Personalized medicine is the core concept of modern medicine and whatever it is called in any newly coined names, it manifests that medicine is practiced in a customized way to help individual persons in any phase of medical intervention such as prevention, classification and prognostic characterization (diagnosis), therapy and rehabilitation/regeneration. The most instrumental among these interventions is therapy which should be obviously personalized. Novel therapy for inhibition of immune checkpoint with monoclonal antibodies were phenomenally successful [1] in treating some (less than 20%) susceptible patients (with melanoma) and raised immediately the importance of in vitro companion diagnostics. In 2016, Food and Drug Administration (FDA) of the USA published a document “Principles for codevelopment of an in vitro companion diagnostic device with a therapeutic product” which mandates the development of in vitro diagnostic (IVD) companion diagnostic devices contemporaneously with the approval of the novel therapeutic products [2]. This is also adopted by other countries, for example, Korea in its publication of similar announcement in 2018 as “Guideline for approval of medicines associated with companion diagnostic devices” by Korean Ministry of Food and Drug Safety [3]. In the USA, 35 novel therapeutic products as new drug application (NDA) or biologic license application (BLA) were approved as companion diagnostics as of December 6, of 2018 [4]. What is interesting is that FDA defined a companion diagnostic as in vitro diagnostic device or an imaging tool to prove safe and effective use of companion therapeutic products. Here the term “effective’ means that companion diagnostic devices can discern whether the companion therapeutic product will take effect on the patients
Here rises another important discipline, in vivo companion diagnostics. Novel therapeutic products, either monoclonal antibodies or small molecules, if given to the patients, will show the characteristic distribution known as pharmacokinetics and the expected effect on the target tissues as pharmacodynamics. Advanced confirmation of successful targeted delivery and/or therapeutic effects in humans can be estimated by pilot in vivo imaging with radionuclide-labeled therapeutic products. This is in vivo companion diagnostics. Voila, this practice is the nuclear imaging belonging to the authentic and classical nuclear medicine. Up until now, nuclear medicine prospered highly while doing these works and was not just called as in vivo companion diagnostics. Notwithstanding the coexistent identity of nuclear medicine as in vivo companion diagnostics, however, in vivo companion diagnostics is unique in its identity to represent the general methods of using nuclear or non-radioactive ones such as ultrasonography. Nuclear imaging is used for the similar purpose of the prediction of desired biodistribution and thus the expected effect on the target tissues/organs by administering the “radiolabeled” novel therapeutic products. In this endeavor, of course, there exists an assumption that the radiolabeled novel therapeutic product is going to behave the same way as unlabeled therapeutic product. Fortunately, nuclear medicine community has evolved to the one which are really capable of producing radionuclide-labeled biological molecules, for example, tositumomab, a murine monoclonal anti-CD20 antibody which once was used as commercialized clinical product for treating patients [5]. The sameness of labeled and unlabeled therapeutic products should have been confirmed by meticulous evaluation beforehand. Microdosing in humans might help perform this comparison study in which collaboration between nuclear medicine and clinical pharmacology/therapeutics are highly warranted. Microdosing is performed either accelerator mass spectrometry (AMS) or positron emission tomography (PET) by the above two disciplines of nuclear medicine and clinical pharmacology, respectively [6–8]. In vivo companion diagnostics shall be the essential contribution of nuclear medicine theranostics.
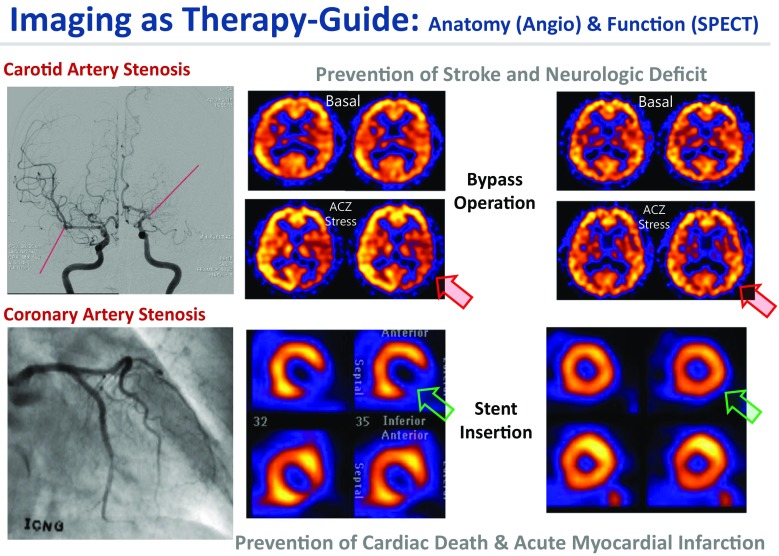
Theranostics is simply combined therapy and diagnostics and thus nuclear theranostics is radionuclide/radiopharmaceutical therapy and nuclear medicine imaging. This concept of theranostics of combined nuclear imaging and therapy emphasizes the existence of successful therapy using radionuclides and radiopharmaceuticals. Nuclear theranostics is broadening its narrow sense of guiding the radionuclide therapy of cancers to the image-guided therapy of other non-communicable diseases such as carotid or coronary artery stenosis. In these vascular diseases, during the past decades, nuclear imaging has well established its utility as guides for the following therapy of angioplasty, stent insertion, and artery bypass graft surgery (Fig. 1). Appropriate uses of exercise EKG, CT coronary angiography, rest/stress myocardial perfusion SPECT, and contrast coronary angiography are very well established. And this establishment had been based not only on the diagnostic accuracy of determining significant stenosis, characterization of the functional significance of culprit coronary arteries, but also more importantly on the stratification of prognostic significance of the anatomical stenosis and “preservation or impairment” of the vascular reserve on myocardial perfusion SPECT. Basal/acetazolamide SPECT has also shown the similar utility determining whether a stenosis in internal carotid or cerebral arteries should be treated by external carotid artery-internal carotid artery (EC-IC) bypass, encephalo-duro-artero-synangiosis (EDAS), or medical treatments. Here the emphasis was put as therapeutic choices on the stent insertion/bypass surgery or EC-IC bypass/EDAS surgery, respectively. Once the patients have definite treatment, the therapy-guiding role of nuclear imaging was seamlessly integrated in the triage of patients in the clinics. We aim at prevention of further cardiac death or acute myocardial infarction in coronary artery disease or prevention of stroke or its sequelae and even death in carotid artery disease. Rest/stress myocardial and brain perfusion SPECT do the pivotal roles in this endeavor.
Fig. 1.
Best examples of nuclear medicine procedures used as in vivo companion diagnostics. Among the non-communicable diseases, brain perfusion SPECT helped choose stent insertion or bypass operation for carotid/cerebral artery stenosis/obstruction and myocardial SPECT predicted major adverse cardiac events (cardiac death and acute myocardial infarction) and helped choose needs of stenting/bypass operation for coronary artery diseases. Anatomical imaging with angiography was used to define the diseases and functional imaging with SPECT was used to define the functional significance
In cancers, nuclear medicine community is progressing rapidly to achieve the similar goal. Fortunately, they invented Lu-177 DOTATATE and similar radiopharmaceuticals and Lu-177 ligands for folate hydrolase 1 (a.k.a. prostate-specific membrane antigen (PSMA)) for neuroendocrine tumors and castration-resistant prostate cancers (Fig. 2), respectively. Encouraged by the successful palliation of metastatic bone cancers by Ra-223, alpha emitters Ac-225 and others are actively investigated to be associated with the somatostatin receptor 2 ligands or PSMA ligands.
Fig. 2.
Summary of theranostics in cancer by nuclear theranostic procedures
In this special issue, we invited the reviews and perspectives of this novel field of nuclear theranostics especially in Asia. Interested readers can refer to the special issue published in Journal of Nuclear Medicine a year ago [9]. We, the editors were interested in the expansion of the discipline of nuclear theranostics not confined to neuroendocrine tumors, castration-resistant prostate cancers and metastatic bone tumors (Fig. 2). Brain theranostics were introduced in the December issue of this Journal, Nuclear Medicine and Molecular Imaging, [10] and possibility of therapeutic use of exosomes (or extracellular vesicles) follows in this issue [11]. Moreover, we invited Asian country representatives to describe their understanding and endeavor to introduce or innovate the nuclear theranostics in his/her own countries most recently.
Bautista from Philippines [12] described her country’s current status that Ga-68 DOTADATE and PSMA PET/CT scans came to be available in January of 2018 and the monumental first neuroendocrine tumor or PSMA therapy took place in the mid-2018 too. Cancer patients in this category of the Philippines no longer seek institutions/hospitals in other countries, such as Malaysia, Australia, or Germany unlike Korea.
Budiawan of Indonesia [13] in a composed way described the situation of Indonesian endeavor, in which the Indonesian leaders struggle to expand the nuclear medicine practice all over the big islands whose next-generation leaders are supplied by the country’s unique education center, Hasan Sadikin General Hospital in Bandung, so far the only nuclear medicine training center in Indonesia. The past success of radioiodine treatment and the prosperity of private sectors as well as the collaboration of his institution and Indonesian Atomic Agency raises hopes of realization of everyday practice of nuclear theranostics
Kinuya of Japan [14] described the recent Japanese efforts to realize targeted radionuclide therapy, another name of nuclear theranostics, in Japan with their advanced science and medicine and also within their national strict regulatory frame. He described the hurdles and obstacles and also the strategy to overcome these, i.e., Japanese National Conference for Nuclear Medicine Theranostics and Japan Foundation of Medical Isotope Development. Further to the concrete efforts by several Japanese pioneers, the nature of personalized medicine is going to embrace omics moving forward to the future.
Al-Ibraheem of Jordan [15] described Jordan’s stride which really encouraged the other Asian country experts. In Jordan, especially in King Hussein Cancer Center in Amman, Jordanian efforts ranged from successful Ga-68 DOTA peptide scan, its use for triage, Lu-177 DOTATAE scan for neuroendocrine tumors, Ga-68 PSMA PET progressively replacing conventional ones, and Lu-177 PSMA therapy being included as a welcome treatment by multidisciplinary genitourinary clinic. He further described the success and hope of I-131 metaiodobenzyl guanidine (MIBG) as the well-trusted classical treatment for neuroblastoma and the related ones.
Bozkurt of Turkey [16] described the advanced and universal practice of nuclear theranostics in Turkey. In addition to the traditional theranostics using I-131 and I-131 MIBG, dozens of centers have performed Lu-177 DOTA peptides or Y-90 peptides therapy, Lu-177 PSMA therapy in 2,500 cases annually or 600 cases annually, respectively. Interestingly, all these treatments are under the supervision of Turkish Atomic Energy Authority and few such as Ra-223 are not covered by national insurance reimbursement system, meaning all the others are covered by it. Based upon this great success, Turkish nationwide move is expected to adopt Ac-225 PSMA and hopefully others too. He phrased an allegory that Turkish move is bridging the advanced European effort to the following Asian struggles.
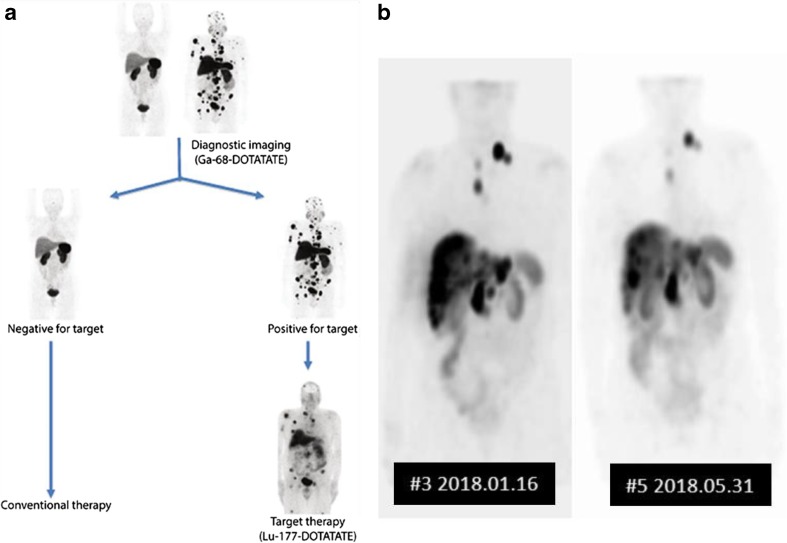
In contrast, in Korea, we are just about to start a clinical trial of Lu-177 DOTATATE therapy, as we failed to establish orphan drug use, compassionate use or any special consideration of radionuclide/radiopharmaceutical therapy/theranostics. Ga-68 DOTATOC is available clinically after passing through Korean Ministry of Food and Drug Safety and New Technology Assessment Committee of Korea. What is missing is the availability of these definitive treatments which are proven and approved in several countries now, and thus 58 patients were sent to a center of Malaysia and treated with Lu-177 DOTATATE last year. Instead, decade ago, Korea encouraged nationwide screening of thyroid and experienced the screening-based “epidemic” of thyroid cancer without changing the mortality [17]. This epidemic provoked hot debate and subsided after the abatement of hype of thyroid screening with ultrasonography for general public. As the phase 3 trial of Lu-177 DOTATAE in neuroendocrine tumors was reported in New Engl J Med in 2017 [18], Koreans, both the physicians and the public, felt urgent, and started the clinical trial. According to the protocol in Fig. 3a, patients coming back from Malaysia, Lu-177 SPECT imaging was done as in Fig. 3b
Fig. 3.
Theranostic use of imaging in the successfully treated cases by nuclear medicine. a Theranostic triage of patients with neuroendocrine tumors. Ga-68 DOTATATE PET is used to choose patients needing Lu-177 DOTATATE treatment. b A case who received Lu-177 DOTATATE treatment and contemporaneous SPECT imaging on the first treatment and the follow-up treatment
The core of nuclear theranostics resides in the capability of nuclear diagnostic imaging to guide therapy and thus as was explained above, nuclear theranostics include rest/stress myocardial perfusion SPECT, despite fractional flow reserve (FFR) to guide further coronary intervention as a biomarker, or basal/acetazolamide brain perfusion SPECT to guide further bypass operations. Yet-to-be intractable cancers await contemporaneous therapy and diagnostic imaging methods and the prerequisite was the discovery/creation of new radiopharmaceuticals. Small molecules in the recent reports such as (1) F-18 Fluoro-2’deoxycytidine for in vivo companion diagnostic imaging associated with anti-tumor chemotherapeutic Fluoro-2’deoxycytines [19], (2) 6-O-[F-18]-Fluoroethylerlotinib or C-11 Erlotinib for in vivo companion diagnostic imaging associated with therapeutic receptor tyrosine kinase inhibitor Erlotinib [20], and (3) no in vivo companion diagnostics but only in vitro companion diagnostics such as BRACAnalysis CDx™ and other devices associated with strikingly promising oral drug poly (ADP-ribose) polymerase (PARP) inhibitor Olaparib and various –paribs [21]. The above two can be followed for their biodistribution and pharmacokinetics in individual patients, but the third one orally administered drug olaparib’s whereabouts are unknown in individual patients. We only know that the patients have germline BRCA mutation which was indicated to take the drugs, olaparib, or the similar drugs. However, people investigate earnestly in vivo companion imaging diagnostics of similar drug, rucaparib using radiotracer technology [22–24].
Peptides, either small or antibody, also called upon companion diagnostic imaging, which was detailed as a review by Krasniqi and colleagues [25]. If the investigators do radionuclide-labeled monoclonal antibody imaging for further guide of alpha/beta emitting radionuclide therapy, it is called immunoPET, and PD-1:PD-L1 immunoPET was the most wanted from the clinical standpoints [26]. Leading immune checkpoint therapy drugs such as nivolumab or pembrolizumab were labeled with Zr-89 for the characterization of biodistribution and thus pharmacokinetics of these antibodies [27]. F-18 or even Tc-99m was also tried for this approach and in all these cases the therapeutic drugs were immune checkpoint inhibitor monoclonal antibodies. Multiple myeloma and CD138 was the target of another immunoPET using Cu-64 [28].
In addition to these small peptides or macromolecules such as monoclonal antibodies and their derivatives, such as affibody, avibody, nanobody, aptide, or even aptamer, little larger particulate materials, nanomaterials were considered as candidates of targeted delivery. By labeling radionuclides on the surface (more commonly) or in the core (less commonly), radionuclide-labeled nanomaterials [29, 30] become very attractive in vivo companion diagnostics. For example, photodynamic effects of nanomaterials are expected to exert therapeutic effects; radio-nanomaterials are exactly the radiopharmaceuticals for nuclear theranostics as well as in vivo companion diagnostics [30, 31]. Classical liposomes can also be easily labeled within them or on the surface with Cu-64 and could be used as in vivo companion diagnostic imaging. Just like Tc-99m HMPAO inside the exosomes [32], Cu-64 was put within the liposome and was validated to reside there within liposomes after systemic administration [33].
These approaches were summarized as a review recently by Notni and Wester [34], while emphasizing the therapeutic radioactive metals Lu-177, Ac-225, and Bi-213, and the final frontier was predicted to be F-18. We need to wait for the time to confirm this prediction and desire the sincere endeavors of our colleague radiochemists and radiopharmacists.
A new disruptive innovation should always be critically evaluated with the concerns by the experts as peers in medicine or by the public including scientists or policy makers. There is woo for promising immune checkpoint inhibitor therapy with associated exciting drugs [35] and also a cautious alert about hasty optimism for genome-guided cancer treatment [36]. Likewise, in vivo companion diagnostics especially with novel biopharmaceuticals or even radio(bio)pharmaceuticals are to be scrutinized prudently in their fit roles in multidisciplinary therapy against non-communicable diseases of significant global burden. In this sense, the satisfaction of the recent progress of nuclear theranostics in several tumors and the too early contentment with the speed of the innovation and development are to be warned. Nevertheless, in vivo companion diagnostics with nuclear theranostics are well based on the long history and trials-and-errors of clinical nuclear medicine and thus, we stand strongly on our own to proceed further to devise or propose the new therapy and theranostics for all the unsolved clinical problems.
In this issue [12–16] and online [37–42] and also the following issues, we will see the current status of state-of-art theranostics in Asian countries, however, beyond the geographic extension of nuclear theranostics, disciplinary expansion above the current obsession with peptides or small molecules, or above a few successful tumors are to be expected with full inspiration in our collective mind of nuclear medicine community. Asian colleagues and also all the colleagues, either current or next-generation leaders, regardless of their nationality with various economic status of their own countries, will develop the novel in vivo theranostics including nuclear theranostics. We are very much open to expand any of our definition of nuclear theranostics, in vivo companion diagnostics to the every other organ/tissue and any disruptive innovative approaches if and only if they solve the current clinical problems. In this special issue, please enjoy the incipient movements inherent in the descriptions of many authors for the near future of our nuclear medicine as theranostics.
Acknowledgments
We thank Dr. Min Seok Suh of Seoul National University for making Fig. 3.
Compliance with Ethical Standards
Conflict of Interest
Dong Soo Lee and Gi Jeong Cheon declare that there is no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 2.Food and Drug Administration of USA. Principles for codevelopment of an in vitro companion diagnostic device with a therapeutic product. 2016 https://www.fda.gov/medicaldevices/productsandmedicalprocedures/invitrodiagnostics/ucm407297.htm. Accessed 8 Dec 2018.
- 3.Korean Ministry of Food and Drug Safety. Guideline for approval of medicines associated with companion diagnostic devices. 2018 http://www.nifds.go.kr/brd/m_15/view.do?seq=12442. Accessed 8 Dec 2018.
- 4.Food and Drug Administration of USA. List of cleared or approved companion diagnostic devices (in vitro and imaging tools). 2018.
- 5.Prasad V. The withdrawal of drugs for commercial reasons: the incomplete story of tositumomab. JAMA Intern Med. 2014;174:1887–1888. doi: 10.1001/jamainternmed.2014.5756. [DOI] [PubMed] [Google Scholar]
- 6.Karlsson C. Exploratory clinical studies (“phase 0 trials”). In Principles of translational science in medicine (Second Edition) 2015 (pp. 229–233).
- 7.Food and Drug Administration of USA. Guidance for industry, investigators, and reviewers: exploratory IND studies 2006 https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM078933.pdf. Accessed 8 Dec 2018.
- 8.Food and Drug Administration of USA. Microdose radiopharmaceutical diagnostic drugs: nonclinical study recommendations guidance for industry. 2018. https://www.fda.gov/downloads/Drugs/.../Guidances/UCM575453.pdf. Accessed 8 Dec 2018.
- 9.Herrmann K, Larson SM, Weber WA. Theranostic concepts: more than just a fashion trend-introduction and overview. J Nucl Med. 2017;58(Suppl 2):1S–2S. doi: 10.2967/jnumed.117.199570. [DOI] [PubMed] [Google Scholar]
- 10.Suh M, Lee DS. Brain theranostics and radiotheranostics: exosomes and graphenes in vivo as novel brain theranostics. Nucl Med Mol Imaging. 2018;52:407–419. doi: 10.1007/s13139-018-0550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang DW. Perspectives in nuclear theranostics using exosome for brain. Nucl Med Mol Imaging. [DOI] [PMC free article] [PubMed]
- 12.Bautista PA. The emergence of theranostics in the Philippines: overcoming challenges and bringing hope. Nucl Med Mol Imaging. 2019;53. [DOI] [PMC free article] [PubMed]
- 13.Budiawan H. Nuclear theranostics in Indonesia: past, present, and future. Nucl Med Mol Imaging. 2019;53. [DOI] [PMC free article] [PubMed]
- 14.Kinuya S. Activities for the development of targeted radionuclide therapy in Japan. Nucl Med Mol Imaging. 2019;53. [DOI] [PMC free article] [PubMed]
- 15.Al-Ibraheem A. Current status of theranostics in Jordan. Nucl Med Mol Imaging. 2019;53. [DOI] [PMC free article] [PubMed]
- 16.Bozkurt F. Nuclear theranostics in Turkey. Nucl Med Mol Imaging. 2019;53. [DOI] [PMC free article] [PubMed]
- 17.Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic”--screening and overdiagnosis. N Engl J Med. 2014;371:1765–1767. doi: 10.1056/NEJMp1409841. [DOI] [PubMed] [Google Scholar]
- 18.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. NETTER-1 trial investigators. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376:125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young CR, Adler S, Eary JF, Lindenberg ML, Jacobs PM, Collins J, et al. Biodistribution, tumor detection and radiation dosimetry of F-18 5-Fluoro-2'-Deoxycytidine (18F-FdCyd) with Tetrahydrouridine in solid tumors. J Nucl Med. [DOI] [PMC free article] [PubMed]
- 20.Shamni O, Grievink H, Itamar B, Mishani E, Abourbeh G. Development of a fluorinated analogue of erlotinib for PET imaging of EGFR mutation–positive NSCLC. Mol Imaging Biol. [DOI] [PubMed]
- 21.Chen Y, Du H. The promising PARP inhibitors in ovarian cancer therapy: from Olaparib to others. Biomed Pharmacother. 2018;99:552–560. doi: 10.1016/j.biopha.2018.01.094. [DOI] [PubMed] [Google Scholar]
- 22.Makvandi M, Xu K, Lieberman BP, et al. A radiotracer strategy to quantify PARP-1 expression in vivo provides a biomarker that can enable patient selection for PARP inhibitor therapy. Cancer Res. 2016;76:4516–4524. doi: 10.1158/0008-5472.CAN-16-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carney B, Kossatz S, Reiner T. Molecular imaging of PARP. J Nucl Med. 2017;58:1025–1030. doi: 10.2967/jnumed.117.189936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kossatz S, Carney B, Farley C, Weber WA, Drain CM, Reiner T. Direct imaging of drug distribution and target engagement of the PARP inhibitor rucaparib. J Nucl Med. 2018;59:1316–1320. doi: 10.2967/jnumed.117.205765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krasniqi A, D’Huyvetter M, Devoogdt N, Frejd FY, Sörensen J, Orlova A, et al. Same-day imaging using small proteins: clinical experience and translational prospects in oncology. J Nucl Med. 2018;59:885–891. doi: 10.2967/jnumed.117.199901. [DOI] [PubMed] [Google Scholar]
- 26.Broos K, Lecocq Q, Raes G, Devoogdt N, Keyaerts M, Breckpot K. Noninvasive imaging of the PD-1: PD-L1 immune checkpoint: embracing nuclear medicine for the benefit of personalized immunotherapy. Theranostics. 2018;8:3559. doi: 10.7150/thno.24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKnight BN, Viola-Villegas NT. 89Zr-ImmunoPET companion diagnostics and their impact in clinical drug development. J Labelled Comp Radiopharm. 2018;61:727–738. doi: 10.1002/jlcr.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailly C, Gouard S, Lacombe M, Remaud-Le Saëc P, Chalopin B, Bourgeois M, et al. Comparison of immuno-PET of CD138 and PET imaging with 64CuCl2 and 18F-FDG in a preclinical syngeneic model of multiple myeloma. Oncotarget. 2018;9:9061. doi: 10.18632/oncotarget.23886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee DS, Im HJ, Lee YS. Radionanomedicine: widened perspectives of molecular theragnosis. Nanomedicine. 2015;11:795–810. doi: 10.1016/j.nano.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Oh SW, Lee DS. Therapeutic/theranostic use of radionanomedicine. In Radionanomedicine 2018 (pp. 431-442). Springer, Cham.
- 31.Ehlerding EB, Grodzinski P, Cai W, Liu CH. Big potential from small agents: nanoparticles for imaging-based companion diagnostics. ACS Nano. 2018;12:2106–2121. doi: 10.1021/acsnano.7b07252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee H, Gaddy D, Ventura M, Bernards N, de Souza R, Kirpotin D, et al. Companion diagnostic 64Cu-liposome positron emission tomography enables characterization of drug delivery to tumors and predicts response to cancer nanomedicines. Theranostics. 2018;8:2300. doi: 10.7150/thno.21670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang DW, Choi H, Jang SC, Yoo MY, Park JY, Choi NE, et al. Noninvasive imaging of radiolabeled exosome-mimetic nanovesicle using 99mTc-HMPAO. Sci Rep. 2015;5:15636. doi: 10.1038/srep15636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Notni J, Wester HJ. Re-thinking the role of radiometal isotopes: towards a future concept for theranostic radiopharmaceuticals. J Labelled Comp Radiopharm. 2018;61:141–153. doi: 10.1002/jlcr.3582. [DOI] [PubMed] [Google Scholar]
- 35.Garber K. A new cancer immunotherapy suffers a setback. Science. 2018;360:588. doi: 10.1126/science.360.6389.588. [DOI] [PubMed] [Google Scholar]
- 36.Kaiser J. Is genome-guided cancer treatment hyped? Science. 2018;360:365. doi: 10.1126/science.360.6387.365. [DOI] [PubMed] [Google Scholar]
- 37.Lin K-H, Chen Y-W, Lee R-C, Wang L-W, Chou F-I, Chang C-W, et al. Nuclear theranostics in Taiwan. Nucl Med Mol Imaging. 2019 In press. [DOI] [PMC free article] [PubMed]
- 38.Bashir H. Nuclear medicine theranostics – perspectives from Pakistan. Nucl Med Mol Imaging. [DOI] [PMC free article] [PubMed]
- 39.Myint K. Dawn of theranostics in Myanmar (dream, reality and constraint). Nucl Med Mol Imaging. [DOI] [PMC free article] [PubMed]
- 40.Pham CP. Efforts in the formation and development of nuclear medicine in Vietnam. Nucl Med Mol Imaging. [DOI] [PMC free article] [PubMed]
- 41.Choudhury PS, Gupta M. Theranostics in India: a particularly exquisite concept or an experimental tool. Nucl Med Mol Imaging. 2019 In press. [DOI] [PMC free article] [PubMed]
- 42.Huang HL, Tong AKT, Thang SP, Yan SX, Lam WWC, Loke KSH, et al. Current status and growth of theranostics in Singapore. Nucl Med Mol Imaging. 2019 In press. [DOI] [PMC free article] [PubMed]