Abstract
BACKGROUND:
Patients with chronic idiopathic urticaria (CIU)/chronic spontaneous urticaria sometimes report systemic complaints (SCs).
OBJECTIVE:
We sought to determine the frequency and characteristics of SCs among patients with CIU, as well as the association of SCs with disease measures, basophil histamine release, and serum tryptase.
METHODS:
Adult patients with CIU were recruited from a university allergy clinic. Patients completed a disease symptom survey and underwent blood sampling for subsequent basophil histamine release and serum tryptase measurement. RESULTS: A total of 155 patients with CIU were surveyed, with 103 reporting SCs with concomitant hives as follows: joint pain or swelling (55.3%), headache/fatigue (47.6%), flushing (42.7%), wheezing (30.1%), gastrointestinal complaints (26.2%), and palpitations (9.7%). Patients with SCs (CIU-SC) were compared with those with no SCs (CIU-NSC). Both groups had similar demographic characteristics (average age in 40s, majority female and white) and basophil histamine release profiles. CIU-SC had significantly greater disease duration (51.5% CIU-SC vs 30.8% CIU-NSC had >4 years duration), emergency department visits (41.7% vs 23.1% had >1 visit in the last year), CIU-related work absences (65% vs 27.5% had >1 day), oral corticosteroid use (84.5% vs 59.6%), quality-of-life impairment (76.1 vs 59.2 SkinDex score), and serum tryptase levels (5.1 ng/mL vs 3.9 ng/mL).
CONCLUSIONS:
Despite similar demographic characteristics and basophil profiles as patients with CIU-NSC, patients with CIU-SC have features of greater disease burden (work absences, emergency department visits, and corticosteroid use), quality-of-life impairment, and baseline serum tryptase levels.
Keywords: Chronic idiopathic urticaria, Chronic spontaneous urticaria, Systemic complaints, Tryptase, Histamine, Quality of life
Urticaria is characterized by pruritic wheals, with or without angioedema, that generally resolve within 24 hours. It is considered chronic if the disease course lasts 6 weeks or longer. An estimated 80% to 90% of patients with chronic urticaria have no identifiable cause of the disease.1 It has been observed that some patients with chronic idiopathic urticaria (CIU), also known as chronic spontaneous urticaria, have associated systemic complaints (SCs) during active wheal flares. These include gastrointestinal symptoms, flushing, joint pain or swelling, cardiovascular manifestations, respiratory symptoms, and other constitutional complaints.2 Of note, similar symptoms are also reported by patients with mast cell activation disorders, and CIU is noted as one of the conditions that can be a mimicker.3 Elevated serum total tryptase level has also been demonstrated in patients with CIU relative to healthy controls.4–6
In addition to mast cells, basophils have previously been shown to have altered function in patients with CIU.7 We have demonstrated that patients with CIU can be divided on the basis of functional response of basophils to anti-IgE antibody stimulation into responders and nonresponders.7 Responders exhibit 10% or greater histamine release, whereas nonresponders exhibit less than 10% histamine release.
At present, there are few studies characterizing the type and frequency of SCs in patients with CIU. There are also limited data on basophil histamine release and tryptase level in this subset of patients with CIU. The aim of this study was 3-fold. First, we examined the frequency and characteristics of SCs among patients with CIU.8 Second, we determined the association of SCs with other CIU disease severity measures. Last, we examined the relationship between SCs and biomeasures of the disease, including basophil histamine release profile and serum tryptase levels.
METHODS
Study subjects
Adults (≥18 years old) diagnosed with CIU were recruited at the Johns Hopkins Asthma and Allergy Center, a tertiary care referral center. Inclusion criteria included an allergist or dermatologist diagnosis of active CIU. Exclusion criteria included use of systemic corticosteroids or other immunomodulatory agents (such as cyclosporine or sulfasalazine) in the month before enrollment due to their potential impact on basophil measures, and diagnosis of another skin disease (such as physical urticaria, atopic dermatitis, or urticarial vasculitis). After informed consent, patients completed a disease severity survey and underwent venipuncture under a Johns Hopkins Hospital institutional review board—approved protocol.
Disease survey
All subjects completed a disease survey containing 5 domains: demographic characteristics, presence of SCs, disease burden, elements of the urticaria severity score, and Skindex-29. Demographic characteristics included age, sex, race, and disease duration. Patients then indicated presence of SCs with concomitant hives in the lifetime of the disease. The SCs evaluated included gastrointestinal complaints, wheezing or breathlessness, palpitations, flushing, joint pain or swelling, and headache or fatigue. Patients who reported SCs with concomitant hives were categorized as CIU-SC, whereas those with no SCs were categorized as CIU-NSC.
The third domain assessed the disease burden. It included the number of systemic corticosteroid tapers, visits to the emergency department, days absent from work or school, and medication use.
The fourth domain contained elements of the urticaria severity score, a validated tool for monitoring urticaria severity.9,10 The elements in the survey included number of wheals present at the time of survey, current itch score, and duration of individual wheals. The number and size of wheals was scored as follows: 0 for no hives; 1 for 1 to 10 small hives (<3 cm); 2 for 10 to 50 small hives or 1 to 10 large hives (≥3cm); 3 for more than 50 small hives or 10 to 50 large hives; and 4 for covered with hives. Itch was rated by a visual analog scale from 0 to 10, indicating no itch to severe itch. Wheal duration of individual wheals was scored as follows: 1 for less than 1 hour, 2 for 1 to 24 hours, and 3 for greater than 24 hours.
The fifth domain is Skindex-29, a 29-question dermatology survey assessing the impact of CIU on the quality of life (QOL) in the past 3 months.10
Basophil histamine release
Basophils from patient venous blood samples were isolated via Percoll density sedimentation to obtain mixed leukocytes with average basophil purity of 1% to 5%. Subsequent histamine release was stimulated by polyclonal goat antihuman IgE (0.01–3 μg/mL), and N-formyl-met-leu-phe (1 μmol/L) for 45 minutes at 37°C in calcium-containing buffers, as described.7 Histamine release (HR) was quantified in cell-free supernatant with an automated fluoro-metric assay. Results were presented as the net percentages of total histamine content in total cell lysates of leukocyte aliquots after spontaneous HR percentages were subtracted from the total HR percentages. CIU histamine response was categorized as follows: HR of 10% or more as responders, HR of less than 10% as nonresponders. In some cases of significant basopenia, HR could not be defined. Total blood histamine content, an indirect measure of blood basophil numbers, was reported for 1 mL of blood.
Serum tryptase
Serum was stored at −20°C until assayed. Serum tryptase levels were measured using the UniCAP Tryptase Fluoro-Enzymatic Immunoassay, performed at Virginia Commonwealth University in Dr Lawrence B. Schwartz’s Laboratory.
Statistical analysis
Comparisons between the CIU-SC and CIU-NSC groups were performed using unpaired t test. Correlation coefficients were found between serum tryptase level and (1) the number of SCs, (2) current wheal size/number score, and (3) current itch. A P value of less than.05 was considered statistically significant.
RESULTS
Patients’ demographic characteristics and measure of disease
A total of 155 patients with a diagnosis of CIU completed surveys and venipuncture for analysis. Patients’ characteristics are presented on the basis of presence or absence of SCs with concomitant hives, CIU-SC and CIU-NSC, respectively (Table I). Most patients in both groups were women, with average age in the 40s, and white. Notably, more than half of the CIU-SC group reported disease duration greater than 4 years, whereas less than a third of the CIU-NSC group reported the same outcome.
TABLE I.
Characteristics of patients with CIU
| Characteristic | CIU-SC (n = 103) | CIU-NSC (n = 52) | P value* |
|---|---|---|---|
| Age (y), mean ± SD, y | 41.4 ± 13.8 | 45.9 ± 13.1 | .05 |
| Sex: female, % | 68.9 | 69.2 | .97 |
| White, % | 79.6 | 78.9 | .95 |
| Disease duration >4 y, % | 51.5 | 30.8 | .01 |
| Number of corticosteroid taper in the past 1 y, mean ± SD | 1.6 ± 1.3 | 0.9 ± 1.2 | .002 |
| Number of corticosteroid taper in CIU lifetime, mean ± SD | 2.3 ± 1.1 | 1.6 ± 1.3 | <.001 |
| > 1 visit to the ED for CIU in the past 1 y, % | 41.7 | 23.1 | <.001 |
| > 1 d of work or school absence in CIU lifetime, % | 65 | 27.5 | <.001 |
| Oral corticosteroids used for CIU, % | 84.5 | 59.6 | .002 |
| Antihistamines used for CIU, % | 99 | 100 | .32 |
| Antileukotrienes used for CIU, % | 45.6 | 38.5 | .40 |
| Antidepressants used for CIU, % | 54.4 | 40.4 | .10 |
ED, Emergency department.
CIU-SC vs CIU-NSC.
As compared with patients with CIU-NSC, patients with CIU-SC had significantly greater use of corticosteroid tapers in the past year, and in the lifetime of their CIU disease. There were also significantly higher frequencies of patients with CIU-SC than patients with CIU-NSC with emergency department visits in the past year and more than 1 day of work or school absences due to CIU.
SCs frequency and characteristics
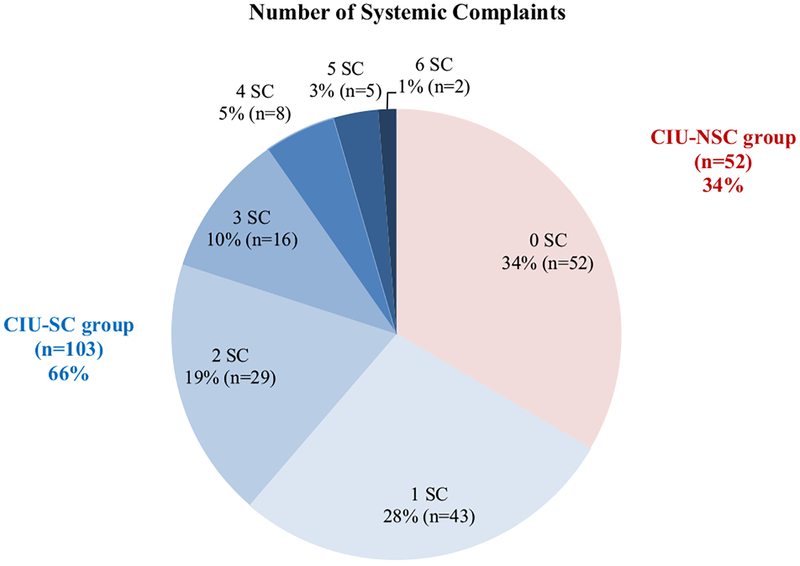
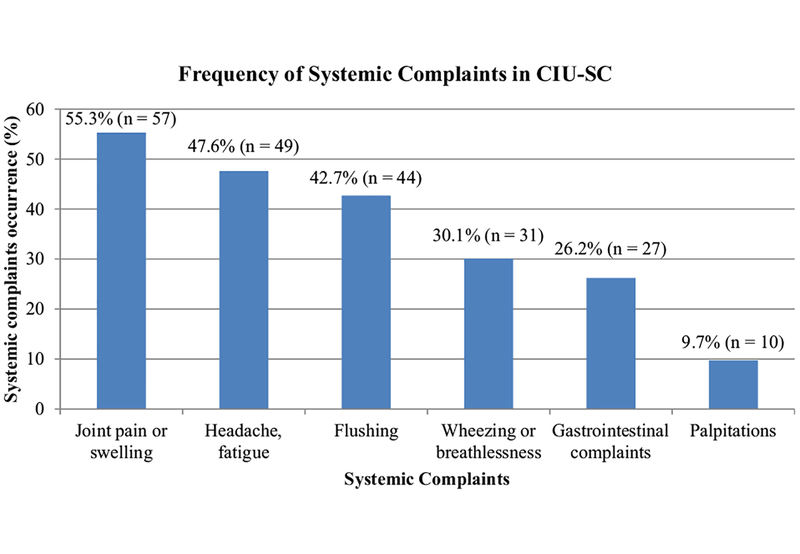
Of the 155 study participants, 103 reported the presence of SCs with hive flares while 52 did not have SCs (Figure 1). The frequency of having 1 SC is 28%, 2 SCs is 19%, and 3 or more SCs is 19%. Among the CIU-SC group, the SCs were distributed as follows (count, frequency): joints pain or swelling (57, 55.3%), headache/fatigue (49, 47.6%), flushing (44, 42.7%), wheezing or breathlessness (31, 30.1%), gastrointestinal complaints (27, 26.2%), and palpitations (10, 9.7%) (Figure 2).
FIGURE 1.
SCs frequency based on number of systems reported. Out of 155 patients with CIU, 52 (34%) reported not having SCs, 43 (28%) reported 1 SC, 29 (19%) reported 2 SCs, 16 (10%) reported 3 SCs, 8 (5%) reported 4 SCs, 5 (3%) reported 5 SCs, and 2 (1%) reported 6 SCs with concomitant hives.
FIGURE 2.
Frequency of individual SCs in the CIU-SC group. Out of 103 patients with CIU with SCs, 57 (55.3%) reported joint pain or swelling, 49 (47.6%) reported headache or fatigue, 44 (42.7%) reported flushing, 31 (30.1%) reported wheezing or breathlessness, 27 (26.2%) reported gastrointestinal complaints, and 10 (9.7%) reported palpitations.
Current disease activity and QOL measure
Current disease activity measures included duration of individual wheals, the number/size of wheals, and the intensity of the itch at the time of the survey. Patients with CIU-SC had significantly higher disease scores than did patients with CIU-NSC in all 3 parameters (Table II). Likewise, Skindex-29 scores were greater among patients with CIU-SC as an indication of greater QOL impairment in the past 3 months (P < .001).
TABLE II.
Current disease activity and QOL measures
| Characteristic | CIU-SC (n = 103) | CIU-NSC (n = 52) |
P value* |
|---|---|---|---|
| Current wheal size/number score, mean ± SD | 1.1 ± 1.2 | 0.6 ± 0.8 | .0005 |
| Current itch score, mean ± SD | 2.7 ± 2.8 | 1.6 ± 2.5 | .02 |
| Wheal duration > 24 h, % | 41.2 | 27.5 | .04 |
| SkinDex, mean ± SD | 76.1 ± 24.1 | 59.2 ± 20.8 | <.001 |
CIU-SC vs CIU-NSC.
Biomeasures of disease: Basophil activity and serum tryptase
Subjects in both CIU-SC and CIU-NSC groups were further subdivided on the basis of their basophil HR profile into responders (HR >10%), nonresponders (HR <10%), or undefined due to basopenia. Both groups had similar frequency of responders and nonresponders (Table III). Within both subsets, no significant differences in blood histamine content were noted between patients with CIU-SC and patients with CIU-NSC, indicating similar numbers of circulating basophils.
TABLE III.
Biomeasures of disease: Serum tryptase and basophil activity
| Characteristic | CIU-SC | CIU-NSC | P value* |
|---|---|---|---|
| Serum tryptase (ng/mL)† | 5.1 ± 10.8 | 3.9 ± 3.5 | .01 |
| Basophil profile not defined | 25.2 (26) | 26.9 (14) | — |
| Frequency, % (n) | |||
| Nonresponder | |||
| Frequency, % (n) | 34.0 (35) | 38.5 (20) | — |
| Histamine concentration (ng/mL blood leukocyte), mean ± SD | 22.1 ± 17.1 | 23.3 ± 16.3 | .80 |
| Responder | |||
| Frequency, % (n) | 40.8 (42) | 34.6 (18) | — |
| Histamine concentration (ng/mL blood leukocyte), mean ± SD | 26.1 ± 15.8 | 27.3 ± 11.2 | .74 |
CIU-SC vs CIU-NSC.
n = 78 for SCs and n = 36 for NSC due to lack of stored serum samples.
A total of 114 subjects of the 155 surveyed had stored serum for serum tryptase measurement (78 CIU-SC and 36 CIUNSC). Serum tryptase values were significantly greater in patients with CIU-SC as compared with patients with CIU-NSC. The highest value was 20 ng/mL in a patient who had 1 SC reported. In contrast, the highest tryptase level in the CIU-NSC group was 8.2 ng/mL.
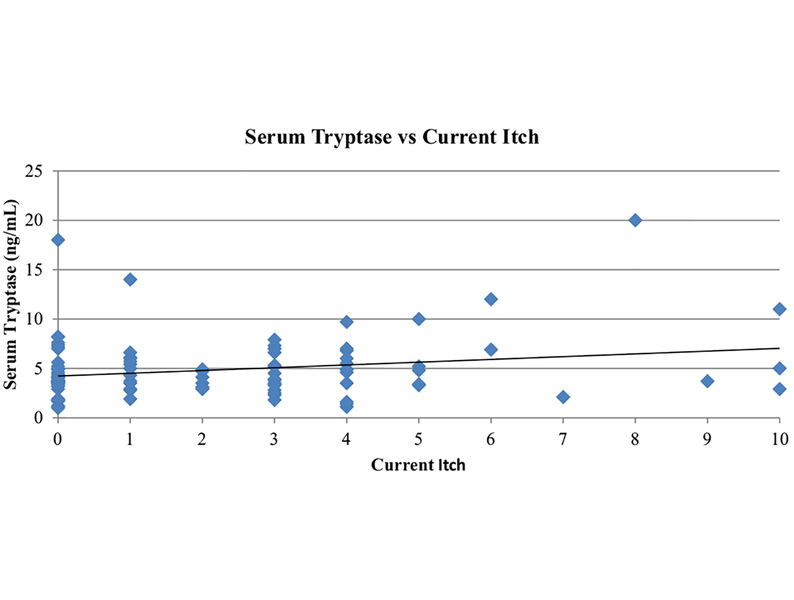
The correlation between serum tryptase levels and (1) number of SCs, (2) current wheal size/number scores, and (3) current itch scores yielded r = 0.06, 0.25, and 0.23, respectively (Table IV). Although the absolute number of SCs correlated poorly with serum tryptase levels, there were significant positive relationships between the current wheal size/number score (P =.01) and current itch to serum tryptase (P = .02) (Figure 3).
TABLE IV.
Correlation coefficients between serum tryptase level and (1) number of SCs, (2) current wheal size/number score, and (3) current itch
| Comparison | Correlation coefficient r | P value |
|---|---|---|
| Number of SCs: Tryptase | 0.06 | .49 |
| Current wheal size/number score: Tryptase | 0.25 | .01 |
| Current itch severity: Tryptase | 0.23 | .02 |
FIGURE 3.
Serum tryptase levels relative to current itch score. A total of 114 subjects (78 CIU-SC and 36 CIU-NSC) had serum sample collected at the time of survey, and the resulting serum tryptase level is plotted against the itch score reported at the time of the survey with correlation coefficient 0.23.
DISCUSSION
To our knowledge, this is the first study to determine the overall incidence of SCs along with urticaria in a university-based CIU patient population. Approximately two-third of this study population reported extracutaneous systemic involvement. A past report of 107 patients with CIU noted that patients with auto-antibodies (anti-IgE and/or anti-FcεRI) had more associated systemic symptoms than those without autoantibodies.2 In that study, the overall frequency of SCs was as follows: headache or lethargy (68.9%), gastrointestinal complaints (40.6%), cardiovascular symptoms (35.8%), joint pain or swelling (34.9%), and flushing (32.1%). In contrast, our study found joint pain or swelling as the most frequent SC, followed by headache or fatigue. However, examination of the joints was not performed at the time of survey to classify joint swelling complaint as intraarticular or periarticular swelling. The reason behind greater joint pain or swelling is unclear, but the high incidence of fatigue could be related to sleep disturbances.11,12 Patients with chronic urticaria report frequent sleep awakenings and difficulty falling asleep, with daytime fatigue reported by 75% of the patients and a higher incidence of sleep-related breathing disorder compared with the general population.13 The resulting daytime fatigue, mood depression, and lower efficiency at school or work also impair these patients.14
The findings of this study are consistent with past reports indicating that chronic urticaria significantly affects QOL, limits the capacity to work, and leads to high prevalence of health care use.11,12,14 This study further suggests that the subset of patients with CIU with SCs suffer from a greater disease burden than do patients without SCs, as demonstrated by greater use of corticosteroids, emergency department visits, more work or school absences, and greater QOL impairment. Patients with SCs also notably had greater current CIU disease activity despite similar background medication to those lacking SCs. It should be noted, however, that this study excluded patients who had recently used systemic corticosteroids or other immunomodula-tory agents in the month before enrollment to allow for accurate assessment of basophil profiles.
It is well recognized that basophils from patients with CIU have altered IgE receptore—mediated degranulation. Ex vivo activation of these basophils is used to segregate patients with CIU on the basis of HR into responders and nonresponders.7 This basophil functional phenotype is stable in active disease and is independent of the presence of autoantibodies (anti-IgE and anti-FceRIa).7,15 We previously demonstrated inverse correlations between total blood leukocyte histamine content, a measure of basophil number in the blood, with current wheal size/number and itch.16 In this study, we found comparable basophil profiles between patients with CIU-SC and patients with CIU-NSC.
Tryptase levels have been shown to be elevated in patients with chronic urticaria compared with healthy controls.4–6,17 Although we observed higher total serum tryptase in the CIUSC patient group, no significant relationship with the overall number of organ systems involved in conjunction with urticaria was found. A recent study of patients with autosomal-dominant vibratory urticaria noted that pruritic hives in response to mechanical stimulation were sometimes accompanied by systemic manifestations such as flushing and headache.18 Interestingly, minimal tryptase elevations occurred in these patients during the episodes of vibratory urticaria, while serum histamine levels were elevated. This suggests that either histamine, or perhaps other mediators such as prostaglandin D2, may be linked to SCs in patients with CIU. In contrast, we did observe positive relationships between serum tryptase values and measures of current skin disease activity such as current itch and current wheal size/number. Our findings that higher tryptase levels are associated with greater skin disease activity are similar to that of Ferrer et al,4 who noted higher tryptase level in more symptomatic patients versus asymptomatic patients at the time of blood sampling.5 In a retrospective study, 18 of 205 patients with CIU had elevated tryptase level (>13.5 μg/L). Of these patients, 8 patients with CIU (6 of which had tryptase >20 μg/L) underwent bone marrow biopsy and had no evidence of systemic mastocytosis.6 Thus, despite modestly greater tryptase levels and greater disease burden in patients with CIU who report SCs, the overall risk of an underlying mast cell disorder remains unclear but appears to be low.
In summary, most patients with CIU reported SCs with concomitant hives, and this subset of patients experienced greater disease burden as well as higher levels of serum tryptase. However, there was no clear difference in basophil measures or use of background medications in these patients except for corticosteroids. This suggests the importance of assessing for SCs in patients with CIU for anticipation of potential greater disease burden. Patients with CIU with SCs may need more effective or aggressive treatment early on to reduce their disease burden, but future studies are required to assess the utility of such an approach.
What is already known about this topic? Patients with chronic idiopathic urticaria can sometimes report systemic symptoms.
What does this article add to our knowledge? Most patients with chronic idiopathic urticaria have systemic complaints with concomitant hives. This subset of patients has greater disease burden and serum tryptase levels.
How does this study impact current management guidelines? Patients with chronic idiopathic urticaria with systemic complaints may require more effective treatment to reduce disease burden.
Abbreviations used
- CIU
Chronic idiopathic urticaria
- HR
Histamine release
- NSC
No systemic complaints
- QOL
Quality of life
- SCs
Systemic complaints
Footnotes
Conflicts of interest: L. B. Schwartz has received travel support from the National Institutes of Health; has received consultancy fees from SanofiAventis, Dyax, ViroPharma, and HELIX; has received research support from CSL Behring, Dyax/Shire, and Merck; shared royalties with VCU Tech Transfer, which received them from ThermoFisher for the tryptase assay; and has received payment to participate in the Atopic Dermatitis in America study from Asthma and Allergy Foundation of America. S. S. Saini has received consultancy fees from AstraZeneca and Teva and receives royalties from UpToDate. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Sheikh J Autoantibodies to the high-affinity IgE receptor in chronic urticaria: how important are they? Curr Opin Allergy Clin Immunol 2005;5:403–7. [DOI] [PubMed] [Google Scholar]
- 2.Sabroe RA, Seed PT, Francis DM, Barr RM, Black AK, Greaves MW. Chronic idiopathic urticaria: comparison of the clinical features of patients with and without anti-FcepsilonRI or anti-IgE autoantibodies. J Am Acad Dermatol 1999; 40:443–50. [DOI] [PubMed] [Google Scholar]
- 3.Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med 2015;373:163–72. [DOI] [PubMed] [Google Scholar]
- 4.Ferrer M, Nuñez-Córdoba JM, Luquin E, Grattan CE, De la Borbolla JM, Sanz ML, et al. Serum total tryptase levels are increased in patients with active chronic urticaria. Clin Exp Allergy 2010;40:1760–6. [DOI] [PubMed] [Google Scholar]
- 5.Hidvégi B, Nagy E, Szabó T, Temesvári E, Marschalkó M, Kárpáti S, et al. Correlation between T-cell and mast cell activity in patients with chronic urticaria. Int Arch Allergy Immunol 2003;132:177–82. [DOI] [PubMed] [Google Scholar]
- 6.Siles R, Xu M, Hsieh FH. The utility of serum tryptase as a marker in chronic spontaneous urticaria. Acta Derm Venereol 2013;93:354–5. [DOI] [PubMed] [Google Scholar]
- 7.Vonakis BM, Vasagar K, Gibbons SP Jr, Gober L, Sterba PM, Chang H, et al. Basophil FcepsilonRI histamine release parallels expression of Src-homology 2-containing inositol phosphatases in chronic idiopathic urticaria. J Allergy Clin Immunol 2007;119:441–8. [DOI] [PubMed] [Google Scholar]
- 8.Doong J, Oliver E, Saini SS. Frequency and characteristics of systemic complaints among chronic idiopathic/spontaneous urticaria patients. J Allergy Clin Immunol 2016;137:AB244. [Google Scholar]
- 9.Jariwala SP, Moday H, de Asis ML, Fodeman J, Hudes G, de Vos G, et al. The Urticaria Severity Score: a sensitive questionnaire/index for monitoring response to therapy in patients with chronic urticaria. Ann Allergy Asthma Immunol 2009;102:475–82. [DOI] [PubMed] [Google Scholar]
- 10.Jáuregui I, Ortiz de Frutos FJ, Ferrer M, Giménez-Arnau A, Sastre J, Bartra J, et al. Assessment of severity and quality of life in chronic urticaria. J Investig Allergol Clin Immunol 2014;24:80–6. [PubMed] [Google Scholar]
- 11.Gattey N, Bahrani B, Hull PR. Chronic spontaneous urticaria: a questionnaire survey. J Cutan Med Surg 2016;20:241–3. [DOI] [PubMed] [Google Scholar]
- 12.Balp MM, Vietri J, Tian H, Isherwood G. The impact of chronic urticaria from the patient’s perspective: a survey in five European countries. Patient 2015;8: 551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perkowska J, Kruszewski J, Gutkowski P, Chciałowski A, Kłos K. Occurrence of sleep-related breathing disorders in patients with chronic urticaria at its asymptomatic or oligosymptomatic stages. Postepy Dermatol Alergol 2016;33:63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vietri J, Turner SJ, Tian H, Isherwood G, Balp MM, Gabriel S. Effect of chronic urticaria on US patients: analysis of the National Health and Wellness Survey. Ann Allergy Asthma Immunol 2015;115:306–11. [DOI] [PubMed] [Google Scholar]
- 15.Eckman JA, Hamilton RG, Gober LM, Sterba PM, Saini SS. Basophil phenotypes in chronic idiopathic urticaria in relation to disease activity and autoantibodies. J Invest Dermatol 2008;128:1956–63. [DOI] [PubMed] [Google Scholar]
- 16.Oliver ET, Sterba PM, Saini SS. Interval shifts in basophil measures correlate with disease activity in chronic spontaneous urticaria. Allergy 2015;70:600–3. [DOI] [PubMed] [Google Scholar]
- 17.Bruno G, Andreozzi P, Magrini L, Graf U, Santangelo G, Zaino S. Mast cell activation in acquired chronic urticaria-angioedema. Sci Total Environ 2001; 270:77–81. [DOI] [PubMed] [Google Scholar]
- 18.Boyden SE, Desai A, Cruse G, Young ML, Bolan HC, Scott LM, et al. Vibratory urticaria associated with a missense variant in ADGRE2. N Engl J Med 2016;374:656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]