Abstract
Construction workers have the highest smoking rate among all occupations (39%). Hispanic/Latino workers constitute a large and increasing group in the US construction industry (over 2.6 million; 23% of all workers). These minority workers have lower cessation rates compared to other groups due to their limited access to cessation services, and lack of smoking cessation interventions adapted to their culture and work/life circumstances. Formative research was conducted to create an intervention targeting Hispanic/Latino construction workers. This paper describes the intervention development and the design, methods, and data analysis plans for an ongoing cluster pilot two-arm randomized controlled trial comparing an Enhanced Care worksite cessation program to Standard Care. Fourteen construction sites will be randomized to either Enhanced Care or Standard Care and 126 participants (63/arm) will be recruited. In both arms, recruitment and intervention delivery occur around “food trucks” that regularly visit the construction sites. Participants at Enhanced Care sites will receive the developed intervention consisting of a single face-to-face group counseling session, 2 phone calls, and a fax referral to Florida tobacco quitline (QL). Participants at Standard Care sites will receive a fax referral to the QL. Both groups will receive eight weeks of nicotine replacement treatment and two follow-up assessments at three and six months. Feasibility outcomes are estimated recruitment yield, barriers to delivering the intervention onsite, and rates of adherence/compliance to the intervention, follow-ups, and QL enrollment. Efficacy outcomes are point-prevalence and prolonged abstinence rates at six month follow-up confirmed by saliva cotinine < 15ng/ml.
Keywords: tailored, worksite, smoking cessation intervention, formative research, cluster randomized clinical trial, feasibly
1. Introduction
Cigarette smoking causes more death and disability among American workers than their workplace environment (e.g., injuries).1 Smoking prevalence among US construction workers (39%) is twice the national average.2 Construction workers are frequently exposed to a wide range of workplace hazards, including exposure to toxins (e.g., carbon monoxide, air pollutants, fibers), many of which interact with smoking to increase workers’ risk for lung cancer and chronic lung disease.3–6 Construction trades remain overwhelmingly male dominated (2.3% women).7,8 Therefore, male construction workers are a high-risk group for smoking-related health problems and should be a prime focus for smoking cessation efforts.
Hispanics/Latinos are one of the fastest growing and largest minority groups in the US.9 Further, the number of Hispanic/Latino workers employed in the construction sector in the US has tripled over a 10-year period to reach 2.6 million workers in 2014, representing nearly a third of the construction workforce.10 Despite the fact that Hispanic/Latino construction workers are at high risk for tobacco-related morbidity and mortality, cessation efforts among them are hindered by their limited access to cessation and health promotion services.11,12 A unique approach to reach out to this population with smoking cessation is through the use of the worksite as an intervention setting.13,14 Potential benefits of such settings include attracting workers who are less likely to seek advice,15 and the provision of a convenient service that will not require extra efforts to access (e.g. appointment, travel).13 Another unique approach developed and tested by our team is to partner with the “food truck” for intervention delivery.16–18 Food trucks visit construction sites several times a day and can thus provide an optimal opportunity to deliver health promotion services.11,19 Our pilot study demonstrated the feasibility and success of recruiting around the “food truck” visits as a means to reach this population with health promotion efforts.11
Despite advances in health promotion programs, promising smoking cessation interventions that are tailored to the culture and work nuances of Hispanic/Latino construction workers are still lacking.20–22 To date, only a few smoking cessation intervention studies have targeted construction workers,22,23 and none of these were tailored to Hispanic/Latino workers.12 This project aims to: 1) develop a culturally sensitive smoking cessation intervention for Hispanic/Latino construction workers, built around formative research; 2) test the intervention for feasibility and potential efficacy in a pilot cluster randomized clinical trial (RCT); and 3) conduct a post-intervention evaluation to fine-tune the intervention before wider testing and dissemination.24 The current report will describe the intervention development and the design, methods, and data analysis plans for its testing in an ongoing pilot cluster RCT.
2. Methods/Design
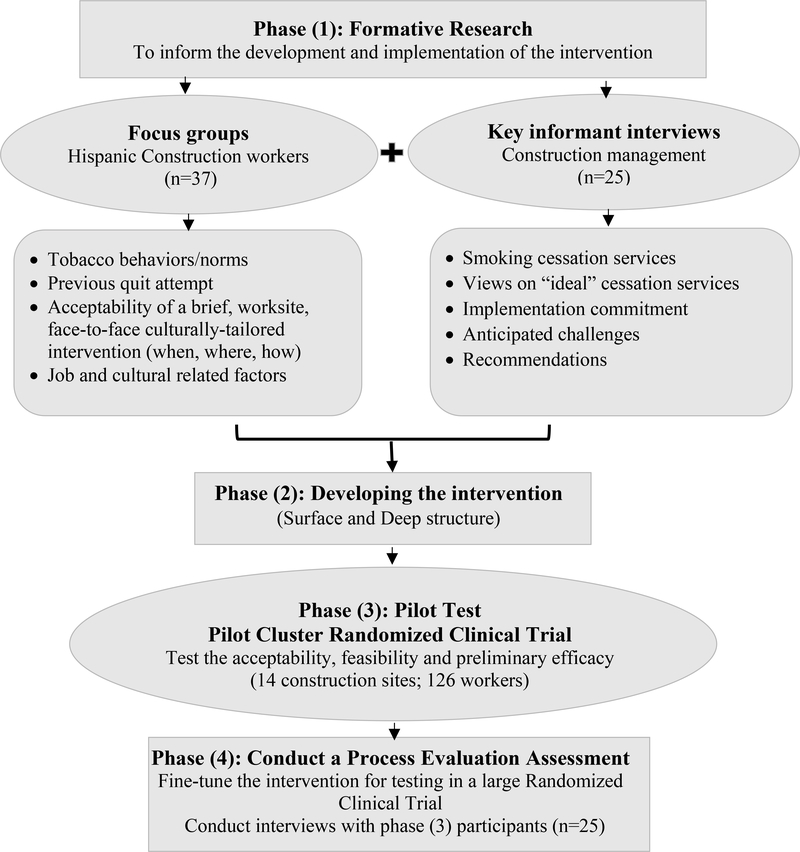
Recently, a novel framework has been recommended to improve cessation services for underserved smokers who are defined as: having a high smoking rate and disproportionate tobacco-related health burden, lacking access to effective treatment; and being understudied in cessation trials.25 This framework stresses the need for innovative cessation strategies among underserved smokers based on adaption of evidence-based treatments to accommodate the cultural, values, attitudes, and behaviors of the target population.25,26 Based on this framework, four phases of cultural adaptation are proposed (Figure 1). Phase (1) involves collecting qualitative data from the target population to help guide changes in the intervention in terms of surface and deep structure. Surface structure refers to matching the intervention content and materials to the social and behavioral characteristics of the target population (e.g., language, location, delivery channel), and deep structure refers to incorporating the core cultural values of the target group to increase saliency of the program impact (e.g., involve family members, get personal, show respect).27 Phase (2) involves the adaptation and modifications of the intervention (content, modality, intensity, delivery). Phase (3) involves pilot testing the culturally adapted intervention for feasibility and initial efficacy. Phase (4) involves measurement of a variety of outcomes in addition to treatment response to fine-tune the intervention for large scale testing. Guided by this framework, this section is divided according to the stages of the study into 4 Phases: Formative Research, Developing the Intervention, Pilot Cluster RCT, and Process Evaluation (Figure 1).
Figure 1.
Study Design
2.1. Phase (1) Formative Research
Our formative work included two studies. The first study was a focus groups conducted among our target population of Hispanic/Latino construction workers who smoke cigarettes, and the second employed key informant interviews conducted with construction management personnel.
2.1.1. Focus Groups
The main objective of this study was to explore the historical, environmental, and social forces that influence our target population and needed to be taken into account during the cultural adaptation process of the smoking cessation intervention. Two primary dimensions of cultural appropriateness of intervention were explored, surface structure and deep structure. Surface structure refers to the tailoring of external intervention components to reflect the characteristics of a target population. We explored five reflections of surface structure: (1) materials (messages designed for counseling), (2) communication channels (how messages are delivered), (3) settings (venues for delivering the intervention), (4) staff (culturally relevant recruiters and educators), and (5) recruitment strategies (methods for recruiting participants). The second dimension, deep structure, reflects a deep understanding of culturally normative practices and beliefs embedded in the target population. We explored three reflections of deep structure: (1) stress due to work environment as triggers for smoking and relapse, (2) ways to increase social support for nonsmoking, and (3) incorporation of cultural contextualization in the intervention such as Familismo (centrality of family) and Simpatia (sympathy).
In 2016, we conducted five semi-structured, 60-minute focus groups with Hispanic/Latino construction workers (n=37) who were ≥ 18 years and reported smoking cigarettes to involve them in the development of an intervention tailored to their cultural, life, and work context.28 Recruitment of participants into the focus groups was done at multiple construction sites in South Florida in partnership with the construction site safety manager and the food truck service. Based on participant language preference, all focus group sessions were conducted in Spanish. Participants were given $25 incentive for their time. The study protocol was approved by the University of Miami Institutional Review Board. Sessions were audio-taped and transcribed to assist in the coding of themes related to smoking cessation. Using a grounded theory approach, data were examined iteratively and data collection continued until saturation is reached, indicating that no new themes are emerging.29
Results indicated that a single face-to-face group cessation intervention format provided at the construction site was more acceptable compared to telephone- or computer- based formats. In terms of the mode of delivery of the intervention, participants were open to using the food truck to deliver the intervention, but stated that the intervention should take place outside workhours (early in the morning, or during the breakfast/lunch break) and with approval and permission from their safety manager. Workers reported tremendous job demands and stress which stimulates smoking. However, workers indicated that their cultural characteristics and social norms are not important for tailoring the intervention (e.g., involving family members in the intervention, getting social support from friends and coworkers, considering their level of acculturation or discrimination).
2.1.2. Key Informant Interviews
Given the important role of the construction site managements in implementing such a workplace focused approach of smoking cessation, we sought to gain insight into their current practices related to smoking cessation services, their views on “ideal” cessation services, implementation commitment, and anticipated challenges. We conducted semi-structured, 45-minute interviews with 25 key personnel at five construction sites in South Florida. Interviews were recorded, transcribed and analyzed thematically using Braun’s six phases approach.30
Participants were all males and represented a range of professions including construction executive, senior/safety manager, and contractor/subcontractor. Results indicated that although employers were concerned about the effects of smoking on productivity loss, safety, and insurance costs, nothing was provided to help smokers quit smoking. The majority considered distributing self-help material with free medications as an appropriate service and recommended providing the service in Spanish during breakfast or lunch breaks. Challenges to integrating services were smokers’ low interest in quitting, time restriction during work hours, and cost. Recommendations to support implementing worksite smoking cessation services were to integrate the intervention in the employment orientation training session, try to mandate the service by local/state government, obtain company approval, and involve key personnel on site such as safety managers/supervisors and subcontractors in providing the service.
2.2. Phase (2) Developing the Intervention
The framework for developing the intervention was based on the social cognitive model facilitators,31,32 our formative data, along with the extant treatment literature for cessation (Table 1).28,33–35
Table 1.
The developed intervention (Enhanced Care group).
| Date | In person Session | Phone Calls | Goal |
|---|---|---|---|
| Day 1 | Recruitment (at worksite) | •Approach potential participant | |
| •Introduce the study and procedures | |||
| •Conduct screening to review eligibility criteria | |||
| •Explain the principle of the consent form | |||
| •Sign the consent form | |||
| •Collect baseline data assessment | |||
| Day 2 | Intervention Session (at worksite) | •Provide the intervention in group session | |
| ➢ Set a goal for smoking reduction in week 1 (up to 30%) | |||
| ➢ Set a quit date | |||
| ➢ Prepare to quit (quitting ritual) | |||
| ➢ Coping with job stress (e.g., deep breathing, reduce caffeine intake, involve the management in the study) | |||
| ➢ Getting social support (co-workers, family) | |||
| ➢ Avoid high risk situations (4 As) | |||
| •Refer participants to the Florida tobacco quitline | |||
| •Distribute 6 weeks of nicotine replacement treatment | |||
| Day 14 | One day before quit date | •Review progress in smoking reduction | |
| •Confirm quit date | |||
| •Review quitting plan | |||
| •Provide support and praise successes | |||
| •Prevent relapse (short term) | |||
| •Complete call 1 Assessment Questionnaire | |||
| Day 30 | Two weeks after quit date | •Review progress | |
| •Provide support and praise successes | |||
| •Prevent relapse (long term) | |||
| •Complete call 2 Assessment Questionnaire | |||
| Day 90 | Three-month follow-up (Assessment 1) | •Conduct the 3-month Assessment Follow up Questionnaire | |
| Day 180 | Six-month follow-up (assessment 2) | •Conduct the 6-month Assessment Follow up Questionnaire | |
| •Meet in person at a mutually agreed-upon location | |||
| Scenario (1): participant quit smoking | •Collect saliva sample to conduct the saliva cotinine test to biochemically confirm smoking abstinence | ||
| Scenario (2): participant did not quit smoking | •Conduct the 6 month Assessment Follow up Questionnaire by phone |
2.2.1. Intervention Format
Based on our qualitative results and evidence suggesting that single-session face-to-face interventions are particularly effective in low income and minority populations that are difficult to reach,36,37 we decided that a worksite-based, single-session, brief (30 min), face-to-face group intervention would be the most appropriate format for our target population. We also believed that this intervention is appropriate for this highly mobile population and has a high potential for dissemination.36,37 However, because we recognize the limitation of providing only a single behavioral session, we decided to add two follow-up phone calls. The first phone call occurs one day before the designated quit date to remind participants about their quit date and to provide more support, and the second call occurs two weeks after the quit date to review progress and prevent relapse. Moreover, to increase the reach and effectiveness of our intervention, we decided to use the fax referral service provided by Florida tobacco Quitline (QL) as a means to connect participants directly with the QL. Tobacco QLs are free to all Floridians, are available in Spanish/English, and allow flexible timing, which makes them suitable for our target population. While QLs have demonstrated efficacy,38 relatively few smokers who are interested in quitting utilize them, particularly those from lower socioeconomic backgrounds.39 An option to facilitate the use of the QL service in Florida is the use of the Fax Referral service (http://tobaccofreeflorida.com/wp-content/uploads/2016/12/TFF_Provider_Referral_Form_ENG.pdf), that allows our study interventionist to complete a form and fax it to the QL. This allows the QL counselor to call smokers to provide the standard QL intervention (up to four phone counseling sessions, plus a free two-week supply of a nicotine replacement treatment [NRT]), and an outcome report to be sent back to the referring party (us) within two weeks (http://tobaccofreeflorida.com/healthcare-providers/). Using the fax referral method will hopefully enhance both quit rates, and participant tracking after referral to the QL. Finally, with the intention of ensuring adherence to the Clinical Practice Guidelines about providing behavioral counseling with medication as a minimum intervention for treating smoking cessation, all participants receive eight weeks of NRT at no cost (6 weeks from us and 2 weeks from the tobacco QL).28
Participants have the ability to receive the intervention in Spanish or English based their preference. The content of the behavioral intervention features three key processes: 1) preparing to quit, 2) the quitting process, and 3) relapse prevention and proper use of NRT.28 Additionally, participants are given a fact sheet of “take home” messages for the most important points of each of the three phases. During the preparation-to-quit discussion, we focus on reducing the number of cigarettes before quit date, stimulus control, and other quit preparation strategies (e.g., disposal of cigarettes before the quit date). We also emphasize the importance of a quit date and discuss when a good quit date would be.28 During the quitting discussion, we emphasize proper use of NRT, reinforce the use of NRT, and discuss what to expect during the first few days of being a nonsmoker and how to cope. Finally, during the relapse prevention discussion, we discuss the 4A’s for preventing relapse (Avoid high temptation situations, Alter those situations you can’t avoid, use Alternatives, and become Active).28 To address job-related stress, participants receive instructions to reduce caffeine intake and practice mindful breathing during their quit attempt.
2.2.2. The intervention delivery methods
Guided by our qualitative data, we are partnering with the construction site management who usually allocates a key contact person (most often the safety manager) to assist us in the study administration. All study activities take place during the breakfast or lunch breaks around the food truck to avoid disturbing the workflow onsite. The research team sets up a table by the lunch truck as a point to meet, recruit, screen, and consent potential participants. To insure privacy and confidentiality, the screening and consenting procedures were done individually (one by one). In addition, the field office of the safety management was made available to our team to provide the intervention in case participants were concerned about their confidentiality. Given the short time available during breaks (20–30 minutes), we divide our procedures into two visits. On the first visit (day 1), we recruit participants, complete the screening, consent those who are eligible, and complete baseline assessment. On day 2, we provide the intervention in a small group and distribute educational materials and NRT to participants.
2.2.3. The Comparison Intervention (Control Group)
Our developed intervention will be compared with the “Standard Care” intervention which consists of fax referral to the Florida QL and provision of eight weeks of free NRT (six weeks provided by the study and two weeks provided by the QL). This “Standard Care” intervention represents the most intensive best practice intervention that our target population is likely to receive in the absence of our “Food Truck” developed intervention. Participants in this treatment group will be informed that the QL is free and that the QL counselor will work with them to develop a specific plan to quit smoking and will arrange the delivery of two free weeks of NRT for their quit attempt. It is important to note that making NRT available to participants in the comparison group will allow matching NRT in the two treatment groups, and testing whether the addition of a brief adapted behavioral intervention, delivered in a convenient setting, improves quit rates over QL referral and provision of NRT.
2.3. Phase (3) Testing the Intervention for Feasibility and Potential Efficacy in a Pilot Two-arm, Cluster Randomized Controlled Trial
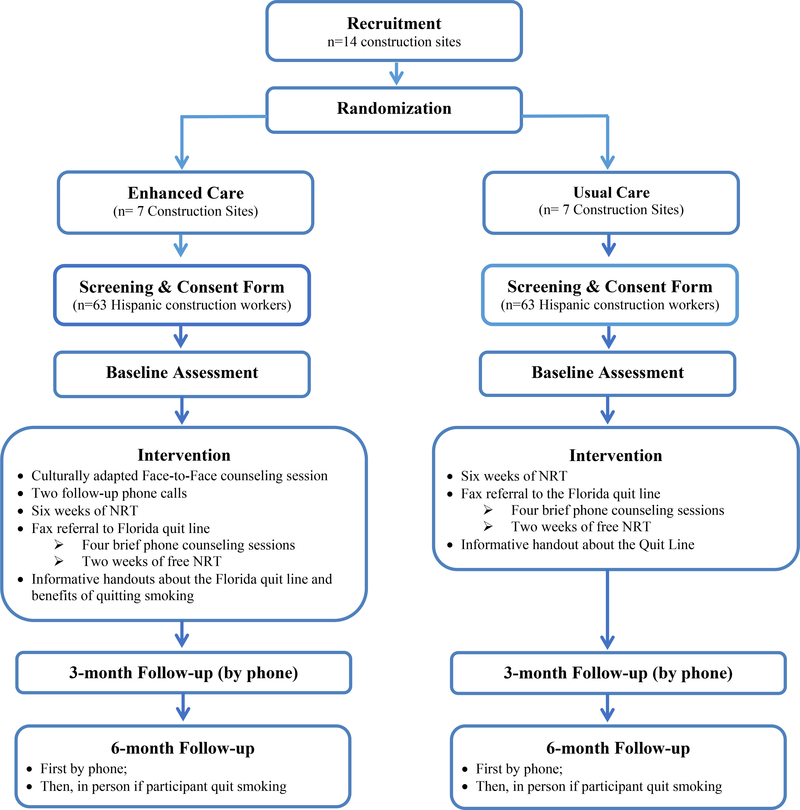
2.3.1. Trial Design
The developed intervention “Enhanced Care” is being tested in a pilot two-arm, cluster RCT (Figure 2). This design is used with the construction site chosen as the unit of allocation because it minimizes the risk of spillover effects from the intervention to the control group that likely would occur if we conducted the randomization at the individual level. Using simple randomization, fourteen construction sites in south Florida are allocated to the Enhanced Care or Standard Care (allocation ratio 1:1). Study participants (n=126; 63/arm) working in these sites receive treatment accordingly. The randomization list was generated using Stata 12 software. Since our intervention involved providing group counseling to stop smoking, it is not possible to blind either participating sites or the interventionist. Workers in both interventions are informed that their construction site is taking part in a smoking cessation study. However, they are not aware of their allocation status. Participants in the “Enhanced Care” group receive our developed intervention. Participants in the “Standard Care” control group receive fax referral to the Florida QL and NRT. Participants in both groups receive two follow-up phone assessments at three, and six months after enrollment. Potential efficacy outcomes will focus on comparing prolonged abstinence (defined as no smoking, not even a puff, after a grace period of two weeks after quit date), and point-prevalent abstinence (defined as self-report of not smoking in the past seven days; not even a puff) confirmed by saliva cotinine level of <15ng/ml at six months. Thus, biochemical validation of smoking status via salivary cotinine analysis is obtained only for those who report prolonged abstinence at the six-month assessment. All study materials are made available in English and Spanish and are administrated by the bilingual Research Assistant based on participant preference. The protocol and consent form have been approved by the Institutional Review Board at University of Miami and the study is registered at the Clinical Trials Registry (#NCT02873377).
Figure 2.
The Cluster Randomization Clinical Trial Study Schema
2.3.2. Recruitment of Construction Sites
We identified several local construction companies in South Florida and contacted them initially by telephone or email to introduce the study, enclosing a one-page brief information leaflet about the study procedure and their role in administering the study. For those who were interested in participating, we organized a meeting with their management, contractors, supervisors, and safety mangers to discuss the study procedures in more details, and asked them to identify a key contact person onsite to be our research partner who assists in the study administration. We also presented the study several times at the monthly Safety Alliance for Excellence meetings aimed at improving construction workplace safety in South Florida (http://www.safetyalliance.org/), and here we invited companies to participate in the study.
2.3.3. Study Participants, and Procedures
Our target sample size is 126 Hispanic/Latino smokers (63/arm). With help from the site’s safety manager, two bilingual health educators are recruiting Hispanic construction during their breakfast or lunch breaks around the food truck. Participants have to be male, ≥ 18 years old, Hispanic/Latino, and have smoked ≥ 5 cigarettes/day for the past year (Table 2). Additionally, they need to have access to a telephone, no plans to move in the next six months, be interested in making a serious quit attempt in the next 30 days, and have no contraindication to NRT. Individuals who decline to participate will still be encouraged to quit smoking and referred to the Florida tobacco QL. Those who are eligible and want to participate will be consented, then undergo a 15 min baseline screening for NRT contraindications, which includes a history of hypersensitivity to nicotine, recent (past month) myocardial infarction, or any history of serious arrhythmias or unstable angina pectoris.28 If there are no contraindications, participants will be given their preferred form of NRT (gum or patch) and will be provided information on side effects and proper use. After screening, participants will complete a baseline assessment to collect information about demographics, job characteristics, job stress,40 acculturation,41, smoking history and nicotine dependence,42 withdrawal symptoms, stages of change,43 quit ladder,44 smoking self-efficacy,45 depression,46 social support,47 exposure to secondhand smoke, quality of life, expired CO, and cotinine saliva (Table 3).48
Table 2.
Inclusion and exclusion criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| • Male | • History of hypersensitivity to nicotine |
| • Hispanic/Latino | • Recent (past month) myocardial infarction |
| • 18 years or older | • History of serious arrhythmias |
| • Smoke at least 5 cigarettes per day for one year | • Unstable angina |
| • Willing to make a serious quit attempt in the next 30 days | • Generalized chronic dermatological disorder |
| • Available on site in the following day | |
| • Have access to a telephone | |
| • Plans to stay in the same home address for the next 6 months |
Table 3.
Measures
| Baseline | Call 1 Assessment | Call 2 Assessment | 3-month follow-up | 6 month follow-up | |
|---|---|---|---|---|---|
| Contact Information | X | ||||
| Demographics | X | ||||
| Residential History | X | ||||
| Acculturation | X | ||||
| Familism | X | ||||
| Identity | X | ||||
| Perceived discrimination | X | ||||
| Job Characteristics | X | ||||
| Job Stress Scale | X | X | X | X | X |
| Smoking history | X | ||||
| Nicotine Dependence (FTND) | X | X | |||
| Use of other tobacco products | X | ||||
| Quit Ladder | X | ||||
| Smoking Self-efficacy/ Temptation | X | X | X | ||
| Hatsukami Withdrawal Scale | X | X | X | ||
| Weight Concern | X | X | X | ||
| Alcohol and Coffee Use | X | X | X | ||
| Depression | X | X | X | ||
| Social Support | X | X | X | X | X |
| Exposure to SHS at work and home | X | X | X | ||
| Quality of Life | X | X | X | ||
| Smoking Status Assessment/ smoking reduction | X | X | X | X | |
| NRT use | X | X | X | X | |
| Self-Report Use of Florida Tobacco Quit line | X | X | X | X | |
| Self-report use of other products or programs to quit smoking | X | X | X | X |
2.3.4. Interventions
As mentioned before, participants in the “Enhanced Care” (our developed intervention) arm receive our developed single face-to-face behavioral group counseling session delivered at the food truck supported by two brief follow-up phone counseling calls, fax referral to the Florida tobacco QL, and eight weeks of free NRT (six weeks provided by the study and two weeks provided by the QL). This intervention will be compared with “Standard Care” intervention which consists of fax referral to the Florida QL and provision of eight weeks of free NRT as in the enhanced care. Justification for our selection to the developed and comparison interventions was described in the formative research section.
2.3.5. Follow-up and Retention
Participants in both arms will receive two follow-up phone calls at three and six months after enrollment to assess their smoking status, as well as get information about concomitant smoking and NRT use, use of additional NRT or cessation drugs (e.g., bupropion), and number and time of total contact with our interventionist and with the QL (Figure 2). Those who report quitting smoking at six months will be visited at a mutually agreed-upon location to obtain a saliva sample to validate their smoking cessation status.49,50 Saliva cotinine is a widely used method to biochemically confirm smoking abstinence with 96–97% sensitivity and 99–100% specificity, and it has a well-tested cut-point of 15 ng/ml to distinguish between nonsmokers highly exposed to secondhand smoke (1–15 ng/ml) and active smokers (>15 ng/ml).49,50 Saliva samples will be collected using NicAlert™ kits and analyzed at the Diabetes Research Institute at the University of Miami by automated gas liquid chromatography.51
To maintain active participation for the entire length of the study, several retention strategies will be used. These include collecting detailed contact information for relatives/friends, who would know the participant’s whereabouts, contacting participants with personalized letters/cards, sending out study-relevant information at three months (e.g., changes in your body after quitting smoking, quitting rewards), and individual case management. In addition, we provided incentives for participants at several occasions to improve adherence to the protocol. Participants receive $20 after completing the intervention and the rest ($30) after completing the final six-month assessments.
2.3.6. Interventionist training
All interventionists underwent an intensive 2 weeks training period. This involved training in human subjects protection, behavior change theories, pharmacotherapies used in smoking cessation, counseling protocol, quality control procedures, NRT adverse events, and role playing of project procedures. The counselors meet weekly throughout the project with an expert in tobacco treatment for quality control, supervision, and case review.
2.3.7. Sample Size
The current study is a pilot study and it is not powered to detect statistically significant effects. Major goals of this pilot study are to explore the feasibility and estimate the intervention’s effect size to help determine sample size needs for a future full-scale RCT.52–54 We will use a two-group comparison of proportions at the two-tailed alpha level. We assume that the intracluster correlation will be low (0.01). With 14 sites (7 in each group) and approximately 9 participants per site (enrolled in multiple cohorts at each site until a target of nine is met) (n=126), this sample size will allow us to detect a range of relative differences from 17% to 22% between the two groups, assuming control proportion between 10% and 50% (a moderate effect size of 0.22), a one-tailed 0.05 alpha, and 80% power. We will include all randomized participants in assessment of endpoints, including participants who do not complete the trial as failures. We realize the limitations of using pilot data to power subsequent studies, related to the inherent imprecision of estimates with small sample sizes.55,56 It is important to note, though, that our sample size is large enough to estimate the effect with a reasonably tight confidence interval,56 and we will not rely exclusively on this effect to estimate power, but will also make use of the extant literature.
2.3.8. Quality Assurance
Following the strategy suggested by Sechrest,57 evaluation of any treatment program should involve satisfactory answers to three questions: 1) was the intervention both standardized and delivered as intended to the participant? 2) if so, did the participants receive it? and 3) if so, was the intervention efficacious in changing behavior? Sechrest’s 3rd question involves the evaluation of outcomes, which is outlined in the Data Analysis section below. However, Sechrest’s 1st and 2nd questions address treatment implementation issues. To ensure standardization of intervention content and delivery, we will use standardized treatment manuals/procedures tailored particularly for this study. Participants will respond to a brief questionnaire at baseline and the three-month follow-up to assess whether key points were learned, including the techniques and information discussed in the intervention sessions. Standard procedures will be used for instrument development, protocol and forms, and data management (e.g. entry, reconciliation, updating, and data security and confidentiality). We will provide on-going training and supervision for quality assurance for the interventionists through weekly staff meetings, review of processes, and tracking reports.
2.3.9. Measures
This pilot study aims to explore the feasibility and establish the potential efficacy of Enhanced Care compared to Standard Care (Table 4). Main feasibility outcomes are to explore: 1) recruitment yield (number of workers available, eligible, and randomized), 2) facilitators and barriers to delivering the intervention at worksite, 3) willingness of participants to be randomized and acceptability of the intervention, 4) follow-up rates and response rates to questionnaires, 5) adherence/compliance rates, 6) the time needed to collect and analyze data, 7) QL enrollment rates (collected from the outcome report provided by the QL as part of the fax referral service), and 8) willingness of the target population to be involved in the research design and conduct.58–60 Potential efficacy outcomes will focus on comparing the two study arms on prolonged abstinence (defined as no smoking, not even a puff, after a grace period of two weeks after quit date), and point-prevalent abstinence (defined as self-report of not smoking in the past 7-days; not even a puff) confirmed by saliva cotinine level of <15ng/ml at six months. For individuals who do not quit entirely, the decrease in smoking (i.e., cigarettes/day) will be assessed.61,62 Relapse is defined as smoking at least once/week on two consecutive weeks.63 Secondary outcome measures will focus on differences in stage of change/readiness to quit,64 and treatment adherence according to intervention assignment.65
Table 4.
Study Outcomes
| Feasibility outcomes | Potential efficacy outcomes |
|---|---|
| • Yield (number of workers available, eligible, and randomized) | • Prolonged abstinence*: defined as no smoking, not even a puff, after a grace period of two weeks after quit date |
| • Facilitators and barriers to delivering the intervention at worksite | • Point-prevalent abstinence*: defined as self-report of not smoking in the past 7-days; not even a puff |
| • Willingness of participants to be randomized and acceptability of the intervention | |
| • Follow-up rates and response rates to questionnaires | |
| • Adherence/compliance rates | |
| • Time needed to collect and analyze data | |
| • QL enrollment rates (collected from the outcome report provided by the QL as part of the fax referral service) | |
| • Willingness of the target population to be involved in the research design and conduct. |
Confirmed by saliva cotinine level of <15ng/ml at 6-month
2.3.10. Data Analysis
The analysis of the acceptability and feasibility outcomes will be mainly descriptive.65–67 Chi-square tests and between-group t-tests will be used to compare between-group differences in baseline characteristics, indices of treatment implementation, adherence, retention, and treatment perceptions. Intraclass correlation coefficients will be used to adjust for clustering within sites.66 This adjustment accounted for within-site clustering of workers characteristics at each of the 14 sites in our trial. Chi-square tests also will be used to compare cessation rates in the two arms. Logistic regression analyses will be conducted to assess baseline predictors of cessation at six months (both prolonged and point-prevalence abstinence). We will also conduct secondary analyses to account for “dose” of treatment on prolonged and point prevalent abstinence at six months independent of treatment assignment. Independent variables for these models will include use of NRT post-randomization (both as a binary yes vs. no and summary of total use) and number/time of total contacts (with both the study interventionist and Florida QL) during the treatment period.
2.4. Phase (4) Post-intervention Evaluation
A post-intervention evaluation interview will be conducted over the phone three months after enrollment with 25 randomly selected participants from the Enhanced Care group. The interviews will be conducted by a staff member who did not previously engage with the participant, to increase truthfulness of reporting. A semi-structured interview will be used to assess the intervention acceptability and perceived helpfulness of methods, and recommendations for improving access and use of the intervention. Participants will receive a $20 incentive by mail for participating. Descriptive analysis will be used to summarize the data, which will be used to refine the intervention prior to wider testing and dissemination (Figure 1).
3. Discussion
The current report describes the development of a worksite based smoking cessation intervention targeting Hispanic/Latino construction workers, as well as the design, methods, and data analysis plans for an ongoing two-arm pilot cluster RCT testing the feasibility and potential efficacy of the developed intervention. Although Hispanic/Latino construction workers are a high risk group for smoking-related health and occupational hazards,1 they still have limited access to interventions tailored to their cultural and work/life circumstances. In addition to focusing on an underserved and difficult to reach population of Hispanic/Latino construction workers, this study is the first to involve these workers and their management in designing and implementing the smoking cessation intervention. We believe that this approach increases the chance of creating an effective intervention that addresses the target population’s needs and is well-integrated into the workplace structure.25,67 Based on feedback from workers and their management, we developed a worksite-based, single-session, brief (30 min), face-to-face group intervention and partnered with the construction site management to deliver the intervention around the food truck during the breakfast or lunch breaks. Although the intervention is not culturally specific, as was requested by our target population, participants have the ability to receive the intervention either in Spanish or English based on their preference. Given the enormous dissemination potential of our intervention in this population, we believe that this pilot trial will provide a formative foundation for more extensive trials and efforts to reduce smoking among Hispanic/Latino construction workers in south Florida and the rest of the US.
There are several important strengths of the current study. First, in designing the study, we used a mixed methods of qualitative and quantitative approach for the development and testing of the intervention. This complementary approach provides an optimal balance between formative assessments needed to obtain our target population’s feedback about designing and adapting the intervention, and an experimental randomized controlled trial that allows us to establish preliminary evidence about the feasibility and potential efficacy of the intervention. Second, to minimize the risk of spillover effects from the intervention to the control group, we used a cluster randomization with the construction site chosen as the unit of allocation where workers in the same site will receive the same intervention. Third, our intervention in the control group “Standard Care” consisting of the “fax referral service” to the state tobacco QL and provision of full dose treatment of NRT represents the most intensive best practice intervention that our target population is likely to receive in the absence of our “Food Truck” developed intervention. Therefore, in addition to testing our developed intervention, this study will establish the potential efficacy of existing but underused resources for improving smoking cessation in this minority group of workers. Finally, the provision of intervention in Spanish will allow for inclusion of a larger, more representative proportion of our target population.
Given the high job demand and workers’ high mobility and turnover we anticipate two main challenges for implementing the study: 1) the time constraints during work hours, which may not provide enough time to complete all study procedures, and 2) the ability to reach all participants at the three- and six-month follow-ups. To meet these challenges we are involving the site safety manger in administering the study procedures to best fit into workers’ schedules and dividing our study procedures into two days to avoid interrupting the workflow on site (e.g., recruiting and consenting workers in day 1, while providing the intervention in day 2). In addition, we are taking extensive retention measures to reach workers for follow-ups and maintain them in the study.
Results from key informant interviews identified another valuable opportunity to deliver smoking cessation intervention to construction workers. The management suggested integrating the intervention into the orientation employment training session. More studies are needed to explore this opportunity given its potential in identifying the smoking status of all workers and helping those who smoke in quitting smoking. In addition, the management recommendations to support implementing a sustainable smoking cessation service in this setting were to “ideally” mandate the intervention by the State/local government to drive demand and cooperation from construction companies. Limited studies have investigated workplace tobacco control policy environment in this sector. Therefore, more efforts are needed to explore this policies at the state level and assess knowledge and commitment to this policy and to identify gaps, challenges, and opportunities for implementation of smoking cessation services in this sector.
Finally, it is important to note that if proven to be effective, our intervention has the potential for immediate dissemination. We have partnered with key stakeholders in the construction industry, union, and government to assist our research team in sharing the study findings with other groups and networks engaged in protecting the health of minority workers such as the Hispanic Contractors Association De Tejas, the U.S. Office of Construction Health and Safety at the National Institute for Occupational Safety and Health, and the Center for Protection of Workers Rights. These entities are committed to disseminating the intervention as part of their outreach activities.
4. Conclusion
This study will be the first to develop and evaluate a novel intervention strategy in a hard-to-reach and underserved population of Hispanic/Latino construction workers. The study aims to tackle major barriers to smoking cessation in Hispanic construction workers, by: 1) increasing their access to smoking cessation through a worksite-based intervention delivered around the food truck; 2) adapting the intervention to their culture, work, and life circumstances by involving them in its development; and 3) involving the construction management in designing and implementing the intervention. Data resulting from this project will inform larger studies of the effectiveness of cessation approaches that have great potential for translation and dissemination in minority construction workers throughout the US.
ACKNOWLEDGMENT
We thank the four participating construction companies: Moss, Coastal, Balfour Beatty, and Turner for their assistance in implementing the study.
FUNDING
This work was supported by the National Cancer Institute at the National Institutes of Health (grant number R21 CA202993). Supplemental funding was provided by the Sylvester Comprehensive Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The study is registered at the Clinical Trials Registry (#NCT02873377).
ABBREVIATIONS
- QL
quit line
- NRT
nicotine replacement treatment
- RCT
Randomized clinical trial
Footnotes
DECLATION OF INTERESTS
The authors declare they have no conflicts of interest.
References
- 1.C. Everett K Reducing the health consequences of smoking 25 years of progress: report of the Surgeon General. 1995.
- 2.Lee DJ, Fleming LE, Arheart KL, et al. Smoking rate trends in U.S. occupational groups: the 1987 to 2004 National Health Interview Survey. J Occup Environ Med. 2007;49(1):75–81. [DOI] [PubMed] [Google Scholar]
- 3.Dong S, Chowdhury R, McCann M, Trahan C, Gittle-man JS. The construction chart book: the U.S. construction industry and its workers, 3rd ed. Silver Spring, MD: CPWR - Center for Construction Research and Training; 2002. [Google Scholar]
- 4.Driscoll T, Steenland K, Imel Nelson D, J. L. Occupational airborne particulates: assessing the environmental burden of disease at national and local levels. Geneva: World Health Organization; 2004. [Google Scholar]
- 5.Driscoll T, Steenland K, Pruss-Ustin A, Nelson DI, Leigh J. Occupational carcinogens: assessing the environmental burden of disease at national and local levels. Geneva: World Health Organization;2004. [Google Scholar]
- 6.Asfar T, Arheart KL, Dietz NA, Caban-Martinez AJ, Fleming LE, Lee DJ. Changes in cigarette smoking behavior among US young workers from 2005 to 2010: the role of occupation. Nicotine Tob Res. 2016;18(6):1414–1423. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Department of Labor BoLS. Employment and earnings. Washington: 1996. [Google Scholar]
- 8.Bureau of Labor Statistics, U.S. Department of Labor. The Construction Chart Book. The Center to Protect Workers’ Rights. Female workers in construction and other industries. In: Washington, D.C: 2000: http://www.cpwr.com/pdfs/pubs/chartbook_02/page%2019.pdf [Google Scholar]
- 9.U.S. Census Bureau. 2012; http://quickfacts.census.gov/qfd/states/00000.html.
- 10.CPWR - The Center for Construction Research and Training. The construction chart book: the US construction industry and its workers. Fifth Edition. April 2013.
- 11.Caban-Martinez A, Clarke T, Davila E, Fleming L, Lee D. Application of handheld devices to field research among underserved construction worker populations: a workplace health assessment pilot study. Environmental Health. 2011;10(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorensen G, Barbeau EM, Stoddard AM, et al. Tools for health: the efficacy of a tailored intervention targeted for construction laborers. Cancer Causes Control. 2007;18(1):51–59. [DOI] [PubMed] [Google Scholar]
- 13.Cahill K, Lancaster T. Workplace interventions for smoking cessation. The Cochrane Library. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiede LP, Hennrikus DJ, Cohen BB, Hilgers DL, Madsen R, Lando HA. Feasibility of promoting smoking cessation in small worksites: an exploratory study. Nicotine & tobacco research. 2007;9(Suppl 1):S83–S90. [DOI] [PubMed] [Google Scholar]
- 15.Klesges RC, Vasey MM, Glasgow RE. A worksite smoking modification competition: potential for public health impact. American Journal of Public Health. 1986;76(2):198–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caban-Martinez AJ, Clarke TC, Davila EP, Fleming LE, Lee DJ. Application of handheld devices to field research among underserved construction worker populations: a workplace health assessment pilot study. Environ Health. 2011;10:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caban-Martinez AJ, Lee DJ, Fleming LE, et al. Cancer health education preferences among Miami-Dade County construction workers. Florida Public Health Review. 2009;6:58–61. [Google Scholar]
- 18.Caban AJ, Lee DJ, Clarke TC, et al. Self-Reported joint and back pain among construction workers: a pilot workplace musculoskeletal assessment. J Muscoskel Res. 2010. June;13(2):49–55. [Google Scholar]
- 19.Caban-Martinez AJ, et al. The “Lunch Truck” experience: active and passive tobacco smoke exposures: a construction workplace health assessment pilot study. Poster Presentation presented at International Conference on Occupational Health; 2012; Cancun, MX. [Google Scholar]
- 20.Lee DJ, Fleming LE, Arheart KL, et al. Smoking rate trends in U.S. occupational groups: the 1987 to 2004 National Health Interview Survey. J Occup Environ Med. 2007;49:75–81. [DOI] [PubMed] [Google Scholar]
- 21.Lee DJ, Fleming LE, McCollister KE, et al. Healthcare provider smoking cessation advice among US worker groups. Tob Control. 2007;16(5):325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorensen G, Barbeau E, Hunt MK, Emmons K. Reducing social disparities in tobacco use: a social-contextual model for reducing tobacco use among blue-collar workers. American Journal of Public Health. 2004;94(2):230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorensen G, Quintiliani L, Pereira L, Yang M, Stoddard A. Work experiences and tobacco use: findings from the gear up for health study. Journal of Occupational and Environmental Medicine. 2009;51(1):87–94. [DOI] [PubMed] [Google Scholar]
- 24.Glasgow RE, Emmons KM. How can we increase translation of research into practice? Types of evidence needed. Annu Rev Public Health. 2007;28:413–433. [DOI] [PubMed] [Google Scholar]
- 25.Borrelli B Smoking cessation: next steps for special populations research and innovative treatments. Journal of Consulting and Clinical Psychology. 2010;78(1):1. [DOI] [PubMed] [Google Scholar]
- 26.Hawkins RP, Kreuter M, Resnicow K, Fishbein M, Dijkstra A. Understanding tailoring in communicating about health. Health Education Research. 2008;23(3):454–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Resnicow K, Soler R, Braithwaite RL, Ahluwalia JS, Butler J. Cultural sensitivity in substance use prevention. Journal of community psychology. 2000;28(3):271–290. [Google Scholar]
- 28.Fiore MC, Jaén CR, Baker TB, et al. , eds. Treating tobacco use and dependence: 2008 update Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services; 2008. Quality AfHRa, ed. [Google Scholar]
- 29.Strauss AJ, Corbin, 1990, Basics of Qualitative Research Grounded theory procedures and techniques. In: Newbury Park, CA: Sage. [Google Scholar]
- 30.Braun V, Clarke V. Using thematic analysis in psychology. Qualitative research in psychology. 2006;3(2):77–101. [Google Scholar]
- 31.Bandura A Health promotion from the perspective of social cognitive theory. Psychology and Health. 1998;13(4):623–649. [Google Scholar]
- 32.Bandura A Social learning theory. Prentice-Hall, Englewood Cliffs, N.J: 1977. [Google Scholar]
- 33.Lancaster T, Stead L. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev. 2005;2. [DOI] [PubMed] [Google Scholar]
- 34.Lancaster T, Stead LF. Self-help interventions for smoking cessation. Cochrane Database Syst Rev. 2005;3(3). [DOI] [PubMed] [Google Scholar]
- 35.Stead LF, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2006;3. [DOI] [PubMed] [Google Scholar]
- 36.Andrews JO, Felton G, Ellen Wewers M, Waller J, Tingen M. The effect of a multi-component smoking cessation intervention in African American women residing in public housing. Res Nurs Health. 2007;30(1):45–60. [DOI] [PubMed] [Google Scholar]
- 37.Curry SJ, Ludman EJ, Graham E, Stout J, Grothaus L, Lozano P. Pediatric-based smoking cessation intervention for low-income women: a randomized trial. Arch Pediatr Adolesc Med. 2003;157(3):295–302. [DOI] [PubMed] [Google Scholar]
- 38.Stead LF, Perera R, Lancaster T. A systematic review of interventions for smokers who contact quitlines. Tob Control. 2007;16 Suppl 1:i3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schauer GL, Malarcher AM, Zhang L, Engstrom MC, Zhu SH. Prevalence and correlates of quitline awareness and utilization in the United States: an update from the 2009–2010 national adult tobacco survey. Nicotine Tob Res. 2014;16(5):544–553. [DOI] [PubMed] [Google Scholar]
- 40.Shea T, De Cieri H. Workplace stress evaluation tools: A Snapshot Review. 2011.
- 41.Ellison J, Jandorf L, Duhamel K. Assessment of the short acculturation scale for Hispanics (SASH) among low-income, immigrant Hispanics. J Cancer Educ. 2011;26(3):478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piper ME, McCarthy DE, Baker TB. Assessing tobacco dependence: a guide to measure evaluation and selection. Nicotine Tob Res. 2006;8(3):339–351. [DOI] [PubMed] [Google Scholar]
- 43.Vilela FA, Jungerman FS, Laranjeira R, Callaghan R. The transtheoretical model and substance dependence: theoretical and practical aspects. Rev Bras Psiquiatr. 2009;31(4):362–368. [DOI] [PubMed] [Google Scholar]
- 44.Biener L, Abrams DB. The contemplation ladder: validation of a measure of readiness to consider smoking cessation. Health Psychology. 1991;10(5):360. [DOI] [PubMed] [Google Scholar]
- 45.Hendricks PS, Wood SB, Baker MR, Delucchi KL, Hall SM. The smoking abstinence questionnaire: measurement of smokers’ abstinence-related expectancies. Addiction. 2011;106(4):716–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reuland DS, Cherrington A, Watkins GS, Bradford DW, Blanco RA, Gaynes BN. Diagnostic accuracy of Spanish language depression-screening instruments. Ann Fam Med. 2009;7(5):455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zimet GD, Dahlem NW, Zimet SG, Farley GK. The multidimensional scale of perceived social support. Journal of personality assessment. 1988;52(1):30–41. [DOI] [PubMed] [Google Scholar]
- 48.World Health Organization. Global Tobacco Surveillance System (GTSS). Tobacco Questions for Surveys. 2011.
- 49.Rebagliato M Validation of self reported smoking. Journal of Epidemiology and Community Health. 2002;56(3):163–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention, National Center for Environmental Health. National report on human exposure to environmental chemicals. Atlanta: 2001. [Google Scholar]
- 51.Jacob Iii P, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. Journal of Chromatography B: Biomedical Sciences and Applications. 1981;222(1):61–70. [DOI] [PubMed] [Google Scholar]
- 52.Stevinson C, Ernst E. A pilot study of Hypericum perforatum for the treatment of premenstrual syndrome. BJOG: An International Journal of Obstetrics & Gynaecology. 2000;107(7):870–876. [DOI] [PubMed] [Google Scholar]
- 53.Ross‐McGill H, Hewison J, Hirst J, et al. Antenatal home blood pressure monitoring: a pilot randomised controlled trial. BJOG: An International Journal of Obstetrics & Gynaecology. 2000;107(2):217–221. [DOI] [PubMed] [Google Scholar]
- 54.Browne RH. On the use of a pilot sample for sample size determination. Statistics in Medicine. 1995;14(17):1933–1940. [DOI] [PubMed] [Google Scholar]
- 55.Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. Journal of psychiatric research. 2011;45(5):626–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kraemer HC, Mintz J, Noda A, Tinklenberg J, Yesavage JA. Caution regarding the use of pilot studies to guide power calculations for study proposals. Archives of General Psychiatry. 2006;63(5):484. [DOI] [PubMed] [Google Scholar]
- 57.Sechrest et al. Evaluation of treatment program. 1979.
- 58.Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. Journal of evaluation in clinical practice. 2004;10(2):307–312. [DOI] [PubMed] [Google Scholar]
- 59.Arain M, Campbell MJ, Cooper CL, Lancaster GA. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC medical research methodology. 2010;10(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharmaceutical Statistics. 2005;4(4):287–291. [Google Scholar]
- 61.Asfar T, Ebbert JO, Klesges RC, Relyea GE. Do smoking reduction interventions promote cessation in smokers not ready to quit? Addictive Behaviors. 2011;36(7):764–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Asfar T, Ebbert JO, Klesges RC, Klosky JL. Use of smoking reduction strategies among U.S. tobacco quitlines. Addictive Behaviors. 2012;37(4):583–586. [DOI] [PubMed] [Google Scholar]
- 63.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43(3):289–294. [DOI] [PubMed] [Google Scholar]
- 64.DiClemente CC, Prochaska JO, Fairhurst SK, Velicer WF, Velasquez MM, Rossi JS. The process of smoking cessation: an analysis of precontemplation, contemplation, and preparation stages of change. Journal of Consulting and Clinical Psychology. 1991;59(2):295–304. [DOI] [PubMed] [Google Scholar]
- 65.Asfar T, Weg MV, Maziak W, Hammal F, Eissenberg T, Ward KD. Outcomes and adherence in Syria’s first smoking cessation trial. Am J Health Behav. 2008;32(2):146–156. [DOI] [PubMed] [Google Scholar]
- 66.Perisic I, Rosner B. Comparisons of measures of interclass correlations: the general case of unequal group size. Stat Med. 1999;18(12):1451–1466. [DOI] [PubMed] [Google Scholar]
- 67.Resnick MD, Bearman PS, Blum RW, et al. Protecting adolescents from harm. Findings from the National Longitudinal Study on Adolescent Health. JAMA. 1997;278(10):823–832. [DOI] [PubMed] [Google Scholar]