Abstract
Introduction
Lymphoscintigraphy is the gold standard for imaging in the diagnosis of peripheral lymphedema. However, there are no clear guidelines to standardize usage across centers, and as such, large variability exists. The aim of this perspectives paper is to draw upon the knowledge and extensive experience of lymphoscintigraphy here in Genoa, Italy, from our center of excellence in the assessment and treatment of lymphatic disorders for over 30 years to provide general guidelines for nuclear medicine specialists.
Method
The authors describe the technical characteristics of lymphoscintigraphy in patients with limb swelling. Radioactive tracers, dosage, administration sites, and the rationale for a two-compartment protocol with the inclusion of subfascial lymphatic vessels are all given in detail.
Results
Examples of lymphoscintigraphic investigations with various subgroups of patients are discussed. The concept of a transport index (TI) for semi-quantitative analysis of normal/pathological lymphatic flow is introduced. Different concepts of injection techniques are outlined.
Discussion
It is past time that lymphoscintigraphy in the diagnosis of lymphatic disorders becomes standardized. This represents our first attempt to outline a clear protocol and delineate the relevant points for lymphoscintigraphy in this patient population.
Keywords: Lymphoscintigraphy, Epifascial and subfascial lymphatic vessels, Semi-quantitative transport index, Limb swelling
General Considerations and Peripheral Lymphedema
The scope of these guidelines is to provide appropriate assistance to specialists in nuclear medicine regarding the indications for, the correct procedures, and interpretation of lymphoscintigraphy. Lymphoscintigraphy is a secure method, minimally invasive, and well-established for the evaluation of lymphatic drainage in lymphatic disorders.
The lymphoscintigraphic method has by now largely replaced the more invasive and technically difficult technique of lymphography [1]. In the limbs, the lymphatic system consists of a superficial compartment in which the lymph flow derives from the skin and the subcutaneous tissue, rising through the lymphatic vessels within the epi-fascial planes to reach the loco-regional lymph node stations, and a deep compartment that drains the subfascial structures, like muscles, bones, and deep blood vessels. In the lower limbs, these two circuits unite in the inguinal region; while in the upper limbs, they join in the axillary region. The two systems of drainage (superficial and deep) are functionally complementary, such that the deep system participates in lymph drainage from the skin during lymphatic obstruction [2].
Lymphedema is a chronic disease, which is often unrecognized or misdiagnosed, leading to late or no treatment. Lymphedema derives from a deficiency of lymphatic transport caused by surgical or traumatic lesions to the lymphatic vessels, by infectious processes, or congenital anomalies. Effective therapies for lymphedema can be suggested to the patient, particularly after adequate characterization of the disease [3]. Lymphedema is a disease with a high prevalence. About 10 million people develop lymphedema secondary to surgical treatments of lymphadenectomy and radiotherapy of numerous neoplasms, recurrent infections, traumatic injuries, or vascular surgeries. Around 90 million people worldwide suffer from lymphedema due to parasitic infections. When chronic venous insufficiency appears to be a contributory factor, lymphedema is estimated to be over 300 million cases [4].
Lymphedema of the upper arm is a frequent complication of surgery and radiotherapy for breast cancer and axillary lymph node dissection, with an estimated frequency of 5–30% [3, 5]. This incidence is principally based on studies that utilize volume and circumference criteria in the first 2–5 years after surgery. Arm volume difference above 100–200 cm3 or a circumference difference of more than 2 cm is used as the threshold for the diagnosis of lymphedema. All of these studies ignore lighter forms of lymphedema, not recognizing a significant number of patients with mild lymphedema, especially in the non-dominant arm, which also could have been 200 cm3 smaller than the dominant arm before surgery [5].
Lymphedema in the lower limbs is generally a primary condition or secondary to surgery and mainly from pelvic lymphadenectomies secondary to prostatic and uterine neoplastic pathology. The reported frequency of secondary lymphedema varies from 10 to 49% [6, 7]. Even the “mild” lymphedema of the leg can cause discomfort to the patient. Advanced leg lymphedema causes severe permanent disability. Lymphedema of the lower extremities can also be associated with genital lymphedema with a certain frequency [8].
In summary, non-infectious lymphedema is a common disease and one can expect an increase in the number of patients rather than the disappearance of this condition in the next few decades. Many of these patients suffer because they have not been properly diagnosed and treated. Early diagnosis can lead to effective treatment and prevention of side effects, including extremity deformity, disuse atrophy, and increased susceptibility to recurrent infections [3, 5–8].
Most of the radionuclide studies of lymphatic flow use particle materials. The agents used include 99mTc-sulfacolloid, 99mTc-nanocolloid human serum albumin, 99mTc-labeled antimony sulfur colloid, or colloidal gold particles, administered in the interstitial space. Particles smaller than a few nanometers generally pass through blood capillaries, while larger particles, of up to about 100 nm, are able to enter lymphatic capillaries and be transported to the lymph nodes. However, even large particles have been detected in the venous blood immediately after subcutaneous injection, probably as a result of direct capillary disruption from the needle. It is believed that the optimal colloidal size for lymphoscintigraphy is about 50–70 nm [9]. Larger particles (> 100 nm) are trapped in the interstitial compartment for a relatively long period, not allowing radioisotopic examination within optimal times.
Indications
Lymphoscintigraphy is a non-invasive procedure for the differentiation of lymphedema from other causes of limb or truncal edema, such as heart failure, lipedema, and deep vein thrombosis [10, 11].
The indications for lymphoscintigraphy include primary lymphatic dysplasia, secondary lymphatic dysplasia, primary lymphedema, congenital lymphedema, secondary lymphedema, and chylous leakage (chylous ascites and chylothorax) [12]. The lymphoscintigraphy technique has also been proposed, by some authors, for the evaluation of thoracic duct abnormalities [13].
Absolute Contraindications
None.
Relative Contraindications
Pregnancy and breastfeeding are relative, not absolute, contraindications. The effective dose of lymphoscintigraphy is 0.014 mSv/Mbq. Based on this report, the global dose to patient it is about 1.0 mSv [14]. The amount of radiopharmaceutical transferred from the interstitium into the blood and from the blood to the milk is very low, and therefore, it is not necessary to interrupt breastfeeding. However, it seems prudent to recommend the suspension of breastfeeding for 24 h after administration of the radiopharmaceutical.
Like all tests using ionizing radiation, a lymphoscintigraphy in pregnancy must be “justified” by an actual benefit to the patient without excessive risk of exposure to the fetus/infant. It is therefore important to evaluate the opportunity to perform a case-by-case examination in a multidisciplinary context, always considering postponing the examination until the end of breastfeeding. The same principle can be applied in pregnant patients [15]. No intolerance reactions to the radiopharmaceutical used have been reported [4].
Preparatory Procedure
Organization Phases (in Conjunction with the Referrer, Usually Without the Patient Present)
Verification of the appropriateness of the referral for lymphoscintigraphy.
There are no particular dietary or pharmacological preparation requirements. The patient is informed, usually by specifically designed information sheets, about the modality of testing, waiting times for the exam, and the eventual delivery of the outcome report.
Collection of anagraphical information, medical history, and previous clinical and instrumental exams related to the patient.
Verification of the ability of the patient to remain in place on the gamma-camera bed for the entire length of the exam.
Recommendations in terms of radio-protection for the patient.
Pre-Injection Phases (with the Patient Present)
Verification of the appropriateness of the procedure and the correct understanding by the patient of the characteristics of the examination and of the procedures to which they will be submitted.
Signing of the informed consent, providing the patient with all of the information related to the lymphoscintigraphic method used (number of injections, timing of acquisitions, physical activity to which they will have to undergo, times and methods of delivery of the report).
Collection of the medical history and anamnestic information, with particular attention to the timing of the onset of edema, its nature, possible surgical operations, trauma, or infective processes.
Removal of compression garments is highly recommended, if possible.
The patient should be warned of a possible, albeit modest and very transitory, local pain resulting from administration of the tracer.
Radiation Protection Precautions
The use of radioactive substances should be “justified” by an actual benefit to patients. One must follow the usual procedures in terms of radiation protection. In the first instance, the patient should be given a radiotracer dose as low as reasonably achievable. In addition, all measures must be taken to reduce the dose received by the exposed health personnel, to the other patients in the waiting room and to other people that the patient may come into contact with at the end of the lymphoscintigraphic examination. This can also include the use of lead shielding.
Radiopharmaceutical Choice and Site of Administration
99mTc NANOCOLL®, colloidal particles of human albumin (at least 95% of the total size between 20 and 80 nm).
Stability: 6 h. Volume: 0.1–0.2 ml per aliquot.
Administered activity: 30–50 MBq per limb and per compartment.
Administration: subcutaneous injections or sub-fascial with insulin syringes and a 25-gauge needle. To guarantee the reproducibility of the exams, it is recommended to use the same type of injection, volume, and activity of the radiotracer, type of exercises, and modality of image acquisition for every patient.
Injection of the tracer should be preceded by disinfection with an iodine solution (especially in patients with advanced stage lymphedema) or alcohol. The use of local anesthetics is not recommended as they can interfere with the radiopharmaceutical washout from the injection site.
For the study of the superficial circulation, both subcutaneous injection and intradermic in the first few interdigital spaces have been proposed. Opinions differ regarding the best injection technique, but available data suggest that the optimal technique may vary depending on the type of tracer used, with colloidal agents obtaining the best results by subcutaneous injection [4, 10, 11, 16]. The subfascial injection of the radiotracer is used for investigations of the deep lymphatic system of the limbs. The injection can be subfascial in the lateral retromalleolar region, under the ulnar styloid, or in the plantar aponeurosis of the hands and feet. The lymphoscintigraphic study of both the two compartments (subfascial followed by epifascial or vice versa) is preferable, as it allows the differentiation of diverse mechanisms of the edema of the limbs [17, 18] (Figs. 1 and 2), particularly as there is significant evidence that peripheral lymphedema can derive from only superficial lymphatic vessels damage, only deep, or combination of the two [19, 20].
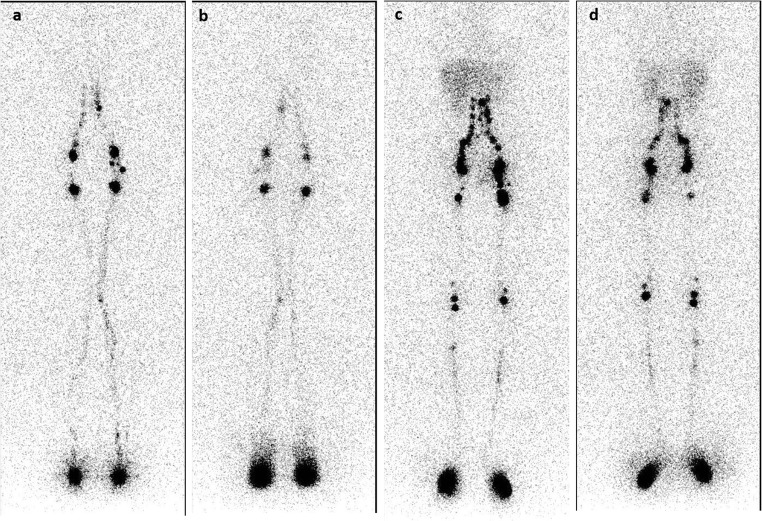
Fig. 1.
Lymphoscintigraphy with 99mTc-Nanocoll®: normal lymphoscintigraphic picture of the lower limbs, obtained 30 min after the injections (early images). Superficial circulation: anterior (a) and posterior views (b). Deep circulation: anterior (c) and posterior (d) views
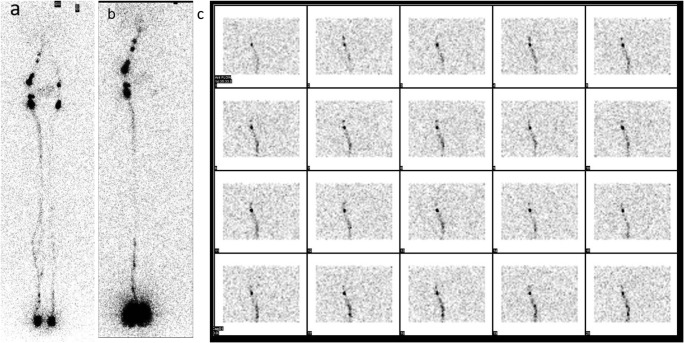
Fig. 2.
Imaging at 2 h. In some cases, lymphatic circulation problems can be mostly confined to one compartment: left lower limb lymphedema with slight deficit of the left limb superficial circulation (a) but a severe insufficiency of the ipsilateral deep circulation (b). The dynamic node uptake of the deep circulation is shown in c with images sampled every 120 s after the subfascial injection. (Transport index (TI) scores are as follows: superficial circuit: left leg TI = 9: K − 3, D − 0, T − 0.3 (8 min), N − 3, and V − 3 and right leg TI = 1.1: K − 1, D − 0, T − 0.1 (2 min), N – 0, and V − 0. Deep circuit: left leg TI = 29.8: K − 8, D − 0, T − 4.8 (120 min), N – 8, and V − 9 and right leg TI = 1.1: K − 1, D − 0, T − 0.1 (2 min), N – 0, and V − 0. Please note that when the lymph nodes are not visualized (as in c), a T value is assigned that is equal to the time taken from injection of the tracer and the late image acquisition—120 min)
Bilateral upper and lower limbs should be studied contemporaneously, such that both arms or both legs are studied in order to provide a comparison, regardless of whether clinically evident swelling is unilateral or bilateral. A “total body” study involves simultaneous administration to the four limbs. In case of an ascending deficit, further subcutaneous administration to the arm or thigh above the obstacle to the lymphatic flow may be useful to verify the functional presence of proximal collectors, in the eventuality of subjecting the patient to surgical treatments such as lymphatic-venous anastomosis.
In case of scrotal or vulvar edema, the study should start with two subcutaneous injections of the tracer into the bottom of the scrotum, lateral to the medial raphe, or into the labia majora. The examination will then be continued with the study of the lower limbs (Fig. 3).
Fig. 3.
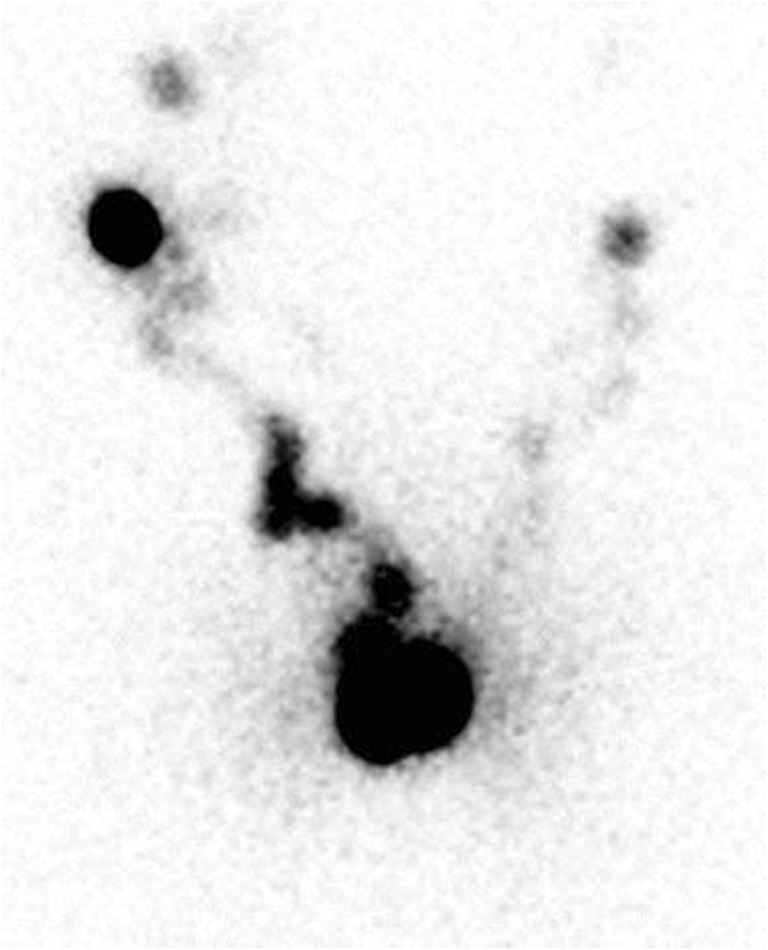
Scrotal edema. Slowing of lymphatic flow from the left hemiscrotum to inguinal lymph node stations. Imaging obtained after 2 h
Acquisition Protocol
Large field of view gamma camera is required, equipped with a parallel hole low energy high-resolution (LEHR) collimator for low energy and high resolution (± 15% window centered on the 140-keV energy peak of 99mTc).
Mandatory acquisition of dynamic images on the lymph glandular districts occurs for about 20 min after radiopharmaceutical administration (60 s/frame, 64 × 64 matrix, zoom 1). Dynamic lymphoscintigraphic evaluation measures variations in lymph obstruction in flow at the lymph nodes or lymphatic vessel displacement. This procedure is mandatory to evaluate changes in lymphatic flow before and after medical, conservative, or surgical treatments. Normal scans show a swift and smooth movement of the radiotracer along the lymphatic pathways of the limbs towards the axilla or inguinal regions. [21–23].
At the end of the dynamic study, acquisition of total body images (speed 10 cm/min) or static planar views on the injection sites and on the loco-regional lymphoglandular districts can be taken (300 s, 128 × 128 matrix, zoom 1) in anterior and posterior projections after 30 min (early acquisition), 2 h, and possibly 4 h (delayed acquisitions) in case of poor radiotracer migration.
Single-photon emission computed tomography/computed tomography—SPECT/CT study (see below) is particularly recommended in the study of diseases of the lymphatic system in the pelvic-abdominal-thoracic districts (Fig. 4).
In the delayed images, the liver uptake value confirms the arrival of the tracer into the hepatic circulation.
Late imaging is also essential for the assessment of “dermal backflow” (Fig. 5) or post-traumatic stagnation.
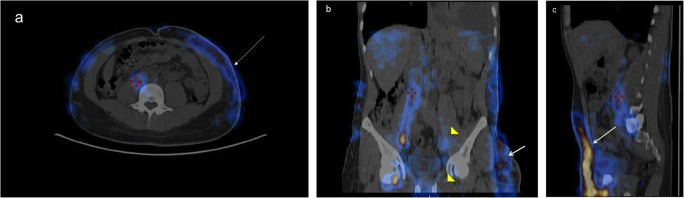
Fig. 4.
SPECT/CT hybrid images where the lymph flow is in blue/yellow. Normal uptake of 99mTc-Nanocoll® by inguinal and iliac lymph nodes on the right. Lack of visualization of left iliac and inguinal lymph nodes (yellow arrow heads in b) and the presence of dermal back flow (white arrows in pictures a, b, and c) to the left flank regions and left hemithorax in a patient with left inguinal lymphodenectomy for Hodgkin’s lymphoma. NB. The red marks are not significant but simply a method of centering the two image sources in order to provide the hybrid image
Fig. 5.
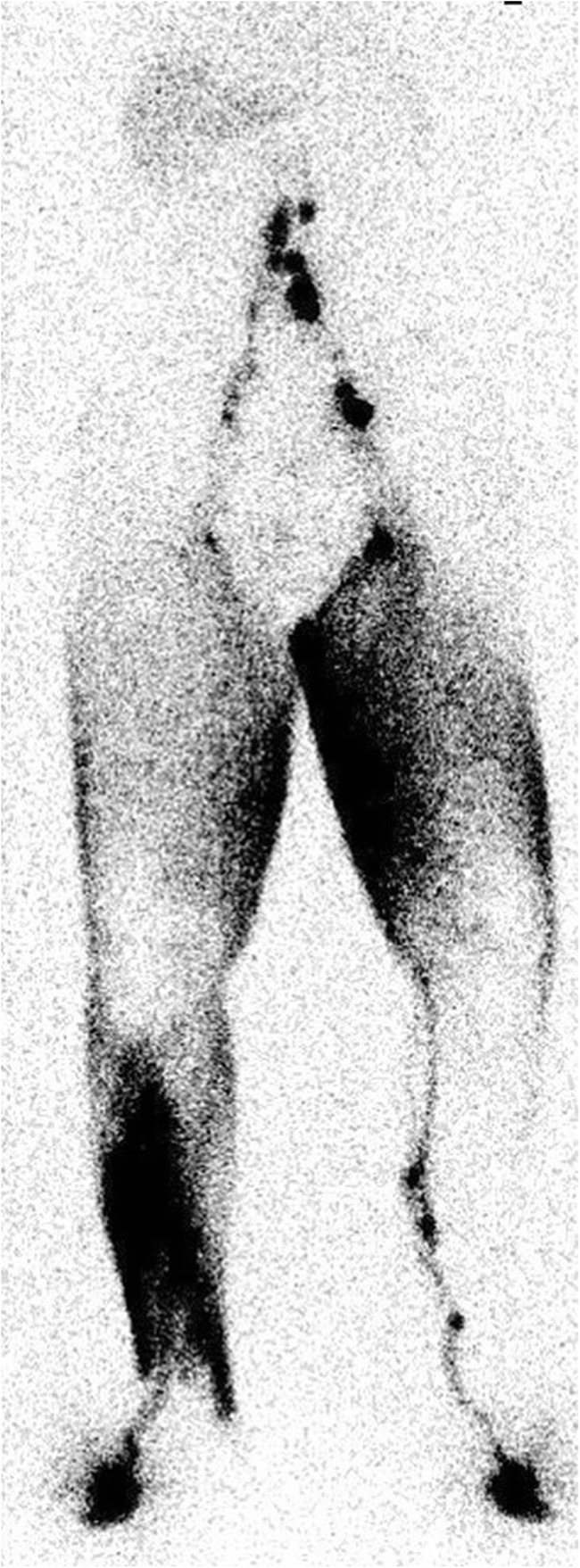
Widespread dermal flow in bilateral lymphedema of the lower limbs resulting from inguinal lymphadenectomy and radiation treatment for cervical carcinoma (delayed imaging at 4 h)
In the interval between early and late acquisition (so after 30 min and before 2 h), it is useful to advise the patient to perform physical activity in order to increase lymphatic flow. In the lower limbs, such stress maneuvers include walking, limb massage, or the exercise bike. In the upper limbs, repeated compressions of a rubber ball have been proposed [4, 17, 24, 25]. If performing a two-compartment lymphoscintigraphy, subfascial/epifascial imaging is completed first and epifascial/subfascial imaging occurs at least 24 h later, preferably 48 h, to allow for washout of the tracer from the first compartment imaged. It does not matter which compartment is examined first.
SPECT/CT study (hybrid images) improves the interpretation and increases the diagnostic accuracy of the examination by providing the exact topographic localization of the sentinel lymph node(s) with respect to the surrounding anatomical structures. With peripheral lymphedema, at the stage IA (Table 1), it can be difficult to diagnosis as there are little or no clinical indications of swelling. However, this type of lymphatic dysfunction can be demonstrated with lymphoscintigraphy, particularly an intravascular lymphostasis is seen, while the lymphatic system seems nearly normal. In cases of superficial lymphatic insufficiency, hybrid images can confirm an epifascial dermal backflow with a normal deep lymphatic system. Hybrid imaging also gives the precise location of blockage to lymphatic flow and may verify which lymph nodes are functional and which are not. For the clinician, this new imaging approach can be used to modify the treatment protocol, resulting in a well-adapted treatment [24].
Table 1.
Clinical and immunohistochemical stages of lymphedema
| Stage I | A. Latent lymphedema, without clinical evidence of edema, but with impaired lymph transport (demonstrable with lymphoscintigraphy) and with initial immunohistochemical alterations of lymph nodes, lymph vessels, and the extracellular matrix |
| B. Initial lymphedema, totally or partially relieved with rest and a draining position, with worsening impairment of lymph drainage capacity and of the immunohistochemical alterations of lymph nodes, lymph vessels, and the extracellular matrix | |
| Stage II | A. Increasing lymphedema, with vanishing lymph transport capacity, relapsing lymphangitis attacks, fibroindurative skin changes, and developing disability |
| B. Column-shaped limb fibrolymphedema, with lymphostatic skin changes, suppressed lymph transport capacity, and worsening disability | |
| Stage III | A. Properly called elephantiasis, with sclera-indurative pachydermitis, papillomatous lymphostatic verrucosis, no lymph transport capacity, and life-threatening disability |
| B. Extreme elephantiasis with total disability |
NB: these stagings were developed by Campisi (2009) and first published in Campisi C, Bellini C, Campisi C, et al. Microsurgery for lymphedema: clinical research and long-term results. Microsurgery. 2010;30(4):256–260
Processing
No processing is required in the case of acquisitions with a planar technique. In the case of tomographic studies, reconstruction using a filtered back-projection method or with iterative methods can be used.
Interpretation
Qualitative or Visual Analysis
The analysis aims to evaluate the kinetic distribution of the radiopharmaceutical as a function of time, the number of lymphatic pathways displayed, the direction of lymphatic drainage, the number of lymph node stations visualized, and their respective lymph nodes displayed. The presence of lymph nodes in atypical sites (“in-transit” lymph nodes) is indicative of slowing of the lymphatic flow is indicative of slowing of the lymphatic flow (Fig. 6).
Fig. 6.

Presence of “in-transit lymph nodes” in the deep lymphatic pathway of the lower left limb (2 h imaging), indicative of lymph stasis. Bilaterally visualization of popliteal nodes and shunt from deep to superficial pathways
Qualitative lymphoscintigraphy (i.e., visual interpretation) is in many cases sufficient to provide a reliable diagnosis but has been shown to have a diagnostic accuracy inferior to a semi-quantitative or qualitative/evaluative combination after decay corrections [24]. Kleinhans developed a transport index to evaluate the effect of transplantation of lymphatic vessels on lymphatic function. Five criteria acquired from visual interpretation were semi-quantified and included in a formula developed by the authors [25, 26] and shown in Table 2 below. The TI can be calculated using the following formula to categorize the lymphatic flow as normal or pathological where: TI = K + D + (0.04 × T) + N + V and K = transport kinetics (scored as 0—normal, 3—mild delay, 5—marked delay, 9—no transport); D = distribution of the tracer (scored as 0—normal, 3—mild dermal diffusion, 5—marked dermal diffusion, 9—absent visualization); T = time to visualize the lymph nodes(min); N = visualization of lymph nodes (scored as 0—normal, 3—mild, 5—poor, 9—absent); and V = visualization of lymph vessels (scored as 0—normal, 3—mild, 5—poor, 9—absent). A score of less than 10 signifies a normal TI and a score equal to or greater than 10 signifies a pathological TI.
Table 2.
Components of the transport index (TI)
| 0 | SCORE 3 | 5 | 9 | |
|---|---|---|---|---|
| Transport kinetics | No delay | Mild delay | Extreme delay | No flow |
| Distribution pattern | Normal | Partial dermal | Diffuse dermal | No flow |
| Time index | (Time in minutes | For appearance of | Regional lymph nodes | ×0.04) |
| Lymph nodes | Normal | Visible, diminished | Barely visible | Not seen |
| Lymph vessels | Normal | Visible, diminished | Barely visible | Not seen |
From: Cambria RA, Gloviczki P, Naessens JM, Wahner HW. Non-invasive evaluation of the lymphatic system with lymphoscintigraphy: a prospective, semiquantitative analysis in 386 extremities. J. of Vasc Surg. 1993; 18: 773–782. Republished with permission
Other quantitative methods are represented by the calculation, after correction for the physical decay of the tracer of the lymphatic transport capacity calculated as the percentual washout of the tracer from the injection site at different times (usually 30 and 120 min) or as the fraction of the injected activity transported to the reference region, usually lymphoglandular after a certain time interval [27, 28]. Quantitative methods are particularly useful in the surgical follow-up of lymphatic disorders, where the degree of pathology/normality of lymphatic flow can be followed over time.
Report
In general, the final report can be divided into five parts: patient identification details, clinical question, the instrumental part dedicated to the description of the procedure, the body of the report, and the conclusions. This report must include all the relevant information described below:
First Part: Identification
This is the part that includes the fields that identify the patient, the structure where the examination took place, the date of the examination, the type of examination, and the radioactive drug administered to the patient. The fields that generally identify the patient are as follows: name, surname, date of birth, and archive number with which the patient is cataloged in the Department of Nuclear Medicine.
Second Part: Clinical Question
This is the part dedicated to the clinical referral question and to the compilation of the summary of the patient’s clinical history.
Third Part: Procedure
This is the part dedicated to the description of the type of the radio drug administration performed, the location of injection, the injected volume, and details of the instrumentation used and of the data acquisition protocol.
Fourth Part: Body of the Text
This is the part where the exam is described. It is necessary to describe:
Delayed or rapid transit of the radiotracer compared to the normal side in unilateral edema.
The quantitative method with transport index is useful in the evaluation of bilateral edema. In this case, the transport index score is reported for each limb (and separately for superficial and deep lymphatic vessels if a two-compartment study is performed) and whether the score is normal or pathological.
Visualization of lymphatic vessels.
Visualization of lymph nodes in physiological anatomical districts.
Visualization of collateral lymphatic vessels.
Presence of abnormal lymph nodes (“in-transit nodes”).
Presence, extension, and location of “dermal backflow.”
Presence of lymphoceles or abnormal lymphoscintgraphic patterns on plain scintigraphy or in combination with SPECT acquisition.
Presence of chylothorax or chyloperitoneum.
Functional or morphological abnormalities of the thoracic duct.
Comparison with previous exams.
Fifth Part: Conclusions
This is the part in which the clinical question is answered in a clear and concise manner. It includes the description of any abnormalities of the lymphatic system, including asymmetric visualization of lymphatic channels, collateral lymphatic channels, interrupted vascular structures, and abnormal visualization of the lymph nodes of the deep lymphatic system (i.e., popliteal lymph nodes after web space injection in the lower extremities, which should only visualize the superficial nodes) expressive of a lymphatic shunt. The lack of visualization of anatomical districts should also be noted, signaling lymph node hypoplasia or aplasia.
The conclusions should help the referring physician to refer the patient, if necessary, to surgical, physical, or medical treatments. If no lymphatic abnormalities are found and a calculated transport index is normal, the conclusion should be that there any clinically evident swelling does not have a lymphatic basis.
Sources of Error
The most common sources of error that can arise during lymphoscintigraphy are the following:
External radioactive contamination.
Failure to use the SPECT/CT technique in pelvic-abdominal-thoracic diseases. The planar technique has, in fact, a very poor sensitivity in these districts.
Patient movement artifacts or instrumentation-related artifacts.
Most centers use a single injection per limb and per compartment (superficial and deep), and this is our recommendation. If two or more injections are used, caution should be exercised in the interpretation of the results. For example, lateral dorsal injections in the hand and foot for the study of the superficial circulation can also visualize the deep lymphatic compartment, with consequent visualization of tributary lymph nodes of the deep pathways of the elbow or popliteal nodes. These results must be carefully interpreted to avoid erroneously assuming that the superficial lymphatic pathways are normal, when in fact, the deep pathways are being visualized.
Conclusions
Lymphoscintigraphy is able to demonstrate the flow of lymph in both the superficial and deep lymphatic pathways and can detect abnormal lymphatic circulation patterns, such as a-, hypo-, or hyperplasia of vessels and nodes and dermal backflow [29–31]. It has been shown to have high sensitivity (96%) and specificity (100%) for lymphedema [20], capable of definitively distinguishing lymphedema from other sources of edema (e.g., venous incompetence), in cases where venous problems have been ruled out. In cases of long-standing venous incompetence and other clinical signs (positive Stemmer sign), lymphoscintigraphy can demonstrate any lymphatic flow problems that may change the diagnosis to flebolymphedema [4].
As much as possible, the diagnostic lymphoscintigraphy protocol should be standardized across patients, in terms of administration of the radiotracer in the same site with the same dosage and same timing of the image acquisition with possible exceptions in selected patients of additional administration of the radiotracer in order to visualize the proximal lymphatic collectors in cases of proximal obstruction.
The inclusion of a semi-quantitative transport index can provide a method to characterize lymphoscintigraphic exams as normal (and therefore, a non-lymphatic source of swelling) or pathological (lymphatic obstruction/abnormality). The addition of a subfascial injection to visualize the deep lymphatic vessels can provide additional information that will allow a diagnosis to be made in the sub-group of patients with only deep lymphatic vessel abnormalities that might have otherwise been classified as normal if only epifascial superficial examination is made. In our experience, we found deep vessel abnormalities in approximately 30% of the patients with normal superficial system. We recommend inclusion of the subfascial examination as part of the general protocol for lymphoscintigraphy in patients with swelling.
Acknowledgements
None.
Conflict of Interest
Giuseppe. Villa, Corrado C Campisi, Melissa Ryan, Francesco Boccardo, Pietro di Summa, Marco Frascio, Gianmario Sambuceti, and Corradino Campisi declare that they have no conflict of interest. There is no source of funding.
Ethical Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
The institutional board of our Institution reviewed the study and approved it as a retrospective study, waiving the need for formal consent. Although no formal consent was required, patients gave their written consent to use their anonymous data.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weissleder H, Weissleder R. Lymphedema: evaluation of qualitative and quantitative lymphoscintigraphy in 238 patients. Radiology. 1988;167:729–735. doi: 10.1148/radiology.167.3.3363131. [DOI] [PubMed] [Google Scholar]
- 2.Bräutigam P, Földi E, Schaiper I, Krause T, Vanscheidt W, Moser E. Analysis of lymphatic drainage in various forms of leg edema using two compartment lymphoscintigraphy. Lymphology. 1998;31:43–55. [PubMed] [Google Scholar]
- 3.Logan V. Incidence and prevalence of lymphoedema: a literature review. J Clin Nurs. 1995;4:213–219. doi: 10.1111/j.1365-2702.1995.tb00209.x. [DOI] [PubMed] [Google Scholar]
- 4.Szuba A, Shin WS, Strauss HW, Rockson S. The third circulation: radionuclide lymphoscintigraphy in the evaluation of lymphedema. J Nucl Med. 2003;44:43–57. [PubMed] [Google Scholar]
- 5.Segerstrom K, Bjerle P, Graffman S, Nystrom A. Factors that influence the incidence of brachial oedema after treatment of breast cancer. Scand J Plast Reconstr Surg Hand Surg. 1992;26:223–227. doi: 10.3109/02844319209016016. [DOI] [PubMed] [Google Scholar]
- 6.Chatani M, Nose T, Masaki N, Inoue T. Adjuvant radiotherapy after radical hysterectomy of the cervical cancer: prognostic factors and complications. Strahlenther Onkol. 1998;174:504–509. doi: 10.1007/BF03038982. [DOI] [PubMed] [Google Scholar]
- 7.Rainwater LM, Zincke H. Radical prostatectomy after radiation therapy for cancer of the prostate: feasibility and prognosis. J Urol. 1988;140:1455–1459. doi: 10.1016/S0022-5347(17)42072-6. [DOI] [PubMed] [Google Scholar]
- 8.Solsona E, Iborra I, Monros JL, Ricos JV, Mazcunan F, Vazquez C. Penile and scrotal lymphedema of radiation origin: its surgical treatment. Actas Urol Esp. 1986;10:45–48. [PubMed] [Google Scholar]
- 9.Moghimi SM, Bonnemain B. Subcutaneous and intravenous delivery of diagnostic agents to the lymphatic system: applications in lymphoscintigraphy and indirect lymphography. Adv Drug Deliv Rev. 1999;37:295–312. doi: 10.1016/S0169-409X(98)00099-4. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida RY, Kariya S, Ha-Kawa S, Tanigaw N. Lymphoscintigraphy for imaging of the lymphatic flow disorders. Tech Vasc Interv Radiol. 2016;19(4):273–276. doi: 10.1053/j.tvir.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Williams WH, Witte CL, Witte MH, McNeill GC. Radionuclide lymphangioscintigraphy in the evaluation of peripheral Lymphoedema. Clin Nucl Med. 2000;25:451–464. doi: 10.1097/00003072-200006000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Berenji GR, Iker E, Glass EC. Lymphoscintigraphic findings in chylous reflux in a lower extremity. Clin Nucl Med. 2007;32:725–728. doi: 10.1097/RLU.0b013e318123eddd. [DOI] [PubMed] [Google Scholar]
- 13.Baulieu F, Baulieu JL, Mesny J, et al. Visualization of the thoracic duct by lymphoscintigraphy. Eur J Nucl Med. 1987;13:264–265. doi: 10.1007/BF00252605. [DOI] [PubMed] [Google Scholar]
- 14.ICRP. Radiation dose to patients from radiopharmaceuticals. ICRP Publication 53. Ann ICRP. 1988;18(1–4). [DOI] [PubMed]
- 15.Keleher A, Wendt R, 3rd, Delpassand E, Stachowiak AM, Kuerer HM. The safety of lymphatic mapping in pregnant breast cancer patients using Tc-99m sulfur colloid. Breast J. 2004;10:492–495. doi: 10.1111/j.1075-122X.2004.21503.x. [DOI] [PubMed] [Google Scholar]
- 16.British Nuclear Medicine Society. BNMS clinical guidelines—lymphoscintigraphy. 2011. https://www.bnms.org.uk/images/Lymphoscintigraphy_2016_NEW.pdf.
- 17.Mostbeck A, Partsch H. Isotope lymphography: possibilities and limits in evaluation of lymph transport. Wien Med Wochenschr. 1999;149:87–91. [PubMed] [Google Scholar]
- 18.Brautigam P, Vanscheidt W, Foldi E, Krause T, Moser E. The importance of the subfascial lymphatics in the diagnosis of lower limb edema: investigations with semiquantitative lymphoscintigraphy. Angiology. 1993;44:464–470. doi: 10.1177/000331979304400606. [DOI] [PubMed] [Google Scholar]
- 19.Stanton AW, Mellor RH, Cook GJ, Svensson WE, Peters AM, Levick JR, et al. Impairment of lymph drainage in subfascial compartment of forearm in breast cancer-related lymphedema. Lymphat Res Biol. 2003;1:121–132. doi: 10.1089/153968503321642615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassanein AH, Maclellan RA, Grant FD, Greene AK. Diagnostic accuracy of lymphoscintigraphy for lymphedema and analysis of false-negative tests. Plast Reconstr Surg Glob Open. 2017;5:e1396. doi: 10.1097/GOX.0000000000001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Godoy JM, Iozzi AJ, Azevedo WF, Jr, Godoy MF. New method to assess manual lymph drainage using lymphoscintigraphy. Nucl Med Rev. 2012;15(2):140–142. [PubMed] [Google Scholar]
- 22.Suga K, Kume N, Matsunaga N, Motoyama K, Hara A, Ogasawara N. Assessment of leg oedema by dynamic lymphoscintigraphy with intradermal injection of technetium-99m human serum albumin and load produced by standing. Eur J Nucl Med. 2001;28(3):294–303. doi: 10.1007/s002590000418. [DOI] [PubMed] [Google Scholar]
- 23.Weiss M, Baumeister RG, Hahn K. Dynamic lymph flow imaging in patients with oedema of the lower limb for evaluation of the functional outcome after autologous lymph vessel transplantation: an 8-year follow-up study. Eur J Nucl Med Mol Imaging. 2003;30(2):202–206. doi: 10.1007/s00259-002-1020-1. [DOI] [PubMed] [Google Scholar]
- 24.Baulieu F, Bourgeois P, Maruani A, Belgrado JP, Tauveron V, Lorette G, et al. Contributions of spect/ct imaging to the lymphoscintigraphic investigations of the lower limb lymphedema. Lymphology. 2013;46:106–119. [PubMed] [Google Scholar]
- 25.Kleinhans E, Baumeister RGH, Hahn D, Siuda S, Bull U, Moser E. Evaluation of transport kinetics in lymphoscintigraphy: follow-up study in patients with transplanted lymphatic vessels. Eur J Nucl Med. 1985;10:349–352. doi: 10.1007/BF00251310. [DOI] [PubMed] [Google Scholar]
- 26.Weiss M, Baumeister RGH, Frick A, Wallmichrath J, Bartenstein P, Rominger A. Primary lymphedema of the lower limb: the clinical utility of single photon emission computed tomography/CT. Korean J Radiol. 2015;16:188–195. doi: 10.3348/kjr.2015.16.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cambria RA, Gloviczki P, Naessen JM, Wahner HW. Noninvasive evaluation of the lymphatic system with lymphoscintigraphy: a prospective, semi-quantitative analysis in 386 extremities. J Vasc Surg. 1993;18:773–782. doi: 10.1016/0741-5214(93)90331-F. [DOI] [PubMed] [Google Scholar]
- 28.Keramida G, Wroe E, Winterman N, Aplin M, Peters AM. Lymphatic drainage efficiency: a new parameter of lymphatic function. Acta Radiol. 2017. [DOI] [PubMed]
- 29.Dylke ES, McEntee MF, Schembri GP, Brennan PC, Bailey E, Ward LC, et al. Reliability of a radiological grading system for dermal backflow in lymphoscintigraphy imaging. Acad Radiol. 2013;20:758–763. doi: 10.1016/j.acra.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 30.Baulieu F, Tauveron V, Erra B, Muller C, Courtehoux M, Venel Y, et al. Lymphoscintigraphy in limb lymphoedema: current methodology and interests. Méd Nucl. 2015;39:26–42. doi: 10.1016/j.mednuc.2015.02.009. [DOI] [Google Scholar]
- 31.O'Donnell TF, Rasmussen JC, Sevick-Muraca EM. New diagnostic modalities in the evaluation of lymphedema. J Vasc Surg: Venous Lymphat Disord. 2017;5:261–273. doi: 10.1016/j.jvsv.2016.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]