Abstract
Introduction
Age-related macular degeneration (AMD) is a leading cause of blindness. After smoking, nutrition is the key modifiable factor in reducing AMD incidence and progression, and no other preventative treatments are currently available. At present, there is an evidence–practice gap of dietary recommendations made by eye care practitioners and those actually practised by patients with AMD. To address this gap, a telephone-delivered dietary intervention tailored to patients with AMD will be piloted. The study aims to improve dietary intake and behaviours in patients with AMD. This type of nutrition-focused healthcare is currently not considered in the long-term management of AMD and represents the first empirical evaluation of a telephone-supported application encouraging adherence to dietary recommendations for AMD.
Methods and analysis
140 participants with AMD will be recruited for this randomised controlled trial. Those lacking English fluency; unwilling to engage in the intervention or provide informed consent were excluded. Following the completion of the baseline questionnaire, participants will be randomised into one of two arms: intervention or wait-list control (70 each in the intervention and control groups). Intervention participants will receive a detailed mail-delivered workbook containing information on healthy eating behaviours that promote optimal macular health, as well as scheduled phone calls over 4 months from an accredited practising dietitian. Descriptive statistics and multivariate stepwise linear regressions analyses will be used to summarise and determine the changes in dietary intakes, respectively. Economic analysis will be conducted to determine intervention feasibility and possibility of a large-scale rollout.
Ethics and dissemination
The study was approved by the University of Sydney Human Research Ethics Committee (HREC) (Reference: HREC 2018/219). Study findings will be disseminated via presentations at national/international conferences and peer-reviewed journal articles.
Trial registration number
ACTRN12618000527268; Pre-results.
Keywords: age-related macular degeneration, nutrition, telephone coaching, behaviour change, eye disease
Strengths and limitations of this study.
First empirical evaluation of a telephone-supported application encouraging adherence to dietary recommendations for age-related macular degeneration (AMD).
Randomised controlled trial with a wait-list control group.
Translational research—applying theoretical knowledge about AMD and diet links to practice.
Recruitment of the majority of participants limited to the location of participating clinics, that is, Southern, South-Western and Western Sydney. Therefore, data may not be representative of the Australian AMD population.
Unable to blind participants and project staff (ie, study coordinator).
Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness in Australia, accounting for 50% of cases among persons aged over 50 years.1 Antivascular endothelial growth factor treatments that can reduce visual loss in those with the neovascular (wet) form of late AMD comes at a high cost, that is, currently constitutes one of the top three Pharmaceutical Benefits Scheme expenditures,2 while the other form of late AMD (dry atrophic) is irreversible and untreatable.3 Loss of central vision in AMD means that everyday activities such as reading and driving cannot be performed.4 The resulting disability involves enormous personal costs and is a substantial burden on healthcare resources.3
Dietary modifications represent one of the only means of preventing the development of AMD as well as slowing its progression.4 5 Current evidence indicates that all patients with AMD, regardless of their disease severity, should be given dietary advice to increase consumption of dark green leafy vegetables, to consume low-glycaemic index (GI) diets and to consume fish at least twice a week.5–10 Independent inverse associations between these dietary factors and risk of AMD development and progression have been observed. For example, the Coimbra Eye Study and Korea National Health and Nutrition Examination Survey found that higher intakes of vegetables, fruit and nuts related to a statistically significant reduction in the risk of AMD development.11 12 This relationship is likely due to fruits and vegetables, particularly the dark green leafy variety, being good sources of carotenoids (eg, lutein and zeaxanthin), and nuts having a high fatty acid content.5 Fish is also a good source of omega-3 fatty acids, and similarly, a reduced risk of AMD development was observed in the Age-Related Eye Disease Study (AREDS) and Nurses Health Study and Health Professionals Follow Uup Study when at least one serving of fish was consumed.13 14 The Blue Mountains Eye Study provided compelling data demonstrating that carriers of certain gene polymorphisms that increases AMD risk by twofold to fourfold, substantially reduced their risk to close to their non-carrier counterparts by regular consumption of fish.15 Dietary intake of wholegrains also shows a beneficial effect against risk of AMD in studies such as The Blue Mountains Eye Study and AREDS. Both studies found that reducing usual dietary GI by approximately 8–10 units was protective against AMD development.5 Moreover, the Melbourne Collaborative Cohort Study identified that higher intakes of wholegrains and fish was associated with a lower risk of late AMD, with 51% reduced odds in participants in upper quartile for intake versus the lowest quartile.16 Another notable outcome from AREDS highlighted that strict adherence to a Mediterranean diet, which promotes an abundance of the above recommended dietary factors, showed a reduced risk of progression to late-stage AMD by 26%.17 A similar inverse relationship between adherence to the Mediterranean diet and advanced AMD was also observed in the European Eye Study.18
Specific nutrients have been identified to have a protective effect on AMD. Landmark population studies—The Rotterdam Study, Blue Mountains Eye Study and Beaver Dam Eye Study—support the inverse relationship between dietary zinc and risk of early and/or late AMD.5 Furthermore, the AREDS showed that taking a supplement containing high doses of zinc, with vitamin C, vitamin E, beta carotene and copper can reduce progression to advanced AMD by 25%.6 7 A follow-up of this study (AREDS 2) found that adding lutein and zeaxanthin (naturally occurring carotenoids) or omega-3 fatty acids to the original AREDS formulation (with beta carotene) had no overall effect on the risk of late AMD. However, the trial found that replacing beta carotene with a 5-to-1 mixture of lutein and zeaxanthin may help further reduce the risk of late AMD.8 9 Based on this literature, patients most susceptible to AMD progression (ie, have moderate AMD or late AMD in one eye) are encouraged to take AREDS-based supplements. Preventative benefits of the supplements or use in mild AMD has not been adequately investigated and therefore not recommended in routine practice.5
Preventive measures through dietary modulation are attractive strategies, because these are more affordable than clinical or drug therapies, do not require specialists for administration and there is strong research evidence showing the benefits of micro- and macro-nutrients with respect to AMD with few, if any, adverse effects.5 Despite this, current research knowledge has identified an evidence–practice gap of dietary recommendations made by eye care practitioners and the recommendations actually practised by patients with AMD.10–19 This is largely due to a lack of clear-cut guidelines for patients and/or practitioners to follow; patients not having sufficient information and/or misconceptions regarding diet and AMD; inadequate explanation and reinforcement by eye care practitioners; and lack of dietitian referral and support.10–20 Hence, it is essential to design effective measures for imparting and disseminating appropriate dietary and supplementation advice for patients with AMD.21 Furthermore, translation efforts in AMD have focused on screening and drug or laser treatments.5 22 Currently, there are no evidence-based interventions designed specifically to improve the dietary behaviours of patients with AMD that are being routinely implemented in clinical practice.5
Telephone-delivered interventions involving regular coaching to support behavioural changes can help overcome some of the patient-centred barriers, address existing gaps in practice and facilitate adoption of complex dietary recommendations.23 This type of nutrition-focused healthcare is currently not considered in the long-term management of AMD.22 Therefore, this study will involve implementing and evaluating this service for patients with AMD. The project aim is to appreciably increase intakes of vegetables, fruit, fish and low GI (rather than high GI) foods and to improve adherence to AREDS-type supplements (where appropriate) among patients with AMD. It will have a two-pronged approach: (1) develop and distribute a resource with evidence-based information on diet and AMD to the patient; and (2) have an accredited practising dietitian provide telephone coaching and support to facilitate and enhance the patients’ adoption of AMD-specific dietary recommendations. An economic assessment to measure the cost-effectiveness of the intervention will also be undertaken.
The primary hypothesis asserts that the intervention will result in a 0.5 serves per day improvement in intakes of vegetables, as well as increases in fruits and fish, and better adherence to AREDS-type supplement usage 3 months postintervention. High participant adherence to dietary counselling sessions (≥70%) and participant satisfaction with the intervention are also expected (~80%).
Methods and analysis
Recruitment
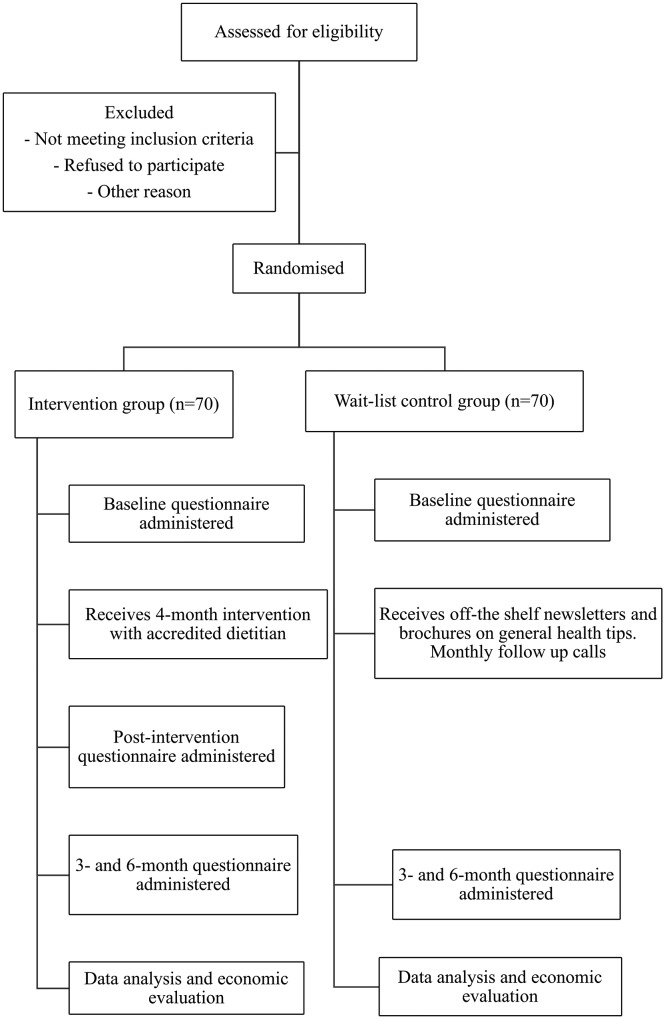
This two-arm randomised controlled trial includes an intervention group and a wait-list control group (figure 1). One hundred and forty study participants will be primarily identified from three eye clinics (Sydney West Retina, Liverpool Retina Associates and Hurstville Retina Consultants). Advertisements through consenting eye clinics will also help to attract participants. Participants involved in other studies run by the centre who have consented to be contacted about other research involvement will also be approached. Patients with any form of AMD will be invited to participate in the study. Exclusion criteria are: (A) lack of sufficient English fluency; (B) unwilling to actively engage in the telephone-delivered coaching sessions over the 4 months; and (C) inability to give informed consent. Participant interests in the study will be screened by the study coordinator who will provide an explanation of the study, participant information sheet and consent form to those eligible to consider their involvement. These documents will outline the purpose and requirements of the study as well as address participant confidentiality including the deidentification and secure storage of data. Eligible patients at participating eye clinics will be approached by the study coordinator postconsultation with their respective ophthalmologists. Interested participants will return the completed consent form prior to baseline questionnaires being administered. Completed questionnaires can be returned to the study coordinator at the clinic or via a reply-paid envelope.
Figure 1.
The flow chart summarises the randomised controlled trial. It illustrates the various stages of the study particularly recruitment, randomisation, intervention, follow-up and economic analysis.
Randomisation
All participants will be randomised into one of two arms: telephone-delivered dietary behaviour intervention or wait-list control (figure 1). The randomisation sequence will be generated centrally using permuted blocks of mixed size to ensure equal numbers while maintaining an unpredictable sequence. Assignments to the intervention or control group will be managed centrally so treating and recruiting staff will not be involved in the process. Treating staff will also be blinded to participant allocations.
Intervention group
Participants will be mailed a detailed workbook that contains information on healthy eating behaviour, which promotes optimal macular health and provides information on goal setting and action plans (table 1). Scheduled phone calls with an accredited practising dietitian will also occur across a 4-month period. The dietitian will evaluate the frequency of calls based on individual needs, with all participants contacted at least monthly. It is expected that a higher frequency of calls (if needed) will occur in the first month to help clarify the information in the dietary resource as well as any other associated misconceptions.
Table 1.
Summary of workbook contents
| Contents | |
| Introduction | Brief explanation of AMD including a definition of the condition, prevalence and risk factors. |
| Role of AMD in diet | Summary of the diet and AMD links, including key dietary messages. |
| Australian dietary guidelines | Outline of the Australian dietary guidelines including the five core food groups and recommended serves. |
| Important foods for AMD | Visual examples of the dietary messages and elaboration on the links to macula health. Information of AMD supplements also included such as the AREDS2 formula. |
| Action plan | Action plan templates included for participants to write down their SMART dietary goals to be achieved through the dietary intervention and in the long term. |
AMD, age-related macular degeneration; AREDS2, Age-Related Eye Disease Study 2; SMART, Specific, Measurable, Attainable, Realistic and Timely.
The dietitian will be trained in motivational interviewing and the constructs of social-cognitive theory that underpins the intervention. This is important for successful dietary counselling and long-term behaviour change as the dietitian will be able to explore the dynamic relationship between the individual (participant), their environment and their behaviour.24 The coaching sessions will be evidence based and involve an interactive approach with the patient that helps identify impediments to behaviour change25 (eg, lack of knowledge and limited cooking skills), as well as methods of teaching and modelling behaviour that empower the patient to achieve and maintain improved dietary behaviours.
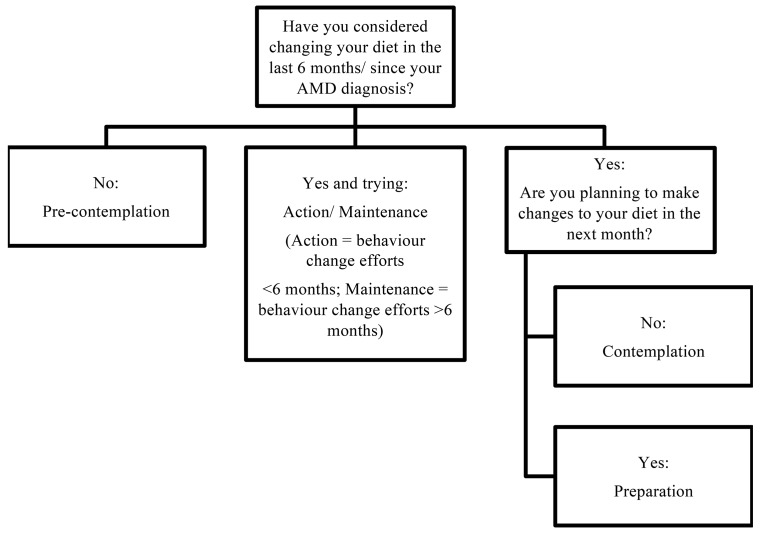
Intervention calls with follow the 4 A’s approach26: (1) assessment (and feedback): evaluation of participant stage of change as well as the adequacy of their diet. Follow-up calls will reassess these areas accordingly to capture any dietary behaviour changes. Stage of change is an important component of the dietary intervention and will help guide the focus of the phone calls (eg, looking at diet-disease relationship, environmental factors or self-confidence). It is categorised as: precontemplation, contemplation, preparation, or action and maintenance (figure 2).27 28 (2) Advice on optimal dietary behaviours: this will be tailored education according to the outcome of the assessment, for example, education on the diet–disease relationship in the precontemplative stage versus education on food preparation/recipes in the action phase. Each monthly call will focus on one recommendation (ie, vegetables, fruit, fish and nuts, or low GI), ordered from highest to lowest priority based on the participant’s needs. (3) Assistance with collaborative goal setting and developing a personalised plan for modifying dietary behaviours: goal setting and empowerment will be important features of the telephone coaching.25 The SMART principle—Specific, Measurable, Attainable, Realistic and Timely—is an effective format for goal setting as it increases motivation through short-term achievements that build towards reaching a long-term goal.29 Participants will choose, in collaboration with the dietitian which dietary goals will be addressed and in what order. These will be adapted, changed or new ones added over the course of the intervention. All participants will be encouraged to work on all goals. The dietary goals will be consistent with the Australian dietary guidelines30 and with the evidence-base on nutrition and AMD links.5 8 9 (4) Arranging of follow-up support in the form of subsequent telephone contacts for the duration of the intervention.
Figure 2.
This figure illustrates the process to determine participant stage of change, that is, precontemplation, contemplation, preparation or action/maintenance. Determining stage of change will contribute towards the intervention delivered to participant’s during their telephone consultations with the dietitian. AMD, age-related macular degeneration.
Participants with moderate or advanced AMD will be advised by the dietitian to continue using AREDS-type supplements, but current or ex-smokers will be advised to use formulations free of beta carotene (table 2). This is due to data showing that beta carotene increases lung cancer risk in smokers.5 Given the lack of side effects associated with lutein/zeaxanthin in most trials, participants will be advised to consume lutein/zeaxanthin containing AREDS-type supplements if they are unsure of their dietary lutein intake or if the dietitian assesses that they are likely to be consuming low levels of dietary lutein. Participants with normal or high dietary lutein intake may require only modified, beta carotene-free AREDS formulation. The results of Age-Related Eye Disease Study 2 (AREDS2) indicate that increased dietary fruit and vegetable intake may provide the additional benefit of adequate lutein/zeaxanthin intake without the need for beta carotene supplementation, thus avoiding the additional risks that beta carotene appears to carry for smokers.5
Table 2.
Dietary recommendations or goals for patients with AMD5 8 9 30
| AMD category | Dietary goals |
| Mild AMD | Consume a diet high in dark green leafy vegetables and raw vegetables (total 5+ serves/day) and fruits (2+ serves/ day). Low-GI foods (eg, basmati rice and rolled oats) to replace high-GI foods. Increase fish/seafood intake (2+ serves/week). Consume a diet with highly bioavailable sources of zinc. Regular inclusion of nuts (eg, pistachio and walnuts) in diet (3+ times/week). |
| Moderate AMD | Use AREDS-based supplements containing lutein/zeaxanthin or are beta carotene free. Dietary advice as per mild AMD. |
| Advanced AMD | Use AREDS-based supplements containing lutein/zeaxanthin or are beta carotene free. Dietary advice as per mild AMD. |
AMD, age-related macular degeneration; AREDS, Age-Related Eye Disease Study.
Control group
Participants will receive a one-page letter to thank them for their continued involvement in the study. They will also receive newsletters with general health tips and relevant off-the-shelf brochures (ie, ‘Nutrition and Supplements’ brochure from the Macular Disease Foundation Australia, and Eat for Health Australian dietary guidelines for adults). Support staff will conduct monthly calls during the intervention to help clarify any questions they have regarding the general dietary information they have received, discuss any general AMD queries and remind participants of the next stages of the study, that is, 3-month and 6-month postintervention follow-up questionnaires. Call duration will be kept to a minimum to reduce the potential of lengthy conversations regarding diet and AMD, which may prompt the participant to make dietary changes. Participants will also be offered the opportunity to receive the dietary counselling at the conclusion of the study after 24 months.
Measurement of covariates
A baseline questionnaire will be administered to all participants. It will cover topics including: demographics, health and lifestyle. The health questions will look at medical and surgical history, hearing, eye health and visual function, while the lifestyle questions address smoking, exercise, diet and basic anthropometry (ie, weight, height and waist circumference). The long-term ‘usual’ dietary information will be captured using a validated 145-item food frequency questionnaire (FFQ),27 while short-term dietary data will be assessed using a 14-question dietary behaviour questionnaire (DBQ) [Radd-Vagenas S, Daniel K, Noble Y, Fiatarone Singh MA, Flood VM. 2018].28
Specifically, the FFQ will help evaluate the average intake over the last 12 months of the five core food groups: dairy, fruits, vegetables, meat, fish and eggs, and breads, cereals and starches, as well as beverages, discretionary foods and supplements. Common food items and their serve sizes are listed under each core food group. Participants select the corresponding frequency that they consume the food on average. Frequency options include: ‘Never’; ‘Less than 1 per month’; ‘1–3 per month’; ‘1 per week’; ‘2–4 per week’; ‘5–6 per week’; ‘1 per day’; ‘2–3 per day’; and ‘4+ per day’. Further questions about fats and oils are also included covering types of fats and oils used (eg, margarine, butter and olive oil), how they are used (eg, on bread or vegetables) and whether fried foods and visible fat on meat is consumed.
However, the DBQ will cover intake in the last week of similar food groups to the FFQ, as well as diet behaviour and self-efficacy questions. Questions are respectively designed in free-response and 5-point Likert scale format. The DBQ aims to provide a comparison of recent dietary intake versus usual long-term intake to be indicated by FFQ responses. This questionnaire will also assess participant confidence in achieving key AMD-linked nutrition goals such as ‘Eating 5 serves of vegetables a day’ and behaviours related to the consumption of nutrient-poor, discretionary food. Baseline understanding of participants’ confidence will contribute to the dietitian’s initial assessment during the intervention of participant stage of change, while the understanding of participant behaviour will assist with the development of strategies to achieve nutrition goals.
For the intervention group, follow-up time-points will be: (1) immediately after the intervention (4 months from baseline); (2) 3 months postintervention (7 months from baseline); and (3) 6 months postintervention (10 months from baseline). Follow-up with the control group will occur: (1) 3 months postintervention (7 months from baseline) and (2) 6 months postintervention (10 months from baseline). The DBQ will be administered immediately after the intervention and 3-month and 6-month postintervention to all participants to capture any recent changes in dietary behaviour. These data will be a useful indicator of the intervention’s effectiveness in terms of short-term dietary improvements and self-efficacy. At 6-month postintervention, the FFQ will be readministered to more accurately identify any changes in dietary intakes since baseline.
Outcome measurement
Questionnaires and data collected throughout the intervention will be used to assess the effectiveness of the intervention. The primary outcome is a 0.5 serves per day change in total vegetable intake. This measure of change is informed by expected dietary improvement asserted by recent data published from the Rotterdam Study reporting that a minimum 200 g of vegetables (2.6 serves) was linked to a reduced risk of AMD and data from an Australian population-based intervention that observed an increase in vegetable intake by roughly 0.5 serves per day from 2.6 serves to 3.2 serves.31 32 Appreciable improvements in preintervention versus postintervention intake levels of dark, green leafy vegetables (spinach, kale and so on) and raw fruit, low GI foods, fish and nuts, as well as appropriate use of AREDS-type supplements are the secondary outcomes.
AMD progression will be monitored through the collection of optical coherence tomography (eg, central macula thickness, presence of fluid and pigment epithelial detachment), and additional information on visual acuity and number of injections received will be documented. All patients recruited from participating eye clinics will have this information collected at baseline and at the final follow-up (6-month postintervention). These data will be used in future investigations to evaluate the clinical outcomes of recommended dietary practices. A reduced risk in AMD progression might not be observed in the short term, that is, at 6-month follow-up. However, reductions in the risk of other comorbidities such as obesity and diabetes could be observed.
Acceptability and feasibility
Practical implications of the intervention will be evaluated via systematically tracking all participant contacts. This includes reporting on: the number of call attempts, completed calls (‘dose’ of intervention received), number of calls completed at the scheduled time (vs via call back), reasons for missed calls and call duration. The call content will be tracked via checklists completed after each call allowing for reporting on the extent to which the intervention content is delivered per protocol and the percentage of participants setting goals for dietary behaviours. Treatment acceptability in the intervention arm will be assessed by questions via a postintervention survey: (1) ‘Overall, how satisfied were you with the program?’, (2) ‘How satisfied were you with the educational content?’, (3) ‘Would you feel confident in recommending this treatment to a friend?’ and (4) ‘Was it worth your time doing the program?’ Participants will respond to the first two questions using a 5-point Likert scale, ranging from ‘Very Satisfied’ to ‘Very Dissatisfied’ and the second two questions with a simple ‘yes/no’ response.
Economic evaluation
A ‘within-trial’ cost-effectiveness analysis will be performed whereby outcomes will be measured as clinically relevant improvements as per the FFQ and DBQ. The perspective taken will be that of the health provider. The cost of resources required to administer the intervention will include staff, training, telephone consults, production and delivery of the dietary messages and consumables such as stationary. Valuation of these variables will be in Australian dollars at 2018 market rates. Further economic analysis such as direct healthcare costs through Medical Benefits Scheme (MBS) and Pharmaceutical Benefits Scheme (PBS) linkage have not been conducted due to time constraints.
Sample size considerations
A total of 140 participants (70 each in the intervention and control group) will be recruited over 4–5 months. This sample size was considered sufficient to achieve a minimum 0.5 serves per day change in vegetable intake (primary outcome) with 80% power as significant at the 5% level and allowing for 10% drop-out rate.33 34
Data collection and analysis plan
Data for all participants will be collected via baseline and follow-up questionnaires. Participants will be prompted to complete and return questionnaires by study staff to promote participant retention. Intervention participants will also have additional data collected by the dietitian throughout the telephone counselling sessions. All data will be double-entered into Research Electronic Data Capture—a secure data management platform. Only staff directly involved in the study will have access to the databases.
Intention-to-treat analysis will be used to include all randomised participants and determine the key outcome measures. Missing data from participants and those lost to follow-up will also be included in the analysis. To assess the effectiveness of the intervention, the analysis will primarily focus on pre-FFQ and post-FFQ data to evaluate participant adoption of the dietary recommendations into their ‘usual’ intake. Specifically, the analysis will involve matching FFQ items to food items from the most recent national nutrient database (NUTTAB 2010) with portion size estimates based on ‘natural’ serve sizes, or age and gender specific data from national nutrition surveys. Weighting for these items has been derived from supermarket literature data and information about availability from the Sydney Markets. The US Department of Agriculture Carotenoid Food Composition database will be used to estimate intakes of α-carotene, β-cryptoxanthin, combined lutein/zeaxanthin and lycopene. Information about fatty acid composition of foods will be obtained from food composition data (NUTTAB 2010). Total omega-3 fatty acid and long-chain omega-3 fatty acid consumption will be assessed. The vitamin and mineral supplement section of the FFQ included questions about brand name, frequency, strength and duration of use for multivitamin preparations and individual supplements (eg, vitamins A, C, E, beta carotene and zinc). Supplement intake will then be converted into μg/day or mg/day. Overall GI for the diet will be calculated by summing the weighted GI of individual foods in the diet and performing weighting proportional to the contribution of individual foods to total carbohydrate intake.
Similar nutrient analyses will also be conducted on applicable DBQ data that capture frequency and serve sizes. Descriptive statistics will report on other data to reflect short-term trends in dietary intake and behaviours at baseline and subsequent follow ups.
Safety measures and end-point
Accredited practising dietitians (DT and VF) have experience in conducting nutritional assessments and providing appropriate nutrition advice and counselling. Rated a low risk study, adverse events are unlikely to occur. However, safety end-points will be indicated where participants report or present with persistent or severe reactions to any dietary modifications resulting in hospitalisation or a prolonged recovery period. In this case, participants will be unblinded to their treating ophthalmologist, and appropriate documentation will be completed. For intervention participants, the dietitian will engage in regular contact for 4 months, and support staff will touch-base at 3-month and 6-month postintervention to monitor whether a safety end-point measure is appropriate.
Patient and public involvement
This study did not have patient or public involvement in the study design. Patients will not be involved in the recruitment or conduct of the study. Overall results will be disseminated to consenting participants at the end of the study.
Ethics and dissemination
The University of Sydney Human Research Ethics Committee has approved this study and assigned a low-risk rating. Results of this study will be disseminated through publications in quality peer-reviewed research journals and through presentations at national and/or international conferences. Any amendments to the protocol will be communicated to the ethics committee and updated on the Australian New Zealand Clinical Trials Registry.
Supplementary Material
Footnotes
Contributors: Author responsibilities were as follows: study concept and design - BG, VF, PM, GL; data collection - DT; and drafting of manuscript - DT, BG, VF, AK, AH, GL, PM. The final version has been approved by all authors.
Funding: This work is supported by National Health and Medical Research Council (NHMRC) grant number APP1150101.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The University of Sydney Human Research Ethics Committee (HREC) has approved this study (reference: HREC 2018/2019).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Taylor HR, Keeffe JE, Vu HT, et al. Vision loss in Australia. Med J Aust 2005;182:565–8. [DOI] [PubMed] [Google Scholar]
- 2.PBS Information Management Section Pharmaceutical Policy Branch. Do H, PBS Expenditure and prescriptions twelve months to 30 June 2013. Canberra, Australia, 2014. [Google Scholar]
- 3. Economics DA, Mitchell P. Eyes on the future. A clear outlook on Age-related Macular Degeneration: Deloitte Access Economics Pty Ltd, 2011. [Google Scholar]
- 4. Lim LS, Mitchell P, Seddon JM, et al. Age-related macular degeneration. Lancet 2012;379:1728–38. 10.1016/S0140-6736(12)60282-7 [DOI] [PubMed] [Google Scholar]
- 5. Broadhead GK, Grigg JR, Chang AA, et al. Dietary modification and supplementation for the treatment of age-related macular degeneration. Nutr Rev 2015;73:448–62. 10.1093/nutrit/nuv005 [DOI] [PubMed] [Google Scholar]
- 6.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 2001;119:1417–36. 10.1001/archopht.119.10.1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis MD, Gangnon RE, Lee LY, et al. Age-Related Eye Disease Study Group. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol 2005;123:1484–98. 10.1001/archopht.123.11.1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chew EY, Clemons TE, Sangiovanni JP, et al. Age-Related Eye Disease Study 2 (AREDS2) Research Group. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol 2014;132:142–9. 10.1001/jamaophthalmol.2013.7376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013;309:2005–15. 10.1001/jama.2013.4997 [DOI] [PubMed] [Google Scholar]
- 10. Shah SU, Pilli S, Telander DG, et al. Survey of patients with age-related macular degeneration: knowledge and adherence to recommendations. Can J Ophthalmol 2013;48:204–9. 10.1016/j.jcjo.2013.01.013 [DOI] [PubMed] [Google Scholar]
- 11. Nunes S, Alves D, Barreto P, et al. Adherence to a Mediterranean diet and its association with age-related macular degeneration. The Coimbra Eye Study-Report 4. Nutrition 2018;51-52:6–12. 10.1016/j.nut.2017.12.010 [DOI] [PubMed] [Google Scholar]
- 12. Kim EK, Kim H, Kwon O, et al. Associations between fruits, vegetables, vitamin A, β-carotene and flavonol dietary intake, and age-related macular degeneration in elderly women in Korea: the Fifth Korea National Health and Nutrition Examination Survey. Eur J Clin Nutr 2018;72:161–7. 10.1038/ejcn.2017.152 [DOI] [PubMed] [Google Scholar]
- 13. Cho E, Hung S, Willett WC, et al. Prospective study of dietary fat and the risk of age-related macular degeneration. Am J Clin Nutr 2001;73:209–18. 10.1093/ajcn/73.2.209 [DOI] [PubMed] [Google Scholar]
- 14. SanGiovanni JP, Chew EY, Clemons TE, et al. Age-Related Eye Disease Study Research Group. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No. 20. Arch Ophthalmol 2007;125:671–9. 10.1001/archopht.125.5.671 [DOI] [PubMed] [Google Scholar]
- 15. Wang JJ, Rochtchina E, Smith W, et al. Combined effects of complement factor H genotypes, fish consumption, and inflammatory markers on long-term risk for age-related macular degeneration in a cohort. Am J Epidemiol 2009;169:633–41. 10.1093/aje/kwn358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amirul Islam FM, Chong EW, Hodge AM, et al. Dietary patterns and their associations with age-related macular degeneration: the Melbourne collaborative cohort study. Ophthalmology 2014;121:1428–34. 10.1016/j.ophtha.2014.01.002 [DOI] [PubMed] [Google Scholar]
- 17. Merle BM, Silver RE, Rosner B, et al. Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: a prospective cohort study. Am J Clin Nutr 2015;102:1196–206. 10.3945/ajcn.115.111047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hogg RE, Woodside JV, McGrath A, et al. Mediterranean diet score and its association with age-related macular degeneration: the European Eye Study. Ophthalmology 2017;124:82–9. 10.1016/j.ophtha.2016.09.019 [DOI] [PubMed] [Google Scholar]
- 19. Gopinath B, Flood VM, Kifley A, et al. Smoking, antioxidant supplementation and dietary intakes among older adults with age-related macular degeneration over 10 years. PLoS One 2015;10:e0122548 10.1371/journal.pone.0122548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ng WT, Goggin M. Awareness of and compliance with recommended dietary supplement among age-related macular degeneration patients. Clin Exp Ophthalmol 2006;34:9–14. 10.1111/j.1442-9071.2006.01141.x [DOI] [PubMed] [Google Scholar]
- 21. Stevens R, Bartlett H, Walsh R, et al. Age-related macular degeneration patients’ awareness of nutritional factors. British Journal of Visual Impairment 2014;32:77–93. 10.1177/0264619613519341 [DOI] [Google Scholar]
- 22. Downie LE, Keller PR. The self-reported clinical practice behaviors of Australian optometrists as related to smoking, diet and nutritional supplementation. PLoS One 2015;10:e0124533 10.1371/journal.pone.0124533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kelly JT, Reidlinger DP, Hoffmann TC, et al. Telehealth methods to deliver dietary interventions in adults with chronic disease: a systematic review and meta-analysis. Am J Clin Nutr 2016;104:1693–702. 10.3945/ajcn.116.136333 [DOI] [PubMed] [Google Scholar]
- 24. Rankin A, Kuznesof S, Frewer LJ, et al. Public perceptions of personalised nutrition through the lens of Social Cognitive Theory. J Health Psychol 2017;22:1233–42. 10.1177/1359105315624750 [DOI] [PubMed] [Google Scholar]
- 25. Dennis SM, Harris M, Lloyd J, et al. Do people with existing chronic conditions benefit from telephone coaching? A rapid review. Aust Health Rev 2013;37:381–8. 10.1071/AH13005 [DOI] [PubMed] [Google Scholar]
- 26. Goldstein MG, Whitlock EP, DePue J. Planning Committee of the Addressing Multiple Behavioral Risk Factors in Primary Care Project. Multiple behavioral risk factor interventions in primary care. Summary of research evidence. Am J Prev Med 2004;27(2 Suppl):61–79. 10.1016/j.amepre.2004.04.023 [DOI] [PubMed] [Google Scholar]
- 27. Campbell MK, DeVellis BM, Strecher VJ, et al. Improving dietary behavior: the effectiveness of tailored messages in primary care settings. Am J Public Health 1994;84:783–7. 10.2105/AJPH.84.5.783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee JE, Lee DE, Kim K, et al. Development of tailored nutrition information messages based on the transtheoretical model for smartphone application of an obesity prevention and management program for elementary-school students. Nutr Res Pract 2017;11:247–56. 10.4162/nrp.2017.11.3.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Round R, Marshall B, Horton K. Planning for effective health promotion evaluation: Victorian Government Department of Human Services, 2005. [Google Scholar]
- 30.National Health and Medical Research Council. Dietary Guidelines for Australian Adults. Canberra: Commonwealth of Australia, 2003. [Google Scholar]
- 31. de Koning-Backus APM, Buitendijk GHS, Kiefte-de Jong JC, et al. Intake of vegetables, fruit, and fish is beneficial for Age-related Macular Degeneration. Am J Ophthalmol 2018:30578–6. 10.1016/j.ajo.2018.09.036 [DOI] [PubMed] [Google Scholar]
- 32. Pollard CM, Miller MR, Daly AM, et al. Increasing fruit and vegetable consumption: success of the Western Australian Go for 2&5 campaign. Public Health Nutr 2008;11:314–20. 10.1017/S1368980007000523 [DOI] [PubMed] [Google Scholar]
- 33.Sealed Envelope Ltd. Power calculator for continuous outcome superiority trial, 2012. [Google Scholar]
- 34. Altman DG. Practical statistics for medical research: Taylor & Francis Ltd, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.