Abstract
Background
Phosphodiesterase 4 is a promising target for developing novel antidepressants. However, prototype phosphodiesterase 4 inhibitors show severe side effects, including nausea and vomiting. N-Isopropyl-3-(cyclopropylmethoxy)-4-difluoromethoxy benzamide (FCPR03) is a novel phosphodiesterase 4 inhibitor with little emetic potential. In the present study, we investigated the inhibitory effect of FCPR03 on chronic unpredictable mild stress-induced, depressive-like behaviors in mice and explored the underlying mechanisms.
Methods
The depression model of mice was established by chronic unpredictable mild stress. Forced swim test, tail suspension test, and sucrose preference test were used to assess depressive-like behaviors. Golgi-staining was utilized to analyze dendritic morphology and spine density. The level of cAMP was measured by enzyme-linked immnosorbent assay assay. Western blot was used to evaluate protein levels of phosphorylated cAMP-response element binding protein, protein kinase B, glycogen synthase kinase-3β, and brain derived neurotrophic factor in both hippocampus and prefrontal cortex. Postsynaptic density protein 95 and synapsin 1 were also detected by western blot in the hippocampi.
Results
Treatment with FCPR03 (0.5–1.0 mg/kg, i.p.) increased consumption of sucrose in the sucrose preference test in mice exposed to chronic unpredictable mild stress. FCPR03 shortened the immobility time in forced swim test and tail suspension test without affecting locomotor activity. Furthermore, chronic unpredictable mild stress decreased the dendritic spine density and dendritic length in the hippocampus. This change was accompanied by decreased expression of postsynaptic density protein 95 and synapsin 1. Interestingly, FCPR03 prevented dendritic spine loss and increased synaptic protein levels. Moreover, the levels of cAMP, phosphorylated cAMP-response element binding protein, and brain derived neurotrophic factor were elevated in chronic unpredictable mild stress-challenged mice after treatment with FCPR03. In addition, FCPR03 also enhanced the phosphorylation of both protein kinase B and glycogen synthase kinase-3β in mice exposed to chronic unpredictable mild stress.
Conclusion
The present study suggests that FCPR03 could prevent both depressive-like behaviors and spine loss induced by chronic unpredictable mild stress in the mice hippocampi.
Keywords: dendritic spines, depression, FCPR03, phosphodiesterase 4, stress
Significance Statement
Phosphodiesterase 4 (PDE4) is a promising target for the treatment of major depression. However, most of the current canonical PDE4 inhibitors, such as rolipram, produce a severe nausea and vomiting response in patients. These intolerable gastrointestinal side effects hinder the clinical application of PDE4 inhibitors for the treatment of neuropsychiatric disorders. FCPR03 is a novel PDE4 inhibitor with little emetic potential. Our previous studies showed that FCPR03 inhibited the neuroinflammatory response in microglial cells and alleviated lipopolysaccharide-induced depressive-like behaviors. In this study, we found that FCPR03 produced an antidepressant-like effect in mice challenged with chronic unpredictable mild stress. The amelioration of the depressive-like effect was accompanied by a decreased loss of dendritic spine. We also identified the involved signaling pathways. Hence, it is reasonable to suppose that FCPR03 is a potential candidate for further preclinical study in the prevention and treatment of depression.
Introduction
Depression is a common mental illness that has become the third largest disease in the world in terms of burden of disease (Vigo et al., 2016). The clinical manifestations of depression include sustained pessimism, loss of pleasure and appetite, sleep disorders, and deficits in attention (H Wang et al., 2015). More seriously, patients suffering from major depression experience greater rates of self-harm and suicide attempts, which make the condition a serious burden to individuals, their families, and the community (De Crescenzo et al., 2017). Despite ongoing research, the treatment of depression is still a tough problem to solve because of its unknown etiology and pathogenesis, and the current state of antidepressants is still not entirely adequate for widespread success in the clinic. Currently available antidepressants, such as selective serotonin reuptake inhibitors and noradrenaline reuptake inhibitors, are effective in about 50% to 70% of patients with major depressive disorders (H Wang et al., 2015). Importantly, however, most of the patients who respond to these medications are inclined to discontinue therapy prematurely due to side effects, such as dizziness, headache, inattention, disturbance of consciousness, sexual dysfunction, and nausea and vomiting (Hieronymus et al., 2018). Other limitations of current antidepressants include a delayed onset of action and an increased risk of suicide (Sung et al., 2013; Hengartner, 2017). Therefore, it is of great significance and urgency to explore the pathogenesis of depression and develop novel effective antidepressants.
Over the past 2 decades, the biological functions of phosphodiesterase 4 (PDE4) have been extensively studied in the central nervous system, and PDE4 itself has come to be viewed as a potential antidepressant target (Jindal et al., 2013; ZZ Wang et al., 2013, 2015; Bolger, 2017). Biologically, PDE4 is capable of specifically catalyzing the hydrolysis of the second messenger cyclic adenosine monophosphate (cAMP) and thus lowering the intracellular concentration of cAMP (Bolger, 2017). The inhibition of PDE4 enhances the accumulation of intracellular cAMP, thus activating downstream signaling pathways. The protein kinase A (PKA)/ cAMP-response element-binding protein (CREB) pathway is a classical signaling pathway involved in the multiple biological functions of cAMP (Bolger, 2017; Guo et al., 2017), especially in the regulation of emotion (Pandey et al., 2005; L Zhou et al., 2016). Postmortem data showed that the phosphorylation of CREB in both the hippocampus and prefrontal cortex was reduced in individuals with major depression, while chronic treatment with antidepressants restored the level of phosphorylated CREB (Dowlatshahi et al., 1998). Separately, the overexpression of CREB in the region of the hippocampal dentate gyrus produced antidepressant-like effects in the forced swimming test in an acquired helplessness animal model (Chen et al., 2001). Furthermore, knocking down the expression of PDE4D in the prefrontal cortex enhanced the phosphorylation of CREB and alleviated depressive-like behaviors (ZZ Wang et al., 2015). PDE4 inhibitors, such as etazolate and rolipram, have also been found to be effective at attenuating depressive-like behaviors in various animal models (Jindal et al., 2013, 2015; Fujita et al., 2017). Our previous studies also revealed that PDE4 modulated the development of negative emotional reactions associated with ethanol abstinence, including depression (Gong et al., 2017). Activated CREB promotes the transcription of brain-derived neurotrophic factor (BDNF), which is a potent trophic factor that maintains the morphology of neurons and mediates synaptic plasticity (ZZ Wang et al., 2013; Liu et al., 2017). The concentration of BDNF in the brain is reduced in patients with major depressive disorder, and antidepressant treatment may improve or normalize cerebral concentrations of BDNF (Sheldrick et al., 2017). Research has shown that cAMP/CREB/BDNF signaling in hippocampal newborn neurons is pivotal in mediating neuroplasticity and contributing to the antidepressant-like effects of PDE4 inhibition (Koch et al., 2009; Li et al., 2009; ZZ Wang et al., 2015). Collectively, such investigations support the idea that the inhibition of PDE4 produces antidepressant-like effects in rodents. Currently, multiple drugs targeting PDE4 have been synthesized. However, most of them possess intolerable gastrointestinal side effects, including nausea and vomiting (Boswell-Smith and Spina, 2007; Chong et al., 2017). For example, the classic prototype PDE4 inhibitor rolipram showed a good antidepressant-like effect in preclinical studies, and its efficiency was better than that of amitriptyline, a tricyclic antidepressant (Scott et al., 1991). Furthermore, it demonstrated a rapid onset of efficacy (Zeller et al., 1984). Unfortunately, dose-dependent gastrointestinal-related adverse reactions eventually rendered it a failure in phase II clinical trials (Scott et al., 1991). Therefore, the development of a novel PDE4 inhibitor with improved clinical efficacy and reduced gastrointestinal side effects is warranted.
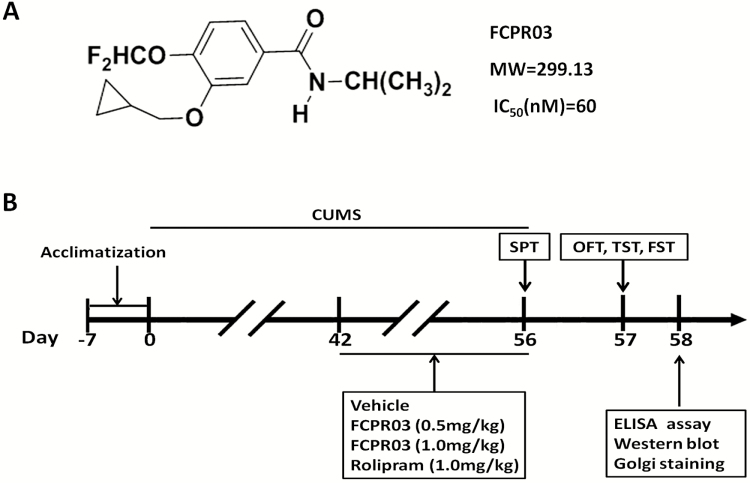
N-Isopropyl-3-(cyclopropylmethoxy)-4-difluoromethoxy benzamide (FCPR03) is a highly selective and novel PDE4 inhibitor with a half maximal inhibitory concentration value of 60 nM for PDE4CAT (the core catalytic domain of human PDE4) that has at least 2000-fold selectivity over other PDE family members (ZZ Zhou et al., 2016). The chemical structure of FCPR03 is shown in Figure 1A. The inhibition of FCPR03 on 4 PDE4 subtypes (PDE4A/B/C/D) has been reported before in our recent work (ZZ Zhou et al., 2016). Rolipram was used as a control. Our recent studies showed that FCPR03 attenuated lipopolysaccharide-induced production of inflammatory factors in microglial cells and alleviated lipopolysaccharide-induced depressive-like behaviors in mice (ZZ Zhou et al., 2016b; Yu et al., 2018). The antiinflammatory effects of FCPR03 were considered to be associated with the activation of the cAMP/PKA/CREB signaling pathway and nuclear factor kappa B inhibition (Zou et al., 2017). Importantly, as compared with the prototype PDE4 inhibitor rolipram, FCPR03 did not cause emesis in beagle dogs (ZZ Zhou et al., 2016). Hence, FCPR03 is a novel, promising PDE4 inhibitor with the potential to be used in the clinic. In this study, we aimed to investigate the antidepressant-like effects of FCPR03 in an animal model and explore the underlying mechanisms. The chronic unpredictable mild stress (CUMS) model is one of the available classic behavioral models that mimics daily hassles and stress levels in humans (Liang et al., 2015). This model has been widely used as a rodent model to evaluate the efficiency and molecular mechanisms of potential antidepressant agents (Zhang et al., 2014; Gao et al., 2017; Wan et al., 2017). We found that FCPR03 significantly alleviated depressive-like behaviors in a CUMS mouse model, and this effect was accompanied with activation of the cAMP/PKA/CREB/BDNF and protein kinase B (Akt)/glycogen synthase kinase-3β (GSK-3β) signaling pathways. Morphologically, FCPR03 enhanced dendritic spine density and dendritic length. These results suggest that FCPR03 is a potential drug for the treatment and prevention of depression.
Figure 1.
Chemical structure of n-isopropyl-3-(cyclopropylmethoxy)-4-difluoromethoxy benzamide (FCPR03) and experimental procedures. (A) Chemical structure of FCPR03 and inhibition (half maximal inhibitory concentration, nM) of FCPR03 on PDE4CAT (the core catalytic domains of human phosphodiesterase 4 [PDE4]). (B) After 7 days of acclimatization, mice were subjected to chronic unpredictable mild stress (CUMS) for 56 days. FCPR03, rolipram, or vehicle were administered (i.p.) once a day for 14 days (from week 7 to week 8). Sucrose preference test (SPT) was performed on day 56, other behavioral tests were carried out on day 57, and the mice were subsequently killed. Enzyme-linked immunosorbent assay (ELISA) assay, biochemical assays, and Golgi staining were performed after behavioral tests. MW, molecular weight; OFT, open field test; TST, tail suspension test; FST, forced swimming test.
Materials and Methods
Animals
Adult male C57BL/6 mice (22–25g) were obtained from Laboratory Animal Center of Southern Medical University (Guangzhou, China). Efforts were made to minimize the number of animals used in the experiment and their suffering during the process of study. Mice were housed in a temperature- and humidity-controlled room (temperature: 22°C–23°C, humidity: 55%–65%), with a 12-hour-light/-dark cycle. Mice had free access to food (standard raw chow) and water and were acclimated in the facility for 7 days prior to initiating the experiments. All experimental protocols were strictly in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Ethics Committee of the Southern Medical University.
Drug Preparation and Treatment
FCPR03 was provided by Neuropharmacology and Drug Discovery Group, Southern Medical University (Zou et al., 2017). Rolipram was purchased from Aladdin (Shanghai, China). FCPR03 and rolipram were diluted with the vehicle (0.5% DMSO, 0.5% carboxymethylcellulose sodium) to obtain working solutions. Drugs were prepared freshly before use. All other chemicals used were of analytical grade.
Experimental Design
The experimental design was laid out in Figure 1B. A total of 40 mice were randomly distributed into 5 groups (n=8): (1) Vehicle; (2) CUMS+vehicle; (3) CUMS+FCPR03 (0.5 mg/kg); (4) CUMS+FCPR03 (1.0 mg/kg); and (5) CUMS+rolipram (1.0 mg/kg). After 1 week of acclimatization, mice in the vehicle group were housed in normal conditions and mice in other groups were subjected to CUMS. After 6 weeks of CUMS procedure, mice were administered (i.p.) with FCPR03 or rolipram once a day for 14 days (from week 7 to week 8). After the procedure, the sucrose preference test (SPT) was performed on day 56. Behavioral tests including open field test (OFT), tail suspension test (TST), and forced swimming test (FST) were performed on day 57. After behavioral tests, on day 58, animals were killed for biochemical analysis and Golgi staining. All experiments were performed in a blinded manner; the experimenters did not know which mice were treated with vehicle, FCPR03, or rolipram.
CUMS Procedure
The CUMS paradigm was adapted as previously described with minor modification (Zhou et al., 2010; Liang et al., 2015). Briefly, mice were randomly exposed to a variety of unpredictable stressors, including (1) 12-hour cage tilt (45°), (2) 30-second tail pinch, (3) overnight illumination, (4) 24-hour food deprivation, (5) 24-hour water deprivation, (6) 5-minute swimming in water at 4°C to 6°C, and (7) white noise for 10 hours. All stressors were applied individually and continuously. Mice in the vehicle group did not receive any kind of stressor and were housed in normal conditions.
Sucrose Preference Test
Experiments were carried out as previously described (Liang et al., 2015) with minor modification. On the first day (day 53), 2 bottles of solution containing 5% sucrose were positioned in each cage. Mice were trained to adapt to a 5% sucrose solution for 24 hours. On the second day (day 54), one of the bottles was replaced with pure water, and the position of the 2 bottles was changed during this period (24 hours). Mice were deprived of water and food for 24 hours before a 12-hour testing session. In the SPT (day 56), each mouse was presented with 1 bottle containing 5% sucrose solution, and the other containing pure water. Sucrose preference was defined as (weight of sucrose solution ingested)/(weight of water ingested + weight of sucrose solution ingested)×100%.
Open Fielf Test
To verify whether the administration of FCPR03 could prompt a change of the spontaneous locomotor activity of mice, an OFT was conducted to measure the spontaneous activity of mice. For this, mice were placed in a white wooden box
(40 cm×30 cm×20 cm). The rear number (the numbers of vertical rearing movements within 5 minutes) and crossing number (the number of times the animal crossed from one square to another within 5 minutes) were counted and recorded for 5 minutes for each animal by use of an open-field experimental video analysis system (Smart 3.0, video tracking system, Panlab, Barcelona, Spain).
Forced Swim Test and Tail Suspension Test
The FST and TST are widely used approaches for assessing depressive-like behaviors in animals. For the FST, mice were placed in a clear glass cylinder (10 cm in diameter×25 cm in height) containing fresh water at 25°C. For the TST, mice were suspended at 20 cm above the floor using adhesive tape placed 3 cm from the tip of the tail. Immobility time in both the FST and TST was recorded by a camera with a video analysis system (Smart 3.0, video tracking system, Panlab, Barcelona, Spain). Immobility time during the last 4 minutes was scored.
Enzyme-Linked Immunosorbent Assay
The level of cAMP in the right hippocampus were measured by an enzyme-linked immnosorbent assay kit (KGE012B, R&D systems, Minneapolis, MN) in accordance with the manufacturer’s protocols. The cAMP levels were normalized to total protein and the samples were assayed in triplicate.
Western Blot
Western-blot analysis was carried out according to previously published protocols, with slight modification (Zeng et al., 2016). The left hippocampi of the mice were homogenized with radioimmunoprecipitation assay lysis buffer (1× radioimmunoprecipitation lysis buffer including 1% protease inhibitor cocktail and 1% phosphatase inhibitor cocktail) containing a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and centrifuged at 12000 g for 10 minutes. Next, the total protein concentration was measured using a BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA). An equal amount of protein was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Protein bands were transferred to polyvinylidene fluoride membranes. Membranes were then incubated in TBST containing 5% skim milk for 2 hours at room temperature to block nonspecific binding sites. Following washing with TBST 3 times, the membranes were incubated overnight at 4°C with the appropriate primary antibodies, including anti-phospho-CREB (1:1000, #9198; Cell Signaling Technology, Danvers, CT), anti-CREB (1:1000, #9197; Cell Signaling Technology), anti-BDNF (1:1000; SAB2108004, Sigma-Aldrich, St.Louis, MO), anti-phospho-Akt (1:1000, #9271S; Cell Signaling Technology), anti-Akt (1:1000, #9272; Cell Signaling Technology) anti-phospho-GSK-3β (1:1000, #9336; Cell Signaling Technology), anti-GSK-3β (1:1000, #12456; Cell Signaling Technology), anti-PSD95 (1:1000, ab18258; Abcam, Cambridge, UK), anti-synapsin1 (1:1000, ab64581; Abcam), or anti-GAPDH (1:10000, ab181602; Abcam). Membranes were subsequently incubated with corresponding secondary antibodies for 2 hours at room temperature. The bands were visualized using a Kodak Digital Science ID (Kodak, Rochester, NY) and quantified with the Image J software (National Institutes of Health, Bethesda, MD).
Golgi Staining and Dendrite Analysis
Golgi staining were determined by a FD Rapid Golgistain Kit (PK401A, FD NeuroTechnologies, Columbia, MD) according to the manufacturer’s protocols. In brief, mouse brains were removed from their skulls as quickly as possible and rinsed immediately in double-distilled water to remove blood from the surface. After rinsing, the brains were impregnated into solutions A and B (equal volume) and stored at room temperature for 3 weeks in the dark. Brains were then transferred into solution C and stored at room temperature for 1 week in the dark. Brains were subsequently sectioned into 100-μm slices using freezing microtome (Leica) and mounted on gelatin-coated glass coverslips with solution C. Following drying at room temperature, the slices were incubated in a mixture solution (solution D:solution E:distilled water=1:1:2) for 10 minutes. The slices were then dehydrated with 50%, 75%, 95%, and absolute ethanol, respectively.
For quantitative analysis, 10 neurons from the CA1 region in the hippocampus were traced using ImageJ. For dendritic spine analysis, pyramidal neurons in the CA1 area were imaged under bright field illumination with a 100× oil immersion objective. Neurons were traced using a laser confocal microscope (LSM880 with Airyscan; Carl Zeiss, Oberkochen, Germany) connected to a desktop installed with the ZEN software. Neurons chosen for tracing contained an isolated, fully stained cell body and demonstrated as fully stained and completed dendritic arbors as possible. The ImageJ software was used to quantify the dendritic tracings. The number of dendritic spines was counted using the NeuronJ plugin for Image J. The total number of spines was counted at a high magnification (×1000). Spine density corresponded to the sum of spines along the dendritic length of 50 μm. Pyramidal neurons from the hippocampal subregion CA1 were selected for the analysis of spine density (G Wang et al., 2013). The spines were counted from the apical dendrites. We assessed spine density in a 2-dimension manner, which is also widely used by other laboratories (Magariños et al., 2011; Allen et al., 2015). This method provides a direct comparison of treatment groups when they are assessed in an identical manner. The total dendritic length and the number of branching points were measured via the Sholl analysis plugin for ImageJ. Specifically, neurons were traced under 400× and Sholl creates a series of concentric circles around the cell body of the neurons. The starting radius was 10.00 µm and the radius interval between circles was set as 10 µm per step. The number of dendrite intersects with the concentric circles was counted.
Statistical Analysis
All data are expressed as mean±SEM, and differences were defined statistically significant only when P<.05. Data were analyzed by 1-way ANOVA followed by Bonferroni posthoc tests. All data were analyzed using SPSS version 19.0 (SPSS Inc., Chicago, IL). Figures were plotted using the GraphPad Prism 5.0 software.
Results
FCPR03 Alleviated Depressive-Like Behaviors in Mice Exposed to CUMS
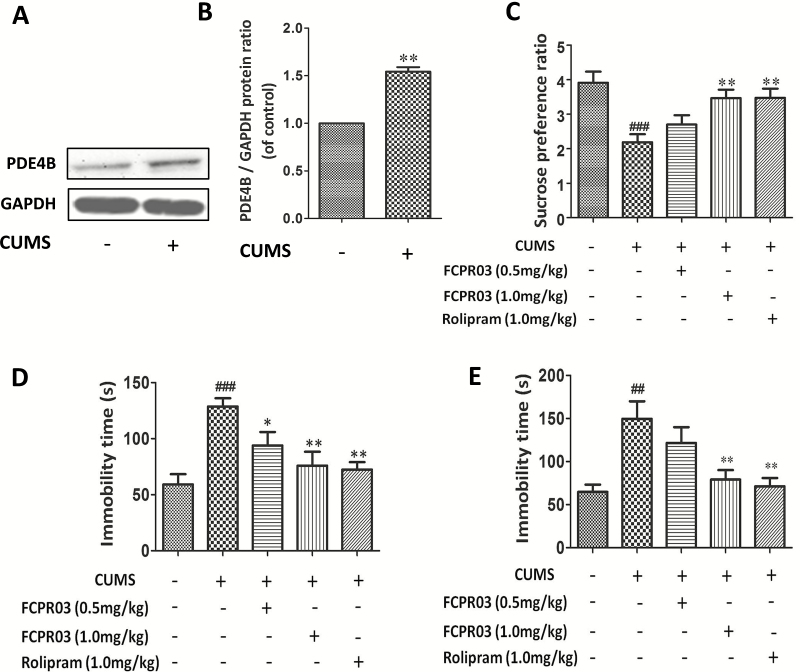
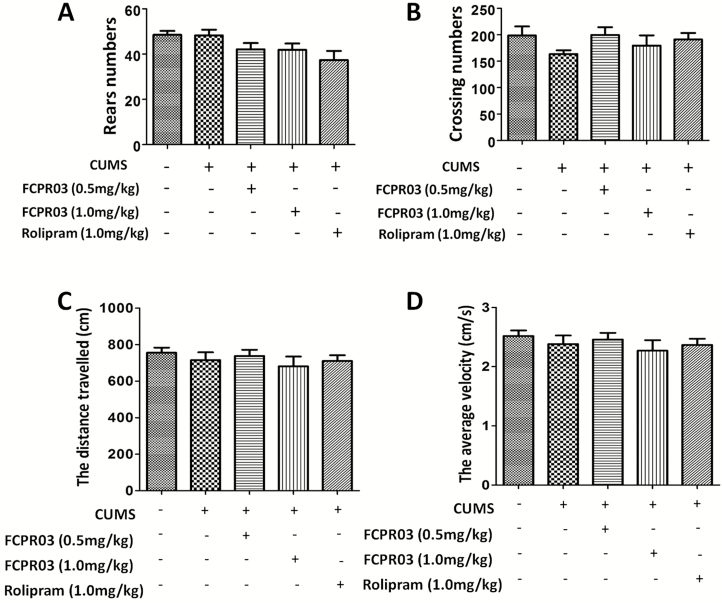
The chemical structure of FCPR03 is illustrated in Figure 1A, while the experimental procedure is shown in Figure 1B. Among 3 PDE4 subtypes (PDE4A, PDE4B, and PDE4C), the PDE4B subtype is highly correlated with depressive-like effects (Zhang et al., 2017). Hence, we first tested the effects of CUMS on PDE4B expression in the hippocampus. As shown in Figure 2A–B, CUMS increased the expression of PDE4B significantly (P<.01). We then investigated whether FCPR03 could ameliorate depressive-like behaviors in mice. FCPR03 (0.5 and 1.0 mg/kg) were administered once a day for 14 days consecutively. The canonical PDE4 inhibitor rolipram was used as a control. The doses of FCPR03 and rolipram used in the current study were selected based on our previous experiments (Zou et al., 2017). SPT, FST, and TST were implemented to assess depressive-like behaviors in the mice. As shown in Figure 2C, 1-way ANOVA showed a significant difference among the experimental groups (F4,35=6.588, P=.0005). The preference for sucrose solution was significantly decreased in the CUMS-challenged mice compared with the vehicle-treated mice (P<.001), confirming depressive-like behaviors in these animals. Treatment with FCPR03 or rolipram restored sucrose preference in CUMS mice. Secondly, as shown in Figure 2D–E, 1-way ANOVA showed significant effects of FCPR03 on immobility time in both FST (F4,35=7.461, P=.0002) and TST (F4,35=6.425, P=.0005) in mice (Figure 2D–E). In comparison with mice in the vehicle group, the CUMS-induced depressive mice exhibited a significant increase in immobility time in FST (P<.0001), while FCPR03 at the doses of 0.5 and 1.0 mg/kg reduced the immobility time in comparison with in the CUMS group (P<.05 and P<.01). Figure 2E shows the effects of FCPR03 on the immobility time tested in the TST. The results of this test revealed a significantly reduced immobility time of FCPR03 at the dose of 1.0 mg/kg. The prototype PDE4 inhibitor rolipram produced a similar effect regarding immobility time in both FST and TST at the dose of 1.0 mg/kg. Third, results presented in Figure 3A–B show the effects of FCPR03 on crossing and rearing activity of OFT. Statistical analysis by 1-way ANOVA showed no significant difference in rearing activity (F4,35=2.542, P= .0570) or crossing activity (F4,35=1.065, P=.3886). We also found that FCPR03 had no effect on the distance travelled (Figure 3C) or the average velocity (Figure 3D) in CUMS-exposed mice. In other words, FCPR03 produced no significant difference in the total locomotor activity number as compared with vehicle-treated mice in this test (P>.05), suggesting that FCPR03 does not cause sedation in mice. Taken together, our results indicate that FCPR03 possesses a potent antidepressant-like effect in mice subjected to CUMS.
Figure 2.
Antidepressant-like effect of n-isopropyl-3-(cyclopropylmethoxy)-4-difluoromethoxy benzamide (FCPR03) in chronic unpredictable mild stress (CUMS)-exposed mice. Mice were exposed to CUMS for 56 days, and the hippocampus extracts were then homogenized. Western blots were used to assess phosphodiesterase 4 (PDE)4B protein levels (A); the corresponding quantification data are shown in (B). Data are expressed as mean±SEM (n=5). ##P<.01 vs vehicle group. After 2 weeks of treatment with FCPR03 (0.5 mg/kg or 1.0 mg/kg) or rolipram (1.0 mg/kg), sucrose preference ratio in the sucrose preference test (SPT) (C) and the immobility times in the forced swim test (FST) (D) and tail suspension test (TST) (E) were measured. Data are expressed as mean±SEM (n=8). ##P<.01, ###P<.001 vs vehicle group, *P<.05, **P<.01 vs CUMS group.
Figure 3.
Effects of n-isopropyl-3-(cyclopropylmethoxy)-4-difluoromethoxy benzamide (FCPR03) or rolipram in chronic unpredicted mild stress (CUMS)-exposed mice in the open field test (OFT). After 2 weeks of treatment with FCPR03 (0.5 mg/kg or 1.0 mg/kg) or rolipram (1.0 mg/kg), the rear numbers (A), crossing numbers (B), distance travelled (C), and average velocity (D) were measured. Data are expressed as mean±SEM (n=8).
FCPR03 Increased Dendritic Complexity and Spine Density in Mice Exposed to CUMS
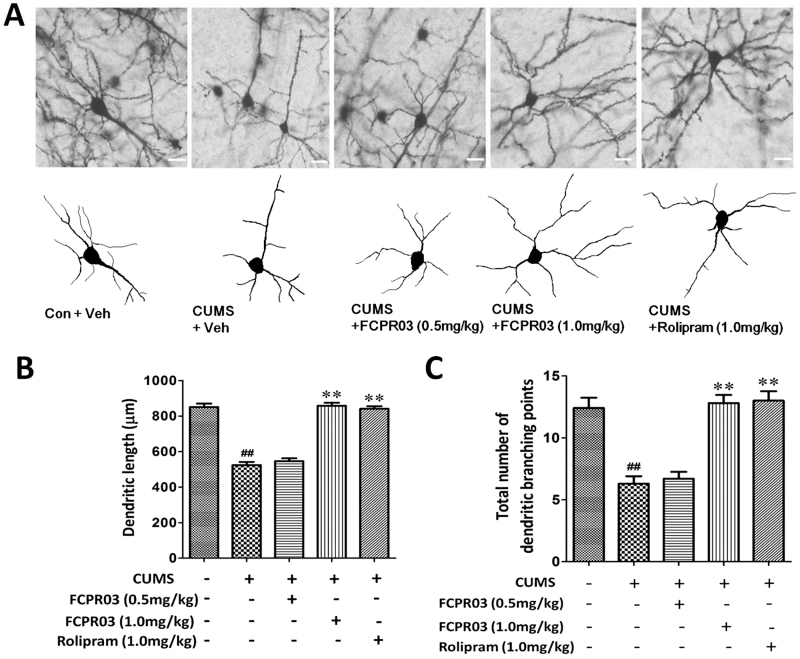
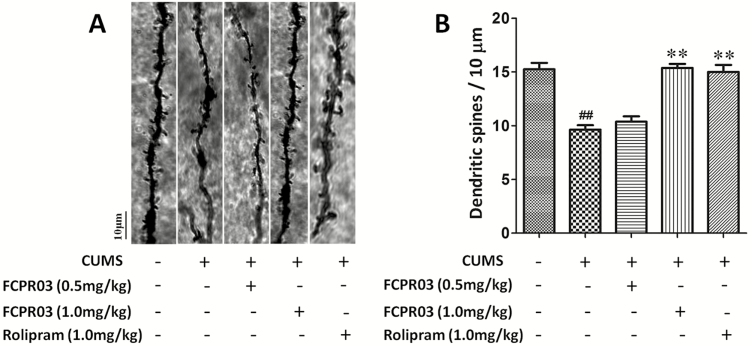
To explore the effects of FCPR03 on the morphology of neuronal dendrites in the hippocampus, Golgi staining was used to determine the dendritic complexity and spine density in the hippocampus in mice. As shown in Figure 4, apparent differences were observed in total dendritic length (F4,20=62.52, P<.0001) and branching points (F4,20=10.15, P=.0001) among different groups. Our results revealed significant decreases of total dendritic length (P<.01) (Figure 4A–B) and branching points (P<.01) (Figure 4A,C) in mice subjected to the CUMS procedure when comparing such with the vehicle group. Treatment with FCPR03 (1.0 mg/kg) or rolipram (1.0 mg/kg) significantly increased the total dendritic length (P<.01) and branching points (P<.01). Using 1-way ANOVA analysis, we also compared dendritic spine density level among different groups, and we observed significant differences in spine density (F4,20=17.99, P<.0001; Figure 5). Notably, the FCPR03-treated group displayed an increased spine density compared with the CUMS group without FCPR03 administration (P<.01). These results suggest that FCPR03 is effective in reversing CUMS-induced decreases of dendritic complexity and spine density.
Figure 4.
n-Isopropyl-3-(cyclopropylmethoxy)-4-difluoromethoxy benzamide (FCPR03) enhanced the dendritic length and total number of dendritic branching points in the hippocampus in chronic unpredictable mild stress (CUMS)-exposed mice. After 2 weeks of treatment with FCPR03 (0.5 mg/kg or 1.0 mg/kg) or rolipram (1.0 mg/kg), Golgi staining was used to assess dendritic length and the total number of dendritic branching points in the hippocampus. Photomicrographs of Golgi staining pyramidal neurons in the hippocampus of mice in each group (A), scale bar=50 μm. The corresponding quantification data of dendritic length and total number of dendritic branching points are shown in (B) and (C). Data are expressed as mean±SEM; n=3. ##P<.01 vs vehicle group, **P<.01 vs CUMS group.
Figure 5.
n-Isopropyl-3-(cyclopropylmethoxy)-4-difluoromethoxy benzamide (FCPR03) enhanced dendritic spines in the hippocampus in chronic unpredictable mild stress (CUMS)-exposed mice. After 2 weeks of treatment with FCPR03 (0.5 mg/kg or 1.0 mg/kg) or rolipram (1.0 mg/kg), Golgi staining was used to assess dendritic spines in the hippocampus. Photomicrographs of dendrite fragments with visible spines from mice in each group (A). The corresponding quantification data of dendritic spines are shown in (B). Data are expressed as mean±SEM (n=3). ##P<.01 vs vehicle group, **P<.01 vs CUMS group.
FCPR03 Enhanced the Levels of Postsynaptic Density Protein 95 and Synapsin 1 in Mice Exposed to CUMS
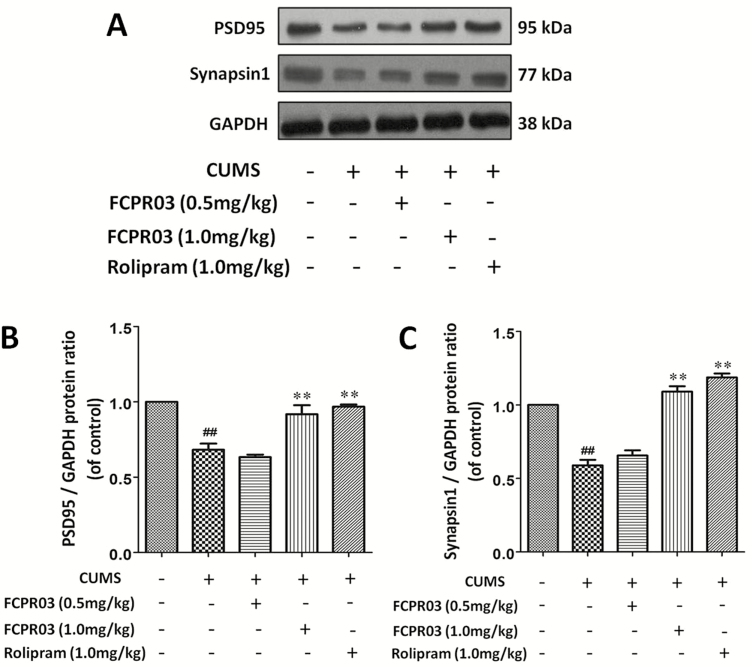
To examine the effects of FCPR03 on the expression of synapse associated proteins in the hippocampi of CUMS-exposed mice, we detected the protein levels of PSD95 and synapsin 1 in the hippocampus in mice. As shown in Figure 6, the results indicated that the levels of PSD95 (P<.01) and synapsin 1 (P<.01) were decreased in mice subjected to the CUMS procedure vs in mice in the vehicle group. Treatment with FCPR03 (1.0 mg/kg) or rolipram (1.0 mg/kg) for 2 weeks significantly increased expression of PSD95 (P<.01) and synapsin 1 (P<.01). These results indicate that the antidepressant-like effect of FCPR03 is associated with the expression of synapse associated proteins, such as PSD95 and synapsin 1.
Figure 6.
n-Isopropyl-3-(cyclopropylmethoxy)-4-difluoromethoxy benzamide (FCPR03) enhanced the protein levels of PSD95 and synapsin 1 in the hippocampus in chronic unpredictable mild stress (CUMS)-exposed mice. After 2 weeks of treatment with FCPR03 (0.5 mg/kg or 1.0 mg/kg) or rolipram (1.0 mg/kg), the hippocampal tissues were homogenized. Western blot was used to assess PSD95 and synapsin 1 (A) protein levels in the hippocampus; the corresponding quantification data are shown in (B–D). Data are expressed as mean±SEM (n=5). ##P<.01 vs vehicle group, **P<.01 vs CUMS group.
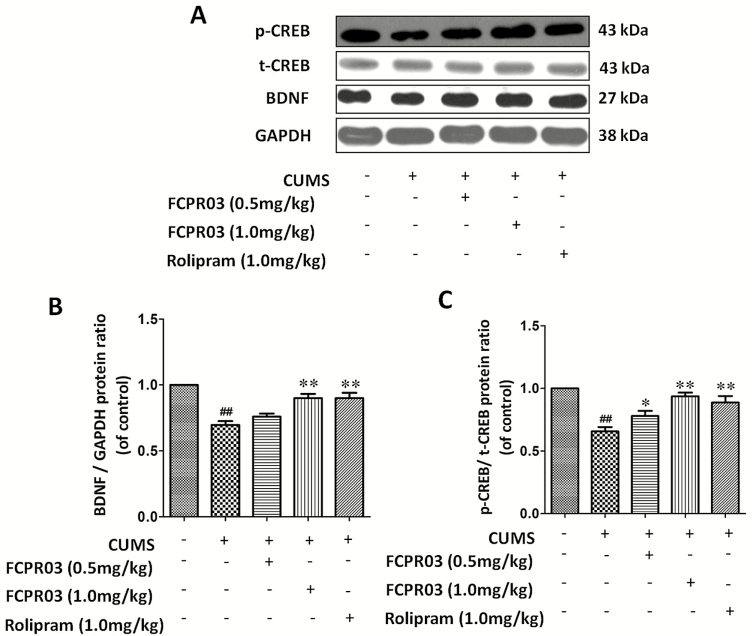
FCPR03 Increased Hippocampal cAMP, Phosphorylated CREB, and BDNF Levels in Mice Exposed to CUMS
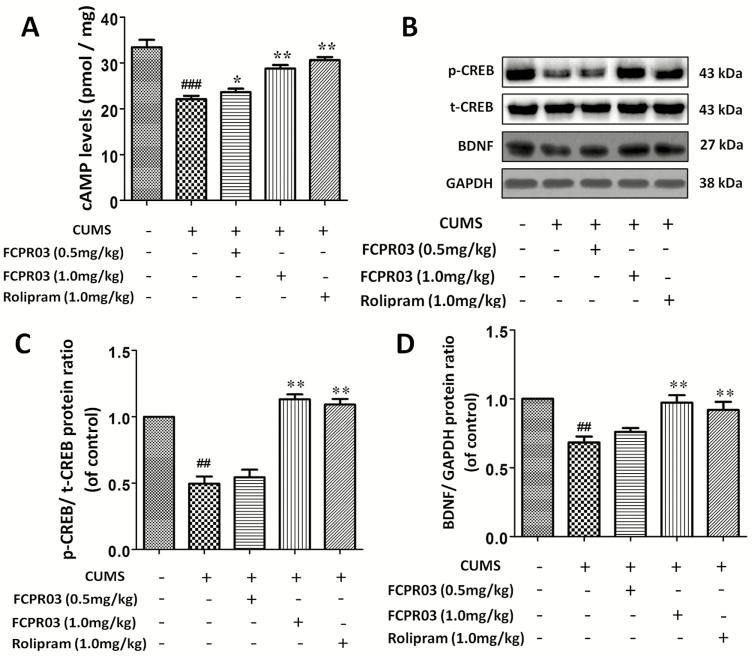
The inhibition of PDE4 is supposed to decrease the degradation of cAMP. FCPR03 is a novel selective PDE4 inhibitor. In the present study, we then investigated the level of cAMP in the hippocampi of mice exposed to CUMS. The results depicted in Figure 7A showed that the level of cAMP was decreased in the CUMS group vs in the vehicle group (F4,15=95.58, P<.0001). However, at doses of 0.5 and 1.0 mg/kg, FCPR03 significantly increased the level of cAMP in the hippocampus. Increased cAMP activates the PKA/CREB signaling pathway (Guo et al., 2017). Hence, we then sought to detect the level of phosphorylated CREB in mice exposed to CUMS. As shown in Figure 7B, we found that CUMS caused a significant reduction of phosphorylated CREB, while FCPR03 reversed the decline of CREB phosphorylation in mice, without altering the total CREB level. BDNF is a downstream target of CREB. Phosphorylated CREB enhances the transcription of BDNF (Guo et al., 2017). Importantly, a deficit of BDNF is involved in the pathology of major depression (Kishi et al., 2017). Having known that FCPR03 increased the phosphorylation of CREB, we then detected the protein level of BDNF in mice exposed to CUMS. We found that CUMS suppressed the expression of BDNF in the hippocampus (Figure 7B,D) tissues in mice. Treatment with FCPR03 at 1.0 mg/kg significantly increased the protein level of BDNF (P<.05) in the hippocampus in CUMS-treated mice. As shown in Figure 7D, 1 mg/kg of rolipram promoted a similar effect to that of 1 mg/kg FCPR03 in the expression of BDNF. These results suggest that the antidepressant-like effect of FCPR03 may involve the cAMP/CREB/BDNF signaling pathway in mice exposed to CUMS.
Figure 7.
n-Isopropyl-3-(cyclopropylmethoxy)-4-difluoromethoxy benzamide (FCPR03) enhanced the level of cyclic adenosine monophosphate (cAMP) and the phophorylated cAMP-response element-binding protein (p-CREB) in the hippocampus in chronic unpredictable mild stress (CUMS)-exposed mice. After 2 weeks of treatment with FCPR03 (0.5 or 1.0 mg/kg) or rolipram (1.0 mg/kg), the hippocampal tissues were homogenized, and an enzyme-linked immunosorbent assay (ELISA) kit was used to assess cAMP levels (A), while western blot was used to assess phosphorylated cAMP-response element-binding protein (CREB), total CREB, and brain-derived neurotrophic factor (BDNF) (B) levels in the hippocampus. The corresponding quantification data are shown in (C) and (D). Data are expressed as mean±SEM (n=5). ##P<.01, ###P<.001 vs vehicle group, *P<.05, **P<.01 vs CUMS group.
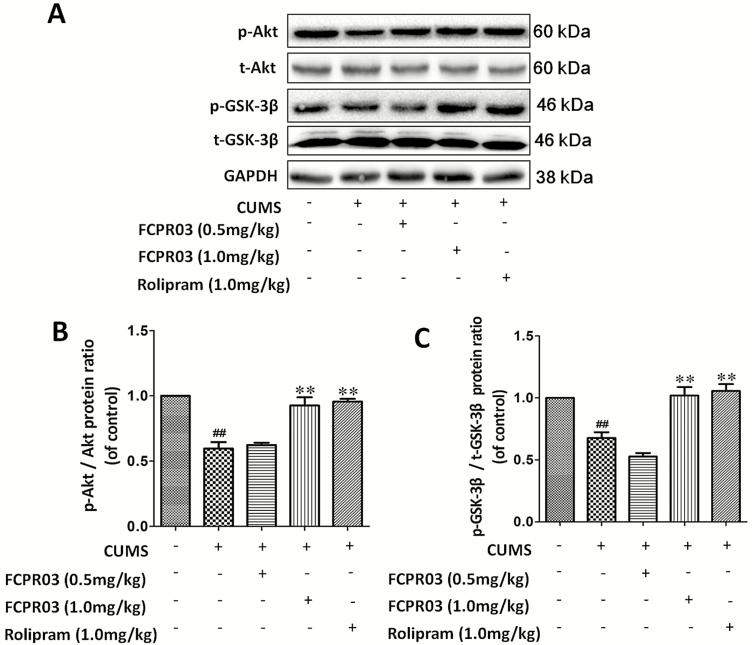
FCPR03 Enhanced the Phosphorylation of Akt and GSK3β in the Hippocampus of Mice Exposed to CUMS
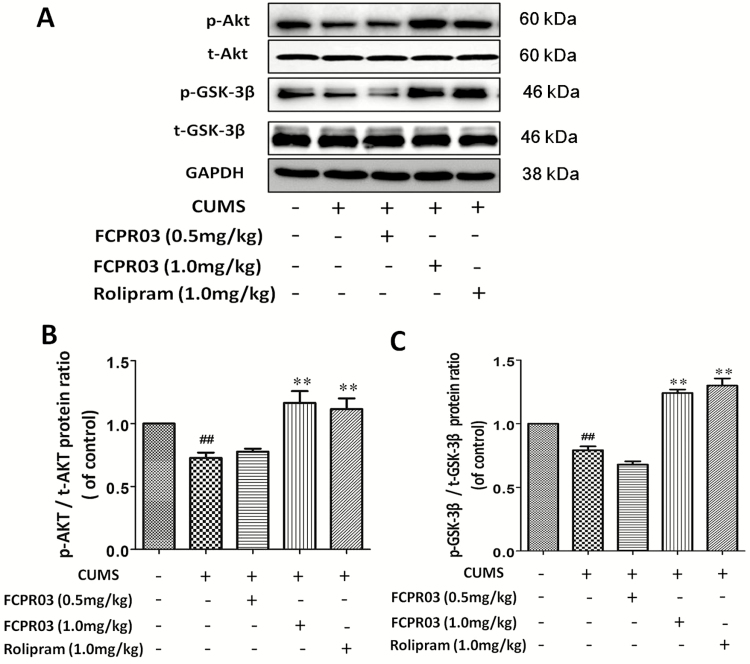
Akt has been shown to inhibit GSK-3β in response to multiple growth factors, including BDNF (Zheng et al., 2012). The inhibition of GSK-3β is also beneficial for the treatment of major depression (Chen et al., 2015). In addition, GSK-3β signaling is involved in the hippocampal neurogenesis mediated by antidepressants (Hui et al., 2014). To investigate the phosphorylation of Akt and GSK-3β after treatment with FCPR03 in the mice exposed to CUMS, we performed western-blot analysis. As shown in Figure 8, CUMS-exposed mice showed a remarkable decrease in the phosphorylation of Akt (P<.01) (Figure 8A–B) and GSK3β (P<.01) (Figure 8A,C). Treatment with 1.0 mg/kg of FCPR03 furthermore increased the phosphorylation of Akt (P<.01), and the influence on the phosphorylation of GSK-3β was similar (P<.01). Moreover, treatment with 1.0 mg/kg of rolipram significantly increased the phosphorylation of Akt (P<.01) and GSK-3β (P<.01). These results suggest that the phosphorylation of Akt and GSK3β is accelerated in the vulnerable hippocampal region following treatment with FCPR03 in mice exposed to CUMS.
Figure 8.
n-Isopropyl-3-(cyclopropylmethoxy)-4-difluoromethoxy benzamide (FCPR03) enhanced the protein levels of phosphorylated protein kinase B (p-Akt) and phosphorylated glycogen synthase kinase-3β (p-GSK-3β) in the hippocampus in chronic unpredictable mild stress (CUMS)-exposed mice. After 2 weeks of treatment with FCPR03 (0.5 mg/kg or 1.0 mg/kg) or rolipram (1.0 mg/kg), the hippocampal tissues were homogenized. Western blot was used to assess phosphorylated Akt, total Akt, GSK-3β, and total GSK-3β protein levels in the hippocampus (A); the corresponding quantification data are shown in (B) and (C). Data are expressed as mean±SEM (n=5). ##P<.01 vs vehicle group, **P<.01 vs CUMS group.
FCPR03 Activated Akt/GSK3β and CREB/BDNF Signaling Pathways in the Prefrontal Cortex in Mice Exposed to CUMS
To test whether CUMS and FCPR03 produce similar biochemical changes in other brain regions, we measured the phosphorylation of Akt, GSK-3β, and CREB and the level of BDNF in the prefrontal cortex in mice subjected to CUMS. As shown in Figure 9, CUMS exposure caused a significant decrease in the phosphorylation of Akt (P<.01) (Figure 9A–B) and GSK3β (P<.01) (Figure 9A,C). Meanwhile, FCPR03 (1.0 mg/kg) remarkably reversed the effect of CUMS (P<.01). Similarly, CUMS decreased the expression of BDNF (P<.01) (Figure 10A–B) and the level of phosphorylated CREB (P<.01) (Figure 10A,C) in the prefrontal cortex of mice. FCPR03 treatment restored the levels of both phospho-CREB and BDNF (Figure 10). Moreover, treatment with the canonical PDE4 inhibitor rolipram (1.0 mg/kg) also increased the phosphorylation of Akt (P<.01), GSK-3β (P<.01), and CREB (P<.01) and enhanced the expression of BDNF (P<.01) in the prefrontal cortex in these mice (Figures 9 and 10). These results suggest that besides the hippocampus, FCPR03 also activates the Akt/GSK-3β and CREB/BDNF signaling pathways in the prefrontal cortexes of mice exposed to CUMS.
Figure 9.
n-Isopropyl-3-(cyclopropylmethoxy)-4-difluoromethoxy benzamide (FCPR03) enhanced the protein levels of phosphorylated protein kinase B (p-Akt) and phosphorylated glycogen synthase kinase-3β (p-GSK-3β) in the prefrontal cortex of chronic unpredictable mild stress (CUMS)-exposed mice. After 2 weeks of treatment with FCPR03 (0.5 mg/kg or 1.0 mg/kg) or rolipram (1.0 mg/kg), the prefrontal cortex tissues were homogenized. Western blot was used to assess phosphorylated cAMP-response element-binding protein (CREB) and brain-derived neurotrophic factor (BDNF) protein levels in the prefrontal cortex (A); the corresponding quantification data are shown in (B) and (C). Data are expressed as mean±SEM (n=5). ##P<.01 vs vehicle group, **P<.01 vs CUMS group.
Figure 10.
n-Isopropyl-3-(cyclopropylmethoxy)-4-difluoromethoxy benzamide (FCPR03) enhanced the protein levels of phosphorylated cAMP-response element-binding protein (p-CREB) and brain-derived neurotrophic factor (BDNF) in the prefrontal cortex in chronic unpredictable mild stress (CUMS)-exposed mice. After 2 weeks of treatment with FCPR03 (0.5 mg/kg or 1.0 mg/kg) or rolipram (1.0 mg/kg), the prefrontal cortex tissues were homogenized. Western blot was used to assess p-CREB and BDNF protein levels in the prefrontal cortex (A); the corresponding quantification data are shown in (B) and (C). Data are expressed as mean±SEM (n=5). ##P<.01 vs vehicle group, *P<.05, **P<.01 vs CUMS group.
Discussion
In this study, we showed that the selective inhibition of PDE4 by FCPR03 produces an antidepressant-like effect in mice exposed to CUMS. This is based on the following observations: (1) treatment with FCPR03 restored sucrose preference in the SPT and FCPR03 also shortened the immobility time tested in both FST and TST, while FCPR03 had no influence on locomotor activity; (2) treatment with FCPR03 significantly enhanced dendritic length, branching points, and dendritic spine density in the hippocampus; (3) inhibition of PDE4 by FCPR03 resulted in an increased expression of PSD95, and synapsin 1 in the hippocampi of mice exposed to CUMS; (4) FCPR03 increased the level of cAMP, the phosphoylation of CREB, and the expression of BDNF in the hippocampi of mice exposed to CUMS; (5) FCPR03 enhanced the phosphorylation of both Akt and GSK-3β; and (6) FCPR03 activated the Akt/GSK-3β and CREB/BDNF signaling pathways in the prefrontal cortex in mice exposed to CUMS. Thus, the present study provided evidence that FCPR03 exhibited its antidepressant-like activity via its regulation on cAMP/CREB/BDNF and Akt/GSK-3β and subsequently enhanced spine density and dendritic morphology.
CUMS is a widely accepted animal model mimicking the etiology of depression in humans (Zhang et al., 2014; Liang et al., 2015; Wan et al., 2017). This animal model is manifested by the loss of pleasure in rodents (i.e., manifested by a decline in sucrose preference in mice) and prolonged duration of immobility in FST and TST (Zhang et al., 2014; Liang et al., 2015). In our experiment, mice indeed developed depressive-like behaviors after the CUMS procedure. Not surprisingly, FCPR03 increased the consumption of sucrose and shortened immobility time in both FST and TST, while FCPR03 had no influence on the locomotor activity. These results confirmed the antidepressant-like effect of FCPR03 in mice. Additionally, the antidepressant-like effect of FCPR03 was similar to the canonical PDE4 inhibitor rolipram. In our previous study, mice were given a single dose of FCPR03, and we found that FCPR03 shortened the immobility time in FST and TST (ZZ Zhou et al., 2016). In another lipopolysaccharide-induced depressive animal model, FCPR03 additionally exhibited an antidepressant-like effect (Yu et al., 2018). Together with these findings, we verified the antidepressant-like effect of FCPR03 on different animal models. Importantly, as compared with canonical PDE4 inhibitors, FCPR03 does not trigger emesis in beagle dogs (ZZ Zhou et al., 2016). Hence, in regards to side effects and drug safety, FCPR03 boasts a higher advantage than do canonical PDE4 inhibitors, such as rolipram. However, compared with existing antidepressants, PDE4 inhibitors have been proven to possess significant antidepressant and cognitive enhancement effects (Bolger, 2017). As patients with depression usually present cognitive impairments (Srisurapanont et al., 2018), the cognitive enhancement property of PDE4 inhibitors makes them more advantageous. Moreover, because of the lack of anticholinergic effects, PDE4 inhibitors may show less anticholinergic and hypotensive side effects. Collectively, FCPR03 is a potential compound for the prevention and treatment of depression. However, we would like to point out that although our study supports the potential application of FCPR03, additional experiments, especially systematically toxicological studies of the compound, are needed in the future.
PDE4 is a well-identified target for the treatment of depression (Li et al., 2009; Wang et al., 2014; Bolger, 2017). The inhibition of PDE4 by its inhibitors or short hairpin RNA increases the concentration of intracellular cAMP, which triggers the downstream signal cascade, including the activation of PKA and CREB and the subsequent production of its downstream target BDNF (Guo et al., 2017; Zou et al., 2017). PKA/CREB/BDNF is a defined canonical pathway responsible for the biological functions of PDE4 (Guo et al., 2017). This signaling pathway has been taken into account as a common mechanism undertaking antidepressant action of different classes of antidepressants (Li et al., 2009; Bolger, 2017). In our study, we also found that FCPR03 had a strong effect on the activation of this pathway in both the hippocampus and prefrontal cortex. Meanwhile, we noticed that Akt and GSK-3β were also phosphorylated in the presence of FCPR03. Currently, little information is available about the role of PDE4 inhibitors in the phosphorylation of GSK-3β. Our recent study reported that the inhibition of PDE4 in SH-SY5Y cells increased the concentrations of cAMP and Epac, which promotes the phosphorylation of Akt at Ser473 (Zhong et al., 2018). In this study, we also found that FCPR03 increased the concentration of cAMP in the brain. Hence, it is highly possible that increased cAMP in the hippocampus enhances the level of Epac and the consequent activation of Akt. GSK-3β is a direct target of Akt, and the activation of Akt by Epac may directly phosphorylate and inactivate GSK-3β at Ser9. Interestingly, it was reported that the pharmacological inhibition of combined PDE4 and GSK-3β in low, subeffective doses elicits synergistic, antipsychotic effects (Lipina et al., 2012). In this condition, the inhibition of PDE4 by FCPR03 is expected to produce its antidepressant-like effect by acting on both cAMP/PKA/CREB/BDNF and cAMP/Epac/Akt/GSK-3β signaling pathways. It is noteworthy that we observed the alterations of these 2 signaling pathways in this study. Further experiments, such as the blockade of these 2 signaling pathways or the knocking down of key proteins involved in these pathways, are still needed to verify the essential role of these in the antidepressant-like effect of FCPR03, which will add immense value to the present communication. The specific inhibition of PKA and Akt will serve a purpose. It is worthwhile to test this idea in future research.
BDNF is a potent neurotrophin maintaining the morphology and neural plasticity of neurons (Finsterwald et al., 2010; Kojima and Mizui, 2017). Deficits in the synaptic plasticity are involved in the pathological process of depression (Li et al., 2009). Chronic stress elevates the level of serum corticosterone, which leads to reduced hippocampal BDNF levels and neurogenesis (Li et al., 2009; H Wang et al., 2015). Antidepressants such as tricyclic antidepressant oxazine and the selective 5-HT reuptake inhibitor fluoxetine increase neurogenesis in the dentate gyrus of the hippocampus (Malberg et al., 2000). Interestingly, the antidepressant-like effect of these drugs was attenuated when neurogenesis or neural plasticity was blocked (Santarelli et al., 2003), suggesting that amelioration of the depressive-like behaviors may be mediated by the stimulation of neurogenesis and neural plasticity in the hippocampus (Santarelli et al., 2003). After adulthood, neurogenesis takes place at an extremely slow rate, and neural plasticity occurs through learning, exercise, and antidepressants use after injury (Kraus et al., 2017). Numerous studies have shown that CREB and BDNF play a key role in the regulation of neurogenesis, neuronal plasticity, and survival (Guo et al., 2017; Kishi et al., 2017; Sheldrick et al., 2017). Our results showed that FCPR03 increased the levels of synapsin 1, and PSD95, which are important proteins in the preservation of the generation, morphology, and functions of synapse (Dorostkar et al., 2014; Kandimalla et al., 2016).
The formation and elimination of dendritic spines rely on the normal expression of synaptic proteins (Lee et al., 2012; Moutin et al., 2012). In our studies, we also found that the dendritic length, branching points, and dendritic spine density were reduced under CUMS, while FCPR03 significantly restored the morphological alterations. Dendritic spines play a pivotal role in neuronal communication and neuroplasticity. Chronic stress impairs the density and organization of spines, while changes in dendrite morphology contribute to the behavioral deficits caused by chronic stress (Licznerski and Duman, 2013). Currently, little is known about the mechanisms underlying the atrophy of dendritic spines and branching in response to chronic stress (Licznerski and Duman, 2013), but it is possible that the inhibition of neurotrophic factor (e.g., BDNF) levels, PKA/CREB/BDNF and Akt/GSK-3β signaling, and synaptic protein synthesis could contribute to the effects of stress. It is largely believed that BDNF controls the dendritic length and branching of neurons (McAllister et al., 1996; Finsterwald et al., 2010), while CREB mediates the positive effects of BDNF on dendritic length and complexity (Finsterwald et al., 2010). In our study, it is predictable that the morphological alterations caused by FCPR03 are produced by the enhanced expression of synaptic proteins. Furthermore, mechanically, FCPR03 regulates cytoskeleton dynamics and modulates dendritic morphology through the activation of the PKA/CREB/BDNF and Akt/GSK-3β signaling pathways. Of note, one of the limitations of this study is that the sample for western-blot analysis was taken from the entire hippocampus, which is a large and heterogeneous region, even in the mouse brain. Hence, to identify specific involved brain regions, protein analysis is better performed in hippocampal subregions, such as CA1, CA3, and DG.
In conclusion, CUMS caused a series of depressive-like behaviors, including the reduction of sucrose intake and increased immobility time in FST and TST in mice. This procedure also inhibited the cAMP/PKA/CREB/BDNF signaling pathway and attenuated the phosphorylation of both Akt and GSK3β. FCPR03 produced beneficial effects on the stressed mice by improving sucrose consumption and reducing immobility time in FST and TST. These behavioral changes were accompanied by amelioration of CUMS-induced alterations in mice. Our results suggest that FCPR03 exhibited an antidepressant-like effect, at least partially, due to its ability to the activation of the cAMP/PKA/CREB/BDNF and Akt/GSK-3β pathways.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (nos. 81301099, 81773698, and 81872735), Foundation for Science and Technology Program of Guangdong Provinces (nos. 2015B020211007, and 2016A020217008), Science and Technology Program of Guangzhou (no. 201604020112) and Guangdong Natural Science Foundation (nos. S2013040014202 and 2018A030313046).
Statement of Interest
None.
References
- Allen AR, Raber J, Chakraborti A, Sharma S, Fike JR(2015)(56)fe irradiation alters spine density and dendritic complexity in the mouse hippocampus. Radiat Res 184:586–594. [DOI] [PubMed] [Google Scholar]
- Bolger GB.(2017)The PDE4 camp-specific phosphodiesterases: targets for drugs with antidepressant and memory-enhancing action. Adv Neurobiol 17:63–102. [DOI] [PubMed] [Google Scholar]
- Boswell-Smith V, Spina D(2007)PDE4 inhibitors as potential therapeutic agents in the treatment of COPD-focus on roflumilast. Int J Chron Obstruct Pulmon Dis 2:121–129. [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Shirayama Y, Shin KH, Neve RL, Duman RS (2001) Expression of the cAMP response element binding protein (CREB) in hippocampus produces an antidepressant effect. Biol Psychiatry 49:753–762. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang M, Waheed Khan RA, He K, Wang Q, Li Z, Shen J, Song Z, Li W, Wen Z, Jiang Y, Xu Y, Shi Y, Ji W(2015)The GSK3B gene confers risk for both major depressive disorder and schizophrenia in the han chinese population. J Affect Disord 185:149–155. [DOI] [PubMed] [Google Scholar]
- Chong J, Leung B, Poole P(2017)Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 9:CD002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Crescenzo F, Serra G, Maisto F, Uchida M, Woodworth H, Casini MP, Baldessarini RJ, Vicari S(2017)Suicide attempts in juvenile bipolar versus major depressive disorders: systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry 56:825–831.e3. [DOI] [PubMed] [Google Scholar]
- Dorostkar MM, Burgold S, Filser S, Barghorn S, Schmidt B, Anumala UR, Hillen H, Klein C, Herms J(2014)Immunotherapy alleviates amyloid-associated synaptic pathology in an alzheimer’s disease mouse model. Brain 137:3319–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlatshahi D, MacQueen GM, Wang JF, Young LT(1998)Increased temporal cortex CREB concentrations and antidepressant treatment in major depression. Lancet 352:1754–1755. [DOI] [PubMed] [Google Scholar]
- Finsterwald C, Fiumelli H, Cardinaux JR, Martin JL(2010)Regulation of dendritic development by BDNF requires activation of CRTC1 by glutamate. J Biol Chem 285:28587–28595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Richards EM, Niciu MJ, Ionescu DF, Zoghbi SS, Hong J, Telu S, Hines CS, Pike VW, Zarate CA, Innis RB(2017)Camp signaling in brain is decreased in unmedicated depressed patients and increased by treatment with a selective serotonin reuptake inhibitor. Mol Psychiatry 22:754–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Zhou JJ, Zhu Y, Wang L, Kosten TA, Zhang X, Li DP(2017)Neuroadaptations of presynaptic and postsynaptic GABAB receptor function in the paraventricular nucleus in response to chronic unpredictable stress. Br J Pharmacol 174:2929–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong MF, Wen RT, Xu Y, Pan JC, Fei N, Zhou YM, Xu JP, Liang JH, Zhang HT(2017)Attenuation of ethanol abstinence-induced anxiety- and depressive-like behavior by the phosphodiesterase-4 inhibitor rolipram in rodents. Psychopharmacology (Berl) 234:3143–3151. [DOI] [PubMed] [Google Scholar]
- Guo H, Cheng Y, Wang C, Wu J, Zou Z, Niu B, Yu H, Wang H, Xu J(2017)FFPM, a PDE4 inhibitor, reverses learning and memory deficits in APP/PS1 transgenic mice via camp/PKA/CREB signaling and anti-inflammatory effects. Neuropharmacology 116:260–269. [DOI] [PubMed] [Google Scholar]
- Hengartner MP.(2017)Methodological flaws, conflicts of interest, and scientific fallacies: implications for the evaluation of antidepressants’ efficacy and harm. Front Psychiatry 8:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieronymus F, Lisinski A, Nilsson S, Eriksson E(2018)Efficacy of selective serotonin reuptake inhibitors in the absence of side effects: a mega-analysis of citalopram and paroxetine in adult depression. Mol Psychiatry 23:1731–1736. [DOI] [PubMed] [Google Scholar]
- Hui J, Zhang J, Kim H, Tong C, Ying Q, Li Z, Mao X, Shi G, Yan J, Zhang Z, Xi G(2014)Fluoxetine regulates neurogenesis in vitro through modulation of GSK-3beta/beta-catenin signaling. Int J Neuropsychopharmacol 18:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal A, Mahesh R, Bhatt S(2013)Etazolate, a phosphodiesterase 4 inhibitor reverses chronic unpredictable mild stress-induced depression-like behavior and brain oxidative damage. Pharmacol Biochem Behav 105:63–70. [DOI] [PubMed] [Google Scholar]
- Jindal A, Mahesh R, Bhatt S(2015)Etazolate, a phosphodiesterase-4 enzyme inhibitor produces antidepressant-like effects by blocking the behavioral, biochemical, neurobiological deficits and histological abnormalities in hippocampus region caused by olfactory bulbectomy. Psychopharmacology (Berl) 232:623–637. [DOI] [PubMed] [Google Scholar]
- Kandimalla R, Manczak M, Fry D, Suneetha Y, Sesaki H, Reddy PH(2016)Reduced dynamin-related protein 1 protects against phosphorylated tau-induced mitochondrial dysfunction and synaptic damage in Alzheimer’s disease. Hum Mol Genet 25:4881–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Yoshimura R, Ikuta T, Iwata N(2017)Brain-derived neurotrophic factor and major depressive disorder: evidence from meta-analyses. Front Psychiatry 8:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch JM, Hinze-Selch D, Stingele K, Huchzermeier C, Goder R, Seeck-Hirschner M, Aldenhoff JB(2009)Changes in CREB phosphorylation and BDNF plasma levels during psychotherapy of depression. Psychother Psychosom 78:187–192. [DOI] [PubMed] [Google Scholar]
- Kojima M, Mizui T(2017)BDNF propeptide: a novel modulator of synaptic plasticity. Vitam Horm 104:19–28. [DOI] [PubMed] [Google Scholar]
- Kraus C, Castrén E, Kasper S, Lanzenberger R(2017)Serotonin and neuroplasticity - links between molecular, functional and structural pathophysiology in depression. Neurosci Biobehav Rev 77:317–326. [DOI] [PubMed] [Google Scholar]
- Lee KF, Soares C, Béïque JC(2012)Examining form and function of dendritic spines. Neural Plast 2012:704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YF, Huang Y, Amsdell SL, Xiao L, O’Donnell JM, Zhang HT(2009)Antidepressant- and anxiolytic-like effects of the phosphodiesterase-4 inhibitor rolipram on behavior depend on cyclic AMP response element binding protein-mediated neurogenesis in the hippocampus. Neuropsychopharmacology 34:2404–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang BF, Huang F, Wang HT, Wang GH, Yuan X, Zhang MZ, Guo HB, Cheng YF, Xu JP(2015)Involvement of norepinephrine and serotonin system in antidepressant-like effects of hederagenin in the rat model of unpredictable chronic mild stress-induced depression. Pharm Biol 53:368–377. [DOI] [PubMed] [Google Scholar]
- Licznerski P, Duman RS(2013)Remodeling of axo-spinous synapses in the pathophysiology and treatment of depression. Neuroscience 251:33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipina TV, Wang M, Liu F, Roder JC(2012)Synergistic interactions between PDE4B and GSK-3: DISC1 mutant mice. Neuropharmacology 62:1252–1262. [DOI] [PubMed] [Google Scholar]
- Liu FG, Hu WF, Wang JL, Wang P, Gong Y, Tong LJ, Jiang B, Zhang W, Qin YB, Chen Z, Yang RR, Huang C(2017)Z-guggulsterone produces antidepressant-like effects in mice through activation of the BDNF signaling pathway. Int J Neuropsychopharmacol 20:485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magariños AM, Li CJ, Gal Toth J, Bath KG, Jing D, Lee FS, McEwen BS(2011)Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus 21:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS(2000)Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20:9104–9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC(1996)Neurotrophin regulation of cortical dendritic growth requires activity. Neuron 17:1057–1064. [DOI] [PubMed] [Google Scholar]
- Moutin E, Raynaud F, Roger J, Pellegrino E, Homburger V, Bertaso F, Ollendorff V, Bockaert J, Fagni L, Perroy J(2012)Dynamic remodeling of scaffold interactions in dendritic spines controls synaptic excitability. J Cell Biol 198:251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Xu T(2005)Deficits in amygdaloid camp-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J Clin Invest 115:2762–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R(2003)Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809. [DOI] [PubMed] [Google Scholar]
- Scott AI, Perini AF, Shering PA, Whalley LJ(1991)In-patient major depression: is rolipram as effective as amitriptyline?Eur J Clin Pharmacol 40:127–129. [DOI] [PubMed] [Google Scholar]
- Sheldrick A, Camara S, Ilieva M, Riederer P, Michel TM(2017)Brain-derived neurotrophic factor (BDNF) and neurotrophin 3 (NT3) levels in post-mortem brain tissue from patients with depression compared to healthy individuals - a proof of concept study. Eur Psychiatry 46:65–71. [DOI] [PubMed] [Google Scholar]
- Srisurapanont M, Mok YM, Yang YK, Chan HN, Della CD, Zainal NZ, Jambunathan S, Amir N, Kalita P(2018)Cognitive complaints and predictors of perceived cognitive dysfunction in adults with major depressive disorder: findings from the cognitive dysfunction in asians with depression (cogdad) study. J Affect Disord 232:237–242. [DOI] [PubMed] [Google Scholar]
- Sung SC, Wisniewski SR, Balasubramani GK, Zisook S, Kurian B, Warden D, Trivedi MH, Rush AJ, CO-MED Study Team (2013)Does early-onset chronic or recurrent major depression impact outcomes with antidepressant medications? A CO-MED trial report. Psychol Med 43:945–960. [DOI] [PubMed] [Google Scholar]
- Vigo D, Thornicroft G, Atun R(2016)Estimating the true global burden of mental illness. Lancet Psychiatry 3:171–178. [DOI] [PubMed] [Google Scholar]
- Wan S, Xu M, Hu L, Yan T, He B, Xiao F, Bi K, Jia Y(2017)Schisandrin rescues depressive-like behaviors induced by chronic unpredictable mild stress via GDNF/ERK1/2/ROS and PI3K/AKT/NOX signaling pathways in mice. Psychiatry Res 257:230–237. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhang J, Lu Y, Lin P, Pan T, Zhao X, Liu A, Wang Q, Zhou W, Zhang HT(2014)Antidepressant-like effects of the phosphodiesterase-4 inhibitor etazolate and phosphodiesterase-5 inhibitor sildenafil via cyclic AMP or cyclic GMP signaling in mice. Metab Brain Dis 29:673–682. [DOI] [PubMed] [Google Scholar]
- Wang G, Cheng Y, Gong M, Liang B, Zhang M, Chen Y, Zhang C, Yuan X, Xu J(2013)Systematic correlation between spine plasticity and the anxiety/depression-like phenotype induced by corticosterone in mice. Neuroreport 24:682–687. [DOI] [PubMed] [Google Scholar]
- Wang H, Quirion R, Little PJ, Cheng Y, Feng ZP, Sun HS, Xu J, Zheng W(2015)Forkhead box O transcription factors as possible mediators in the development of major depression. Neuropharmacology 99:527–537. [DOI] [PubMed] [Google Scholar]
- Wang ZZ, Yang WX, Zhang Y, Zhao N, Zhang YZ, Liu YQ, Xu Y, Wilson SP, O’Donnell JM, Zhang HT, Li YF(2015)Phosphodiesterase-4D knock-down in the prefrontal cortex alleviates chronic unpredictable stress-induced depressive-like behaviors and memory deficits in mice. Sci Rep 5:11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZZ, Zhang Y, Liu YQ, Zhao N, Zhang YZ, Yuan L, An L, Li J, Wang XY, Qin JJ, Wilson SP, O’Donnell JM, Zhang HT, Li YF(2013)RNA interference-mediated phosphodiesterase 4D splice variants knock-down in the prefrontal cortex produces antidepressant-like and cognition-enhancing effects. Br J Pharmacol 168:1001–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Zou Z, Zhang X, Peng W, Chen C, Ye Y, Xu J, Wang H(2018)Inhibition of phosphodiesterase 4 by FCPR03 alleviates lipopolysaccharide-induced depressive-like behaviors in mice: involvement of p38 and JNK signaling pathways. Int J Mol Sci 19:513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller E, Stief HJ, Pflug B, Sastre-y-Hernández M(1984)Results of a phase II study of the antidepressant effect of rolipram. Pharmacopsychiatry 17:188–190. [DOI] [PubMed] [Google Scholar]
- Zeng B, Li Y, Niu B, Wang X, Cheng Y, Zhou Z, You T, Liu Y, Wang H, Xu J(2016)Involvement of PI3K/akt/foxo3a and PKA/CREB signaling pathways in the protective effect of fluoxetine against corticosterone-induced cytotoxicity in PC12 cells. J Mol Neurosci 59:567–578. [DOI] [PubMed] [Google Scholar]
- Zhang C, Xu Y, Zhang HT, Gurney ME, O’Donnell JM(2017)Comparison of the pharmacological profiles of selective PDE4B and PDE4D inhibitors in the central nervous system. Sci Rep 7:40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Luo J, Zhang M, Yao W, Ma X, Yu SY(2014)Effects of curcumin on chronic, unpredictable, mild, stress-induced depressive-like behaviour and structural plasticity in the lateral amygdala of rats. Int J Neuropsychopharmacol 17:793–806. [DOI] [PubMed] [Google Scholar]
- Zheng W, Wang H, Zeng Z, Lin J, Little PJ, Srivastava LK, Quirion R(2012)The possible role of the Akt signaling pathway in schizophrenia. Brain Res 1470:145–158. [DOI] [PubMed] [Google Scholar]
- Zhong J, Yu H, Huang C, Zhong Q, Chen Y, Xie J, Zhou Z, Xu J, Wang H(2018)Inhibition of phosphodiesterase 4 by FCPR16 protects SH-SY5Y cells against MPP+-induced decline of mitochondrial membrane potential and oxidative stress. Redox Biol 16:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Jin H, Lin HB, Yang XM, Cheng YF, Deng FJ, Xu JP(2010)Antidepressant effect of the extracts from Fructus Akebiae. Pharmacol Biochem Behav 94:488–495. [DOI] [PubMed] [Google Scholar]
- Zhou L, Ma SL, Yeung PK, Wong YH, Tsim KW, So KF, Lam LC, Chung SK(2016)Anxiety and depression with neurogenesis defects in exchange protein directly activated by camp 2-deficient mice are ameliorated by a selective serotonin reuptake inhibitor, prozac. Transl Psychiatry 6:e881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZZ, Cheng YF, Zou ZQ, Ge BC, Yu H, Huang C, Wang HT, Yang XM, Xu JP(2016)Discovery of N-alkyl catecholamides as selective phosphodiesterase-4 inhibitors with anti-neuroinflammation potential exhibiting antidepressant-like effects at non-emetic doses. ACS Chem Neurosci 8:135–146. [DOI] [PubMed] [Google Scholar]
- Zou ZQ, Chen JJ, Feng HF, Cheng YF, Wang HT, Zhou ZZ, Guo HB, Zheng W, Xu JP(2017)Novel phosphodiesterase 4 inhibitor FCPR03 alleviates lipopolysaccharide-induced neuroinflammation by regulation of the camp/PKA/CREB signaling pathway and NF-κb inhibition. J Pharmacol Exp Ther 362:67–77. [DOI] [PubMed] [Google Scholar]