Significance
Glutathione is a major antioxidant and redox regulator in cells. In addition to its essential roles in redox homeostasis, it functions as cofactors for a multitude of enzymes. We show here that the glutathione cycle molds the activity of synaptic glutamate, the major excitatory neurotransmitter in the central nervous system. Deficits in glutathione have been linked to multiple neurodegenerative and neuropsychiatric disorders. Accordingly, agents that restore glutathione-glutamate homeostasis may afford therapeutic benefit.
Keywords: glutathione, glutamate, neurotransmission, mEPSC, acivicin
Abstract
Glutamate is the most abundant excitatory neurotransmitter, present at the bulk of cortical synapses, and participating in many physiologic and pathologic processes ranging from learning and memory to stroke. The tripeptide, glutathione, is one-third glutamate and present at up to low millimolar intracellular concentrations in brain, mediating antioxidant defenses and drug detoxification. Because of the substantial amounts of brain glutathione and its rapid turnover under homeostatic control, we hypothesized that glutathione is a relevant reservoir of glutamate and could influence synaptic excitability. We find that drugs that inhibit generation of glutamate by the glutathione cycle elicit decreases in cytosolic glutamate and decreased miniature excitatory postsynaptic potential (mEPSC) frequency. In contrast, pharmacologically decreasing the biosynthesis of glutathione leads to increases in cytosolic glutamate and enhanced mEPSC frequency. The glutathione cycle can compensate for decreased excitatory neurotransmission when the glutamate-glutamine shuttle is inhibited. Glutathione may be a physiologic reservoir of glutamate neurotransmitter.
Glutamate is the most abundant excitatory transmitter in the central nervous system, utilized at 50–70% of cortical synapses (1, 2). Glutamate participates in diverse physiological processes, such as developmental plasticity and long-term potentiation as well as brain diseases: epilepsy, stroke, amyotrophic lateral sclerosis, Alzheimer’s disease, Parkinson’s disease, and schizophrenia (3). Glutathione is a tripeptide of glutamate, cysteine, and glycine, occurring in neurons at concentrations of 0.2–2 mM; it is the most abundant low molecular weight thiol of bacteria, plant, and animal cells (4–6). As such, it regulates critical cellular processes such as metabolism of estrogens, prostaglandins, leukotrienes, and xenobiotic drugs. Glutathione is well known as an antioxidant agent, providing a major line of defense against oxidative and other forms of stress, largely as a cofactor for the glutathione peroxidase and S-transferase enzyme families (7–10).
Glutathione metabolism is governed by the glutathione cycle (Fig. 1A and SI Appendix, Fig. S1), in which glutamate is added and liberated at discrete steps (4, 11). Glia serve as a major supplier of cysteine for neuronal glutathione synthesis, and 50–60% of a glutamate neurotransmitter is derived from the glutamine-glutamate shuttle between neurons and glia, with smaller amounts of glutamate transmitter derived from glycolysis (12–14). However, this shuttle is not the only means to replenish supply of neuronal glutamate; when it is inhibited, neurons quickly restore glutamate neurotransmission by an ill-defined endogenous mechanism, suggesting that neurons might be making use of a storage buffer of glutamate (15). We hypothesize that the glutathione cycle may be one such glutamate reserve, especially considering its high concentration and short half-life.
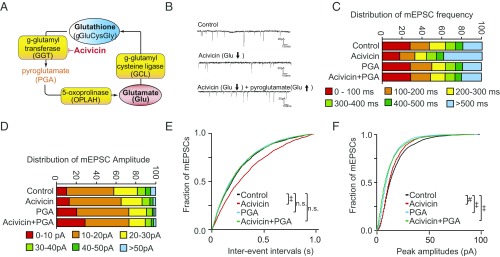
Fig. 1.
Blocking efflux of glutamate from the glutathione cycle decreases mEPSC. (A) Schematic representation of the glutathione cycle and inhibition of GGT by acivicin, which is upstream of the liberation of glutamate. Details appear in SI Appendix, Figs. S1 and S2. (B) Representative mEPSC traces in primary cortical neurons treated 24 h with vehicle, 25 μM acivicin, and/or 5 μM PGA. Acivicin decreased mEPSC frequency, which can be recovered by pyroglutamate. (C) Distribution of mEPSC frequency in cortical neurons treated with acivicin. (D) Distribution of mEPSC amplitude in cortical neurons treated with acivicin. (E) Cumulative probability plots of mEPSC frequency. Acivicin, which decreases efflux of glutamate from the glutathione cycle, decreased mEPSC frequency, a reflection of presynaptic drive. Pyroglutamate restores glutamate and presynaptic drive (see also SI Appendix, Fig. S2A) (n.s., not significant). (F) Cumulative probability plots of mEPSC amplitude. Acivicin decreased mEPSC amplitude to a smaller degree than frequency. (#P < 0.01, ‡P < 0.0001 by Steel–Dwass all pairs test.)
We previously reported that in addition to its support of antioxidant function, the glutathione cycle also serves as a reservoir of intracellular neural glutamate (16). Decreasing the liberation of glutamate from the glutathione cycle leads to decreased cortical neuron glutamate, while decreasing the utilization of glutamate increases total glutamate by about 25%. These shifts in glutamate pools could be achieved without increasing oxidative stress or cell death. In the present study, we sought to further test this concept by determining if shunting glutamate from the glutathione cycle can shape excitatory neurotransmission. We employed selective inhibitors of different steps of the glutathione cycle and the glutamine-glutamate shuttle and show that glutathione serves as a source for a material portion of glutamatergic neurotransmission.
Results
Inhibition of Glutathione Metabolism Depletes Neuronal Glutamate and Affects Excitatory Transmission.
To test the hypothesis that glutathione is a significant reservoir for glutamate, we treated neuronal cells with molecular inhibitors targeting enzymes of the glutathione metabolic cycle: acivicin, l-buthionine sulfoximine (BSO), and sulforaphane. Glutathione and glutamate were quantified by Ellman’s procedure and glutamate oxidase methods (17, 18). If glutathione constitutes a glutamate reservoir, then inhibiting gamma-glutamyl transferase (GGT) with acivicin should lead to decreased cellular glutamate as this enzyme is upstream of the ultimate liberation of glutamate from the cycle (Fig. 1A and SI Appendix, Fig. S1). Acivicin decreased intracellular glutathione about 25%, consistent with prior reports (SI Appendix, Fig. S2B) (19). GGT acts on glutathione to generate a γ-glutamyl amino acid or glutamate, and glycine-cysteine, depending upon whether an amino acid or water is used as an acceptor (20). As GGT is a membrane protein, acivicin increases extracellular glutathione levels and decreases glycine-cysteine, which is the source of rate-limiting cysteine in the intracellular synthesis of glutathione (21).
As we had previously demonstrated in cell lines (16), total glutamate and glutathione levels declined in primary cortical neurons treated with acivicin (SI Appendix, Fig. S2 A and B). To confirm the specificity of this effect, shRNA targeting of GGT also decreased glutamate levels (SI Appendix, Fig. S2C). As an additional test of the specificity of the effect, we find that the decrease in glutamate brought by acivicin could be rescued by administration of pyroglutamate (5-oxoproline), a downstream metabolite in the glutathione pathway that is a precursor of glutamate (SI Appendix, Fig. S1). Pyroglutamate selectively repleted glutamate, but not glutathione (SI Appendix, Fig. S2 A and B).
To determine if glutamate availability from the glutathione cycle (Fig. 1A) could shape excitatory transmission, we measured the frequency of miniature excitatory postsynaptic currents (mEPSCs), a reflection of presynaptic drive. Acivicin treatment (24 h) significantly decreased mEPSC frequency (Fig. 1 B, C, and E) and amplitudes to a smaller degree (Fig. 1 B, D, and F). To demonstrate specificity of the effect, we sought to rescue the decreased mEPSC frequency by pretreating with pyroglutamate (PGA) (Fig. 1A). Pyroglutamate rescued the effect on mEPSC frequency (Fig. 1 C and E) but not amplitude, consistent with it being a presynaptic precursor of glutamate. As we previously demonstrated, acivicin treatment at these concentrations did not elicit significant oxidative stress or affect cell viability (16).
Inhibition of Glutamate Cysteine Ligase Depletes Neuronal Glutathione, Elevates Glutamate, and Increases Excitatory Neurotransmission.
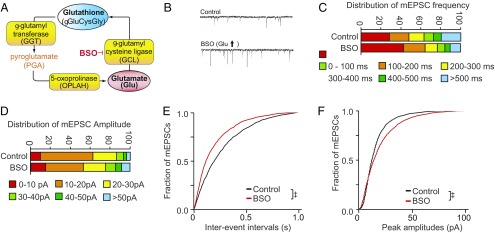
Glutamate cysteine ligase (GCL) is the rate-limiting step for glutathione synthesis and utilizes glutamate as a substrate. Inhibition of GCL with BSO depleted glutathione rapidly, reflecting its short half-life (1–4 h) as a substrate of multiple enzymes (SI Appendix, Fig. S3B). BSO treatment increased neuronal glutamate (SI Appendix, Fig. S3A), consistent with our prior findings in cell lines (16). This was confirmed by shRNA to GCLC, the target of BSO, which increased glutamate levels (SI Appendix, Fig. S3C). To further test the role of GCL in modulating glutathione and glutamate, we utilized sulforaphane, which increases GCL expression through activation of the Nuclear factor-erythroid 2 p45-related factor 2 (Nrf2) pathway (22). While BSO inhibition of GCL decreased glutathione and increased glutamate, sulforaphane had reciprocal effects: It induced GCL and decreased glutamate while increasing glutathione (SI Appendix, Fig. S3 D–F). We previously demonstrated that these doses and durations of treatment do not cause detectable changes in oxidative stress or neuronal viability (16). BSO was utilized to test whether increasing the liberation of glutamate from the glutathione cycle could shape excitatory transmission. The BSO-induced increase in glutamate was accompanied by an increase in mEPSC frequency (Fig. 2 B, C, and E) and a smaller increase in amplitude (Fig. 2 B, D, and F).
Fig. 2.
Efflux of glutamate from the glutathione cycle can increase mEPSC. (A) Schematic representation of the glutathione cycle and inhibition of GCL by BSO, which shuttles free glutamate into glutathione. Details appear in SI Appendix, Figs. S1 and S3. (B) Representative mEPSC traces in primary cortical neurons treated 24 h with vehicle or 200 μM BSO. (C) Distribution of mEPSC frequency in cortical neurons treated with BSO. (D) Distribution of mEPSC amplitude in cortical neurons treated with BSO. (E) Cumulative probability plots of mEPSC frequency. BSO, which increases efflux of glutamate from the glutathione cycle, increased mEPSC frequency, a reflection of presynaptic drive (see also SI Appendix, Fig. S4). (F) Cumulative probability plots of mEPSC amplitude in cortical neurons treated with BSO. (‡P < 0.0001 by Steel–Dwass all pairs test.)
The Glutathione Cycle Can Complement the Glutamate-Glutamine Shuttle and Influence Excitatory Neurotransmission Under Conditions of Glutamine Restriction.
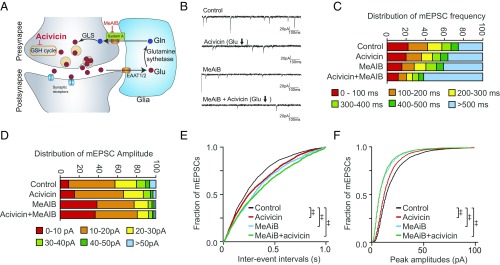
The glutamate-glutamine shuttle (SI Appendix, Fig. S4) between neurons and glia contributes 50–60% of a glutamate neurotransmitter (12, 13, 23) with intracellular sources such as glycolysis supplying the remainder. In the shuttle, glutamate is converted to glutamine in astrocytes, and then imported to neurons by system A transporters, where it is converted to glutamate intracellularly by phosphate-activated glutaminase (24). However, glutaminase knockout mice (25) or blockade of system A transporters with methylaminoisobutyric acid (MeAIB) (15) fail to block excitatory neurotransmission. We explored whether the glutathione cycle can complement the actions of the glutamate-glutamine shuttle by blocking import of glutamine by the system A transporter (15), which imports glutamine into neurons, after which it is converted to glutamate (Fig. 3A).
Fig. 3.
The glutathione cycle supports excitatory transmission, but to a smaller degree than glutamine-derived glutamate. (A) Scheme of glutamate-glutamine cycling and blockade of system A glutamine transporters by MeAIB. Details appear in SI Appendix, Fig. S4. (B) Representative traces of mEPSC recordings in primary cortical neurons treated with 25 μM acivicin (24 h) and/or 25 mM MeAIB (2 h). (C and D) Distribution of mEPSC frequency and amplitude in cortical neurons treated with acivicin and/or MeAIB. (E and F) Cumulative probability plots of mEPSC frequency and amplitude. MeAIB decreased mEPSC frequency to a greater degree than acivicin, with both treatments having additive effects for presynaptic drive frequency. (‡P < 0.0001 by Steel–Dwass all pairs test.)
As expected, MeAIB decreased the average mEPSC frequency (Fig. 3 B, C, and E). Acivicin, which diminishes the availability of glutathione-derived glutamate, decreased average mEPSC frequency significantly, although not to the same extent as MeAIB (Fig. 3E). mEPSC frequency declined even further when acivicin and MeAIB were coadministered, consistent with glutamate derived from the glutathione cycle contributing to maintenance of excitatory neurotransmission when glutamine supply is restricted (Fig. 3 C and E). mEPSC amplitude distributions were similarly diminished by acivicin, but to a smaller degree, although combinations of acivicin and MeAIB did not further impair the effect of MeAIB alone (Fig. 3 D and F).
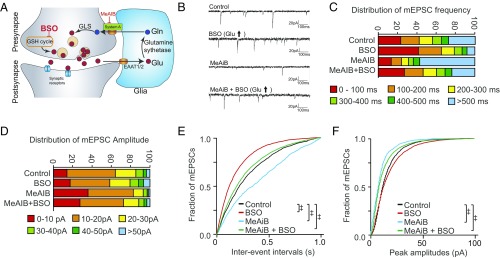
Glutamate Derived from the Glutathione Cycle Rescues Excitatory Postsynaptic Currents During Glutamine Limitation.
We next examined whether glutamate from the glutathione cycle could rescue mEPSC when glutamine supply was restricted (Fig. 4A). BSO, which augments glutamate levels, also increased mEPSC frequency, while glutamine restriction by MeAIB decreased mEPSC frequency (Fig. 4 B, C, and E). However, administration of BSO rescued the decreased mEPSC frequency brought by MeAIB (Fig. 4 B, C, and E, green line), suggesting the glutathione cycle may compensate for decreased availability of glutamine, an established source of glutamate neurotransmitter. BSO also significantly improved the decrease in mEPSC amplitudes by MeAIB, although to a smaller degree than its effect upon mEPSC frequency (Fig. 4 D and F), consistent with a more predominant presynaptic effect.
Fig. 4.
The glutathione cycle can rescue restrictions of glutamine-derived glutamate. (A) Scheme of glutamate-glutamine cycling and blockade of system A glutamine transporters by MeAIB. Details appear in SI Appendix, Fig. S4. (B) Representative traces of mEPSC recordings in primary cortical neurons treated with 25 μM BSO (24 h) and/or 25 mM MeAIB (2 h). (C and D) Distribution of mEPSC frequency and amplitude in cortical neurons treated with BSO and/or MeAIB. (E and F) Cumulative probability plots of mEPSC frequency and amplitude. MeAIB, which restricts glutamine-derived glutamate, inhibits mEPSC frequency (blue). Decreases in mEPSC frequency induced by MeAIB were rescued by pretreatment with BSO (green), which increased mEPSC frequency (red). BSO also improved the decrease in mEPSC amplitudes by MeAIB. (‡P < 0.0001 by Steel–Dwass all pairs test.)
Discussion
We previously reported that the glutathione cycle may serve as a reservoir of total neuronal glutamate (16). Treatments that decrease the liberation of glutamate from the glutathione cycle lead to decreased intracellular glutamate, whereas decreasing utilization of glutamate or glutathione synthesis increases it (16). We now report that shunting of glutamate derived from glutathione can shape excitatory neurotransmission. Inhibiting GCL, which utilizes glutamate to synthesize glutathione, leads to increased glutamate and mEPSC frequency. Decreasing the release of glutamate from the glutathione cycle by blocking GGT leads to diminished mEPSC. We demonstrated specificity of the effect of acivicin, which decreases glutamate liberation from glutathione, by rescuing with pyroglutamate, the immediate metabolic precursor of glutamate in the glutathione cycle. We have previously demonstrated that these fluxes of cytosolic glutamate can occur without significant increases in oxidative stress or altered cell viability (16). Thus, the glutathione pathway is poised to contribute glutamate without impacting neuronal viability unless there are massive, sustained deficits (16, 26). Our studies also reveal a modest effect of glutathione cycle inhibitors on mEPSC amplitude, implying possible postsynaptic effects. These could include an increase in the number or activity of postsynaptic receptors or may reflect increases in the number of neurotransmitter (glutamate) molecules or vesicles. While changes in mEPSC amplitude are often postsynaptically driven, increases in cytoplasmic glutamate can increase mEPSC amplitude, while decreased filling of vesicles decreases mEPSC amplitude (27–30). Another mode by which GSH could potentially modulate neurotransmission is by its effects on redox-regulated presynaptic proteins such as synaptosomal-associated protein 25 (SNAP-25) and N-ethylmaleimide-sensitive factor (NSF) (31, 32). Glutathione has also been reported to modulate the redox-sensitive site of NMDA receptors (33). These changes could be operational in neurodegenerative diseases, reflecting an imbalance in redox balance modulated by GSH. The redox effects of thiol compounds have been analyzed in modulation of GABA- and glycine-evoked currents in rat retinal ganglion cells (34). In addition to effects on redox-modulated synaptic proteins, depletion of GSH can alter steady-state nitrosylation (35). More generally, GSNO is a major source of NO bioactivity in the brain and its depletion will inhibit the activity of GSNOR resulting in increased nitrosylation (nitrosative stress) (36, 37) and possibly altered neurotransmission. Further studies on the potential redox effects of GSH need to be conducted to ascertain whether this aspect plays a role in neurotransmission.
The glutathione pathway may also supply glutamate when glia-derived glutamine is blocked by MeAIB, which inhibits system A transporters. While glia-derived glutamine provides 50–60% of a glutamate neurotransmitter, when the pathway is blocked with MeAIB, excitatory transmission abruptly decreases but rapidly recovers, consistent with endogenous neuronal sources of glutamate neurotransmitter (15). We suggest glutathione is one such endogenous source, as impairing glutamate liberation from the cycle further diminishes synaptic activity by MeAIB, while increasing glutamate availability can rescue the impairment by MeAIB. A reservoir capacity of glutathione may be utile during periods of sustained synaptic activity. Future studies on the readily releasable glutamate pool in the presence of the GSH-glutamate cycle inhibitors would yield a more detailed picture of glutamate dynamics. The rapidly releasable pool is the maximum number of vesicles that can be released in 2 or 3 s and is thought to coincide with those vesicles that are docked to the active zone and primed for release. They may represent the pool of glutamate most recently recruited for neurotransmission and could be relevant to the present study involving acute effects of drugs affecting the GSH-glutamate cycle. Whole-cell recording techniques using methods described previously may be utilized to estimate the readily releasable pool size can be measured here after manipulating GSH-associated enzymes (38).
Alterations in neuronal glutamate levels, such as by fluxes to and from a glutathione reservoir, might have an impact upon glutamate neurotransmitter. Vesicular glutamate transporters have a much lower affinity for glutamate 0.5–3.5 mM than plasma membrane transporters GLT1/EAAT2, whose Km is 4–40 μM (39, 40). Furthermore, as glutamate neurotransmitter typically does not saturate postsynaptic receptors, modest impacts upon release frequency may influence synaptic strength (27, 29, 41, 42). “Phasic” axons that fatigue in their glutamate neurotransmitter release have lower glutamate levels than “tonic” glutamate axons with greater glutamate levels (43). Glutamine levels are similar in both, suggesting that significant reservoirs of glutamate exist in neurons independent of glutamine. Our findings also affirm prior reports that directly increasing intracellular glutamate concentration in presynaptic terminals leads to greater excitatory postsynaptic currents (27, 29). In our specific approach, we suggest that the glutathione cycle may be one such source of this glutamate. Localized glutathione synthesis would be expected to have an even more pronounced effect, and it has been suggested that nonsoma areas contain more glutathione (44). Interestingly, our studies reveal the presence of the enzyme GGT in synaptosomes, suggesting that there may be accentuated exchange of glutathione to glutamate near vesicles (SI Appendix, Fig. S5). Metabolic flux analysis would yield additional information regarding flux of glutathione and glutamate under various conditions.
These findings may be relevant to human disease. Glutamatergic dysfunction has been implicated in schizophrenia by multiple lines of evidence (45–52). Several investigators have reported aberrant glutathione levels in schizophrenia patients, including medication naïve subjects (53–57). Mice lacking the modifier subunit of GCL have a 60% reduction in glutathione, accompanied by abnormal cortical gamma synchrony, decreased parvalbumin interneurons, and behavioral phenotypes relevant to schizophrenia (58–60). Despite substantial glutathione deficits, the mice are outwardly healthy. Additionally, rare deficiencies of glutathione cycle enzymes (gamma-glutamylcysteine ligase, glutathione synthetase, 5-oxoprolinase, and gamma-glutamyl transferase) have all been associated with neuropsychiatric and cognitive impairments, although detailed phenomenological characterization has not been reported (9, 61, 62). A role for glutathione as a glutamate reservoir may be a bridge between distinct lines of research that implicate glutamatergic dysfunction and aberrant glutathione levels in neuropsychiatric conditions such as schizophrenia.
Our model may have mechanistic implications to interpret magnetic resonance spectroscopy studies in human subjects, in which total regional brain glutamate may be determined (63, 64), although it is unknown if this affects synaptic activity. Seven-Tesla (7T) proton magnetic resonance spectroscopy studies have shown that glutamate levels were significantly lower in first episode psychosis subjects, whereas glutamine levels were unaltered (65). This study also revealed lower levels of glutathione in the anterior cingulate cortex and thalamus, which supports the idea of origin of glutamate from glutathione (16).
We suggest that two drugs available for human use, sulforaphane, which increases glutathione, and pyroglutamate, which is converted to glutamate in the glutathione cycle, may be therapeutically beneficial. Sulforaphane (66) is a potent inducer of the Nrf2 transcription factor, has blood–brain barrier penetration (67), and might expand the size of the glutathione reservoir by our observation that it increases expression of GCL, the rate-limiting step in glutathione biogenesis. Our recent study in human subjects revealed that sulforaphane elevates peripheral glutathione levels and those of other brain metabolites (68). Sulforaphane has also been reported to improve symptoms of autistic spectrum disorder (69). Pyroglutamate is a glutamate precursor whose CSF concentration is 120 μM (70), rivaling the 400 μM extracellular glutamine concentration (basal glutamate is 2–3 μM). Oral administration of pyroglutamate has been found to benefit age-associated memory impairment (71), alcoholic encephalopathy (72), and delirium induced by anticholinergic medication (73). Pyroglutamate may be a promising therapeutic candidate for cognitive dysfunction in schizophrenia and other conditions with glutathione disturbances.
Materials and Methods
Cell Culture and Reagents.
Dissociated cortical neuron cultures from Sprague–Dawley rats were prepared as described (74). Primary cortical neurons were maintained in Neurobasal medium (Life Technologies Corporation) supplemented with 1x B-27 (Life technologies).
Measurement of Glutathione.
Total and oxidized glutathione were measured using 5–5′-dithiobis (2-nitrobenzoic acid). Additional details of reagents and methods are available in SI Appendix.
Supplementary Material
Acknowledgments
We thank Dr. Minori Koga for experimental support and discussions, Yukiko Lema for assistance in preparing the manuscript, and Brian Lee and Dr. David Linden for helpful discussions. Support was provided by National Institutes of Health, National Institute of Neurological Disorders and Stroke Grant K08NS057824; Johns Hopkins Brain Science Institute and National Association for Research on Schizophrenia and Depression Young Investigator Award (to T.W.S.); National Institute of Mental Health Grant MH-092443; National Institutes of Health Silvio O. Conte Center Grant MH-094268, NIH Grants MH-084018, MH-105660, and MH-107730; US Public Health Service Grant MH18501 (to S.H.S.); and foundation supports from the Stanley Foundation and S/R (A.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1817885116/-/DCSupplemental.
References
- 1.Reis HJ, et al. Neuro-transmitters in the central nervous system & their implication in learning and memory processes. Curr Med Chem. 2009;16:796–840. doi: 10.2174/092986709787549271. [DOI] [PubMed] [Google Scholar]
- 2.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 3.Mattson MP. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann N Y Acad Sci. 2008;1144:97–112. doi: 10.1196/annals.1418.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forman HJ, Zhang H, Rinna A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoyama K, Watabe M, Nakaki T. Regulation of neuronal glutathione synthesis. J Pharmacol Sci. 2008;108:227–238. doi: 10.1254/jphs.08r01cr. [DOI] [PubMed] [Google Scholar]
- 6.Janáky R, Cruz-Aguado R, Oja SS, Shaw CA. Handbook of Neurochemistry and Molecular Neurobiology. Springer; Berlin: 2007. Glutathione in the nervous system: Roles in neural function and health and implications for neurological disease; pp. 347–399. [Google Scholar]
- 7.Dickinson DA, Forman HJ. Glutathione in defense and signaling: Lessons from a small thiol. Ann N Y Acad Sci. 2002;973:488–504. doi: 10.1111/j.1749-6632.2002.tb04690.x. [DOI] [PubMed] [Google Scholar]
- 8.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 9.Ballatori N, et al. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem. 2009;390:191–214. doi: 10.1515/BC.2009.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chinta SJ, et al. Inducible alterations of glutathione levels in adult dopaminergic midbrain neurons result in nigrostriatal degeneration. J Neurosci. 2007;27:13997–14006. doi: 10.1523/JNEUROSCI.3885-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meister A. Selective modification of glutathione metabolism. Science. 1983;220:472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- 12.Hamberger AC, Chiang GH, Nylén ES, Scheff SW, Cotman CW. Glutamate as a CNS transmitter. I. Evaluation of glucose and glutamine as precursors for the synthesis of preferentially released glutamate. Brain Res. 1979;168:513–530. doi: 10.1016/0006-8993(79)90306-8. [DOI] [PubMed] [Google Scholar]
- 13.Thanki CM, Sugden D, Thomas AJ, Bradford HF. In vivo release from cerebral cortex of [14C]glutamate synthesized from [U-14C]glutamine. J Neurochem. 1983;41:611–617. doi: 10.1111/j.1471-4159.1983.tb04785.x. [DOI] [PubMed] [Google Scholar]
- 14.Kanai Y, Hediger MA. The glutamate/neutral amino acid transporter family SLC1: Molecular, physiological and pharmacological aspects. Pflugers Arch. 2004;447:469–479. doi: 10.1007/s00424-003-1146-4. [DOI] [PubMed] [Google Scholar]
- 15.Kam K, Nicoll R. Excitatory synaptic transmission persists independently of the glutamate-glutamine cycle. J Neurosci. 2007;27:9192–9200. doi: 10.1523/JNEUROSCI.1198-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koga M, et al. Glutathione is a physiologic reservoir of neuronal glutamate. Biochem Biophys Res Commun. 2011;409:596–602. doi: 10.1016/j.bbrc.2011.04.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akerboom J, et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J Neurosci. 2012;32:13819–13840. doi: 10.1523/JNEUROSCI.2601-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 19.Dringen R, Kranich O, Hamprecht B. The gamma-glutamyl transpeptidase inhibitor acivicin preserves glutathione released by astroglial cells in culture. Neurochem Res. 1997;22:727–733. doi: 10.1023/a:1027310328310. [DOI] [PubMed] [Google Scholar]
- 20.Keillor JW, Castonguay R, Lherbet C. Gamma-glutamyl transpeptidase substrate specificity and catalytic mechanism. Methods Enzymol. 2005;401:449–467. doi: 10.1016/S0076-6879(05)01027-X. [DOI] [PubMed] [Google Scholar]
- 21.Dringen R, Pfeiffer B, Hamprecht B. Synthesis of the antioxidant glutathione in neurons: Supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci. 1999;19:562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinkova-Kostova AT, Abramov AY. The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med. 2015;88:179–188. doi: 10.1016/j.freeradbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reimer RJ, Chaudhry FA, Gray AT, Edwards RH. Amino acid transport system A resembles system N in sequence but differs in mechanism. Proc Natl Acad Sci USA. 2000;97:7715–7720. doi: 10.1073/pnas.140152797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rector FC, Jr, Seldin DW, Copenhaver JH. The mechanism of ammonia excretion during ammonium chloride acidosis. J Clin Invest. 1955;34:20–26. doi: 10.1172/JCI103058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masson J, et al. Mice lacking brain/kidney phosphate-activated glutaminase have impaired glutamatergic synaptic transmission, altered breathing, disorganized goal-directed behavior and die shortly after birth. J Neurosci. 2006;26:4660–4671. doi: 10.1523/JNEUROSCI.4241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: A major physiologic cytoprotectant. Proc Natl Acad Sci USA. 2002;99:16093–16098. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishikawa T, Sahara Y, Takahashi T. A single packet of transmitter does not saturate postsynaptic glutamate receptors. Neuron. 2002;34:613–621. doi: 10.1016/s0896-6273(02)00692-x. [DOI] [PubMed] [Google Scholar]
- 28.Wu XS, et al. The origin of quantal size variation: Vesicular glutamate concentration plays a significant role. J Neurosci. 2007;27:3046–3056. doi: 10.1523/JNEUROSCI.4415-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamashita T, Ishikawa T, Takahashi T. Developmental increase in vesicular glutamate content does not cause saturation of AMPA receptors at the calyx of Held synapse. J Neurosci. 2003;23:3633–3638. doi: 10.1523/JNEUROSCI.23-09-03633.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Q, Petersen CCH, Nicoll RA. Effects of reduced vesicular filling on synaptic transmission in rat hippocampal neurones. J Physiol. 2000;525:195–206. doi: 10.1111/j.1469-7793.2000.t01-1-00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foley TD, Clark AR, Stredny ES, Wierbowski BM. SNAP-25 contains non-acylated thiol pairs that can form intrachain disulfide bonds: Possible sites for redox modulation of neurotransmission. Cell Mol Neurobiol. 2012;32:201–208. doi: 10.1007/s10571-011-9748-4. [DOI] [PubMed] [Google Scholar]
- 32.Lowenstein CJ, Tsuda H. N-ethylmaleimide-sensitive factor: A redox sensor in exocytosis. Biol Chem. 2006;387:1377–1383. doi: 10.1515/BC.2006.173. [DOI] [PubMed] [Google Scholar]
- 33.Sucher NJ, Lipton SA. Redox modulatory site of the NMDA receptor-channel complex: Regulation by oxidized glutathione. J Neurosci Res. 1991;30:582–591. doi: 10.1002/jnr.490300316. [DOI] [PubMed] [Google Scholar]
- 34.Pan ZH, Bähring R, Grantyn R, Lipton SA. Differential modulation by sulfhydryl redox agents and glutathione of GABA- and glycine-evoked currents in rat retinal ganglion cells. J Neurosci. 1995;15:1384–1391. doi: 10.1523/JNEUROSCI.15-02-01384.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amal H, et al. Shank3 mutation in a mouse model of autism leads to changes in the S-nitroso-proteome and affects key proteins involved in vesicle release and synaptic function. Mol Psychiatry. July 9, 2018 doi: 10.1038/s41380-018-0113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: A current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seth D, Stamler JS. The SNO-proteome: Causation and classifications. Curr Opin Chem Biol. 2011;15:129–136. doi: 10.1016/j.cbpa.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- 39.Takamori S. VGLUTs: ‘Exciting’ times for glutamatergic research? Neurosci Res. 2006;55:343–351. doi: 10.1016/j.neures.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 40.Liguz-Lecznar M, Skangiel-Kramska J. Vesicular glutamate transporters (VGLUTs): The three musketeers of glutamatergic system. Acta Neurobiol Exp (Warsz) 2007;67:207–218. doi: 10.55782/ane-2007-1649. [DOI] [PubMed] [Google Scholar]
- 41.Otis TS. Vesicular glutamate transporters in cognito. Neuron. 2001;29:11–14. doi: 10.1016/s0896-6273(01)00176-3. [DOI] [PubMed] [Google Scholar]
- 42.Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66:337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shupliakov O, Atwood HL, Ottersen OP, Storm-Mathisen J, Brodin L. Presynaptic glutamate levels in tonic and phasic motor axons correlate with properties of synaptic release. J Neurosci. 1995;15:7168–7180. doi: 10.1523/JNEUROSCI.15-11-07168.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slivka A, Mytilineou C, Cohen G. Histochemical evaluation of glutathione in brain. Brain Res. 1987;409:275–284. doi: 10.1016/0006-8993(87)90712-8. [DOI] [PubMed] [Google Scholar]
- 45.Akbarian S, et al. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J Neurosci. 1996;16:19–30. doi: 10.1523/JNEUROSCI.16-01-00019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao XM, et al. Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: Effects of schizophrenia. Am J Psychiatry. 2000;157:1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- 47.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai G, et al. Abnormal excitatory neurotransmitter metabolism in schizophrenic brains. Arch Gen Psychiatry. 1995;52:829–836. doi: 10.1001/archpsyc.1995.03950220039008. [DOI] [PubMed] [Google Scholar]
- 49.Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: The final common pathway on the road to schizophrenia? Brain Res Bull. 2010;83:108–121. doi: 10.1016/j.brainresbull.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koga M, Serritella AV, Sawa A, Sedlak TW. Implications for reactive oxygen species in schizophrenia pathogenesis. Schizophr Res. 2016;176:52–71. doi: 10.1016/j.schres.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 51.Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet. 2016;388:86–97. doi: 10.1016/S0140-6736(15)01121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Landek-Salgado MA, Faust TE, Sawa A. Molecular substrates of schizophrenia: Homeostatic signaling to connectivity. Mol Psychiatry. 2016;21:10–28. doi: 10.1038/mp.2015.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19:220–230. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Do KQ, et al. Schizophrenia: Glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12:3721–3728. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- 55.Matsuzawa D, et al. Negative correlation between brain glutathione level and negative symptoms in schizophrenia: A 3T 1H-MRS study. PLoS One. 2008;3:e1944. doi: 10.1371/journal.pone.0001944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao JK, Leonard S, Reddy R. Altered glutathione redox state in schizophrenia. Dis Markers. 2006;22:83–93. doi: 10.1155/2006/248387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nucifora LG, et al. Reduction of plasma glutathione in psychosis associated with schizophrenia and bipolar disorder in translational psychiatry. Transl Psychiatry. 2017;7:e1215. doi: 10.1038/tp.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steullet P, et al. Redox dysregulation affects the ventral but not dorsal hippocampus: Impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J Neurosci. 2010;30:2547–2558. doi: 10.1523/JNEUROSCI.3857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kulak A, Cuenod M, Do KQ. Behavioral phenotyping of glutathione-deficient mice: Relevance to schizophrenia and bipolar disorder. Behav Brain Res. 2012;226:563–570. doi: 10.1016/j.bbr.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 60.das Neves Duarte JM, et al. N-acetylcysteine normalizes neurochemical changes in the glutathione-deficient schizophrenia mouse model during development. Biol Psychiatry. 2012;71:1006–1014. doi: 10.1016/j.biopsych.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 61.Ristoff E, Larsson A. Patients with genetic defects in the gamma-glutamyl cycle. Chem Biol Interact. 1998;111-112:113–121. doi: 10.1016/s0009-2797(97)00155-5. [DOI] [PubMed] [Google Scholar]
- 62.Njålsson R, et al. Genotype, enzyme activity, glutathione level, and clinical phenotype in patients with glutathione synthetase deficiency. Hum Genet. 2005;116:384–389. doi: 10.1007/s00439-005-1255-6. [DOI] [PubMed] [Google Scholar]
- 63.Cai K, et al. Magnetic resonance imaging of glutamate. Nat Med. 2012;18:302–306. doi: 10.1038/nm.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rae CD. A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem Res. 2014;39:1–36. doi: 10.1007/s11064-013-1199-5. [DOI] [PubMed] [Google Scholar]
- 65.Anna M, et al. 7 Tesla proton magnetic resonance spectroscopy in first episode psychosis. JAMA Psychiatry. January 9, 2019 doi: 10.1001/jamapsychiatry.2018.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keum YS. Regulation of the Keap1/Nrf2 system by chemopreventive sulforaphane: Implications of posttranslational modifications. Ann N Y Acad Sci. 2011;1229:184–189. doi: 10.1111/j.1749-6632.2011.06092.x. [DOI] [PubMed] [Google Scholar]
- 67.Zhao J, Kobori N, Aronowski J, Dash PK. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci Lett. 2006;393:108–112. doi: 10.1016/j.neulet.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 68.Sedlak TW, et al. Sulforaphane augments glutathione and influences brain metabolites in human subjects: A clinical pilot study. Mol Neuropsychiatry. 2018;3:214–222. doi: 10.1159/000487639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singh K, et al. Sulforaphane treatment of autism spectrum disorder (ASD) Proc Natl Acad Sci USA. 2014;111:15550–15555. doi: 10.1073/pnas.1416940111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eckstein JA, Ammerman GM, Reveles JM, Ackermann BL. Analysis of glutamine, glutamate, pyroglutamate, and GABA in cerebrospinal fluid using ion pairing HPLC with positive electrospray LC/MS/MS. J Neurosci Methods. 2008;171:190–196. doi: 10.1016/j.jneumeth.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 71.Grioli S, Lomeo C, Quattropani MC, Spignoli G, Villardita C. Pyroglutamic acid improves the age associated memory impairment. Fundam Clin Pharmacol. 1990;4:169–173. doi: 10.1111/j.1472-8206.1990.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 72.Sinforiani E, et al. Reversibility of cognitive disorders among chronic alcoholics in phases of withdrawal. Effect of arginine pyroglutamate. Minerva Psichiatr. 1985;26:339–346. [PubMed] [Google Scholar]
- 73.Blin O, et al. Effects of dimethylaminoethanol pyroglutamate (DMAE p-Glu) against memory deficits induced by scopolamine: Evidence from preclinical and clinical studies. Psychopharmacology (Berl) 2009;207:201–212. doi: 10.1007/s00213-009-1648-7. [DOI] [PubMed] [Google Scholar]
- 74.Hayashi-Takagi A, et al. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010;13:327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.