on intraepithelial γδT cells focuses on the skin and intestine epithelia, our knowledge on these cells in the gingiva is still incomplete. In this study, we demonstrate that even though the gingiva develops after birth, the majority of gingival γδT cells are fetal thymus-derived Vγ6+ cells, and to a lesser extent Vγ1+ and Vγ4+ cells. Furthermore, we show that γδT cells are motile and locate preferentially in the epithelium adjacent to the biofilm. Vγ6+ cells represent the major source of IL-17–producing cells in the gingiva. Chimeric mice and parabiosis experiments indicated that the main fraction of gingival γδT cells is radioresistant and tissue-resident, persisting locally independent of circulating γδT cells. Notably, gingival γδT cell homeostasis is regulated by the microbiota as the ratio of Vγ6+ and Vγ4+ cells was reversed in germ-free mice, and their activation state was decreased. As a consequence, conditional ablation of γδT cells results in elevated gingival inflammation and subsequent alterations of oral microbial diversity. Taken together, these findings suggest that oral mucosal homeostasis is shaped by reciprocal interplays between γδT cells and local microbiota.
The establishment of oral mucosal homeostasis is essential for human health (1, 2). Besides functioning as a physical barrier protecting the underlying tissues from microbial invasion and mechanical forces, the oral epithelium is known to have an immunological role. Oral epithelial cells are capable of producing antibacterial peptides and a variety of cytokines that regulate antimicrobial defense (3, 4). Gingival epithelial cells also produce IL-6 due to on-going damage from mastication, which drives homeostatic Th17 responses in the gingiva (5). Microbial colonization of the oral cavity was found to up-regulate expression of cytokines and chemokines that are considered to be involved in tissue homeostasis (6). Moreover, shortly after birth the microbiota induces the expression of growth arrest-specific protein 6 (GAS6) in the outermost layers of the oral epithelium; GAS6 in turn down-regulates the activation of epithelial cells and plays a critical role in the establishment of oral homeostasis (7). Besides epithelial cells, tissue-resident leukocytes colonize the oral epithelium and maintain immunological balance with the microbes on the other side of the epithelium. Langerhans cells (LCs), a special subset of antigen-presenting cells exclusively residing in stratified epithelia, were recently reported to regulate oral mucosal immunity and to prevent spontaneous alveolar bone loss (8, 9). Thus, both hematopoietic and nonhematopoietic cells that constitute the epithelium are shaping homeostasis in the oral mucosa.
γδT cells are innate T lymphocytes residing in high abundance in epithelial surfaces, including the oral epithelium (10, 11). This is reminiscent of the skin and intestine, where intraepithelial γδT cells represent the majority of epithelial T cells and were shown to function as local sentinels (12). γδT cells can be divided into different subgroups based on the V-segment they express in the variable region of the T cell receptor (TCR) γ-chain. These subgroups are differentially localized among tissues, secrete distinct cytokines, and develop at different periods in the thymus (13, 14). While Vγ1+ and Vγ4+ γδT cells arise from both embryonic and adult thymus, Vγ5+ and Vγ6+ γδT cells develop as natural effector T cells only in the embryonic thymus and display a restricted TCR repertoire (15, 16). Because of their preactivated effector phenotype and highly specific tissue residence, γδT cells can rapidly respond to perturbations in mucosal tissue integrity and homeostasis (14). Studies suggested that γδT cells could directly recognize microbial antigen and respond by production of effector cytokines, such as IL-17A (17, 18). Intraepithelial γδT cells can also limit transepithelial pathogen invasion and contribute to immune protection in mucosal tissues (19). On the other hand, γδT cells can enhance the pathogenesis of inflammatory diseases, such as psoriasis-like dermatitis and spondyloarthritis (20, 21).
Whereas the aforementioned observations highlight the immunological function of intraepithelial γδT cells, most research on these cells focused on the skin and intestinal epithelium, while our knowledge on γδT cells in the oral epithelium is incomplete. The oral epithelium shares many features with epithelia of the skin and intestine, yet has its own distinctive characteristics. On a structural aspect, the oral mucosa possesses a stratified squamous epithelium similar to the epidermis and not a single-layer epithelium that covers the intestine. However, whereas the epidermis is not accessible to cells from the circulation at steady state, the oral epithelium, akin to the intestine, is constantly replenished by circulating leukocytes (9). The oral mucosa also deals with a unique microbial challenge, the persistent dental biofilm, where the gingival epithelium serves as a protective barrier that monitors it (1). Therefore, immune surveillance in the gingiva is complex and homeostasis is often disrupted, resulting in both oral and systemic medical pathologies (2). Thus, understanding the role of γδT cells in gingival homeostasis is an important task. Furthermore, because the gingiva is created only after birth (i.e., simultaneously with tooth eruption), the contribution of embryonic- versus adult-derived γδT cells to the overall gingival γδT cell population is intriguing. This study investigated the development and steady-state function of gingival γδT cells in correlation to the oral microbiota.
Results
Intraepithelial γδT Cells of the Gingiva Are Motile and Localize Close to the Biofilm.
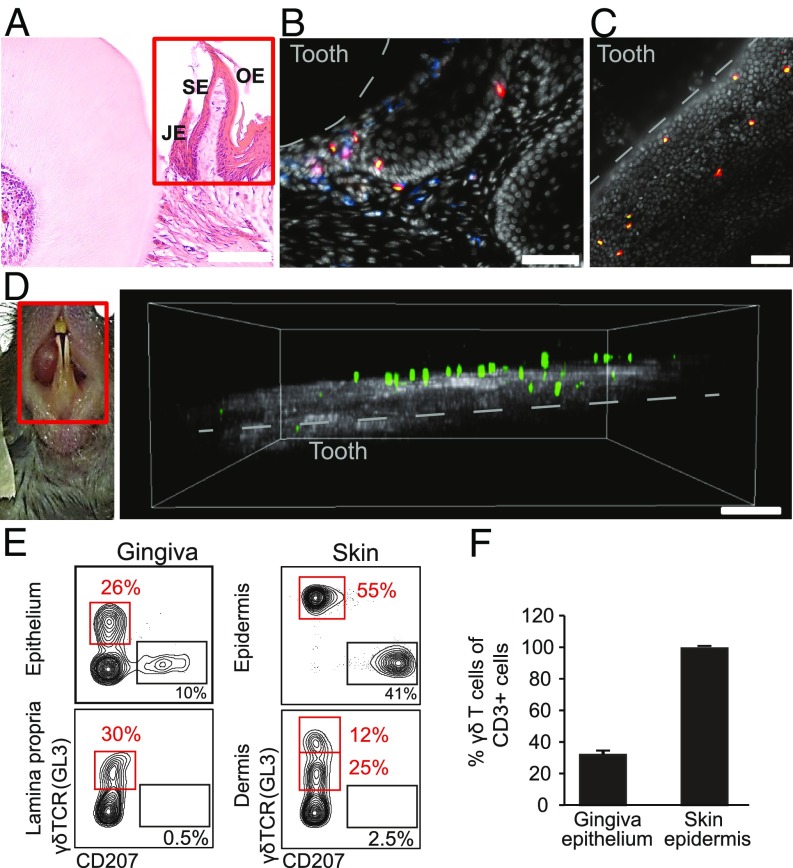
The stratified gingival epithelium can be divided into three sections: the junctional epithelium that is attached to the tooth surface, the sulcular epithelium lining the gingival sulcus, and the oral epithelium covering the external surface of the gingiva. To visualize the distribution of intraepithelial γδT cells in the various specialized gingival epithelia, we performed immunofluorescence microscopy on gingival cross-sections of TcrdH2BeGFP reporter mice expressing the GFP in the nuclei of γδT cells. As demonstrated in Fig. 1 A and B, γδT cells were mainly located in the junctional epithelium in close proximity to the dental biofilm. Furthermore, whereas γδT cells in the oral and sulcular epithelium mostly reside in the basal layers of the epithelium, they were also detected in suprabasal layers of the junctional epithelium. γδT cells were also observed in the lamina propria underlying the epithelium, and most of the cells were positioned close the basal membrane. By whole-mount immunofluorescence microscopy staining on gingival epithelial sheets, we detected round-shaped CD3+ γδT cells close to the epithelial margin (Fig. 1C). In contrast, γδT cells in the skin epidermis were evenly distributed and had a characteristic dendritic-like shape (22) (SI Appendix, Fig. S1A). We next applied in vivo two-photon laser-scanning microscopy on gingival tissues of the incisors using narcotized TcrdH2BeGFP mice. The large majority of γδT cells were localized in the gingival epithelium above the second harmonic signal, which was generated by collagen structures of the connective tissue (Fig. 1D). This setting also identified γδT cells within the connective tissue, as GFP+ cells were detected very close to the second harmonic signal. Importantly, two-photon laser-scanning in vivo microscopy analysis revealed that most gingival intraepithelial γδT cells were motile (Movie S1), and thus permanently screening their tissue of residency. We next performed flow cytometric analysis of γδT cells in the gingival epithelium and lamina propria. Because the gingival epithelium and its connective tissue structurally resemble the skin epidermis and dermis, we compared gingival γδT cells to those residing in the skin. Consistent with previous findings, langerin/CD207+ cells were exclusively located in both epithelia (9). Concurring with the histology results, γδT cells were found in both the epithelium and lamina propria of the gingiva similar to the skin compartments (Fig. 1E). Nevertheless, TCR expression levels on intraepithelial γδT cells were lower in the gingiva in comparison with the skin epidermis. Next, we found that in contrast to the epidermis in which γδT cells represent the dominating T cell subset, intraepithelial gingival γδT cells represented about 30% of the T cell population (Fig. 1F). Taken together, these data indicate that the gingiva contains a clear population of intraepithelial γδT cells that is morphologically and quantitatively different from skin epidermal γδT cells. Moreover, gingival intraepithelial γδT cells are motile and reside predominantly in the junctional epithelium, suggesting a role for these cells in steady-state monitoring of the oral biofilm.
Fig. 1.
Visualization of γδT cells in the oral mucosa. (A) H&E histological staining of cross sections of the maxilla of adult TcrdH2BeGFP mice. The red rectangle specifies the gingival mucosa. JE, junctional epithelium; OE, oral epithelium; SE, sulcular epithelium. One representative of three independent analyses. (Scale bar, 100 µm.) (B) Immunofluorescence staining of cross sections of the maxilla of adult TcrdH2BeGFP mice (γδT cells in green/eGFP) with mAbs directed against CD45 (blue), CD3 (red), and with DAPI (white) for nuclear visualization. Representative image of four independent experiments. (Scale bar, 50 µm.) (C) Immunofluorescence whole mount staining from gingival epithelial layers of adult TcrdH2BeGFP mice with mAbs directed against CD3 (red) and with DAPI (white). Representative image from four independent experiments. Dotted white line indicates the location of the tooth surface. (Scale bar, 50 µm.) (D) Two-photon microscopy on gingival tissues of the lower incisors (Left; red rectangle indicates imaging area) of adult TcrdH2BeGFP mice demonstrating the localization of γδT cells (green/eGFP) close to collagen structures (gray) (Right). Representative of three independent experiments. (Scale bar, 70 μm.) (E and F) Epithelial and lamina propria/dermal layers were prepared from the gingiva or skin, and stained with antibodies against CD45, γδTCR, and langerin. (E) Representative FACS plots present the frequencies of γδT cells in each tissue. (F) Bar graphs depict percentages of γδT cells among total CD3+ cells in the gingiva and skin epithelium (n = 3). Data are representative of four independent experiments.
Gingival γδT Cells Have an Activated IL-17–Secreting Phenotype.
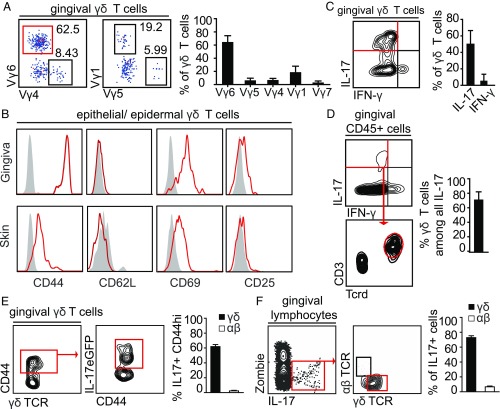
We next sought to characterize the effector phenotype and activation status of gingival γδT cells using flow cytometry. First, we analyzed γδT cell subsets in the gingiva based on their V-segment expression in the TCR γ-chain. Of total gingival γδT cells in adult mice, 63 ± 2.3% were Vγ6+ (Fig. 2A) and were localized in the epithelium as well as the lamina propria (SI Appendix, Fig. S2A). Other subsets included Vγ1+ (17.3 ± 2.6%), Vγ4+ (7.3 ± 1%), Vγ5+ (6.5 ± 1.1%), and Vγ7+ (3.2 ± 0.8%) γδT cells, taken together representing >97% of gingival γδT cells (Fig. 2A). In comparison, the main subsets in peripheral lymph nodes were Vγ1+ and Vγ4+ γδT cells, while Vγ6+ γδT cells only represented a minor population (SI Appendix, Fig. S2B). Next, we examined the expression of activation markers on gingival intraepithelial γδT cells compared with epidermal γδT cells. Elevated expression of CD44 and CD69 combined with the lack or low expression of CD62L and CD24 indicated an activated phenotype of intraepithelial as well as lamina propria γδT cells in the gingiva (Fig. 2B and SI Appendix, Fig. S2C).
Fig. 2.
Characterization of γδT cells in the oral mucosa. (A) FACS plots show γ-chain usage by gingival γδT cells from adult TcrdH2BeGFP mice. Bar graphs depict mean frequencies + SEM of γ-chain types of gingival γδT cells. Data are pooled from two to three independent experiments (n = 3–7 mice per experiment). (B) Expression of selected typical surface markers on γδT cells in the gingiva and skin epithelium. Gray filled histograms represent isotype controls (Ctl). Data representative of three independent experiments (n = 5 mice in each experiment). (C and D) Isolated gingival lymphocytes were stimulated ex vivo with phorbol myristate acetate (PMA) and ionomycin. Representative FACS plots of three independent experiments (n = 3–4 mice per experiment). Bar graphs present the mean frequencies + SEM of pooled data from six experiments. FACS plots and bar graphs show γδT cells expressing IL-17 or IFN-γ and the relative contribution of γδT cells to IL-17–expressing total leukocytes in the tissue. (E and F) IL-17 production analyzed directly ex vivo in gingival γδT cells from Il17-eGFP reporter mice. (E) IL-17+ cells among CD44hi γδT cells. Bar graph depicts means + SEM of pooled frequencies from two independent experiments (n = 4 mice per experiment). (F) Frequencies of γδT cells and αβT cells among all IL-17+ cells in the gingival tissue, two independent experiments (n = 4 mice per experiment). Bar graph shows mean frequency + SEM of pooled data from two independent experiments.
Further phenotypic analysis demonstrated that CD25 (IL-2 receptor-α chain) was slightly up-regulated on intraepithelial γδT cells of the gingiva compared with the epidermis, whereas expression of CD122 (IL-2 receptor-β chain) was not altered. Expression of the CD103 integrin was elevated on intraepithelial γδT cells in both the gingiva and skin (SI Appendix, Fig. S2C). Next, intracellular cytokine staining revealed that 50.6 ± 5.3% of gingival effector γδT cells produced IL-17 and only 5.6 ± 2.6% secreted IFN-γ after ex vivo stimulation (Fig. 2C). Furthermore, Vγ6+ γδT cells were the predominant IL-17–producing cells (SI Appendix, Fig. S2D). Concurring with this cytokine profile, the majority of gingival γδT cells showed a typical γδT17 phenotype (CD27−, NK1.1− CD44hi) (SI Appendix, Fig. S2F). Gating on IL-17 production among all gingival CD45+ leukocytes demonstrated that γδT cells are the main source of IL-17 in the gingiva (70.7 ± 3.5% of IL-17–expressing cells) (Fig. 2D). Because we observed that γδT cells secrete IL-17 after ex vivo stimulation, we asked whether these cells would produce this proinflammatory cytokine in vivo within the gingiva of Il17eGFP reporter mice. Similar to ex vivo stimulation, 60% of γδT cells in the gingiva of Il17eGFP mice were CD44hi and GFP+, indicative of in vivo IL-17 secretion at steady state, whereas αβT cells barely produced IL-17 (∼2% of CD44hi αβT cells) (Fig. 2E). After gating on all IL-17+ cells in the gingiva, γδT cells represented the main subset of IL-17–producing cells in this setting (Fig. 2F). In contrast to the gingiva, merely 12.8% among CD44hi γδT cells in peripheral lymph nodes produced IL-17 in IL17eGFP mice (SI Appendix, Fig. S2F). However, the IL-17 secretion level in lymph nodes was nearly tripled (33.6 ± 3.8%) when the cells were stimulated ex vivo (SI Appendix, Fig. S2G). To sum up, the large majority of gingival γδT cells expresses Vγ6 and has a preactivated phenotype representing the main IL-17–producing lymphocyte subset in this oral tissue.
Gingival γδT Cells Are Partially Dependent on CCR6 and IL-23R Signaling.
IL-23 signaling has been suggested to be crucial for the development and maintenance of IL-17–producing αβT cells (23, 24). Hence, we asked whether IL-23 receptor (IL-23R) signaling is also required for the maintenance and proliferation of gingival γδT cells. Using heterozygous Il23rgfp/+ knockin mice, we found that IL-23R–expressing γδT cells were almost exclusively Vγ6+ T cells (SI Appendix, Fig. S3A). In IL23rgfp/gfp mice lacking IL-23R, we observed a significantly reduced number of γδT cells in the gingiva while αβT cells were not affected, concurring with our earlier observations that gingival αβT cells are not IL-17 producers (SI Appendix, Fig. S3B). In particular, Vγ6+ T cells expressing IL-23R were dramatically reduced but not absent in the gingiva of Il23rgfp/gfp mice lacking IL-23R signaling (SI Appendix, Fig. S3C). Next, we investigated whether the chemokine receptors, CCR6 and CX3CR1, played a role in the homing of gingival γδT17 cells, as suggested for other tissues (25–28). To this end, we used CX3CR1 and CCR6 GFP knockin transgenic mice. As demonstrated in SI Appendix, Fig. S3D, epidermal γδT cells homogenously expressed CX3CR1. In contrast, only 18% of the γδT cells in the gingival epithelium expressed CX3CR1. However, deletion of the receptor CX3CR1 in Cx3cr1gfp/gfp mice had no impact on the frequencies of γδT cells in the gingival epithelium, as well as in the skin epidermis (SI Appendix, Fig. S3E). As for CCR6, while no γδT cells in the epidermis express this receptor, nearly 25% of gingival γδT cells expressed CCR6 (SI Appendix, Fig. S3F). The absence of CCR6 in Ccr6gfp/gfp mice led to a significant reduction in intraepithelial γδT cells in the gingiva, but not in skin (SI Appendix, Fig. S3G). In conclusion, the homeostasis of Vγ6+ T cells in the gingiva is partially dependent on IL-23R signaling, while CCR6 drives homing of part of the γδT cells to the gingival epithelium.
γδT Cells Reside in the Prospective Gingival Epithelium During Embryogenesis and Their Numbers Increase Postnatally but Decline with Age.
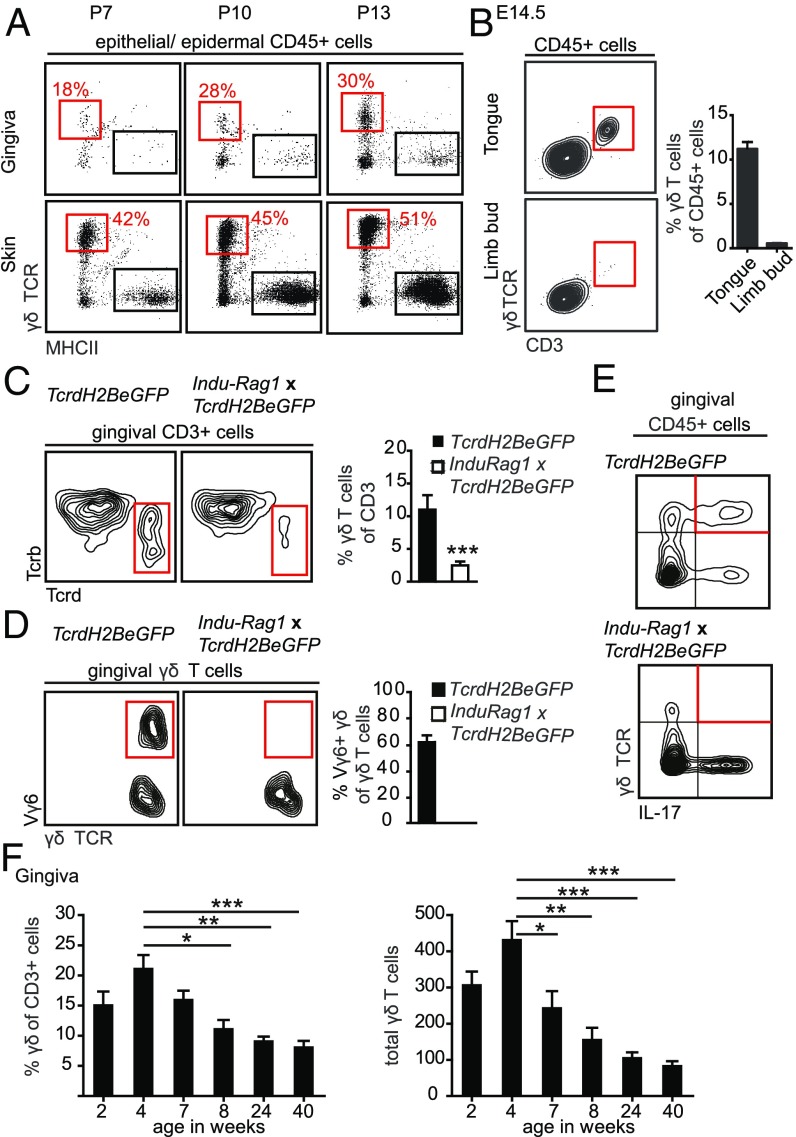
The gingiva is a special tissue because it emerges postnatally during the process of tooth eruption, starting in mice during the second week after birth. To track the development of gingival intraepithelial γδT cells, we first collected the epithelium of the prospective gingiva at postnatal day (P)7, as well as during gingiva development at days P10 and P13. A small population of intraepithelial γδT cells was detectable at P7 and the frequency of these cells increased relatively fast in the epithelium later on (Fig. 3A). In fact, the frequencies of γδT cells on P13 were similar to those observed in adult mice (∼25–30% of total leukocytes in the epithelium). In contrast to the gingiva, γδT cells in the epidermis were established already at day P7. Because Vγ6+ γδT17 cells might have entered the oral mucosa already during embryogenesis, we examined γδT cells in the tongue, an oral tissue that is accessible for analysis at embryonic day (E)14.5. Indeed, a clear population of CD3+ γδT cells was observed in the tongue mucosa at this early developmental stage (Fig. 3B). A small population of γδT cells was also found in the limb bud representing the developing skin (Fig. 3B).
Fig. 3.
Oral γδT cells develop prenatally, expand postnatally, but decline with age. (A) FACS plots show frequencies of epithelial γδT cells in the gingiva and skin at indicated days postnatally (P). Five to six mice were analyzed in each experiment, two independent experiments. (B) Representative FACS plots and bar graph demonstrating the presence of γδT cells in the tongue and limb buds on day E14.5 (three independent experiments; eight embryos were analyzed in each experiment). (C) γδT cell populations among CD3+ cells in gingival tissues form TcrdH2BeGFP and Indu-Rag1×TcrdH2BeGFP adult mice. Representative FACS plots from three independent experiments. Bar graph depicts the mean frequencies + SEM of γδT cells among total gingival CD3+ T cells. (Data pooled from three independent experiments, n = 7–8 mice per experiment.) (D) Frequencies of Vγ6+ cells from total gingival γδT cells of TcrdH2BeGFP and Indu-Rag1×TcrdH2BeGFP mice. Bar graph demonstrates mean frequencies + SEM of Vγ6+ cells among total gingival γδT cells. Data were pooled from two independent experiments (n = 8 mice per experiment). (E) FACS plots show γδT cells producing IL-17 from total gingival leukocytes isolated from TcrdH2BeGFP and Indu-Rag1×TcrdH2BeGFP mice and stimulated ex vivo with PMA and ionomycin. (Three independent experiments, n = 8–10 mice per experiment.) (F) Frequencies and total numbers of murine gingival γδT cells throughout life. Data pooled from three independent experiments are shown and presented as the mean values + SEM (n = 6–9 mice per experiment). *P < 0.05, **P < 0.01, ***P < 0.001.
To investigate whether gingival γδT cells develop in embryonic thymus or arise from postnatal development, we used the Indu-Rag1×TcrdH2BeGFP mice (29). These mice lack B and T lymphocytes due to the absence of recombination-activating gene 1 (Rag1), but maturation of B and T cells can be initiated in adult mice upon administration of tamoxifen. Seven weeks after tamoxifen treatment, Indu-Rag1×TcrdH2BeGFP mice had reduced frequencies of γδT cells in the gingiva (Fig. 3C), whereas αβT cells were completely restored (SI Appendix, Fig. S4A). Furthermore, Vγ6+ γδT cells were completely absent in these mice, confirming that their development is restricted to the embryonic thymus (Fig. 3D). Interestingly, γδT cells present in the gingiva of tamoxifen-treated Indu-Rag1×TcrdH2BeGFP adult mice failed to produce IL-17 upon ex vivo stimulation, but this task was taken over by other non-γδT lymphocytes in the gingiva (Fig. 3E). Next, we examined the frequencies of gingival γδT cells throughout life, because age is known to have a major impact on oral homeostasis (30). Whereas frequencies and absolute numbers of γδT cells increased in the gingiva during the first 4 wk after birth, a sharp and significant reduction was observed in 8-wk-old mice (Fig. 3F). The frequency and numbers of γδT cells gradually declined afterward, and in 40-wk-old mice gingival γδT cells were reduced three- to fourfold in comparison with 4-wk-old mice (Fig. 3F). Accordingly, total numbers of Vγ6+ cells declined in 40-wk-old mice compared with 4-wk-old mice (SI Appendix, Fig. S4B). However, the frequencies of these cells among all γδT cells were not altered, which suggests that non-Vγ6+ cells were also reduced with age. Analysis of gingival intraepithelial γδT cells in aged (18-mo-old) mice showed a significant reduction in their frequencies compared with 2-mo-old adult mice (SI Appendix, Fig. S4C). In contrast, the frequency of epidermal γδT cells was not decreased in aged mice. In conclusion, γδT cells, predominantly Vγ6+ cells, enter the oral epithelium during embryogenesis and increase during weaning until 4 wk of age. Later, aging is accompanied with a gradual loss of γδT cells.
Gingival Intraepithelial γδT Cells Comprise Radioresistant and Tissue-Resident Populations.
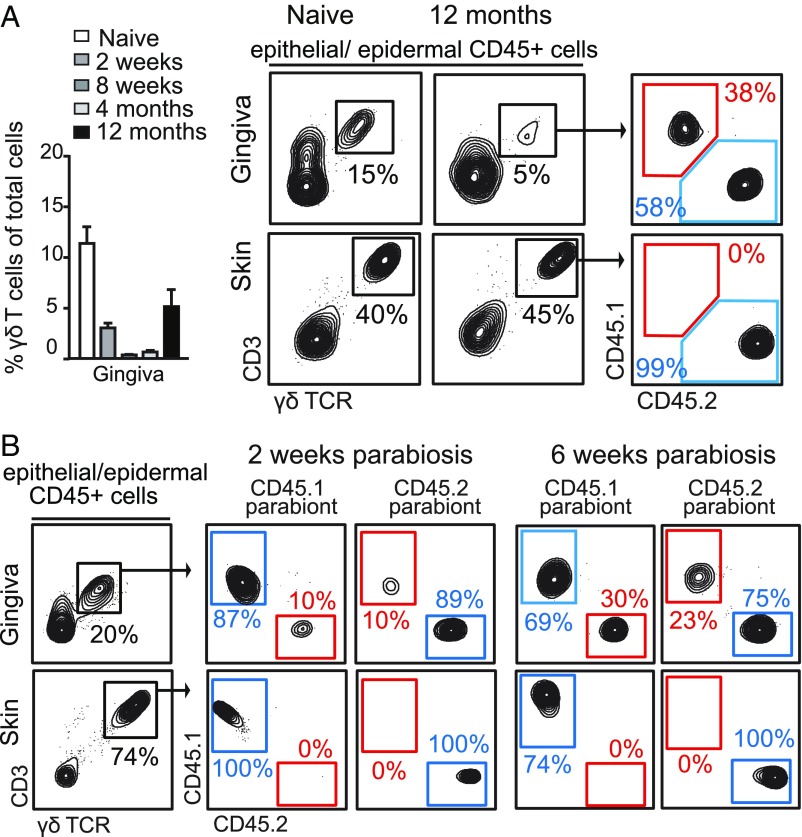
To investigate the homeostasis and turnover of gingival γδT cells, we first evaluated the kinetics of these cells following continuous administration of BrdU. Seven days after BrdU treatment, about 30% of the intraepithelial γδT cells were BrdU+ and levels doubled after 14 d of treatment (SI Appendix, Fig. S5). In contrast, epidermal γδT cells showed only negligible BrdU-labeling levels (SI Appendix, Fig. S5). Next, we reconstituted lethally irradiated CD45.2+ B6 mice with bone marrow purified from congenic CD45.1+ mice. Two weeks after irradiation, a significant decrease in the frequencies of gingival intraepithelial γδT cells was detected, which was further reduced 8-wk postirradiation (Fig. 4A). The population of γδT cells then slowly expanded in the gingival epithelium, reaching about half of the original frequencies 12 mo after the irradiation. Importantly, the majority of reconstituting γδT cells (∼58%) developed from the host, whereas donor-derived γδT cells constituted only 38% of the population (Fig. 4A). Thus, gingival intraepithelial γδT cells contain a radioresistant population and an additional population arriving via the circulation. To directly examine the origin of intraepithelial γδT cells, we generated parabiotic mice by pairing surgically CD45.2+ B6 mice with congenic CD45.1+ mice, so the mice shared the same blood circulation but had separate organs. Two weeks after parabiosis ∼10% of the γδT cells originated from circulating cells, while their frequencies increased to 20–30% after 6 wk (Fig. 4B). Because in parabiotic mice the circulating leukocytes from both parabionts are evenly divided, the contribution of circulating cells to intraepithelial γδT cells in this setting was in fact 40–60%. As an expected control for the parabiosis experiments, no contribution from the circulation was found in epidermal γδT cells. Taken together, these data thus suggest that gingival intraepithelial γδT cells contain a population of tissue-resident and radioresistant cells with self-renewing capability. An additional population comprising approximately half of the intraepithelial γδT cells originated from the circulation, and replaced constitutively and independently of the tissue-resident population.
Fig. 4.
Oral γδT cells comprise both radioresistant tissue-resident cells and radiosensitive circulating cells. (A) Lethally irradiated CD45.2+ B6 mice were transplanted with bone marrow purified from congenic CD45.1+ B6 mice. Representative FACS plots demonstrate the contribution of donor (CD45.1+ cells) to γδT cells in the gingiva and skin epithelium 12 mo after the transplantation. Bar graphs depict the frequencies of γδT cells in the gingival epithelium at various times after the bone marrow transplantation (n = 4–6 mice per group for each time point). FACS plots represent the results of one of two independent experiments with similar results, and graphs present the mean values + SEM. (B) CD45.1+ B6 and CD45.2+ B6 mice were joined surgically for the generation of parabiotic pairs, and homeostasis of γδT cells was analyzed 2 and 6 wk after parabiosis in each parabiont. Representative flow cytometry plots from four independent experiments (n = 3–4 pairs per time point).
The Microbiota Regulates the Frequency, Vγ Chain Subset Composition, and Activation of Gingival γδT Cells.
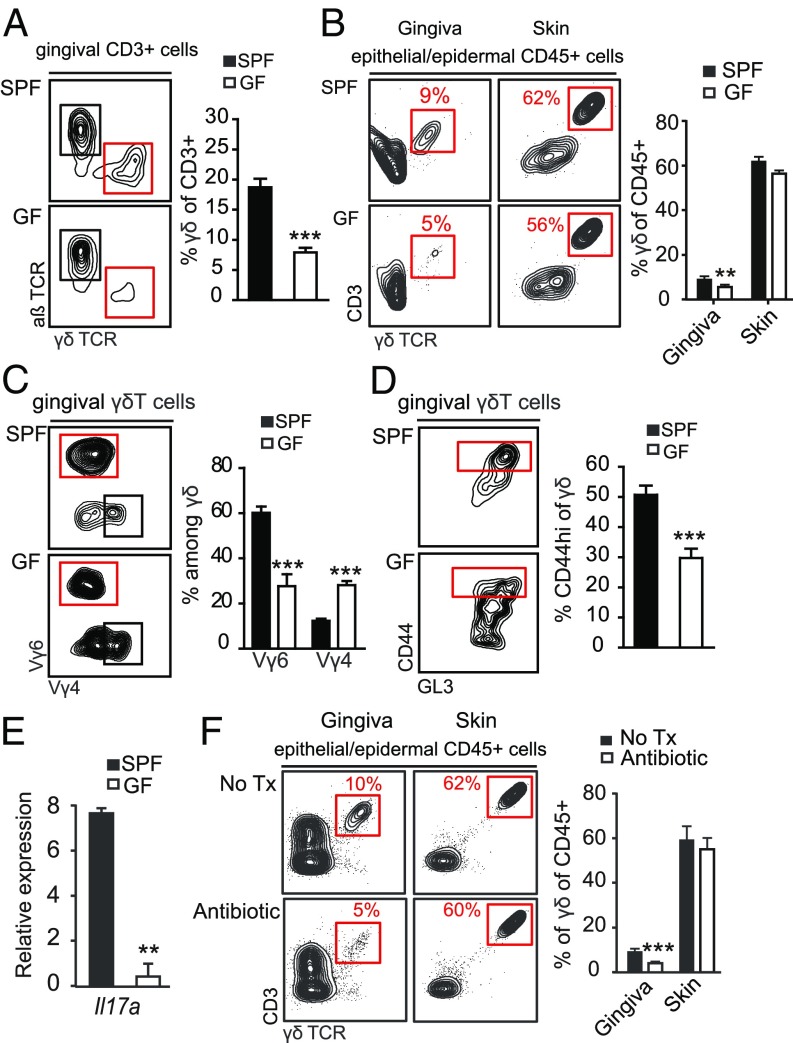
The localization of intraepithelial γδT cells in the junctional epithelium, as well as their expansion after birth, suggest an interplay with the microbiota. To examine this issue directly, we analyzed γδT cells in adult germ-free (GF) mice. The absence of the microbiota resulted in decreased frequencies and total numbers of gingival γδT cells (Fig. 5A and SI Appendix, Fig. S6A) but not of skin intraepithelial γδT cells (Fig. 5B). In contrast, the frequencies of αβT cells were elevated in the gingiva of GF mice compared with specific pathogen-free (SPF) mice (SI Appendix, Fig. S6B). Moreover, among the γδT cells, the frequency and number of the Vγ6+ subset was greatly reduced, whereas the relative frequencies but not the numbers of Vγ4+ T cells were elevated (Fig. 5C and SI Appendix, Fig. S6A). Further analysis revealed that the frequencies as well as total numbers of CD44hi γδT cells were reduced in the gingiva of GF mice in comparison with SPF mice, suggesting a lower activation state of these cells in the absence of the oral microbiota (Fig. 5D and SI Appendix, Fig. S6A). The activation status of conventional αβT cells in the gingiva was not significantly reduced (SI Appendix, Fig. S6B). Concurring with the reduction in Vγ6+ and CD44hi γδT cells found in GF mice, IL-17 levels were reduced in the gingiva of these mice compared with the SPF group (Fig. 5E). Interestingly, in GF mice we did not observe a reduction in frequencies or activation status of total γδT cells in cervical lymph nodes that drain the oral mucosa (SI Appendix, Fig. S6C). Nevertheless, the frequency of the Vγ6+ subset was significantly reduced, whereas at the same time the proportion of Vγ4+ T cells increased in the mucosa-draining lymph nodes of GF mice (SI Appendix, Fig. S6C). To examine whether γδT cells are also responsive to changes in the microbiota in an adult host after the establishment of the microbiota, we next treated adult B6 mice with broad-spectrum antibiotic in the drinking water for 2 mo. A significant reduction in the frequency of intraepithelial γδT cells was detected in the gingiva but not epidermis of antibiotic-treated mice in comparison with the untreated control group (Fig. 5F). Taken together, these findings suggest that development and function of gingival γδT cells is critically regulated by the microbiota. Moreover, gingival Vγ6+ T cells appeared to be most responsive to the oral microbiota.
Fig. 5.
Commensal microbiota regulate the development and activation state of oral γδT cells. Gingival tissues were collected and processed from adult SPF and GF mice for flow cytometry analysis. (A) FACS plots and graphs depict the frequencies of γδT cells in the whole gingiva. Data are representative of four independent experiments. Bar graphs present the mean values + SEM of pooled data from four independent experiments (n = 7–10 mice per experiment). (B) Epithelial tissues were collected from the gingiva and skin of SPF and GF B6 mice. Representative FACS plots show the frequencies of γδT cells in each tissue. Bar graph presents the percentages of γδT cells among CD45+ cells as the mean values + SEM (n = 4 mice, three independent experiments). (C) Percentages of Vγ6+ and Vγ4+ γδT cells and (D) levels of CD44hi γδT cells in the gingiva of GF and SPF mice. FACS plots are representative of four independents experiments. Bar graphs present the mean values + SEM of pooled data from four independent experiments (n = 7–10 mice per experiment). (E) Expression of Il-17a mRNA in the gingiva of GF or SPF mice. Results of one of two independent experiments are shown as the mean values + SEM (n = 5 mice per group). (F) Adult SPF B6 mice were treated with a broad-spectrum antibiotic mixture in the drinking water for 2 mo. Representative FACS plots and graph demonstrate the percentages of γδT cells in gingival and ear skin epithelial tissues of antibiotic-treated mice and control group (n = 5). Results of two independent experiments are shown as the mean values + SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
Conditional Ablation of γδT Cells Elevates the Inflammatory Milieu in the Gingiva.
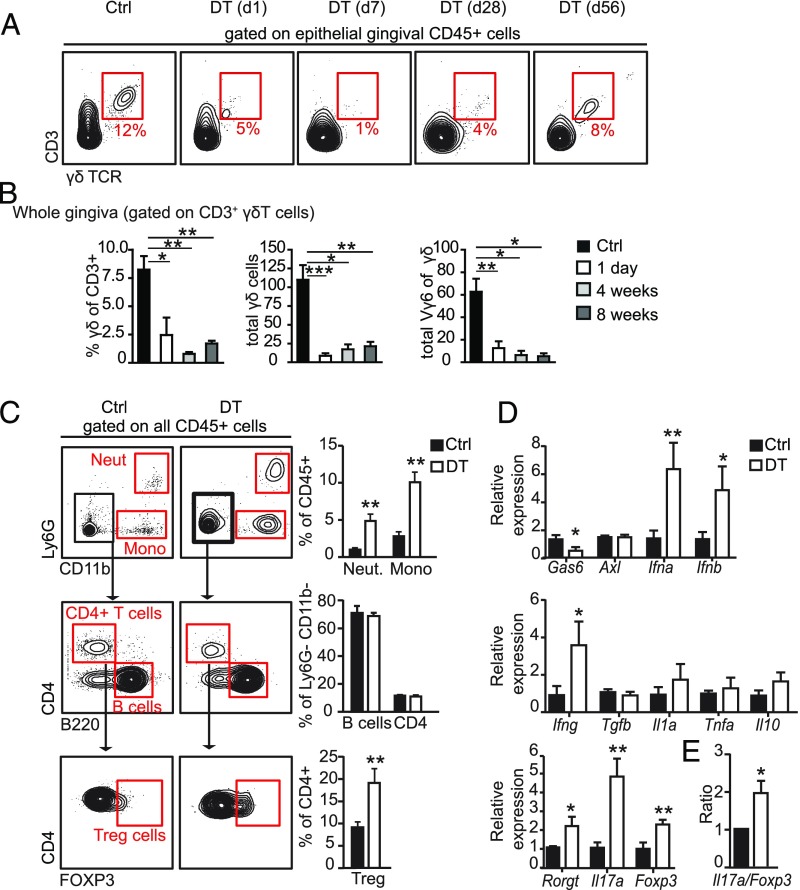
We next addressed whether γδT cells play a role in the maintenance of immunological homeostasis in the oral mucosa. To this end, we used the Tcrd-GDL knockin mice, which express simultaneously three reporter genes (GDL; Gfp-Dtr-Luc) specifically expressed in γδT cells under the control of successfully rearranged TCR δ genes. The expression of diphtheria toxin (DT) receptor in Tcrd-GDL mice allows the depletion in vivo of γδT cells upon administration of DT. First, we examined the repopulation kinetics of γδT cells upon a single administration of DT to adult Tcrd-GDL mice. Depletion of gingival intraepithelial γδT cells was evident 24 h after DT treatment (Fig. 6A). In the following weeks, the γδT cells gradually reappeared in the gingival epithelium, but failed to reach their original frequencies as far as 8 wk after DT treatment. We also analyzed the repopulation of γδT cells in the whole gingiva while focusing on the Vγ6+ T cell subset. Similar slow repopulation kinetics were detected for total gingival γδT cells; nevertheless, the repopulating cells appeared to be non-Vγ6+ cells (Fig. 6B). Next, to elucidate the impact of γδT cell ablation on oral immunity, we depleted these cells for 5 mo by injecting mice with DT on a weekly basis. Of note, we previously reported that such prolonged administration of DT into wild-type mice had no impact on gingival immunity and associated pathologies (8, 31). Tcrd-GDL mice receiving the PBS vehicle were used as a negative control. Flow cytometry analysis of gingival tissues isolated from long-term depleted mice revealed major changes in innate leukocytes. Specifically, the frequencies of neutrophils and monocytes were increased in DT-treated mice (Fig. 6C). Whereas the levels of total CD4+ T cells and B cells were not altered by the DT treatment, higher levels of FOXP3-expressing CD4+ T cells (T regulatory cells) were found in DT-treated mice compared with control group (Fig. 6C).
Fig. 6.
Prolonged ablation of γδT cells increases gingival inflammation. (A) Repopulation kinetics of γδT cells in the gingiva and ear skin following single administration of DT to Tcrd-GDL mice. FACS plots demonstrate the frequencies of epithelial γδT cells; at least three mice were analyzed at each time point. (B) Bar graphs represent the mean + SEM of pooled data from three independent experiments (n = 4–10 mice per experiment) of frequencies of γδT cells among CD3 as well as total numbers of γδT cells and Vγ6+ γδT cells in the gingiva. Three to 10 mice were analyzed at each time point. (C and D) Adult Tcrd-GDL mice were injected intraperitoneally with DT on a weekly basis for 5 mo; gingival tissues were then collected and analyzed. (C) Representative FACS plots and graphs demonstrate the alterations in the frequencies of various leukocytes subsets in the gingiva due to the absence of γδT cells. (D) Relative expression of the indicated genes was quantified by qRT-PCR. Graphs present the transcript levels normalized to control group (Ctrl) depicted as the mean values + SEM (n = 5–6). (E) Il17a/Foxp3 expression level ratio in DT-treated and control groups presented as the mean values + SEM (n = 5). Representative data of two independent experiments are presented. *P < 0.05, **P < 0.01, ***P < 0.001.
We next quantified in the gingiva the expression of certain gene groups using qRT-PCR. As demonstrated in Fig. 6D, mRNA levels of the ligand Gas6 but not its receptor Axl were reduced in DT-treated mice in comparison with control mice. We recently showed that GAS6/AXL signaling down-regulates IFN I signaling (IFN-α/β) in the gingival epithelium, which is crucial for oral homeostasis (7). In line with this view, DT-treated mice expressed significantly higher levels of Ifna and Ifnb mRNA (Fig. 6D). We further quantified the mRNA levels of inflammation-associated cytokines such as Ifng, Tgfb, Il1a, and Il10. Among these cytokines, the level of Ifng was elevated in DT-treated mice, whereas the rest were left unchanged (Fig. 6D). Because the magnitude of IL-17 versus FOXP3 in mucosal sites was shown to represent the immunological equilibrium of the tissue (32, 33), we also measured their relative expression. Elevated mRNA levels of Il17a, Foxp3, as well as the mRNA of Rorgt, a transcription factor controlling IL-17 secretion, were found in DT-treated mice (Fig. 6D). Such elevated secretion of IL-17 is likely to be mediated by other leukocytes, in line with our finding using tamoxifen-treated Indu-Rag1×TcrdH2BeGFP mice (Fig. 3 C–E). Furthermore, the ratio of Il17a versus Foxp3 expression was almost two times higher in DT-treated mice in comparison with control mice (Fig. 6E). This suggests that, while γδT cells are the principal producers of gingival IL-17A in normal mice (Fig. 2D), other CD45+ cells were covering up this task after their depletion in a less balanced manner. Collectively, conditional ablation of γδT cells results in an elevated inflammation in the gingiva, suggesting an important role for these cells in regulating immunological homeostasis in the oral mucosa.
The Absence of γδT Cells Induces Oral Microbial Dysregulation.
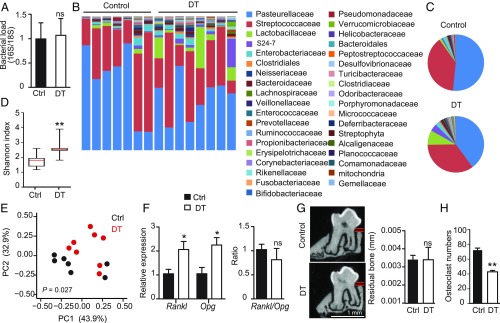
Because alteration in oral mucosal immunity is likely to affect local microbiota, we examined whether the prolonged ablation of γδT cells induced changes in the load and diversity of oral microbiota. To avoid nonspecific microbial variations, aged and sex-matched Tcrd-GDL mice were housed under similar conditions, before randomly dividing the mice into DT or PBS-treated groups that were housed later in separate new sterile cages. The oral microbiota was probed in these groups of mice after 5 mo of treatment by extracting DNA from oral swabs, and the ratio of ribosomal 16S/18S genes was calculated to compare the total bacterial load. As depicted in Fig. 7A, we found no alteration in the overall load of oral bacteria between the two groups. To examine if prolonged ablation of γδT cells affected bacterial diversity, we performed a taxonomic analysis of the oral microbiota. Indeed, significant changes in the composition of oral microbiota were detected between DT-treated mice and control mice (Fig. 7 B and C). α-Diversity analysis indicated higher taxa richness in DT-treated Tcrd-GDL mice compared with controls (Fig. 7D), while the taxa presented in these mice significantly varied as indicated by unweighted β-diversity (Fig. 7E). Specifically, among the high-abundance bacterial families, the Lactobacillaceae family (Firmicutes phylum) was significantly increased in DT-treated mice (Fig. 7 B and C and SI Appendix, Fig. S7A). The Streptococcaceae family (Firmicutes phylum) was not altered by the depletion of γδT cells, whereas the Pasteurellaceae family was reduced (Proteobacteria). Within the low-abundance families, a significant increase in the levels of various families was observed in the absence of γδT cells, among them the Porphyromonadaceae family (Bacteroidetes), which include the inflammophilic oral pathogen Porphyromonas gingivalis (6) (SI Appendix, Fig. S7B). Of note, further analysis revealed that the microbial dysregulation appeared after the immunological shift in the gingiva. Ten days after γδT cell depletion expression of proinflammatory genes was altered, whereas no changes were observed in the oral microbiota (SI Appendix, Fig. S7 C and D).
Fig. 7.
Ablation of γδT cells alters the relative diversity of oral microbiota. Adult Tcrd-GDL mice were injected intraperitoneally with DT on a weekly basis for 5 mo. (A) Total oral bacterial load was determined in oral swabs taken from the mice by qRT-PCR of the 16S rRNA gene. Bar graphs present the 16S/18S ratio in each group as the mean values + SEM (n = 7–8 per group). (B–E) Relative abundance of taxa in oral swabs sampled from DT-treated adult Tcrd-GDL mice and controls (Ctrl) upon prolonged DT treatment. Histograms represent the distribution of sequences in operational taxonomic units (OTUs) assigned to each class. Histograms for individual mice (B) as well as pie charts representing the mean distribution of OTU (C) are provided. (D) α-diversity plot representing taxa richness in samples of both groups of mice (rarified to 10,000 reads). Data represent the mean values + SEM (n = 7–8 mice per group). (E) Principal coordinates analysis of weighted UniFrac distances based on 16S rRNA of DT-treated mice versus littermate control. Taxonomic data from one of two independent experiments are shown. (F) Relative expression of Rankl and Opg as well as Rankl/Opg ratio in DT treated and control mice depicted as the mean values + SEM (n = 5). (G) Representative μCT sections of the second upper molar indicate the distance between the cementoenamel junction and alveolar bone crest. Graph demonstrates 3D quantification of the residual alveolar bone volume 5 mo after DT treatment presented as the mean values + SEM (n = 6–7 per group). (H) Osteoclast numbers identified as tartrate-resistant acid phosphatase-positive cells in gingival sections taken from DT and control groups 2 wk after the treatment. Representative bone loss data of two independent experiments are shown. *P < 0.05, **P < 0.01.
Because oral microbial shift and elevated gingival inflammation are associated with an accelerated level of spontaneous alveolar bone loss (7), we analyzed bone resorption in our system. First, we quantified the mRNA levels of Rankl (receptor activator of NF-κΒ ligand) and Opg (osteoprotegrin), which are known to promote or prevent bone resorption, respectively (34). As depicted in Fig. 7F, both molecules were equally overexpressed in the tissues of DT-treated and control groups, suggesting that bone remodeling was not altered. In line with this expression pattern, bone loss could not be found in the alveolar bone by 3D analysis using µCT (Fig. 7G). Moreover, we found reduced osteoclast numbers around the alveolar bone as early as 2 wk after DT treatment (Fig. 7H), suggesting that γδT cells regulate osteoclast development. Taken together, these data suggest that inducible ablation of γδT cells altered microbial richness and diversity in the oral cavity. Although such microbial dysregulation, together with the elevated inflammation described earlier (Fig. 6 C and D), are expected to facilitate periodontal destruction, spontaneous alveolar bone loss was not increased due to the absence of γδT cells.
Discussion
This study characterized γδT cells of the gingiva, an immunologically challenging tissue monitoring the persistent dental biofilm and preserving oral homeostasis. Our complementary data, which were independently obtained in two different laboratories with similar results, demonstrate that mutual interplay between activated γδT cells and the microbiota shapes gingival homeostasis. First, in vivo ablation of γδT cells altered gingival immunity and caused microbial shift in the oral cavity. Second, the majority of intraepithelial γδT cells are localized in the junctional epithelium, in which they can get access to suprabasal layers close to the dental biofilm. Third, the frequencies of γδT cells but not αβT cells were reduced in GF mice, as well as their ability to secrete IL-17. Moreover, the substantial loss of Vγ6+ γδT cells observed in the gingiva of GF mice, while Vγ4+ γδT cells were unaffected, demonstrated the capacity of the microbiota to shape gingival γδT cell subsets. In contrast to the gingiva, the microbiota has little or no impact on the homeostasis of both intestinal and skin intraepithelial γδT cells (35, 36). Nevertheless, akin to the gingiva, the microbiota plays an important role in the activation of these cells in both compartments (12, 36). Thus, the gingiva represents a unique site in which homeostatic development of intraepithelial γδT cells is clearly modulated by the microbiota. This is in line with a study reporting reduced levels of IL-17–producing γδT cells in the cervical lymph nodes of GF mice, by a mechanism involving cell-to-cell contact between γδT cells and CD103+ dendritic cells (37). Interestingly, we demonstrated previously that CD103 is also expressed by a subset of mucosal LCs, a special type of dendritic cell located in the gingival epithelium (9). Moreover, the frequencies of these gingival CD103+ LCs are specifically reduced in GF or antibiotic-treated mice, and ablation of LCs alters the load and diversity of the oral microbiota (8). Because gingival LCs mainly reside in the junctional epithelium in close proximity to intraepithelial γδT cells, it is likely that CD103+ LCs represent the genuine dendritic cell subset interacting with and regulating the development of intraepithelial gingival γδT cells.
Whereas intraepithelial (dendritic) γδT cells of the skin uniquely express Vγ5, we found that γδT cells in the gingival epithelium rather resembled dermal γδT cells and were mainly Vγ6+, but to some extent expressed also Vγ1 and Vγ4. This could be attributed, in part, to the accessibility of the gingival epithelium to circulating Vγ1+ and Vγ4+ cells that keep developing after birth (15). It is also conceivable that the entry of αβT cells to the gingival epithelium impacts local γδT cell frequencies. However, upon irradiation, γδT cells only slowly repopulated the gingival epithelium and host-derived cells outcompeted donor-derived γδT cells in these experiments. Vγ6+ cells probably constituted the majority of these host-derived γδT cells as they were shown to be radioresistant, while the radiosensitive Vγ4+ cells likely originated from the donor bone marrow, as reported previously in the skin dermis (38, 39). The repopulation advantage of host-derived Vγ6+ cells was proposed to stem from their capacity to proliferate directly in the tissue, whereas Vγ4+ cells cannot do so (40). Vγ6+ cells were also reported to express CCR6 (26); thus, the observed reduction of γδT cells in the gingiva of CCR6-deficent mice may be attributed, in part, to a decrease of Vγ6+ cells. This suggests that Vγ1+ and Vγ4+ T cells generated after birth cannot compensate the absence of embryonic Vγ6+ cells. Notably, the reduced numbers of γδT cells that we detected in Tamoxifen-treated Indu-Rag1×TcrdH2BeGFP and IL23Rgfp/gfp mice confirmed the hypothesis that tissue-resident IL-17–producing Vγ6+ cells are a major population of γδT cells in the gingival mucosa. This is also supported by the slow repopulation kinetics detected upon in vivo depletion, because Vγ6+ cells are not generated in adult mice. An additional support to this assumption is that following 6 wk of parabiosis, about half of gingival γδT cells were replaced in each parabiont, indicating that γδT cells developed in adult mice can replace only non-Vγ6 cells. Furthermore, the inability of such circulating cells to fully restore gingival γδT cells 8 wk after depletion and as late as 1 y after irradiation, strongly suggested that they cannot compensate the loss or absence of Vγ6+ cells. Thus, we propose that embryonic Vγ6+ cells and adult Vγ1+ and Vγ4+ are independently maintained in the gingiva throughout life. The observed peak of Vγ6+ cell numbers and frequencies after weaning may be in part due to increased mechanical stress and mucosal tissue injury due to mastication, as described for gingival Th17 cells (5). However, the sharp decline of Vγ6+ cells in GF mice compared with SPF mice in two independent GF facilities, would strongly advocate for a cardinal role of the oral microbiota in maintaining gingival γδT cell homeostasis.
Interestingly, a recent work reported that Vγ4+ and Vγ1+ rather than Vγ6+ cells are the major subsets of γδT cells in adult gingiva, while the majority of gingival γδT cells are radiosensitive (41). These observations contradict our data and could be simply explained by differences in the microbiota or housing conditions, such as diet, which was shown to increase the frequencies of the radiosensitive Vγ4+ and Vγ1+ subsets (41). However, the complementary data obtained independently by two different laboratories in the present study suggest that other factors could lead to these opposing results. For example, in the study of Krishnan et al. (41), the Vγ subsets characterized in adult gingiva represent only 55% of total γδT cells, whereas in our study above 97% of these cells were identified. It is possible that the frequencies of Vγ6+ cells were underestimated by Krishnan et al., due to the very delicate staining protocol required to identify this particular subset. Indeed, our parabiosis, chimera, and tamoxifen-induced Rag1 expression experiments strongly indicate that embryonic-derived Vγ6+ cells represent the majority of gingival γδT cells in adult mice. Furthermore, the use of TcrdH2BeGFP reporter mice allowed us to identify more precisely the γδT cells compared with staining with GL3 antibody. An additional major difference between both studies is related to the impact of the microbiota on gingival γδT cells. Krishnan et al. reported no alteration in the composition of the Vγ subsets in GF mice, although the total numbers of gingival γδT cells were reduced compared with SPF mice. We similarly revealed a decrease in gingival γδT cells, which was attributed to a significant reduction of the Vγ6+ subset. This is in agreement with a recent study reporting that oral microbiota expanded IL-17–producing Vγ6+ cells systemically (37). Unfortunately, Krishnan et al. did not examine the Vγ6+ cells in GF mice and, moreover, they limited their analysis to the first week after birth, which might cloak the large impact of the microbiota on Vγ6+ cells.
Conditional ablation of γδT cells resulted in an elevated gingival inflammation and significant modifications in the diversity of oral microbiota. Similar microbial changes were reported in IL17R-deficient mice, demonstrating expansion of Lactobacillus, reduction of Pasteurellaceae, while Streptococcaceae was not affected (37). This further supports the importance of steady-state IL-17 signaling by γδT cells, the main IL-17–producing cells in the oral mucosa. Interestingly, in contrast to these observations, in Tcrd−/− mice no change was found in the load and richness of oral microbiota compared with wild-type mice; however, an overgrowth of Aggregatibacter species was reported (41). This echoes the fundamental difference between Tcrd−/− mice and Tcrd-GDL mice with regards to the impact of γδT cells on the homeostasis of oral microbiota (42). Other lymphocytes developing in the empty niche of genuine γδT cells in Tcrd−/− mice might be less suited to balance gingival homeostasis, possibly because they cannot produce regulatory factors, such as Areg in addition to proinflammatory IL-17 (41), and thereby facilitate periodontal bone loss. In any case, expansion of Lactobacillaceae directly reflects the immunological status of the gingiva of γδT cells-depleted mice, because this family is capable of using inflammation byproducts for anaerobic respiration facilitating their growth in an inflamed environment (43–45). This could also explain the outgrowth of Aggregatibacter species in Tcrd−/− mice, although the immunological status of the gingiva in these mice was not addressed by Krishnan et al. (41).
We have recently shown a similar shift in the oral microbiota using Gas6−/− mice, a key homeostatic regulator of the oral mucosa (7), which was also down-regulated in the present study upon depletion of γδT cells. Such activity of GAS6 involved down modulation of IFN I signaling in mucosal tissues (7, 46), and the substantial elevation of Ifna/b mRNA levels in γδT cell-depleted mice suggest that this regulatory pathway is also affected in our system. Of note, the Porphyromonadaceae family was also augmented in the absence of γδT cells. This is highly relevant to oral health, as this family includes the keystone oral pathogen P. gingivalis that flourishes in periodontal inflammation and is closely associated with tissue destruction (6). Nevertheless, despite the higher gingival inflammation and microbial dysregulation observed in γδT cell-depleted mice, which are strongly associated with destructive immunity (1), we did not observe an accelerated alveolar bone loss. This could be explained by our findings that the bacterial load and RANKL/OPG ratio were not altered upon depletion, suggesting that the nature of gingival inflammation rather than its magnitude is relevant for the alveolar bone loss process. Alternatively, the reduction in osteoclast numbers around the alveolar bone suggests that γδT cells might regulate osteoclast differentiation regardless to the overall inflammatory level of the gingiva. However, the overall lack of effect on the alveolar bone might suggest that bone resorption activity of the residual osteoclasts is increased due to local inflammation (47), thus compensating the reduction in their numbers. It is also possible that depletion of γδT cells is affecting osteoblasts, because osteoblast function was shown to be compromised under inflammatory conditions and therefore bone formation is decreased (48).
In contrast to our study, a recent work employing Tcrd−/− mice reported that γδT cells are essential for protection against age-associated periodontal bone loss (41). Nevertheless, Tcrd−/− mice, unlike the Tcrd-GDL mice used in our study, lack γδT cells during pre- and postnatal development, and it is likely that Tcrd−/− mice compensate the loss of these cells by recruiting other effector cells to the gingiva. Indeed, we showed recently that the lack of γδT cells in constitutive Tcrd−/− mice is functionally compensated by other lymphocytes taking over genuine γδT cell functions (42). Additionally, we demonstrate here that ablation of embryonic-derived γδT cells resulted in a development of other IL-17–expressing leukocytes. These might be less suited to balance gingival homeostasis, possibly because they can produce regulatory factors, such as Areg, in addition to proinflammatory IL-17 (41), and thereby facilitate periodontal bone loss. Tcrd-GDL mice, on the other hand, develop normally and thus represent more adequately the role of γδT cells in age-associated bone loss. It should be mentioned that the lack of effect on bone volume in Tcrd-GDL mice could not be explained by an insufficient depletion period of only 8 wk, because we and others have reported that natural bone loss can be detected during this time (2, 8).
In conclusion, this study demonstrates the subtle and critical role of gingival intraepithelial γδT cells in oral mucosal homeostasis. This task is mediated by γδT cells developing both pre- and postnatally, which are maintained independently in the tissue. Furthermore, the development and homeostasis of gingival γδT cells is shaped by the microbiota, a unique phenomenon that does not occur in the intestine and skin epithelia to this extent. Therefore, targeting γδT cells is a promising strategy to modulate and improve oral health.
Materials and Methods
All animal protocols were approved by the Hebrew University Institutional Animal Care and Use Committee as well as in accordance with institutional guidelines of the Hannover Medical School, approved by the Lower Saxony State Office for Consumer Protection and Food Safety animal care and use committee. GF or SPF adult B6 mice were maintained in sterile isolators at the Weizmann Institute or at the central animal facility at Hannover Medical School, the studies were approved by the Institutional Animal Care and Use Committee of the Weizmann Institute of Science or Hannover Medical School, respectively.
Detailed information on antibodies, reagents, mice, and ethical approvals is described in SI Appendix. Details of parabiosis experiments, chimeric mice, immunofluorescence staining, isolation, and processing of gingival and skin γδT cells, two-photon microscopy, conditional ablation of γδT cells in vivo, RNA extraction, and qRT-PCR, induction of Rag1 enzyme expression in adult mice, BrdU incorporation assays, microbiota analysis, bone loss quantification, and statistical analysis are provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank Tiago Amado and Bruno Silva-Santos for kindly providing B6.129S4-Ifngtm3.1Lky Il17atm1Bcgen (MGI:5426367, here IL17eGFP) reporter mice. This work was supported by the German–Israeli Foundation for Scientific Research and Development Grant 1432 (to A.-H.H. and I.P.) and Deutsche Forschungsgemeinschaft Grants PR727/4-1 and SFB900-B8 (to I.P.). A. Wilharm was supported by the Hannover Biomedical Research School, and Y.T. was supported by the Faculty of Dental Medicine, Hebrew University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1818812116/-/DCSupplemental.
References
- 1.Moutsopoulos NM, Konkel JE. Tissue-specific immunity at the oral mucosal barrier. Trends Immunol. 2018;39:276–287. doi: 10.1016/j.it.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajishengallis G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greer A, Zenobia C, Darveau RP. Defensins and LL-37: A review of function in the gingival epithelium. Periodontol 2000. 2013;63:67–79. doi: 10.1111/prd.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eskan MA, Rose BG, Benakanakere MR, Lee MJ, Kinane DF. Sphingosine 1-phosphate 1 and TLR4 mediate IFN-beta expression in human gingival epithelial cells. J Immunol. 2008;180:1818–1825. doi: 10.4049/jimmunol.180.3.1818. [DOI] [PubMed] [Google Scholar]
- 5.Dutzan N, et al. On-going mechanical damage from mastication drives homeostatic Th17 cell responses at the oral barrier. Immunity. 2017;46:133–147. doi: 10.1016/j.immuni.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajishengallis G, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nassar M, et al. GAS6 is a key homeostatic immunological regulator of host-commensal interactions in the oral mucosa. Proc Natl Acad Sci USA. 2017;114:E337–E346. doi: 10.1073/pnas.1614926114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capucha T, et al. Sequential BMP7/TGF-β1 signaling and microbiota instruct mucosal Langerhans cell differentiation. J Exp Med. 2018;215:481–500. doi: 10.1084/jem.20171508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capucha T, et al. Distinct murine mucosal Langerhans cell subsets develop from pre-dendritic cells and monocytes. Immunity. 2015;43:369–381. doi: 10.1016/j.immuni.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Chien YH, Meyer C, Bonneville M. γδ T cells: First line of defense and beyond. Annu Rev Immunol. 2014;32:121–155. doi: 10.1146/annurev-immunol-032713-120216. [DOI] [PubMed] [Google Scholar]
- 11.Conti HR, et al. Oral-resident natural Th17 cells and γδ T cells control opportunistic Candida albicans infections. J Exp Med. 2014;211:2075–2084. doi: 10.1084/jem.20130877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nielsen MM, Witherden DA, Havran WL. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol. 2017;17:733–745. doi: 10.1038/nri.2017.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carding SR, Egan PJ. Gammadelta T cells: Functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 14.Prinz I, Silva-Santos B, Pennington DJ. Functional development of γδ T cells. Eur J Immunol. 2013;43:1988–1994. doi: 10.1002/eji.201343759. [DOI] [PubMed] [Google Scholar]
- 15.Haas JD, et al. Development of interleukin-17-producing γδ T cells is restricted to a functional embryonic wave. Immunity. 2012;37:48–59. doi: 10.1016/j.immuni.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Sumaria N, et al. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J Exp Med. 2011;208:505–518. doi: 10.1084/jem.20101824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng S, et al. Infection with respiratory syncytial virus influences FasL-mediated apoptosis of pulmonary γδ T cells in a murine model of allergen sensitization. J Asthma. 2014;51:360–365. doi: 10.3109/02770903.2013.878954. [DOI] [PubMed] [Google Scholar]
- 18.Zeng X, et al. γδ T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen-specific interleukin-17 response. Immunity. 2012;37:524–534. doi: 10.1016/j.immuni.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edelblum KL, et al. γδ Intraepithelial lymphocyte migration limits transepithelial pathogen invasion and systemic disease in mice. Gastroenterology. 2015;148:1417–1426. doi: 10.1053/j.gastro.2015.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chowdhury AC, Chaurasia S, Mishra SK, Aggarwal A, Misra R. IL-17 and IFN-γ producing NK and γδ-T cells are preferentially expanded in synovial fluid of patients with reactive arthritis and undifferentiated spondyloarthritis. Clin Immunol. 2017;183:207–212. doi: 10.1016/j.clim.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Ramírez-Valle F, Gray EE, Cyster JG. Inflammation induces dermal Vγ4+ γδT17 memory-like cells that travel to distant skin and accelerate secondary IL-17-driven responses. Proc Natl Acad Sci USA. 2015;112:8046–8051. doi: 10.1073/pnas.1508990112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuziel WA, et al. Regulation of T-cell receptor gamma-chain RNA expression in murine Thy-1+ dendritic epidermal cells. Nature. 1987;328:263–266. doi: 10.1038/328263a0. [DOI] [PubMed] [Google Scholar]
- 23.McGeachy MJ, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stritesky GL, Yeh N, Kaplan MH. IL-23 promotes maintenance but not commitment to the Th17 lineage. J Immunol. 2008;181:5948–5955. doi: 10.4049/jimmunol.181.9.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fraticelli P, et al. Fractalkine (CX3CL1) as an amplification circuit of polarized Th1 responses. J Clin Invest. 2001;107:1173–1181. doi: 10.1172/JCI11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haas JD, et al. CCR6 and NK1.1 distinguish between IL-17A and IFN-gamma-producing gammadelta effector T cells. Eur J Immunol. 2009;39:3488–3497. doi: 10.1002/eji.200939922. [DOI] [PubMed] [Google Scholar]
- 27.Hirota K, et al. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoneda O, et al. Membrane-bound form of fractalkine induces IFN-gamma production by NK cells. Eur J Immunol. 2003;33:53–58. doi: 10.1002/immu.200390007. [DOI] [PubMed] [Google Scholar]
- 29.Düber S, et al. Induction of B-cell development in adult mice reveals the ability of bone marrow to produce B-1a cells. Blood. 2009;114:4960–4967. doi: 10.1182/blood-2009-04-218156. [DOI] [PubMed] [Google Scholar]
- 30.Liang S, Hosur KB, Domon H, Hajishengallis G. Periodontal inflammation and bone loss in aged mice. J Periodontal Res. 2010;45:574–578. doi: 10.1111/j.1600-0765.2009.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arizon M, et al. Langerhans cells down-regulate inflammation-driven alveolar bone loss. Proc Natl Acad Sci USA. 2012;109:7043–7048. doi: 10.1073/pnas.1116770109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 33.Lochner M, et al. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med. 2008;205:1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cochran DL. Inflammation and bone loss in periodontal disease. J Periodontol. 2008;79(Suppl 8):1569–1576. doi: 10.1902/jop.2008.080233. [DOI] [PubMed] [Google Scholar]
- 35.Bandeira A, et al. Localization of gamma/delta T cells to the intestinal epithelium is independent of normal microbial colonization. J Exp Med. 1990;172:239–244. doi: 10.1084/jem.172.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naik S, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleming C, et al. Microbiota-activated CD103+ DCs stemming from microbiota adaptation specifically drive γδT17 proliferation and activation. Microbiome. 2017;5:46. doi: 10.1186/s40168-017-0263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray EE, Suzuki K, Cyster JG. Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. J Immunol. 2011;186:6091–6095. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai Y, et al. Differential developmental requirement and peripheral regulation for dermal Vγ4 and Vγ6T17 cells in health and inflammation. Nat Commun. 2014;5:3986, and erratum (2016) 7:11354. doi: 10.1038/ncomms11354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Brien RL, Born WK. Dermal γδ T cells—What have we learned? Cell Immunol. 2015;296:62–69. doi: 10.1016/j.cellimm.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krishnan S, et al. Amphiregulin-producing γδ T cells are vital for safeguarding oral barrier immune homeostasis. Proc Natl Acad Sci USA. 2018;115:10738–10743. doi: 10.1073/pnas.1802320115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandrock I, et al. Genetic models reveal origin, persistence and non-redundant functions of IL-17-producing γδ T cells. J Exp Med. 2018;215:3006–3018. doi: 10.1084/jem.20181439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith PM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winter SE, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467:426–429. doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winter SE, et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science. 2013;339:708–711. doi: 10.1126/science.1232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gough DJ, Messina NL, Clarke CJ, Johnstone RW, Levy DE. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity. 2012;36:166–174. doi: 10.1016/j.immuni.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mbalaviele G, Novack DV, Schett G, Teitelbaum SL. Inflammatory osteolysis: A conspiracy against bone. J Clin Invest. 2017;127:2030–2039. doi: 10.1172/JCI93356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walsh NC, et al. Osteoblast function is compromised at sites of focal bone erosion in inflammatory arthritis. J Bone Miner Res. 2009;24:1572–1585. doi: 10.1359/jbmr.090320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.