Abstract
Background
This study aimed to compare the efficacy and safety of bolus norepinephrine, phenylephrine, and ephedrine in parturient with preeclampsia who had hypotension during cesarean delivery under spinal anesthesia.
Material/Methods
One hundred and sixty-six parturient women with preeclampsia who had a baseline systolic blood pressure (SBP) <80% during spinal anesthesia for cesarean section were divided into three treatment groups; bolus norepinephrine 4 μg (group N) (n=56), phenylephrine 50 μg (group P) (n=55), and ephedrine 4 mg (group E) (n=55). Primary outcomes included overall SBP and heart rate (HR) until delivery. Secondary outcomes included the incidence of tachycardia (HR >120 bpm), bradycardia (HR <60 bpm), hypertension (SBP >120% baseline), number of boluses of vasopressor required and episodes of hypotension, maternal side effects, and neonatal outcome.
Results
Overall HR in group N was significantly increased compared with group P (80.5±12 vs. 76.6±6.9 bpm; P=0.04), and significantly lower compared with group E (80.5±12 vs. 84.9±7.1 bpm; P=0.02). Parturients in group N had fewer episodes of bradycardia compared with group P (3.6% vs. 21.8%; RR=0.26l; 95% CI, 0.07–0.73; P=0.004) and fewer episodes of tachycardia compared with group E (16.1% vs. 36.4%; RR 0.54; 95% CI, 0.29–0.90; P=0.02).
Conclusions
A bolus dose of norepinephrine showed similar efficacy to phenylephrine but improved maternal and neonatal safety in parturients with preeclampsia with hypotension during cesarean section under spinal anesthesia.
MeSH Keywords: Ephedrine, Norepinephrine, Pre-Eclampsia, Treatment Outcome, Phenylephrine
Background
Preeclampsia in parturients, or women before giving birth, is associated with significant morbidity in between 5–7% of cases. Preeclampsia is characterized by the abnormal development of maternoplacental blood vessels, resulting in increased vascular vasomotor responsiveness and the potential for placental hypoperfusion [1]. Currently, spinal anesthesia and cesarean section are a standard treatment for women with preeclampsia in the absence of an indwelling epidural catheter or contraindications to neuraxial anesthesia [2]. Although hypotension is less frequent and easier to treat in patients with preeclampsia when compared with normotensive parturients, hypotension during spinal anesthesia is undesirable in the presence of fetoplacental hypoperfusion. Therefore appropriate intervention for spinal hypotension is necessary and may include fluid loading, lateral positioning, and the use of vasopressors [3].
Phenylephrine and ephedrine are two of the most commonly used vasopressors for the treatment of hypotension. Phenylephrine is a pure α-adrenergic receptor agonist with no β agonism properties, can restore the spinal anesthesia-induced decrease of systemic vascular resistance, and is currently recommended as first-line vasopressor treatment during cesarean section with spinal anesthesia [4]. However, phenylephrine is associated with a dose-dependent decrease in heart rate (HR) and cardiac output. Ephedrine is also a commonly used vasopressor, with a sympathomimetic activity that exerts positive inotropic and chronotropic effects on the heart via stimulation of α-adrenergic and β-adrenergic receptors and is favorable for maintaining uterine blood flow. However, the use of ephedrine has potential adverse outcomes including supraventricular tachycardia, tachyphylaxis, reactive hypertension, and fetal acidemia [5]. Severe fetal acidemia, or fetal acidosis, typically defined as a pH <7.20 in the umbilical artery, can lead to poor neonatal outcomes [6].
Recently, the vasopressor, norepinephrine, has attracted increasing attention in obstetric anesthesia. Norepinephrine has a weak β-receptor agonist activity and no properties of α-receptor agonism. Therefore, norepinephrine might have less tendency to decrease HR, resulting in improved maintenance of cardiac output when compared with phenylephrine. Also, norepinephrine has been shown to be likely to increase HR compared with ephedrine, reducing the risk of tachycardia-related maternal arrhythmia. Current literature indicates that the time of onset for the activity of for norepinephrine is less than 60 seconds [7], which is more rapid than for ephedrine, which takes 2 or 3 minutes. Also, as a catecholamine, norepinephrine does not readily cross the placenta, and maternofetal transfer has been shown to be 11.6±0.6% in a in vitro perfused human placental [8].
In low-risk normotensive parturients, several studies have now supported the efficacy and safety of norepinephrine in the management of maternal hypotension using different dosing regimens and schedules [9–13]. However, there is limited information available for the use of norepinephrine in parturients with preeclampsia and hypotension.
Therefore, this study aimed to compare the efficacy and safety of bolus norepinephrine, phenylephrine, and ephedrine in parturient women with preeclampsia who had hypotension during cesarean delivery with spinal anesthesia. The comparative clinical study included the measurement of maternal hemodynamics, vasopressor requirements, and maternal side-effects, neonatal Apgar scores, and umbilical artery pH and blood gases.
Material and Methods
Subjects and ethics
This study was approved by the Clinical Research Ethics Committee of Nanjing Medical University, Nanjing, China (No 2018-79) and was registered in the Chinese Clinical Trial Registry (ChiCTR1800019408). The study was conducted between January to June 2018 at a maternal and child healthcare hospital in Nanjing, China.
Inclusion criteria
Parturient women were recruited according to the following inclusion criteria: American Society of Anesthesiologists (ASA) status I or II, singleton, non-laboring, scheduled for spinal anesthesia and diagnosed with pre-eclampsia. The cut-off blood pressure (BP) value for pre-eclampsia is ≥140/90 mmHg at least twice with an interval of 4 h, comorbid with 24 h proteinuria ≥300 mg or ≥1+ with a dipstick. The blood pressure (BP) value for severe pre-eclampsia is ≥160/110 mmHg, comorbid with one or more of the following abnormalities: thrombocytopenia, cerebral or visual disturbance; pulmonary edema; liver function impairment; and impairment of renal function [14]. Eligible parturients were invited to participate in the study immediately after entering the operating room.
Exclusion criteria
Exclusion criteria included the following: a diagnosis of chronic hypertension or comorbid chronic hypertension with preeclampsia; comorbidity with diabetes mellitus or cardiovascular disease; twin gestation; suspected fetal compromise. For those not willing to participate in the study, standard obstetric procedures were used, and their data were not collected.
Allocation to the treatment groups
Allocation to the treatment groups was determined using computer-generated random numbers, sealed in an opaque envelope, and held by one of the researchers. Just before spinal anesthesia, an allocation number was used to determine which vasopressor would be given. In cases of spinal anesthesia failure or when no spinal hypotension was administered, the assigned number was automatically allocated to the next subject. Both patient and anesthesiologist were unaware of the study drug allocation.
Intraoperative monitoring and patient management
After obtaining written informed consent, the parturient was positioned in a supine position having a wedge under their right hip to achieve left uterine displacement (LUD). The antecubital vein was cannulated with an 18G indwelling needle to establish vascular access. A BSM 2351K monitor (Nihon Kohden, Tomioka, Japan) was attached to detect blood pressure (BP), heart rate (HR), and pulse oximetry, with the average of three consecutive readings was taken as the baseline value.
Spinal anesthesia was performed with patients in a left lateral position. Hyperbaric bupivacaine 0.5% was injected through a 25-G spinal needle, in a volume of 2.0–2.2 ml, based on the patient’s height, at the L2–3 or L3–4 intervertebral space. The parturient was then returned to the LUD position. Immediately preceding intrathecal injection, Ringer’s lactate solution was infused at a maximum rate of 10 ml/kg.
BP and HR were recorded every minute from intrathecal injection until delivery. Women were randomly allocated to receive bolus norepinephrine 4 μg (Group N), phenylephrine 50 μg (Group P), or ephedrine 4 mg (Group E) to rescue maternal hypotension, defined as systolic blood pressure (SBP) <80% of baseline. One researcher for all patients prepared either norepinephrine, phenylephrine or ephedrine and diluted by normal saline to 4 μg/ml, 50 μg/ml, or 4 mg/ml, respectively. Intravenous atropine 0.5 mg was injected for bradycardia (HR <60 bpm) comorbid with hypotension, or for HR <50 bpm irrespective of blood pressure. The study endpoint was set at delivery. An umbilical artery blood sample was collected from a double-clamped cord, and blood gas was immediately analyzed using a GEM Premier 3000 system (Synergy Medical Systems LLP, Mumbai, India). One pediatrician evaluated the neonatal Apgar score at one minute and at five minutes.
Primary and secondary outcomes
The primary outcome was the overall maternal SBP and HR throughout the observational period. Secondary outcomes measured included the incidence of tachycardia (HR >120 bpm), bradycardia (HR <60 bpm), and hypertension (SBP >120% of baseline); number of vasopressor boluses required; number of hypotension episodes (from the emergence of hypotension until its recovery to the defined threshold); maternal side effects, including nausea, vomiting, dizziness and shivering; and neonatal outcome, including Apgar scores at one minute and five minutes; umbilical artery blood gas and pH. Nausea was a self-reported sensation to vomit, while vomiting was recorded in case of rhythmic abdominal muscle contraction whether gastric contents were expulsed or not, both collectively defined as intraoperative nausea and vomiting (IONV). An independent researcher recorded all hemodynamic variables, as well as maternal and neonatal outcome in this study. Sensory dermatome block during anesthesia was determined by pinprick testing, and if adequate, it reached the T5 distribution, and surgery commenced. During anesthesia and surgery, all parturients breathed air spontaneously, and additional oxygen was given only when the pulse oximeter reading was <95%.
Calculation of sample size
An undesired difference between women receiving norepinephrine and ephedrine is the frequency of tachycardia. In a pilot study with a sample size of 20, tachycardia occurred at a rate of 15% and 40% for norepinephrine and ephedrine treatments, respectively, in parturients with preeclampsia. Using an α set at 0.05, a β set at 0.20, and the power of the test (1-β) at 0.80, a minimum of 49 cases per group were needed to detect a statistically significant difference in tachycardia. Considering potential dropouts or missing data, the sample size was increased to approximately 55 in each group. Also, the sample size of the phenylephrine group was set to 55, a number that was adequate to detect differences in bradycardia between the norepinephrine and phenylephrine groups, based on the pilot study data. Bradycardia occurred at a rate of 5% and 30% after norepinephrine and phenylephrine treatments, resulting in a minimum of 36 cases per group required for the statistically significant difference in the between-group analysis.
Statistical analysis
Data were expressed as the mean ± standard deviation (SD), the median, and the interquartile range (IQR), or percentage (%). Intergroup univariate data were assessed for normality using the D’Agostino-Pearson omnibus normality test, and one-way analysis of variance (ANOVA) followed by a two-sample t-test or Mann-Whitney test. Nominal data between groups were analyzed using a chi-squared (χ2) test or Fisher’s exact test. Standardized SBP and the heart rate (HR) between groups were also analyzed using a two-step summary measure described by Matthews et al. [15]. Standardized SBP and HR were first obtained via calculation of the average area under the curve (AUC). Then, derived data were compared using standard intergroup analysis with a t-test or Mann-Whitney test. Statistical analysis was conducted with GraphPad Prism version 7.0 (GraphPad Inc., San Diego, CA) or Microsoft Excel 2010 (Microsoft Corporation, USA). A P-value <0.05 was considered to be statistically significant.
Results
Figure 1 shows the flowchart of parturient enrollment, allocation, follow-up, and analysis. A total of 368 parturients initially enrolled. Of these, 56, 55 and 55 parturients in groups N, P, and E, respectively, were analyzed after a strict exclusion and follow-up. Forty-two patients in group N, 40 in group P, and 40 in group E were receiving antihypertensive treatment. There were ten patients in group N, 12 patients in group P, and eight patients in group E who were diagnosed with severe preeclampsia, of whom six, five, and five patients, respectively, were receiving magnesium sulfate as prophylaxis against seizures. Table 1 presents the demographic characteristics of the study participants, which showed that all variables were similar among three study groups.
Figure 1.
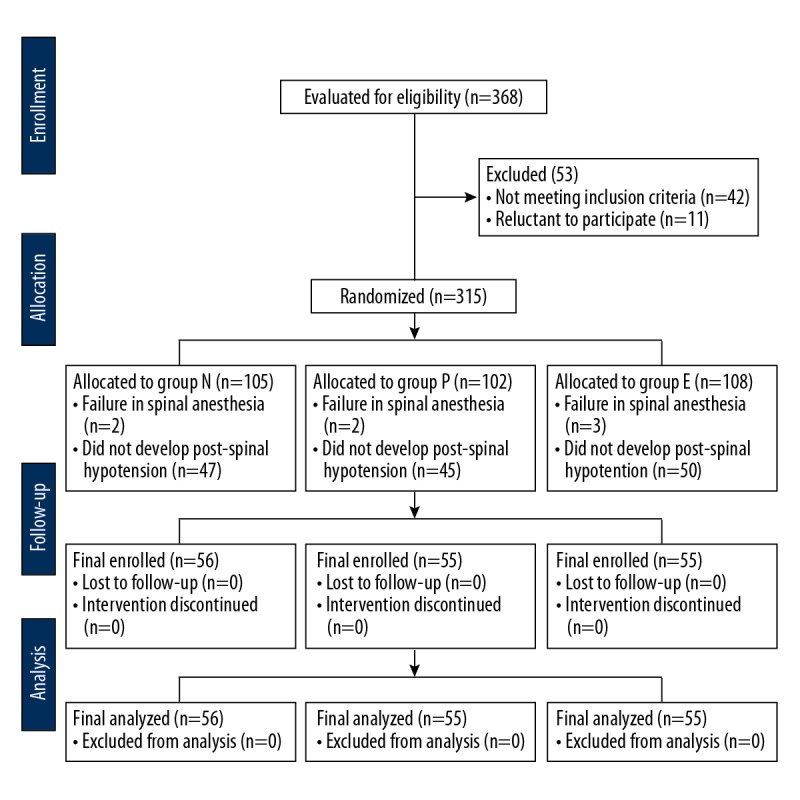
Flowchart of the enrolment of parturients, group allocation, follow-up, and data analysis.
Table 1.
Demographic characteristics and surgical times.
| Demographic characteristics | Group N (n=56) | Group P (n=55) | Group E (n=55) |
|---|---|---|---|
| Age (year) | 32±4.1 | 32±4.4 | 32±4.4 |
| Height (cm) | 162±5.1 | 162±4.7 | 163±4.3 |
| Weight (kg) | 76.5±8.1 | 78.5±9.2 | 76.7±8.4 |
| Gestational age (day) | 274±9 | 273±12 | 273±3 |
| Repeated cesarean delivery | 32 (57%) | 36 (65.5%) | 37 (67%) |
| Severe pre-eclampsia | 10 (17.8%) | 12 (21.8%) | 8 (14.5%) |
| Block dermatome (at 5 min) | T5 (T5–T6) | T5 (T5–T6) | T5 (T5–T6) |
| Block dermatome (at 15 min) | T4 (T3–T5) | T4 (T4–T4) | T4 (T4–T4) |
| Fasting time (hour) | 11±4 | 11±3 | 11±3 |
| Volume of cohydration (ml) | 759±92 | 767±93 | 740±79 |
| Estimated blood loss (ml) | 485±161 | 485±152 | 479±149 |
| Time interval | |||
| Induction to delivery (s) | 650±115 | 634±80 | 679±162 |
| Incision to delivery (s) | 246±77 | 250±81 | 281±107 |
| Uterine incision to delivery (s) | 57±36 | 58±42 | 58±33 |
Values are expressed as mean ±SD, number (%), or median (IQR).
In groups N, P, and E, the baseline SBPs were 149±5.7, 150±4.8, and 148±5.5 mmHg and the baseline HRs were 84.4±6.9, 84.3±5.1, and 85.4±7.5 bpm, respectively. No intergroup differences were observed (Table 2). Although the SBP was lower in group E compared with groups N and P at the 5 or 6 minutes after spinal anesthesia, no statistical difference was detected and overall SBP over time was also similar among groups (Figure 2A). The standardized HR over time was higher in group N compared with group P (80.5±12 vs. 76.6±6.9 bpm, P=0.04), with a difference of −4.0±1.9 mmHg (95% confidence interval [CI], −7.7 to −0.2). The standardized HR was lower in group N compared with group E (80.5±12 vs. 84.9±7.1 bpm; P=0.02), with a difference of 4.4±1.9 mmHg (95% CI, 0.62–8.19) (Figure 2B). The incidence of tachycardia, defined as HR >120 bpm was consistently lower in group N compared with group E (16.1% vs. 36.4%; relative risk [RR]=0.54; 95% CI, 0.29–0.90; P=0.02). The incidence of bradycardia, defined as HR <60 bpm, was lower in group N compared with group P (3.6% vs. 21.8%; RR=0.26; 95% CI, 0.07–0.73; P=0.004). No other hemodynamic variables tested had statistically significant differences, including the incidence of hypertension, the number of vasopressor boluses, the number of hypotensive episodes, and the time to first bolus post-intrathecal injection. SBP and HR changes are shown in Figure 2A, 2B for the first 12 minutes post-spinal anesthesia, a time point with data available for most parturients.
Table 2.
Maternal hemodynamic variables and drug consumption.
| Hemodynamic variables | Group N (n=56) | Group P (n=55) | Group E (n=55) |
|---|---|---|---|
| Baseline SBP (mmHg) | 149±5.7 | 150±4.8 | 148±5.5 |
| Baseline HR (bpm) | 84.4±6.9 | 84.3±5.1 | 85.4±7.5 |
| Standardized SBP over time (mmHg) | 125.1±8.5 | 124.2±6.6 | 123.1±6.8 |
| Standardized HR over time (bpm) | 80.5±12*# | 76.6±6.9# | 84.9±7.1 |
| Tachycardia | 9 (16.1%)# | 8 (14.6%)# | 20 (36.4%) |
| Bradycardia | 2 (3.6%)* | 12 (21.8%)# | 1 (1.8%) |
| Hypertension | 0 | 0 | 0 |
| Number of vasopressor boluses | 3 (2–3) | 3 (2–3) | 3 (2–3) |
| Number of hypotensive episodes | 2 (1.25–3) | 2 (2–3) | 2 (2–3) |
| Time to first bolus (min) | 5.1±2.0 | 5.7±1.7 | 5.6±2.0 |
Values are expressed as mean ±SD, number (%), or median (IQR). SBP – systolic blood pressure; HR – heart rate.
P<0.05 compared to group P.
P<0.05 compared to group E.
Figure 2.
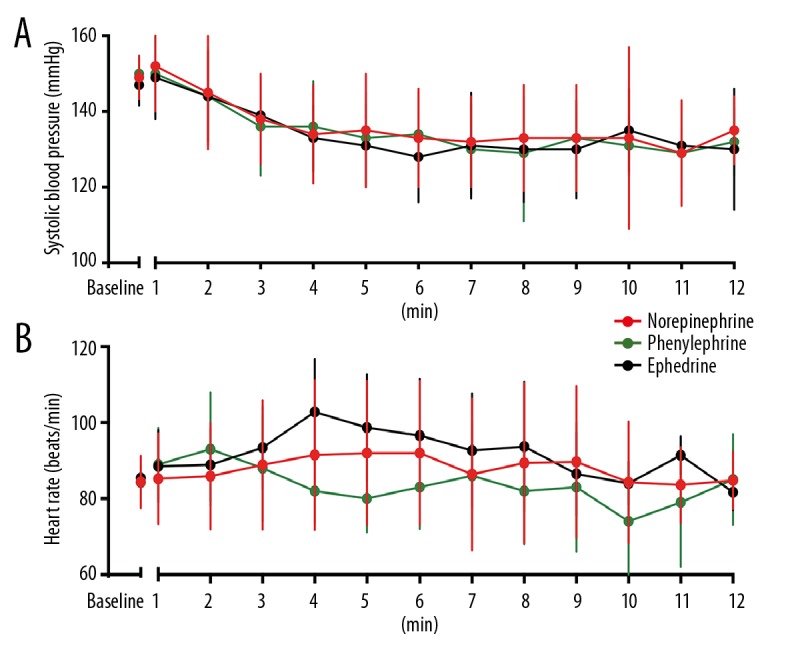
Serial changes in systolic blood pressure (A) and heart rate (B). Serial values for the first 12 measurements when data were available for most parturients. Data are shown as the mean ± standard deviation (SD).
For maternal side effects (Table 3), fewer reports of intraoperative nausea and vomiting (IONV) were observed for parturients in group N compared with group E (5.4% vs. 20%; RR=0.39; 95% CI, 0.14–0.90; P=0.02). No differences in dizziness or shivering were observed between groups N and E. The observed incidence of maternal side effects was similar between groups N and P. Neonatal outcome, including birth weight, Apgar scores, and umbilical artery blood gas and pH, are shown in Table 4. Due to insufficient blood samples, inadequate anticoagulation, or equipment failure, umbilical artery blood gas was not performed in eight, eight, and 10 subjects in groups N, P, and E, respectively. Apgar scores of <7 at 1 min were observed for four, five, and five neonates, in groups N, P, and E, respectively. No neonate had an Apgar score of <9 at 5 min. Although pH-value of umbilical cord blood was higher in group N compared with group E (7.32±0.02 vs. 7.31±0.03l difference −0.02±0.005; 95% CI, −0.03 to −0.005; P=0.006), the entire range of values in group E remained within the normal range. Also, no neonate had fetal acidosis, defined as umbilical artery pH <7.20. Group N also had a higher base excess (BE) (0.2±1.9 vs. −0.2±1.6; difference 1.5±0.50; 95% CI, 0.54–2.5; P=0.003), a lower HCO3− (22.2±1.5 vs. 24.1±5.8; difference −1.8±0.87; 95% CI, −3.6 to −0.12; P=0.037), and lactate (1.3±0.3 vs. 1.8±0.5; difference −0.54±0.08; 95% CI, −0.70 to −0.37; P <0.001) compared with group E, respectively. However, no differences in PO2, PCO2, or glucose were detected between groups N and E. There was no difference in uterine arterial blood gas between groups N and P.
Table 3.
Maternal side effects.
| Group N (n=56) | Group P (n=55) | Group E (n=55) | |
|---|---|---|---|
| Nausea | 2 (3.6%) | 3 (5.5%) | 5 (9.1%) |
| Vomiting | 1 (1.8%) | 1 (1.8%) | 6 (11%) |
| IONV (nausea + vomiting) | 3 (5.4%)# | 4 (7.3%) | 11 (20%) |
| Dizziness | 0 | 1 (1.8%) | 2 (3.6%) |
| Shivering | 4 (7.1%) | 2 (3.6%) | 3 (5.5%) |
Values are expressed as number (%). IONV – intraoperative nausea and vomiting.
P<0.05 compared to group E.
Table 4.
Neonatal outcomes.
| Group N (n=56) | Group P (n=55) | Group E (n=55) | |
|---|---|---|---|
| Birth weight (g) | 3402±428 | 3446±485 | 3492±453 |
| Apgar score (0–10) | |||
| 1-minute | 9 (7.25–9) | 9 (7–9) | 9 (7–9) |
| 5-minute | 10 (9–10) | 10 (9–10) | |
| 1-minute Apgar <7 | 4 (7.1%) | 5 (9.1%) | 5 (9.1%) |
| 5-minute Apgar <9 | 0 | 0 | 0 |
| UA blood gas analysis | n=48 | n=47 | n=45 |
| pH | 7.32±0.02# | 7.32±0.02# | 7.31±0.03 |
| pH <7.2 | 0 | 0 | 0 |
| PO2, mmHg | 14.5±5.8 | 13.5±4.4# | 15.2±5.2 |
| PCO2, mmHg | 50.9±4.1 | 50.8±4.4 | 50.4±6.7 |
| HCO3− (mEq/L) | 22.2±1.5# | 21.8±1.1# | 24.1±5.8 |
| BE | 0.2±1.9# | −0.2±1.6# | −1.3±2.9 |
| Glucose (mmol/L) | 3.5±0.7 | 3.3±0.8 | 3.4±0.8 |
| Lactate (mmol/L) | 1.3±0.3# | 1.2±0.2# | 1.8±0.5 |
Values are expressed as mean ±SD, number (%), or median (IQR). UA – umbilical artery; BE – base excess
P<0.05 compared to group E.
Discussion
The aims of this study were to compare the efficacy and safety of bolus norepinephrine, phenylephrine, and ephedrine in parturient women with preeclampsia who had hypotension during cesarean delivery with spinal anesthesia. The study included 166 parturient women with preeclampsia who had a baseline systolic blood pressure (SBP) <80% during spinal anesthesia for cesarean section and were allocated into three treatment groups, the bolus norepinephrine (4 μg) group (N), the phenylephrine (50 μg) group (P), and the ephedrine (4 mg) group (E). The results showed that treatment with bolus norepinephrine (4 μg), phenylephrine (50 μg), and ephedrine (4 mg) were all effective for managing spinal hypotension in women with preeclampsia. Norepinephrine may be associated with fewer cases of bradycardia compared with phenylephrine; simultaneously, fewer cases of tachycardia and maternal intraoperative nausea and vomiting (IONV), and increased pH, base excess (BE), and reduced HCO3−, lactate in umbilical artery blood compared with ephedrine.
The results from available clinical trials that have studied the use of vasopressors to rescue spinal hypotension in parturients with preeclampsia used bolus phenylephrine or ephedrine and have shown that phenylephrine administration restored mean arterial pressure, but not did not significantly increase maternal cardiac output [16]. Small doses of ephedrine can also be used [17], which may restore spinal anesthesia-induced decrease of peripheral vascular resistance [18] and provide a favorable effect on uteroplacental circulation [19]. Comparative studies have shown that phenylephrine and ephedrine were similarly effective in rescuing spinal hypotension, with no differences observed in neonate Apgar scores and umbilical artery pH in the presence of uteroplacental insufficiency [20]. However, phenylephrine might present more favorable BE and umbilical artery oxygen saturation compared with ephedrine. Despite extensive research, it remains unclear which drug is the better choice for the management of women with preeclampsia. Many obstetricians prefer both phenylephrine and ephedrine due to their safety, efficacy, and ease of use.
Recently, norepinephrine has been proposed as a promising vasopressor for treatment of maternal spinal hypotension in low-risk normotensive pregnancies without obvious maternal or neonatal adverse outcome. Compared with phenylephrine, norepinephrine is associated with fewer cases of bradycardia and a greater cardiac output [10]. Also, either computer controlled closed loop feedback [9] or manually controlled variable rate infusion of norepinephrine [21] provided a more accurate blood pressure control compared with an equivalent dose of phenylephrine infusion or norepinephrine bolus without increasing maternal or neonatal adverse outcome.
However, before this study, the feasibility of norepinephrine had not been explored for women with preeclampsia with uteroplacental insufficiency. Na et al. [22] compared maternal norepinephrine in pregnant women with preeclampsia and normotensive pregnant women and found that parturients with preeclampsia had prominently increased levels of norepinephrine, raising the concern regarding whether women with preeclampsia would remain sensitive to exogenous norepinephrine. In this study, intermittent boluses were applied rather than the continuous infusion of norepinephrine or ephedrine to rescue spinal hypotension. Although prophylactic infusion is a recommended paradigm for rescuing spinal hypotension to minimize hemodynamic fluctuation and maternal side effects [23], it may be related to a higher incidence of reactive hypertension. Also, patients with preeclampsia have a lower incidence of spinal hypotension that requires fewer vasopressors [3]. Therefore, a prophylactic infusion paradigm may not be reasonable.
The relative potency of norepinephrine and phenylephrine when used to rescue the first episode of spinal hypotension in normotensive women is estimated to be nearly 13: 1 [24], while phenylephrine versus ephedrine is estimated 80: 1 [25]; thus a potency ratio of approximately 1000: 1 is indirectly obtained for norepinephrine and ephedrine. As the commonly used clinical dose for phenylephrine is 100 μg to rescue maternal hypotension in normotensive women, smaller dosing was selected of norepinephrine 4 μg or ephedrine 4 mg for hypotensive patients who had preeclampsia, both being equivalent to phenylephrine 50 μg. The number of top-up doses required was the same for groups N, P, and E (median=3, IQR: 2–3), indicating a similar efficacy in all three vasopressors to rescue maternal hypotension. Equivalent dosing is important to ensure that dosing bias does not influence the comparison of clinical efficacy. In one recently published study, Ali et al. [26] compared a 5 μg bolus dose of norepinephrine with a 10 mg bolus dose of ephedrine to maintain arterial blood pressure during cesarean section with spinal anesthesia. Because this dose of norepinephrine halved the potency of ephedrine, it was not unexpected that more norepinephrine boluses were required.
A recent study compared a 10 μg dose of norepinephrine with 5 mg of ephedrine to treat anesthesia-induced hypotension in hypertensive patients undergoing spinal surgery, and showed that this dose of norepinephrine had twice the potency of ephedrine, resulting in fewer hypotensive events, the need for fewer vasopressor doses to rescue the first episode of hypotension, and fewer doses in total [27]. In the present study, despite a similar efficacy for the maintenance of SBP, there was a significant difference in maternal HR after treatment of the three vasopressors used. The overall HR was the highest in parturients receiving ephedrine, followed by norepinephrine, and was lowest with phenylephrine, which was consistent with their respective pharmacological properties. All hemodynamic variables, as well as maternal and neonatal outcome in this study were recorded by an independent researcher, to minimize possible investigator bias. For maternal outcome, norepinephrine treatment was observed to result in fewer cases of IONV when compared with ephedrine. The etiology of IONV is recognized to be multifactorial and reactive treatment of established hypotension, as used in the present study, has previously been reported to be related to an increased incidence of IONV when compared with prophylactic vasopressor infusion before the onset of hypotension [28]. In 2004, Ngan Kee et al. compared phenylephrine infusion regimens based on three different BP thresholds and showed that for optimal management, phenylephrine should be adjusted to maintain maternal BP at near-baseline values [29]. Importantly, ephedrine has been shown to have a duration of onset of between two and three minutes, resulting in slower correction of hypotension when compared with norepinephrine or phenylephrine, which act within 60 seconds after injection [30].
There may have been several factors that contributed to the development of IONV, including maternal demographics and a previous history of IONV or motion sickness, operative procedures, use of perioperative opioids, or peritoneal traction [31]. However, these details were not collected in the present study, and their possible involvement in the IONV difference found between the groups was not explored, which should be regarded as a limitation that requires further study. In this study, the neonatal Apgar scores were measured as an indicator of neonatal well-being in the first minutes after birth, as well as umbilical artery blood gas and pH, which is useful to assess fetal condition immediately before delivery. There were no observed differences in Apgar score at one minute or five minutes among the three study groups, and no differences in umbilical artery pH between groups N and P. However, a higher umbilical artery pH value was observed in women receiving norepinephrine and phenylephrine when compared with those treated with ephedrine (7.32±0.02 and 7.32±0.02 versus 7.31±0.03). However, the entire range of pH-values in group E was still within the normal range, and no neonate experienced fetal acidosis, defined as pH value <7.2, which is the lower limit of normal [32]. Also, in a recently published clinical trial that compared treatment with bolus phenylephrine and ephedrine for spinal hypotension in women with severe preeclampsia, fetal acid-base status was found to be independent of the use of vasopressor [33]. Therefore, the observed lower pH value for women receiving ephedrine is unlikely to have clinical significance. Also, in women receiving norepinephrine and phenylephrine, there was a higher BE, and lower HCO3−, lactate, which are measures of fetal acid-base status and metabolic markers. It might be assumed that such differences mainly resulted from a greater placental transfer of ephedrine due to its higher lipid solubility when compared with catecholamines such as norepinephrine or phenylephrine, and the followed stimulation of fetal β-adrenergic receptors to increase fetal metabolism [34].
An significant concern when using a vasopressor with an α-agonist is the reduction in uteroplacental blood flow. Previous studies have shown phenylephrine is associated with a lower umbilical artery or umbilical venous PO2 when compared with ephedrine, possibly attributable to its greater vasoconstriction property, resulting in a reduction of uteroplacental perfusion and an increase in oxygen extraction [35]. However, norepinephrine restored the decreased peripheral vascular resistance less than phenylephrine [9]. In 2010, Minzter et al. [36] reported that norepinephrine had no effect on fetal arterial perfusion pressure and fetoplacental microcirculation was not compromised. In the present study, no difference in fetal umbilical artery PO2 was detected, suggesting that norepinephrine might not compromise fetal oxygen supply when compared with ephedrine in a bolus regimen. A bolus of norepinephrine (4 μg) has a similar efficacy for rescuing maternal hypotension but was associated with fewer cases of bradycardia compared with phenylephrine (50 μg), as well as more infrequent maternal tachycardia, IONV, a greater neonatal acid-base status, and umbilical artery pH compared with ephedrine (4 mg).
This study had several limitations. Uterine arterial blood flow was not measured to directly observe the effect of vasopressors on uteroplacental perfusion, which is an important consideration for women with preeclampsia. Secondly, other than BP, the other contributors to an increased incidence of IONV observed in women receiving ephedrine were not fully verified. Finally, the study endpoint was set at delivery, and whether or not norepinephrine has similar efficacy and safety for hemodynamic management throughout surgery required further investigation.
Conclusions
A bolus dose of norepinephrine (4 μg) showed similar efficacy for the maintenance of systolic blood pressure (SBP) when compared with bolus doses of phenylephrine (50 μg) and ephedrine (4 mg) in parturient women with preeclampsia with hypotension during spinal anesthesia and cesarean section. However, there was improved maternal safety for norepinephrine (4 μg) when compared with phenylephrine (50 μg) and improved maternal and neonatal safety when compared with ephedrine (4 mg). Therefore, bolus norepinephrine may act as a promising alternative to phenylephrine or ephedrine to rescue maternal hypotension in parturients with preeclampsia during spinal anesthesia and cesarean section.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by the Young Talents Project of Maternal and Child Health Care in Jiangsu Province (FRC201787)
References
- 1.Steegers EA, von Dadelszen P, Duvekot JJ, et al. Pre-eclampsia. Lancet. 2010;376:631–44. doi: 10.1016/S0140-6736(10)60279-6. [DOI] [PubMed] [Google Scholar]
- 2.Henke VG, Bateman BT, Leffert LR. Focused review: Spinal anesthesia in severe preeclampsia. Anesth Analg. 2013;117:686–93. doi: 10.1213/ANE.0b013e31829eeef5. [DOI] [PubMed] [Google Scholar]
- 3.Aya AG, Mangin R, Vialles N, et al. Patients with severe preeclampsia experience less hypotension during spinal anesthesia for elective cesarean delivery than healthy parturients: A prospective cohort comparison. Anesth Analg. 2003;97:867–72. doi: 10.1213/01.ANE.0000073610.23885.F2. [DOI] [PubMed] [Google Scholar]
- 4.Kinsella S, Carvalho B, Dyer R, et al. International consensus statement on the management of hypotension with vasopressors during caesarean section under spinal anaesthesia. Anaesthesia. 2018;73:71–92. doi: 10.1111/anae.14080. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter M, Mowbray P, Desira W, et al. Fetal and maternal effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology. 2002;97:1582–90. doi: 10.1097/00000542-200212000-00034. [DOI] [PubMed] [Google Scholar]
- 6.Malin GL, Morris RK, Khan KS. Strength of association between umbilical cord pH and perinatal and long-term outcomes: Systematic review and meta-analysis. BMJ. 2010;340:c1471. doi: 10.1136/bmj.c1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mets B. Should norepinephrine, rather than phenylephrine, be considered the primary vasopressor in anesthetic practice? Anesth Analg. 2016;122:1707–14. doi: 10.1213/ANE.0000000000001239. [DOI] [PubMed] [Google Scholar]
- 8.Sodha RJ, Proegler M, Schneider H. Transfer and metabolism of norepinephrine studied from maternal-to-fetal and fetal-to-maternal sides in the in vitro perfused human placental lobe. Am J Obstet Gynecol. 1984;148:474–81. doi: 10.1016/0002-9378(84)90729-4. [DOI] [PubMed] [Google Scholar]
- 9.Ngan Kee WD, Lee SW, Ng FF, et al. Randomized double-blinded comparison of norepinephrine and phenylephrine for maintenance of blood pressure during spinal anesthesia for cesarean delivery. Anesthesiology. 2015;122:736–45. doi: 10.1097/ALN.0000000000000601. [DOI] [PubMed] [Google Scholar]
- 10.Ngan Kee WD, Khaw KS, Tam YH, et al. Performance of a closed-loop feedback computer-controlled infusion system for maintaining blood pressure during spinal anaesthesia for caesarean section: A randomized controlled comparison of norepinephrine versus phenylephrine. J Clin Monit Comput. 2017;31:617–23. doi: 10.1007/s10877-016-9883-z. [DOI] [PubMed] [Google Scholar]
- 11.Vallejo M, Attaallah A, Elzamzamy O, et al. An open-label randomized controlled clinical trial for comparison of continuous phenylephrine versus norepinephrine infusion in prevention of spinal hypotension during cesarean delivery. Int J Obstet Anesth. 2017;29:18–25. doi: 10.1016/j.ijoa.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Sharkey AM, Siddiqui N, Downey K, et al. Comparison of intermittent intravenous boluses of phenylephrine and norepinephrine to prevent and treat spinal-induced hypotension in cesarean deliveries: Randomized controlled trial. Anesth Analg. 2018 doi: 10.1213/ANE.0000000000003704. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Chen D, Qi X, Huang X, et al. Efficacy and safety of different norepinephrine regimens for prevention of spinal hypotension in cesarean section: A randomized trial. Biomed Res Int. 2018;2018 doi: 10.1155/2018/2708175. 2708175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 15.Matthews JN, Altman DG, Campbell MJ, et al. Analysis of serial measurements in medical research. BMJ. 1990;300:230–35. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dyer RA, Piercy JL, Reed AR, et al. Hemodynamic changes associated with spinal anesthesia for cesarean delivery in severe preeclampsia. Anesthesiology. 2008;108:802–11. doi: 10.1097/01.anes.0000311153.84687.c7. [DOI] [PubMed] [Google Scholar]
- 17.Aya AG, Vialles N, Tanoubi I, et al. Spinal anesthesia-induced hypotension: A risk comparison between patients with severe preeclampsia and healthy women undergoing preterm cesarean delivery. Anesth Analg. 2005;101:869–75. doi: 10.1213/01.ANE.0000175229.98493.2B. [DOI] [PubMed] [Google Scholar]
- 18.Tihtonen K, Koobi T, Yli-Hankala A, et al. Maternal haemodynamics in pre-eclampsia compared with normal pregnancy during caesarean delivery. BJOG. 2006;113:657–63. doi: 10.1111/j.1471-0528.2006.00931.x. [DOI] [PubMed] [Google Scholar]
- 19.Burns SM, Cowan CM, Wilkes RG. Prevention and management of hypotension during spinal anaesthesia for elective Caesarean section: aA survey of practice. Anaesthesia. 2001;56:794–98. doi: 10.1046/j.1365-2044.2001.02058-5.x. [DOI] [PubMed] [Google Scholar]
- 20.Emmanuel A, Adams S, Lombard C, et al. A randomised comparison of bolus phenylephrine and ephedrine for the management of spinal hypotension in patients with severe preeclampsia and fetal compromise. Int J Obstet Anesth. 2018;33:23–31. doi: 10.1016/j.ijoa.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Ngan Kee WD, Lee SWY, Ng FF, et al. Prophylactic norepinephrine infusion for preventing hypotension during spinal anesthesia for cesarean delivery. Anesth Analg. 2018;126:1989–94. doi: 10.1213/ANE.0000000000002243. [DOI] [PubMed] [Google Scholar]
- 22.Na KH, Choi JH, Kim CH, et al. Altered expression of norepinephrine transporter and norepinephrine in human placenta cause pre-eclampsia through regulated trophoblast invasion. Clin Exp Reprod Med. 2013;40:12–22. doi: 10.5653/cerm.2013.40.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinsella SM, Carvalho B, Dyer RA, et al. International consensus statement on the management of hypotension with vasopressors during caesarean section under spinal anaesthesia. Anaesthesia. 2018;73:71–92. doi: 10.1111/anae.14080. [DOI] [PubMed] [Google Scholar]
- 24.Ngan Kee WD. A random-allocation graded dose-response study of norepinephrine and phenylephrine for treating hypotension during spinal anesthesia for cesarean delivery. Anesthesiology. 2017;127:934–41. doi: 10.1097/ALN.0000000000001880. [DOI] [PubMed] [Google Scholar]
- 25.Saravanan S, Kocarev M, Wilson RC, et al. Equivalent dose of ephedrine and phenylephrine in the prevention of post-spinal hypotension in Caesarean section. Br J Anaesth. 2006;96:95–99. doi: 10.1093/bja/aei265. [DOI] [PubMed] [Google Scholar]
- 26.Selim MF. Norepinephrine versus ephedrine to maintain arterial blood pressure during spinal anesthesia for cesarean delivery: A prospective double-blinded trial. Anesth Essays Res. 2018;12:92–97. doi: 10.4103/aer.AER_204_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins N, Fitzgerald PC, van Dyk D, et al. The effect of prophylactic phenylephrine and ephedrine infusions on umbilical artery blood pH in women with preeclampsia undergoing cesarean delivery with spinal anesthesia: A randomized, double-blind trial. Anesth Analg. 2018;126:1999–2006. doi: 10.1213/ANE.0000000000002524. [DOI] [PubMed] [Google Scholar]
- 28.Habib AS. A review of the impact of phenylephrine administration on maternal hemodynamics and maternal and neonatal outcomes in women undergoing cesarean delivery under spinal anesthesia. Anesth Analg. 2012;114:377–90. doi: 10.1213/ANE.0b013e3182373a3e. [DOI] [PubMed] [Google Scholar]
- 29.Ngan Kee W, Khaw K, Ng F. Comparison of phenylephrine infusion regimens for maintaining maternal blood pressure during spinal anaesthesia for Caesarean section. Br J Anaesth. 2004;92:469–74. doi: 10.1093/bja/aeh088. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Shen X, Liu S, et al. The efficacy and safety of norepinephrine and its feasibility as a replacement for phenylephrine to manage maternal hypotension during elective cesarean delivery under spinal anesthesia. Biomed Res Int. 2018;2018 doi: 10.1155/2018/1869189. 1869189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jelting Y, Klein C, Harlander T, et al. Preventing nausea and vomiting in women undergoing regional anesthesia for cesarean section: challenges and solutions. Local Reg Anesth. 2017;10:83–90. doi: 10.2147/LRA.S111459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernard M, Brown H, St Pierre J, et al. Umbilical cord blood gases for term healthy newborns. Am J Perinatol. 1990;7:157–59. doi: 10.1055/s-2007-999470. [DOI] [PubMed] [Google Scholar]
- 33.Dyer RA, Emmanuel A, Adams SC, et al. A randomised comparison of bolus phenylephrine and ephedrine for the management of spinal hypotension in patients with severe preeclampsia and fetal compromise. Int J Obstet Anesth. 2018;33:23–31. doi: 10.1016/j.ijoa.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Ngan Kee W, Khaw K, Tan P, et al. Placental transfer and fetal metabolic effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology. 2009;111:506–12. doi: 10.1097/ALN.0b013e3181b160a3. [DOI] [PubMed] [Google Scholar]
- 35.Khaw KS, Lau TK, Ng FF, et al. Randomised double-blinded comparison of phenylephrine vs. ephedrine for maintaining blood pressure during spinal anaesthesia for non-elective Caesarean section. Anaesthesia. 2008;63:1319–26. doi: 10.1111/j.1365-2044.2008.05635.x. [DOI] [PubMed] [Google Scholar]
- 36.Minzter BH, Johnson RF, Paschall RL, et al. The diverse effects of vasopressors on the fetoplacental circulation of the dual perfused human placenta. Anesth Analg. 2010;110(3):857–62. doi: 10.1213/ANE.0b013e3181c91ebc. [DOI] [PubMed] [Google Scholar]


