Abstract
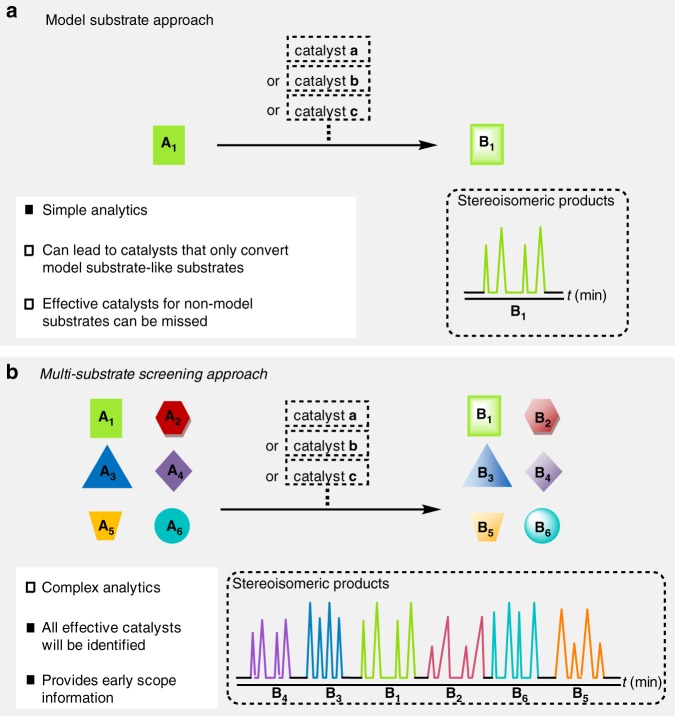
When developing a synthetic methodology, chemists generally optimize a single substrate and then explore the substrate scope of their method. This approach has led to innumerable and widely-used chemical reactions. However, it frequently provides methods that only work on model substrate-like compounds. Perhaps worse, reaction conditions that would enable the conversion of other substrates may be missed. We now show that a different approach, originally proposed by Kagan, in which a collection of structurally distinct substrates are evaluated in a single reaction vessel, can not only provide information on the substrate scope at a much earlier stage in methodology development, but even lead to a broadly applicable synthetic methodology. Using this multi-substrate screening approach, we have identified an efficient and stereoselective imidodiphosphorimidate organocatalyst for scalable Diels–Alder reactions of cyclopentadiene with different classes of α,β-unsaturated aldehydes.
Investigation of a reaction scope usually starts with the optimization for a model substrate. Here, the authors apply a time-efficient multi-substrate screening approach to identify a general organocatalyst for the Diels–Alder reaction of cyclopentadiene with α,β-unsaturated aldehydes.
Introduction
The Diels–Alder reaction is one of the most powerful transformations in chemical synthesis and generates six-membered cyclic products with up to four stereogenic centers. Among the most notable advances of this reaction has been the development of catalytic asymmetric versions. In addition to a variety of chiral Lewis acid catalysts1–3, organic molecules also catalyze Diels–Alder reactions stereoselectively4–6. Moreover, the Diels–Alder reaction between α,β-unsaturated aldehydes and dienes has attracted particular attention due to not only the synthetic utility of aldehydes, but also its wide applicability in the synthesis of drugs, natural products, and fragrances7. For example, chiral secondary amines catalyze highly enantioselective Diels–Alder reactions of β-monosubstituted enals8–13. However, these catalysts typically give moderate diastereoselectivities and cannot readily be used with α-substituted enals14–16. In addition, most reported catalysts that convert α-substituted enals display a limited scope in terms of tolerated substituents1–3,14–20. Despite intense research in this area, a general catalyst of the Diels–Alder reaction that can accommodate a broad range of structurally distinct enals with cyclopentadiene has yet to be developed.
Realizing limitations of the model substrate approach (Fig. 1a), Kagan et al. suggested a catalyst screening that involves the utilization of a pooled collection of different substrates as an alternative method (Fig. 1b)21. The concept has originally been exemplified in the asymmetric reduction of ketones and has subsequently been applied to identify chiral ligands for metal-catalyzed asymmetric transformations such as additions of diethylzinc, hydroformylations, and hydrogenations22–25. The two main advantages of multi-substrate screenings are that information about the scope of a catalyst is revealed early on, and that catalysts that perform suboptimally with the model substrate but excellently with others, can still be identified. Challenges of multi-substrate screenings include the difficulty of developing an accurate analytical assay that can simultaneously differentiate all substrates and all stereoisomeric products. Furthermore, unproductive interactions between substrates and products as well as kinetic competition may complicate the analytics and exacerbate the readout of the assay. Nonetheless, the multi-substrate screening approach has not previously led to a general and broadly applicable catalyst and we became interested in applying it to a challenging synthetic problem. We report here the use of a multi-substrate screening approach for the development of a general catalyst of the asymmetric [4+2] cycloaddition of diverse α,β-unsaturated aldehydes with cyclopentadiene.
Fig. 1.
Two approaches to identify a selective catalyst. a Model substrate approach vs. b multi-substrate screening approach
Results
Assay development
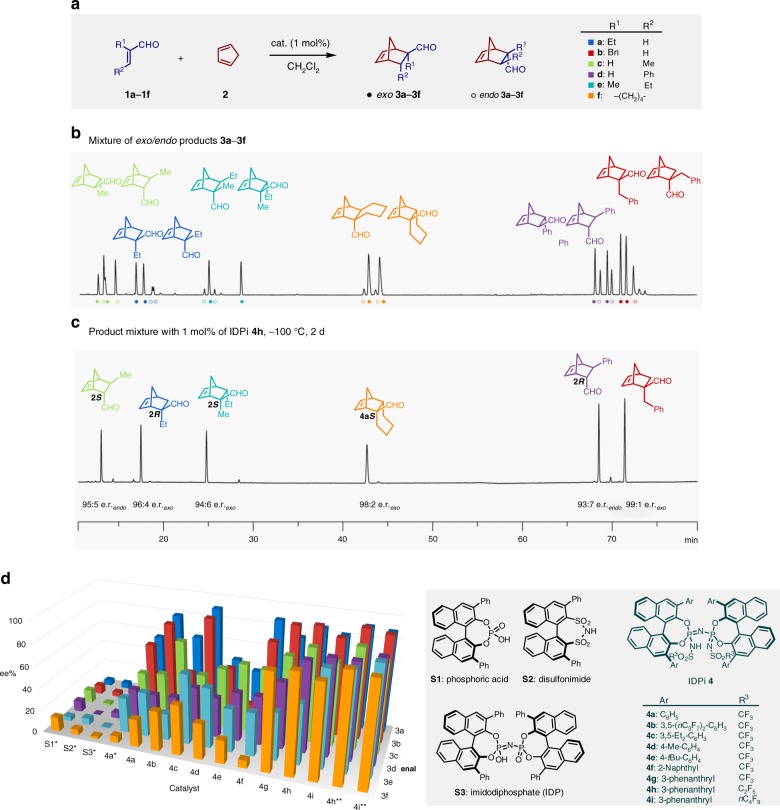
We initiated our studies by establishing an assay to screen several structurally distinct enal dienophiles simultaneously (Fig. 2a). Six representative α,β-unsaturated aldehydes were selected according to the position, nature, and size of the substituents (1a–1f), and to enable product separation on a single chiral stationary gas chromatography (GC) phase. In addition to α- and β-monosubstituted enals 1a–1d, substrates 1e and 1f were chosen as acyclic and cyclic α,β-disubstituted enals. When a representative of a particular substrate class led to product peak overlaps, a minor modification quickly provided baseline separation. For example, the ethyl-substituted aldehyde 1a was employed instead of its methyl analog to circumvent product peak overlap with cycloadduct 3c during GC separation. Gas chromatography with 2,3-dimethyl-6-tert-butyldimethylsilyl-β-cyclodextrin as chiral stationary phase was applied to measure the stereoisomeric ratios of all possible products in the crude mixture. A chromatogram with 23 distinct peaks (endo 3b could not be separated under the described condition) was established and used as the platform for the multi-substrate screening (Fig. 2b).
Fig. 2.
Multi-substrate screening of Diels–Alder reactions of α,β-unsaturated aldehydes and cyclopentadiene. a Investigated reactions. b GC chromatogram of the stereoisomeric product mixture. c Chromatogram of the product mixture using catalyst 4h. d Graphical representation of a subset of the multi-substrate screening. *Reactions at room temperature. **Reactions at −100 °C. For detailed reaction conditions and results, see Supplementary Note 2
Catalyst screening with multiple substrates
With this robust analytical protocol established, we carried out a simultaneous catalyst screening using pooled substrates. Different Brønsted acid catalysts including phosphoric acid S126,27, disulfonimide S228,29, and imidodiphosphate S330 all provided the desired cycloaddition products, albeit sometimes with moderate reactivity and generally with poor stereoselectivities (Fig. 2d and Supplementary Table 1). A remarkable improvement of reactivity and stereoselectivity was observed using highly acidic and confined imidodiphosphorimidate (IDPi) catalyst 4a31–34, and further enhancement of enantiomeric ratios in all reactions was observed at −78 °C. Thus, we focused on screening IDPi catalysts for the stereoselective cycloaddition of α,β-unsaturated aldehydes (1a–1f) and cyclopentadiene 2 (Fig. 2d, for detailed results on the optimization of reaction conditions, see the Supplementary Table 2).
From screening a wide range of IDPi catalysts representatively depicted in Fig. 2d, we found that catalyst 4c showed excellent stereoselectivity exclusively with α-substituted enals (3a: 92:8 exo/endo, 95.5:4.5 e.r.exo; 3b: 91:9 exo/endo, 94.5:5.5 e.r.exo), while aldehydes having β-substituents (1c–1f) were not converted efficiently and stereoselectively (3c: 10:90 exo/endo, 73:27 e.r.endo; 3d–3f: < 5% conv.). In contrast, catalyst 4e imparted higher enantioselectivities for β-monosubstituted dienophiles 1c and 1d (3c: 6:94 exo/endo, 81:19 e.r.endo; 3d: 2:98 exo/endo, 72:28 e.r. endo). Notably, the most favorable results were obtained using IDPi catalysts involving polycyclic aromatic substituents at the 3,3′-positions of the 1,1′-bi-2-naphthol (BINOL) backbone. For example, catalyst 4g gave relatively high stereoselectivities when converting enals 1a–1f. Further investigations to tune the chiral environment of the catalyst by modifying the inner substituent R3 improved the enantioselectivities of the major diastereomers (4g–4i)33,34. Finally, we were delighted to find that catalysts 4h and 4i showed excellent reactivity and stereoselectivity for all pooled α,β-unsaturated aldehydes (1a–1f), achieving full consumption of all dienophiles with only 1 mol% of the catalyst (Figs. 2c, d, for detailed reaction conditions and results, see the Supplementary Tables 2–5).
Discussion
Importantly, individual experiments using aldehydes 1a–1f separately corresponded well to the multi-substrate experiments, providing consistently high yields and stereoselectivities. Moreover, the optimized catalytic conditions were efficient for reacting a broad range of α,β-unsaturated aldehydes (Table 1). Variations of the size of the α-substituent were well tolerated, giving exo-enriched cycloadducts with excellent enantioselectivities (3a, 3b, 3g–3j; 96:4–99:1 exo/endo, 93:7–99:1 e.r.exo). In contrast, dienophiles bearing an aromatic substituent at the β-position yielded highly enantioenriched endo products (3d, 3p–3t; 3:97–1:99 exo/endo, 94:6–97:3 e.r.endo) while β-aliphatic substituents gave moderate to good diastereoselectivities (3c, 3k–3n; 25:75–4:96 exo/endo, 91:9–96:4 e.r.endo). Consistent with the observation during the multi-substrate screening, α,β-disubstituted substrates furnished exo-products in good yields with high diastereo- and enantioselectivities (3e, 3f, 3u and 3v). Acrolein afforded product 3w in relatively good yield and stereoselectivity. The reaction was also tolerant of functional groups such as an alkene, a silyl ether, halides, and heteroarenes at the dienophiles, maintaining high yields and stereoselectivities (3h–3j, and 3o–3t). Notably, all dienophiles (1a–1w) underwent the asymmetric Diels–Alder reaction with cyclopentadiene 2 with generally good to excellent diastereoselectivity and enantioselectivity in the presence of either catalysts 4h or 4i.
Table 1.
Substrate scope of the Diels–Alder reaction

|
Reactions were performed with α,β-unsaturated aldehydes 1 (0.3 mmol), cyclopentadiene 2 (5.0 equiv.) and IDPi catalyst 4h (1 mol%) in CH2Cl2 (0.3 mL) at −100 °C or −110 °C for 2–5 days. All yields are those of isolated products. Diastereomeric ratios (exo/endo) were determined by 1H nuclear magnetic resonance (NMR) analysis and enantiomeric ratios (e.r.) were determined by GC or high pressure liquid chromatography (HPLC) analysis. The relative and absolute configurations of cycloadducts were determined by comparison of the data with those reported. aCatalyst 4i was used. See the Supplementary Methods.
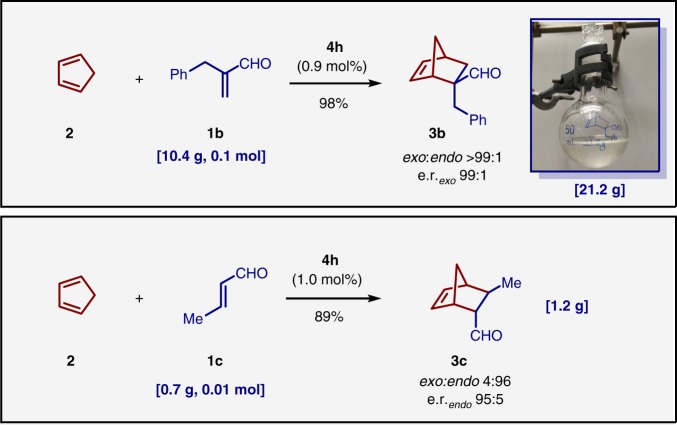
Furthermore, efficiency and preparative utility of our Brønsted acid catalyzed reaction was demonstrated by large scale experiments with aldehydes 1b and 1c (Fig. 3). A decagram scale reaction using 0.1 mol of aldehyde 1b and only 0.9 mol% of catalyst 4h was performed to afford 21 g of product 3b (98% yield, exo/endo, > 99:1, 99:1 e.r.exo). A 0.01 mol scale reaction of aldehyde 1c and cyclopentadiene 2 with 1 mol% of catalyst 4h furnished 1.2 g of product 3c in high yield and stereoselectivities (89% yield, exo/endo, 4:96, 95:5 e.r.endo). In both cases, catalyst 4i was recovered by flash column chromatography and subsequent re-acidification (97% and 92%).
Fig. 3.
Large scale experiments. Two gram scale experiments were conducted with each cyclopentadiene and aldehyde 1b and aldehyde 1c, respectively. 21.2 g of pure product 3b was obtained
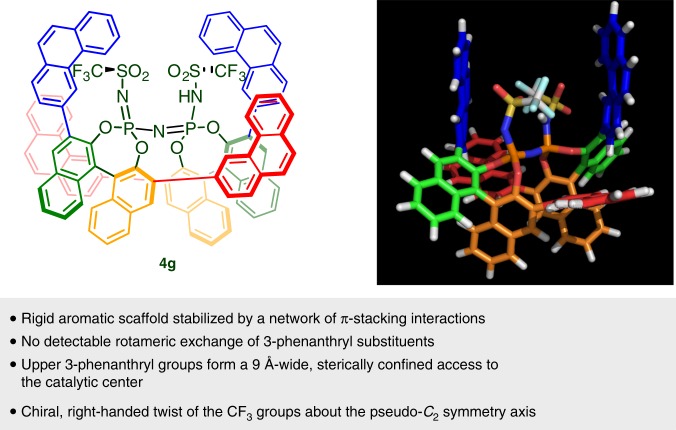
To gain structural insight regarding the activity and stereoselectivity of the 3-phenanthryl-substituted IDPis, a 10-ns molecular dynamics simulation of 4g was performed with distance restraints based on carefully evaluated nuclear Overhauser effect (NOE) contacts. The assembly of the BINOL backbone and its substituents bears strong similarity with other IDPi crystal structures28,30,31, and defines a rigid, narrow and chiral access to the catalytic center. The important structural features are summarized in Fig. 4 (see Supplementary Note 5, Supplementary Discussions 1, 2 and Supplementary Data 1 for details).
Fig. 4.
The solution structure of catalyst 4g. Structural characteristics of our catalysts were revealed by using NMR-based models
In conclusion, we report the discovery of a general, and scalable Brønsted acid catalyst of the asymmetric Diels–Alder reaction between structurally diverse α,β-unsaturated aldehydes and cyclopentadiene (2,3-dimethylbuta-1,3-diene could also be used, as described in the Supplementary Methods). While the optimal catalysts 4h and 4i, in this particularly fortuitous case, may have also been identified using single substrate approaches, other more specialized catalysts such as acid 4c could have easily been missed. Our findings deliver a powerful Diels–Alder catalyst and suggest that multi-substrate screenings can aid in identifying broadly useful and highly stereoselective catalysts of challenging carbon–carbon bond forming reactions.
Methods
General procedure for the asymmetric Diels–Alder reaction
To a solution of the catalyst (1–3 mol%) in anhydrous CH2Cl2 (0.3 mL), immersed in a liquid nitrogen-ethanol slush at −116 °C, were added aldehyde 1 (0.3 mmol, 1.0 equiv) and diene 2 (5.0 equiv). The resulting mixture was stirred at the described temperature. Upon completion of the reaction, NEt3 (50 μL) was added and the reaction mixture was warmed to ambient temperature. After removal of the solvent, the crude mixture was purified by column chromatography to afford product 3.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
Generous support from the Max Planck Society, the Deutsche Forschungsgemeinschaft (Leibniz Award to B.L. and Cluster of Excellence RESOLV, EXC 1069), and the European Research Council (Advanced Grant “C–H Acids for Organic Synthesis, CHAOS”) is gratefully acknowledged. We further thank the Alexander von Humboldt Foundation for a fellowship to H.K., the Max Planck Society-Fundación Bunge y Born for a stipend for G.G., Dr. Philip S. J. Kaib and Arno Döhring for technical support, and Dr. Monika Lindner and Dr. René Pretorius for manuscript reading. Excellent service from our GC department (especially Sylvia Ruthe, Jutta Rosentreter, and Veronika Dietl) is gratefully acknowledged.
Author contributions
H.K., G.G., J.A., P.K. and J.O. jointly conducted the experiments described in this manuscript and analyzed the data. H.K. and B.L. wrote the manuscript with contributions by all other authors. J.B.L., P.G. and C.F. conducted the NMR studies. BL designed and oversaw the project.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files.
Competing interests
A patent application on the IDPi catalysts has been filed by B.L. The remaining authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-019-08374-z.
References
- 1.Kagan HB, Riant O. Catalytic asymmetric Diels–Alder reactions. Chem. Rev. 1992;92:1007–1019. doi: 10.1021/cr00013a013. [DOI] [Google Scholar]
- 2.Corey EJ. Catalytic enantioselective Diels–Alder reactions: methods, mechanistic fundamentals, pathways, and applications. Angew. Chem. Int. Ed. 2002;41:1650–1667. doi: 10.1002/1521-3773(20020517)41:10<1650::AID-ANIE1650>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 3.Johnson JS, Evans DA. Chiral bis(oxazoline) copper(II) complexes: versatile catalysts for enantioselective cycloaddition, aldol, Michael, and carbonyl ene reactions. Acc. Chem. Res. 2000;33:325–335. doi: 10.1021/ar960062n. [DOI] [PubMed] [Google Scholar]
- 4.Erkkilä A, Majander I, Pihko PM. Iminium catalysis. Chem. Rev. 2007;107:5416–5470. doi: 10.1021/cr068388p. [DOI] [PubMed] [Google Scholar]
- 5.Shen J, Tan CH. Brønsted-acid and Brønsted-base catalyzed Diels–Alder reactions. Org. Biomol. Chem. 2008;6:3229–3236. doi: 10.1039/b809505c. [DOI] [PubMed] [Google Scholar]
- 6.Denmark SE, Beutner GL. Lewis base catalysis in organic synthesis. Angew. Chem. Int. Ed. 2008;47:1560–1638. doi: 10.1002/anie.200604943. [DOI] [PubMed] [Google Scholar]
- 7.Funel JA, Abele S. Industrial applications of the Diels–Alder reaction. Angew. Chem. Int. Ed. 2013;52:3822–3863. doi: 10.1002/anie.201201636. [DOI] [PubMed] [Google Scholar]
- 8.Ahrendt KA, Borths CJ, MacMillan DWC. New strategies for organic catalysis: the first highly enantioselective organocatalytic Diels−Alder reaction. J. Am. Chem. Soc. 2000;122:4243–4244. doi: 10.1021/ja000092s. [DOI] [Google Scholar]
- 9.Bonini BF, et al. Aziridin-2-yl methanols as organocatalysts in Diels–Alder reactions and Friedel–Crafts alkylations of N-methyl-pyrrole and N-methyl-indole. Tetrahedron.: Asymmetry. 2006;17:3135–3143. doi: 10.1016/j.tetasy.2006.11.028. [DOI] [Google Scholar]
- 10.Gotoh H, Hayashi Y. Diarylprolinol silyl ether as catalyst of an exo-selective, enantioselective Diels−Alder reaction. Org. Lett. 2007;9:2859–2862. doi: 10.1021/ol071009+. [DOI] [PubMed] [Google Scholar]
- 11.He H, et al. Camphor sulfonyl hydrazines (CaSH) as organocatalysts in enantioselective Diels−Alder reactions. Org. Lett. 2008;10:2421–2424. doi: 10.1021/ol8005826. [DOI] [PubMed] [Google Scholar]
- 12.Langlois Y, Petit A, Rémy P, Scherrmann MC, Kouklovsky C. Camphor-derived sulfonylhydrazines: catalysts for Diels–Alder cycloadditions. Tetrahedron Lett. 2008;49:5576–5579. doi: 10.1016/j.tetlet.2008.07.012. [DOI] [Google Scholar]
- 13.Kano T, Tanaka Y, Maruoka K. exo-Selective asymmetric Diels−Alder reaction catalyzed by diamine salts as organocatalysts. Org. Lett. 2006;8:2687–2689. doi: 10.1021/ol060621i. [DOI] [PubMed] [Google Scholar]
- 14.Ishihara K, Nakano K. Design of an organocatalyst for the enantioselective Diels−Alder reaction with α-acyloxyacroleins. J. Am. Chem. Soc. 2005;127:10504–10505. doi: 10.1021/ja053368a. [DOI] [PubMed] [Google Scholar]
- 15.Sakakura A, Suzuki K, Nakano K, Ishihara K. Chiral 1,1′-binaphthyl-2,2′-diammonium salt catalysts for the enantioselective Diels−Alder reaction with α-acyloxyacroleins. Org. Lett. 2006;8:2229–2232. doi: 10.1021/ol060490l. [DOI] [PubMed] [Google Scholar]
- 16.Kano, T., Tanaka, Y., Osawa, K., Yurino, T. & Maruoka, K. Catalytic enantioselective construction of all-carbon quaternary stereocenters by an organocatalytic Diels–Alder reaction of α-substituted α,β-unsaturated aldehydes. Chem. Commun. 0, 1956–1958 (2009). [DOI] [PubMed]
- 17.Hatano M, et al. Enantioselective Diels–Alder reactions with anomalous endo/exo selectivities using conformationally flexible chiral supramolecular catalysts. Angew. Chem. Int. Ed. 2011;50:12189–12192. doi: 10.1002/anie.201106497. [DOI] [PubMed] [Google Scholar]
- 18.Momiyama N, Konno T, Furiya Y, Iwamoto T, Terada M. Design of chiral bis-phosphoric acid catalyst derived from (R)-3,3′-Di(2-hydroxy-3-arylphenyl)binaphthol: catalytic enantioselective Diels–Alder reaction of α,β-unsaturated aldehydes with amidodienes. J. Am. Chem. Soc. 2011;133:19294–19297. doi: 10.1021/ja2081444. [DOI] [PubMed] [Google Scholar]
- 19.Sakakura A, Yamada H, Ishihara K. Enantioselective Diels–Alder reaction of α-(acylthio)acroleins: a new entry to sulfur-containing chiral quaternary carbons. Org. Lett. 2012;14:2972–2975. doi: 10.1021/ol300921f. [DOI] [PubMed] [Google Scholar]
- 20.Hatano M, Goto Y, Izumiseki A, Akakura M, Ishihara K. Boron tribromide-assisted chiral phosphoric acid catalyst for a highly enantioselective Diels–Alder reaction of 1,2-dihydropyridines. J. Am. Chem. Soc. 2015;137:13472–13475. doi: 10.1021/jacs.5b08693. [DOI] [PubMed] [Google Scholar]
- 21.Gao X, Kagan HB. One-pot multi-substrate screening in asymmetric catalysis. Chirality. 1998;10:120–124. doi: 10.1002/chir.19. [DOI] [Google Scholar]
- 22.Brouwer AJ, van der Linden HJ, Liskamp RMJ. Combinatorial chemistry for ligand development in catalysis: synthesis and catalysis screening of peptidosulfonamide tweezers on the solid phase. J. Org. Chem. 2000;65:1750–1757. doi: 10.1021/jo991628z. [DOI] [PubMed] [Google Scholar]
- 23.Duursma A, Minnaard AJ, Feringa BL. One-pot multi-substrate enantioselective conjugate addition of diethylzinc to nitroalkenes. Tetrahedron. 2002;58:5773–5778. doi: 10.1016/S0040-4020(02)00574-4. [DOI] [Google Scholar]
- 24.Bernsmann H, et al. PipPhos and MorfPhos: privileged monodentate phosphoramidite ligands for rhodium-catalyzed asymmetric hydrogenation. J. Org. Chem. 2005;70:943–951. doi: 10.1021/jo048374o. [DOI] [PubMed] [Google Scholar]
- 25.Satyanarayana T, Kagan HB. The multi-substrate screening of asymmetric catalysts. Adv. Synth. Catal. 2005;347:737–748. doi: 10.1002/adsc.200505057. [DOI] [Google Scholar]
- 26.Akiyama T, Itoh J, Yokota K, Fuchibe K. Enantioselective Mannich‐type reaction catalyzed by a chiral Brønsted acid. Angew. Chem. Int. Ed. 2004;43:1566–1568. doi: 10.1002/anie.200353240. [DOI] [PubMed] [Google Scholar]
- 27.Uraguchi D, Terada M. Chiral Brønsted acid-catalyzed direct Mannich reactions via electrophilic activation. J. Am. Chem. Soc. 2004;126:5356–5357. doi: 10.1021/ja0491533. [DOI] [PubMed] [Google Scholar]
- 28.García‐García P, Lay F, García‐García P, Rabalakos C, List B. A powerful chiral counteranion motif for asymmetric catalysis. Angew. Chem. Int. Ed. 2009;48:4363–4366. doi: 10.1002/anie.200901768. [DOI] [PubMed] [Google Scholar]
- 29.James T, van Gemmeren M, List B. Development and applications of disulfonimides in enantioselective organocatalysis. Chem. Rev. 2015;115:9388–9409. doi: 10.1021/acs.chemrev.5b00128. [DOI] [PubMed] [Google Scholar]
- 30.Čorić I, List B. Asymmetric spiroacetalization catalysed by confined Brønsted acids. Nature. 2012;483:315–319. doi: 10.1038/nature10932. [DOI] [PubMed] [Google Scholar]
- 31.Kaib PSJ, Schreyer L, Lee S, Properzi R, List B. Extremely active organocatalysts enable a highly enantioselective addition of allyltrimethylsilane to aldehydes. Angew. Chem. Int. Ed. 2016;55:13200–13203. doi: 10.1002/anie.201607828. [DOI] [PubMed] [Google Scholar]
- 32.Lee S, Kaib PSJ, List B. Asymmetric catalysis via cyclic, aliphatic oxocarbenium ions. J. Am. Chem. Soc. 2017;139:2156–2159. doi: 10.1021/jacs.6b11993. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, et al. Catalytic asymmetric [4+2]-cycloaddition of dienes with aldehydes. J. Am. Chem. Soc. 2017;139:13656–13659. doi: 10.1021/jacs.7b08357. [DOI] [PubMed] [Google Scholar]
- 34.Tsuji N, et al. Activation of olefins via asymmetric Brønsted acid catalysis. Science. 2018;359:1501–1505. doi: 10.1126/science.aaq0445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files.