Abstract
GRAS genes are suggested to be grouped into plant-specific transcriptional regulatory families that have been reported to participate in multiple processes, including plant development, phytohormone signaling, the formation of symbiotic relationships, and response to environmental signals. GRAS genes have been characterized in a number of plant species, but little is known about this gene family in Citrus sinensis. In this study, we identified a total of 50 GRAS genes and characterized the gene structures, conserved motifs, genome localizations and cis-elements within their promoter regions. According to their structural and phylogenetic features, the identified sweet orange GRAS members were divided into 11 subgroups, of which subfamily CsGRAS34 was sweet orange-specific. Based on publicly available RNA-seq data generated from callus, flower, leaf and fruit in sweet orange, we found that some sweet orange GRAS genes exhibited tissue-specific expression patterning. Three of the six members of subfamily AtSHR, particularly CsGRAS9, and two of the six members of subfamily AtPAT1 were preferentially expressed in leaf. Moreover, protein-protein interactions with CsGRAS were predicted. Gene expression analysis was performed under conditions of phosphate deficiency, and GA3 and NaCl treatment to identify the potential functions of GRAS members in regulating stress and hormone responses. This study provides the first comprehensive understanding of the GRAS gene family in the sweet orange genome. As such, the study generates valuable information for further gene function analysis and identifying candidate genes to improve abiotic stress tolerance in citrus plants.
Introduction
C. sinensis is an extremely important fruit crop in many countries. The release of the whole-genome sequence of sweet orange provides an opportunity to comprehensively analyze numerous known gene families1,2. GRAS proteins, which are plant-specific transcription factors, contain a variable N-terminal domain and five distinct highly conserved motifs in the C-terminus: LRІ, VHIID, LRII, PFYRE and SAW3,4. The intrinsically disordered N-terminal regions are likely responsible for the specific function of each GRAS gene5. GRAS proteins, were classified into ten subfamilies based on their structural characteristics: DELLA, AtLAS, AtSCR, AtSHR, AtPAT1, HAM, LISCL, AtSCL3, SCL4/7 and DLT5, and were found to play crucial roles in diverse fundamental processes of plant growth and development5.
The most widely known biological function of the GRAS family is establishing radial root patterning. For instance, AtSCR (scarecrow), mainly expressed in the cortex/endodermal initial cells, is involved in radial root patterning and the distal specification of the quiescent center (QC)6,7. Analysis of the short-root (shr) mutant showed that the AtSHR protein is also required for asymmetric cell division, responsible for formation of ground tissue (endodermis and cortex) as well as specification of endodermis in Arabidopsis root8. AtSCARECROW-LIKE23 (AtSCL23), a mobile protein, controls movement of SHR and acts redundantly with SCR to specify endodermal fate in the root meristem9. GRAS proteins function in axillary meristem initiation, shoot meristem maintenance and male gametogenesis. Tomato Lateral suppressor (Ls), Arabidopsis LATERAL SUPPRESSOR (LAS) and rice monoclum 1 (MOC1) are orthologous proteins regulating axillary meristem initiation and outgrowth10–12. TaMOC1, a putative MOC1 ortholog in wheat, is associated with wheat spikelet development13. In the petunia mutant hairy meristem (ham), shoot apical meristems fail to retain their undifferentiated character14. LlSCL (Liliumlongiflorum Scarecrow-like) is involved in transcriptional regulation during microsporogenesis within the lily anther15.
The GRAS gene family also participates in phytohormone signaling pathways such as gibberellin acid (GA), jasmonic acid (JA) and brassinosteroid (BR) signal transduction. For example, AtGAI (GA-insensitive), AtRGA (repressor of GA), AtRGL1 (RGA-like 1) and AtRGL2 (RGA-like 2), well-known members of the DELLA subfamily of GRAS proteins, function as negative regulators of GA responses16–19. Overexpression of OsGAI in rice and tobacco modulates GA-dependent multiple responses such as increasing the number of tillers in rice20. AtSCL3 (scarecrow like 3) is a positive regulator of the GA response pathway21. AtRGL3 (RGA-like 3) regulates jasmonic acid (JA) signaling22. DLT (dwarf and low-tillering) and OsGRAS19 act as positive regulators in brassinosteroid signaling in rice23,24. In addition, GRAS proteins are involved in the formation of symbiotic relationships. OsSLR1, the AtGAI homolog in rice, not only acst as an intermediate of the GA-signal transduction pathway but also participates in arbuscular mycorrhizal symbiosis in plants by forming a DELLA complex with DIP1 (DELLA Interacting Protein 1). Nodulation Signaling Pathway1 (NSP1) and NSP2 are involved in early Nod-factor signaling by forming a complex in the model legume Medicago truncatula. Within this complex, NSP1 binds directly to early nodulin gene ENOD11 promoters through the novel cis-element AATTT25–27. The GRAS-type transcriptional regulators NSP1 and NSP2 were also required in carotenoid isomerase gene DWARF27 co-opted in rhizobium symbiosis and strigolactone (SL) biosynthesis in M. truncatula and rice28,29. Phosphorus (Pi), is an important macronutrient for all plants, including citrus. Low phosphate (Pi) availability exists in both natural and agricultural ecosystems. In response to Pi deficiency, plants modify their root architecture to improve Pi acquisition by reducing growth of primary roots and increasing the number and length of lateral roots (LRs) and root hairs30–32. Pi deficiency negatively impactes growth and development of citrus. Pi deficient plants exhibite reduced flowering, bronzed and smaller leaves, smaller fruit with reduced juice, and weak branches. Poncirus trifoliata (L.) Raf is relative to citrus and widely used as a root stock of citrus33. A majority of genes is involved in response to Pi deficiency34.
GRAS proteins also function in responding to environmental signals such as light and abiotic/biotic stress. SCL21 (SCARECROW-LIKE21) and PAT1 (PHYTOCHROME A SIGNAL TRANSDUCTION1) are positive regulators of phytochrome A (phyA) signal transduction for several high-irradiance responses35,36. AtSCL13 is a positive regulator of continuous red light signaling downstream of phytochrome B (phyB)37. SCL14 serves as a transcriptional coactivator of TGA transcription factors and regulates the induction of genes involved in the detoxification of harmful chemicals38. OsCIGR1 and OsCIGR2 act as transcriptional regulators in the early events of the elicitor-induced defense response in rice39. OsGRAS23, a rice GRAS transcription factor, positively regulates rice drought tolerance via the induction of a number of stress-responsive genes40. VaPAT1, one GRAS gene of Vitis amurensis, is induced by cold, drought and high salinity but repressed by exogenous gibberellic acid. VaPAT1 overexpression in Arabidopsis increases tolerance to cold, drought and high salinity, with higher levels of proline and soluble sugar under stress treatment in seedlings41. PeSCL7, a poplar GRAS/SCL gene, is also induced by drought and high salt, and repressed by gibberellic acid (GA) treatment. Compared with wide-type plant, Arabidopsis overexpressing PeSCL7 shows higher tolerance to drought and salt treatment2.
To date, the GRAS gene family has been identified and characterized in rice42, Arabidopsis43, Chinese cabbage44, Populus45, pine46, Prunusmume47, tomato48, tobacco49, grapevine50 and tea plant51. However, there is little information on the identification and functional characterization of GRAS proteins in citrus, the most important evergreen perennial fruit tree. Here we present the first detailed and comprehensive analyses of GRAS gene family in the whole genome of sweet orange. The present work identified 50 putative CsGRAS genes in C. sinensis, together with analyzing their gene characters and chromosome distribution. Then, we discerned phylogenetic relationships between C. sinensis, Arabidopsis and rice, noting main biological functions of GRAS proteins from recent studies. Subsequently, we performed RNA-Seq and qRT-PCR analysis to confirm the tissue expression patterns. Gene-gene interations with CsGRAS were also analyzed. Next, their transcript abundance in response to Pi deficiency treatments in P. Trifoliata (L.) Raf was investigated by both RNA-Seq and qRT-PCR analyses. In addition, the transcriptional level of CsGRAS genes in response to GA3 and NaCl was examined. This study provides details of the GRAS gene family and facilitates the further functional characterization of GRAS genes in Citrus.
Result and Discussion
Structural Analysis of CsGRAS Family
In order to run a complete search for identifying GRAS genes in the genome of sweet orange, all annotated proteins of the genome from sweet orange annotation project database of Huazhong Agricultural University (http://citrus.hzau.edu.cn/orange/) and the phytozome C. sinensis (v1.1) database (http://phytozome.jgi.doe.gov/-/pz/portal.html) were considered analyzed. The Hidden Markov Model (HMM) profile of the GRAS domain (PF03514) (http://pfam.sanger.ac.uk/) was then employed as a query to search the database using the program HMM3.0 with the default E-value. After determining the integrity of the GRAS domain of GRAS proteins by using the online program SMART (http://smart.embl-heidelberg.de/) and sequence alignment, 50 genes (Table S1) were assigned as GRAS genes in C. sinensis, and named from CsGRAS1 to CsGRAS50 based on the coordinate order on C. sinensis chromosomes starting at the top of chromosome 1 from top to bottom.
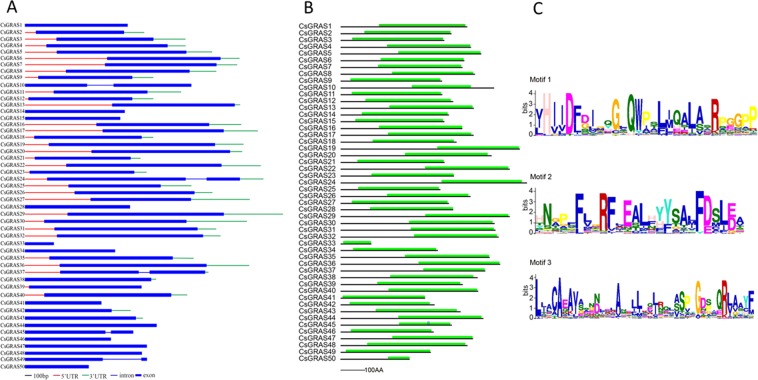
For gene families, the patterns of exon/intron positions may play important roles in the process of evolution52. To examine the diversity of exon/intron patterns in CsGRAS genes, we conducted an exon/intron organization analysis in 50 CsGRAS genes (Fig. 1A). As in Prunus mume and Arabidopsis, many CsGRAS genes (45 genes) were intron-less, and 5 genes had one intron. Moreover, the results also showed that genes in the same branch may showed similar exon/intron organization. The number of GRAS genes in citrus (50 genes) was close to the number of GRAS genes in P. mume (46 GRAS genes), Chinese cabbage (48 GRAS genes), tomato (53 GRAS genes) and Oryza sativa (57 GRAS genes), which is more than that in Arabidopsis (33 GRAS genes), pine (32 GRAS genes). It was, however, less than that of Populus (106 GRAS genes), Malus domestica (127 GRAS genes), Medicago truncatula (75 GRAS genes), and Musa acuminate (73 GRAS genes) (Lu et al. 2015).In order to study the structure of CsGRAS proteins, SMART (http://smart.embl-heidelberg.de/) was employed to identify the GRAS domain in the 50 proteins. All of the CsGRAS proteins showed the GRAS domain, and the GRAS domains were located at the end of the CsGRAS proteins (Fig. 1B). In order to identify the conserved motifs between the 50 CsGRAS proteins, the MEME motif search tool was used to find the 3 conserved motifs in the GRAS domain (Fig. 1C). The 3 conserved motifs were located at the middle part, near the end part and the beginning, respectively. Collectively, GRAS domains had high similarity in the 50 CsGRAS proteins.
Figure 1.
Structure of CsGRAS genes and CsGRAS proteins. (A) The gene structure based on the sequences of GRAS downloaded from orange genome database (http://citrus.hzau.edu.cn/orange/). (B) The protein structures based on the presence of GRAS domain as identified by SMART (http://smart.embl-heidelberg.de/). (C) Three conserved LOGOs for GRAS domain using the MEME algorithm (http://meme-suite.org/tools/meme).
Phylogenetic analysis of GRAS proteins in C. sinensis with Arabidopsis and rice
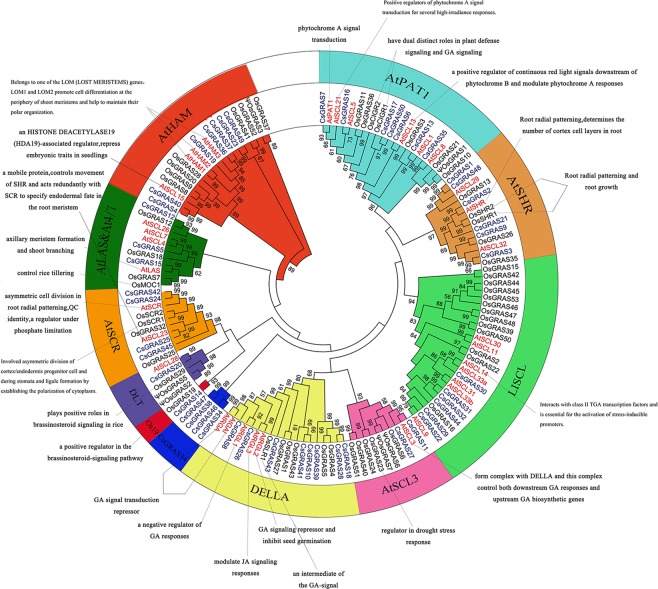
To reconstruct the evolutionary history of the GRAS gene family in the studied plant species, we built a phylogenetic tree from the alignment of 149 full-length GRAS protein sequences in sweet orange (49 GRAS proteins), Arabidopsis (33 GRAS proteins) and rice (59 GRAS proteins) using NJ method (Fig. 2). In particular, CsGRAS33 was not included in phylogenetic tree analysis, because its protein sequence was too short with length of 136 amino acid (Table S1). This tree showed that CsGRAS proteins of the 3 species could be divided into 11 subfamilies. These subfamilies were designated according to earlier previous studies5,42 or named according to one of their members in the case of newly identified subfamilies. The eleven subfamilies were AtHAM, AtLAS & AtSCL4/7, AtSCR, DLT, Os19, CsGRAS34, DELLA, AtSCl3, LISCL, AtSHR, and AtPAT1. Remarkably, the CsGRAS34 subfamily, consisting of CsGRAS47/CsGRAS38/CsGRAS46/CsGRAS34, was distinguished from other GRAS genes since they form an individual clade, which suggested that this individual clade was specific to sweet orange, and there was no homolog member in rice. This meant that they were the result of duplication after the ancestors of Arabidopsis and citrus segregated. Os19 belonged to sweet orange and a rice-specific subfamily containing only two members (OsGRAS19 and CsGRAS14) that form a small unique monophyletic clade. In general, the majority of subfamilies harbored GRAS members from each of the three species (Fig. 2). However, no Arabidopsis gene existed in Os19 subfamily, indicating lineage-specific gene loss in Arabidopsis. Coincidently, a similar result occurred in combined phylogenetic analysis of GRAS protein in Populus45. Notably, the subfamily CsGRAS34 was sweet orange-specific, implying that it had been gained in the sweet orange lineage after divergence from the most recent common ancestor with Arabidopsis and rice or entirely lost from the latter two lineages. It was tempting to speculate that this subfamily had specialized roles in the adaptive evolution of sweet orange. Considering the conserved characteristics of structure and function, we summarized the main biological functions of GRAS proteins in plant development, using information from recent studies (Fig. 2).
Figure 2.
The phylogenetic tree of GRAS proteins among sweet orange, Arabidopsis and rice. Combined phylogenetic analysis of GRAS proteins from C. sinensis (Cs), A. thaliana (At) and O. sativa (Os). The GRAS proteins are clustered into 11 subgroups, marked by different colors. The main biological functions of some GRAS proteins, which are experimentally characterized along with some representative references, are shown in the phylogenetic tree.
Classification of orthologous GRAS will facilitate the future study of their functions in citrus, as evidenced by Ls/LAS/MOC110,11, AtSHR/OsSHR1/OsSHR2/BdSHR8,53,54 and AtGAI/AtRGA/OsSLR116,17,55. Thus, according to previous reports on AtSHR, AtSCR6–8,53,54,56, CsGRAS2, CsGRAS24 and CsGRAS42 are thought to be related to root patterning. CsGRAS7, the homolog of AtPAT1, may be connected with the phytochrome A-specific signaling pathway35. AtGAI and AtRGA function as a negative regulator of GA responses16,17, so it could be deduced that CsGRAS8 and CsGRAS26 are possibly involved in GA responses. CsGRAS15, the homology of Ls/LAS/MOC1, was associated with axillary meristem formation10–12. CsGRAS14, Os19 family member, possibly participates in brassinosteroid-signaling24. These results suggested the potential function of CsGRAS proteins which may have similar roles to other GRAS proteins of rice and Arabidopsis in the same subfamilies.
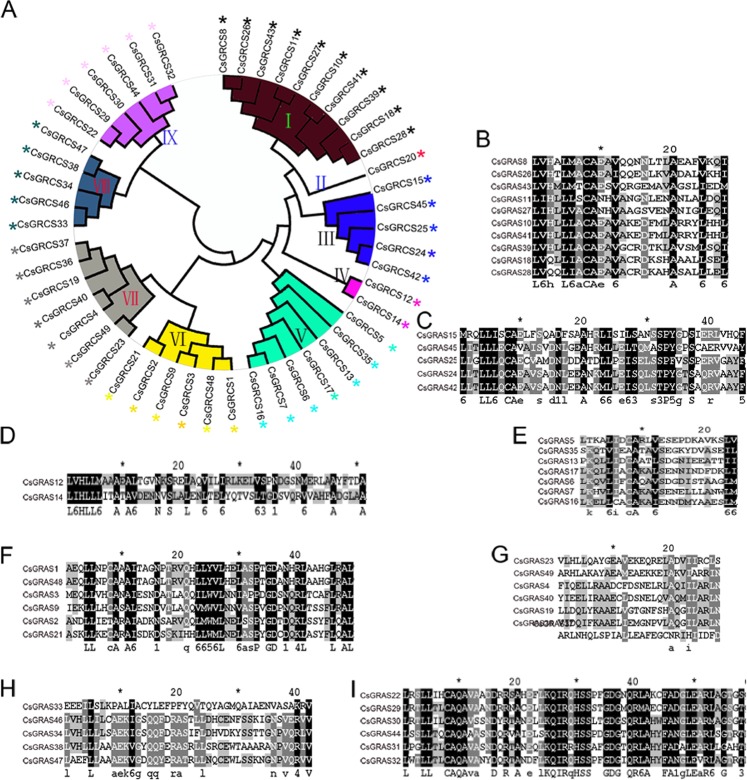
In order to study phylogenetic relationship between members of CsGRAS proteins, we also constructed a phylogenetic tree with GRAS domain sequences of 49 CsGRAS proteins. 9 subfamilies were generated, which were similar to a phylogenetic tree with full-length GRAS protein sequences of CsGRAS proteins, OsGRAS proteins and AtGRAS proteins (Fig. 3A). For example, CsGRAS proteins of subgroup IX were in LISCL family, and CsGRAS proteins of subgroup IV belonged to AtSHR family. To examine the conserved amino acids of GRAS domains in each subgroup, we constructed a multiple sequence alignment of GRAS domains of CsGRAS proteins in each subgroup. GRAS domain sequences in each subgroup showed high homology with each other. Moreover, the GRAS domain sequences of CsGRAS proteins in subgroup I (Fig. 3B), III (Fig. 3C), IV (Fig. 3D), V (Fig. 3E), VI (Fig. 3F), VIII (Fig. 3H) and IX (Fig. 3I) were significantly conserved within each subgroup, indicating that the proteins within each group may perform similar functions. In contrast, subgroup VII (Fig. 3G) proteins displayed reduced sequence similarity, suggesting these proteins had distinct origins and functions.
Figure 3.
The phylogenetic analysis and Multiple sequence alignment of CsGRAS proteins. (A) Unrooted neighbour-joining phylogenetic tree of the CsGRAS family. The phylogenetic tree was generated using the MEGA 5.1 software (Tamura et al. 2011). (B–I) Conserved sequence of multiple sequence alignment of the CsGRAS proteins in subgroup I (B), subgroup III (C), subgroup IV (D), subgroup V (E), subgroup VI (F), subgroup VII (G), subgroup VIII (H), and subgroup IX (I) obtained using GneneDoc and ClustalX.
The goal of GRAS gene family genome-wide analysis in sweet orange was to make comprehensive predictions of citrus GRAS gene function. The location of a CsGRAS gene in the same branch of the phylogenetic tree as say the homologous gene in Arabidopsis or rice could give an approximate indication of the function of the CsGRAS gene in citrus. For example, the orthologous protein of AtPAT1, CsGRAS7, may therefore be involved in phyA signaling. The SCR-SHR complex controlled endodermis development in Arabidopsis9; however, citrus are woody plants with multiple cortex layers and are different to the single cortex in Arabidopsis. Therefore, the mechanism of SCR-SHR in citrus may be dissimilar to that in Arabidopsis. PtSHR2B in Populus functions in the regulation of phellem and periderm formation57; therefore, the homologous gene in citrus, CsGRAS2, may similarly function in phellem and periderm formation. The PPI network provided further information on the molecular function of the CsGRAS protein and confirmed predictions from the phylogenetic tree analysis. The protein which had protein-protein interaction with CsGRAS6 is calmodulin. Since CsGRAS6 belonged to AtPAT1 subfamily, it may also be involved in phyA or phyB signaling.
Chromosomal distribution and gene duplication of CsGRAS genes
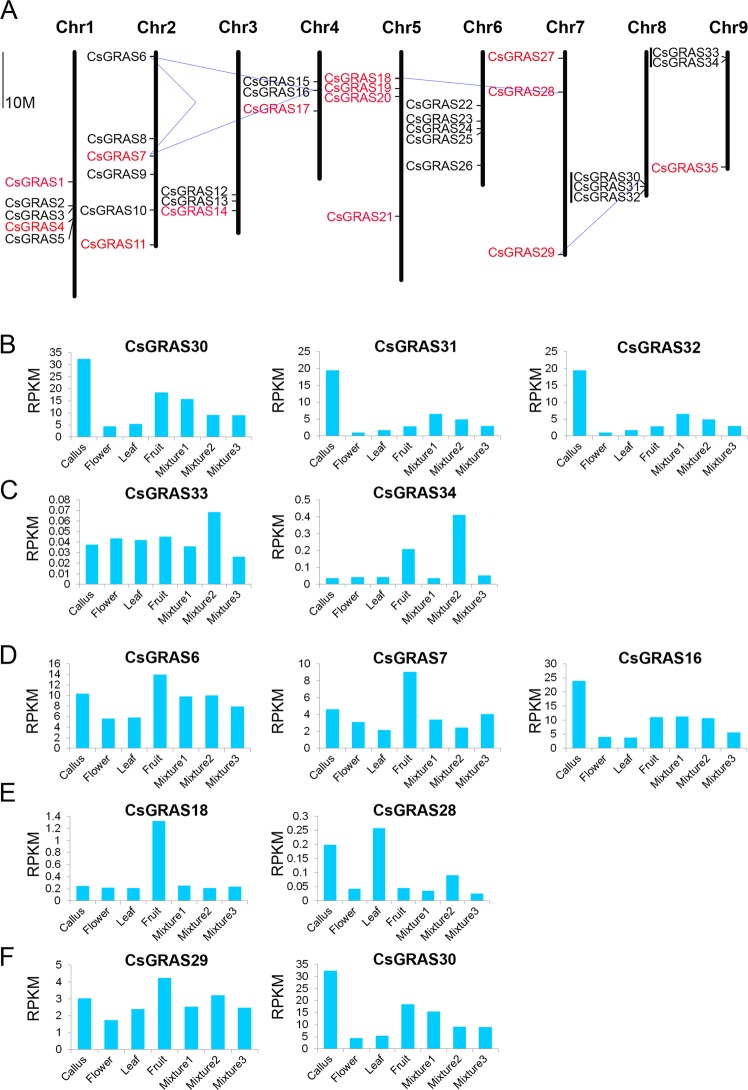
The physical positions of GRAS genes were obtained from the sweet orange annotation project database of Huazhong Agricultural University and the phytozome Citrus sinensis v1.1 Citrus sinensis database. The position and transcriptional direction of each gene are shown (Fig. 4A), and the exact positions on C. sinensis chromosome pseudomolecules are given in Table S1. The results show that 35 CsGRAS genes are distributed on 9 chromosomes, and that the tandem duplication genes, (CsGRAS30, CsGRAS31 and CsGRAS32; CsGRAS33 and CsGRAS34) are located on chromosome 8 and 9, as shown by connected dark lines in Fig. 4A. The segmental duplication genes are located on chromosome 2, 4, 5, 7 and 8, and indicated by connected blue lines (CsGRAS6, CsGRAS7 and CsGRAS16; CsGRAS18 and CsGRAS28; CsGRAS29 and CsGRAS30) (Fig. 4A). The expression patterns of segmentally duplicated and tandemly duplicated CsGRAS genes were examined by using data from the Citrus sinensis annotation project database of Huazhong Agricultural University. The chromosome distribution of GRAS genes showed tandem repeats and segmental repeats, indicating that the derivation of GRAS genes in C. sinensis was generated by generic duplication and recombination.
Figure 4.
Genomic distribution of CsGRAS genes and expression patterns of CsGRAS duplicated genes. (A) The scale on the left is 10 megabases (10 Mb). Chromosome number is indicated at the top of each chromosome. Black and red show the forward and backward direction of transcription. The CsGRAS genes representing segmentally duplicated genes are connected by blue lines and the tandem duplicated genes are highlighted by dark lines on the left. The position of each CsGRAS gene on chromosome pseudomolecules in base pairs are given in Table S1 in electronic supplementary material. The expression levels of tandem duplicated GRAS genes (B,C) and segmentally duplicated GRAS genes (D–F) were analysed in C. sinensis annotation project database of Huazhong Agricultural University (http://citrus.hzau.edu.cn/orange/).
It was found that one pair of tandem duplication genes and two pairs of segmental duplication genes had similar expression patterns. All of the three tandem duplication genes, CsGRAS30, CsGRAS31 and CsGRAS32, had higher transcription levels in callus, but lower expression levels in other tissues (Fig. 4B). CsGRAS33 was expressed in the 4 tissues with similar expression levels, whereas CsGRAS34 showed higher transcriptional level in fruit (Fig. 4C). Moreover, CsGRAS6, CsGRAS7 and CsGRAS16 showed higher transcription accumulation in callus and fruit than in flower, leaf, and mixtures (Fig. 4D). Similarly, both of CsGRAS29 and CsGRAS30 also showed higher expression in callus and fruit (Fig. 4F). However, CsGRAS18 and CsGRAS28 displayed significantly different expression patterns (Fig. 4D). These results indicate that copies of duplicated genes with similar expression patterns might be functionally redundant, maintaining their functions during evolution, while the duplicated genes with different expression patterns may play distinct roles, suggesting that some members may have changed their function during the course of evolution58.
Expression profiling of CsGRAS genes
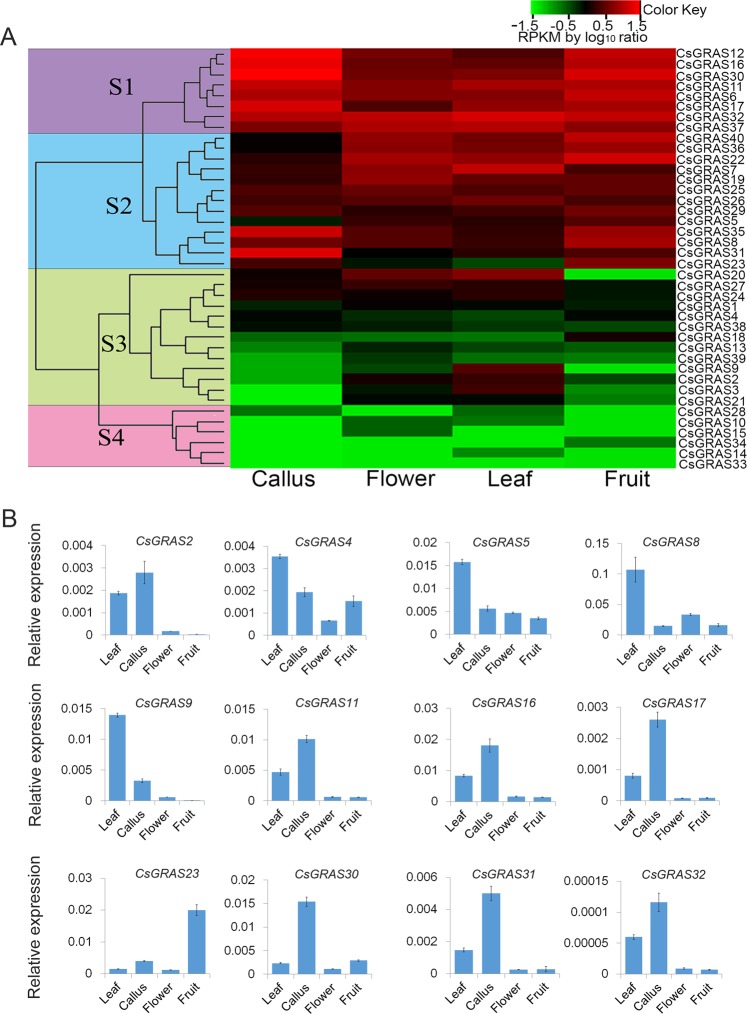
To investigate the transcript accumulation of CsGRAS genes in the sweet orange, the expression profiling covering 4 tissues in C. sinensis were analyzed using Illumina GAII sequencer data from Citrus sinensis annotation project database of Huazhong Agricultural University1. The red or green colors represented the higher or lower relative abundance of each transcript in each sample, respectively, compared to the median expression value of that gene in the whole sample set. The expression patterns of CsGRAS genes could be classified into two major groups (Fig. 5A). Group 1, which contained subgroup S1 and S2, shows high transcript accumulations in the tissues analyzed. Some genes, such as CsGRAS7, CsGRAS25, CsGRAS26 and CsGRAS35, had high expression level in four tissues. However, some genes in group 1 displayed high expression signals and preferential expression in some tissues, like CsGRAS17 and CsGRAS31. CsGRAS5 was abundantly expressed in leaf and callus, but showed low expression level in flower and fruit. CsGRAS31 showed high transcriptional level in callus, but lower in leaf, flower and fruit. Group 2 contained 19 genes which could be divided into two subgroups, S3 and S4. Subgroup S4, which had low expression levels in nearly all tissues, especially in callus and fruit, consisted of 6 genes (CsGRAS10, CsGRAS14, CsGRAS15, CsGRAS28, CsGRAS33 and CsGRAS34). CsGRAS33 exhibited the lowest expression signals in all tissues. Subgroup S3 had 13 CsGRAS genes, all of which showed relatively low expression levels in most tissues analyzed, but higher in particular tissues, such as CsGRAS2, CsGRAS3 and CsGRAS9 in leaves, and CsGRAS18 in fruit. CsGRAS20, CsGRAS24 and CsGRAS27 showed lower expression level in fruit, but higher in the 3 other tissues.
Figure 5.
The expression pattern of 40 GRAS genes in sweet orange. (A) This heatmap was classified to 4 series. The data came from RNA-seq data (RPKM) and was transformed by log10 fold change. (B) Relative expression of 12 CsGRAS genes in 4 tissues of sweet orange, including callus, flower, leaf and fruit. Error bars denotes the standard deviation calculated from three independent experiments, with the Citrus actin gene as internal control.
The expression patterns of 35 CsGRAS genes were further confirmed by real-time PCR analysis (Figs 5B and S1). The expression levels of CsGRAS2, CsGRAS4, CsGRAS5, CsGRAS8, CsGRAS9 and CsGRAS20 were enriched in leaf (Fig. 5B). Moreover, seven genes including CsGRAS11, CsGRAS 16, CsGRAS 17, CsGRAS 30, CsGRAS 31 and CsGRAS32 showed the highest expression level in callus (Fig. 5B), and CsGRAS23 showed a high expression level in fruit (Fig. 5B). These results were consistent with RNA-seq data analysis. However, CsGRAS1, CsGRAS24 and CsGRAS 27 were highly expressed in callus (Fig. S1), which differed from the RNA-seq results. Taken together, the GRAS genes showed varied expression patterning in citrus tissues, implying multiple roles in citrus development.
Interaction analysis between GRAS proteins in C. sinensis
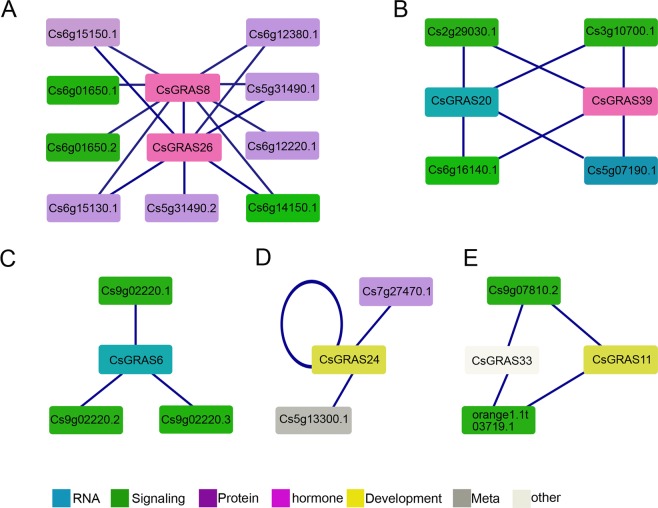
In order to identify the function of CsGRAS genes, the PPI (protein-protein interact) networks of CsGRAS protein were built by C. sinensis annotation project database of Huazhong Agricultural University. The results showed that the PPI networks can been divided to 5 groups (Fig. 6). CsGRAS8 and CsGRAS26 were predicted to interact with each other (Fig. 6A), both of which were DELLA proteins (Fig. 2). Excluding Cs6g01650.1, other predicted interaction proteins with CsGRAS8 and CsGRAS26 belonged to F-box protein families which determine substrate specificity in the ubiquitin-proteasome pathway (Table S2). This indicates that CsGRAS8 and CsGRAS26 may be involved in the regulation of protein degradation. The DLT subfamily member CsGRAS20 and the DELLA protein CsGRAS39 had interactions with Cs2g29030.1, Cs6g16140.1, Cs3g10700.1 and Cs5g07160.1, all of which were calmodulins (Table S2). CsGRAS6 belonged to the AtPAT1 subfamily (Fig. 2), which is associated with phyA signaling36. Cs9g02220.1, a phyB1, and its other two transcripts interacted with CsGRAS6, indicating CsGRAS6 may function in phyB signaling. The AtSCR family member CsGRAS24 had interactions with Cs5g13300.1, Cs7g27470.1 and itself. Cs5g13300.1 is probably a rhamnose biosynthetic enzyme 1 (Table S2), and Cs7g27470.1 is elongation factor 1-alpha 1, indicating CsGRAS24 may be involved in metabolism. CsGRAS11 belonged to the AtSCL3 subfamily which functions in stress response and the GA response pathway21. CsGRAS33 belonged to the CsGRAS34 subfamily, of which little is known. Both interact with two calmodulin, indicating that CsGRAS33 may function in signaling via calmodulin. In short, CsGRAS family proteins may be involved in similar pathways with their homologous genes, but might also play different roles from homologous genes in Arabidopsis and rice.
Figure 6.
The protein-protein interactions (PPI) of GRAS protein in sweet orange. Orthologous-based and domain-based methods were employed to predict PPI network in sweet orange. The colors represent different functions of protein in rectangle. (A) The interacted proteins with CsGRAS8 and CsGRAS26. (B) The interacted proteins with CsGRAS20 and CsGRAS39. (C) The interacted proteins with CsGRAS6. (D) The interacted proteins with CsGRAS24. (E) The interacted proteins with CsGRAS11 and CsGRAS33.
The expression data of 8 GRAS proteins and 20 proposed interacted proteins were downloaded from RNA-seq library in website http://citrus.hzau.edu.cn/orange/ (Fig. S2). The expression patterns of GRAS8 and GRAS26 were consistent, the same as which of Cs6g15130.1 and Cs6g15150.1, Cs6g01650.1 and Cs6g01650.2, Cs5g31490.1 and Cs5g31490.2 (Fig. S2A). The expression patterns of GRAS20 and Cs3g10700.1 were similar in tissues of callus, flower and leaf (Fig. S2B). The genes coded GRAS6 and Cs9g02220.1 were expressed almost similarly in all the tissues checked (Fig. S2C). But the gene expression of GRAS24 is much different with which of two proposed interacted proteins (Fig. S2D). The genes coded GRAS11 and its proposed interacted proteins Cs9g07810.2 and orange1.1t03719.1 expressed much higher in callus than the other three tissues (Fig. S2E). The similar expression patterns suggested the interaction between these GRAS proteins and proposed interacted proteins was possible. However, the predicted protein interaction needs further experiments like as yeast two hybrids, pull down or Co-IP for testing.
Cis-element analysis in the promoters of CsGRAS genes
In response to phytohormones and stress factors, cis-elements in promoter regions affect gene expression to regulate plant development and adaption to environmental changes. In order to obtain more information about functions of CsGRAS genes in citrus, 50 promoters of CsGRAS genes were analyzed using the online software PlantCARE.
Various types of cis elements were identified in the promoter regions, including stress responses elements related to heat and drought, and hormone response elements related to ethylene and ABA signaling (Table S3). In addition, each promoter of CsGRAS genes contains more than one response element. 35 promotors of 50 CsGRAS genes include an anaerobic stress cis-element, many CsGRAS promoters contain cis-elements for drought stress, SA, JA, and ABA, and numerous promoters also contain heat, fungi, ethylene, GA and IAA cis-elements. In addition, several promoters have the low temperature, anoxic and wound cis-elements. This suggests that CsGRAS genes may play important roles in many developmental processes and are involved in various stress responses.
Expression profiles of CsGRAS genes in response to phosphorus deficiency in the root of Pt
In agricultural production, sweet orange is generally used as a scion. Pt is the main rootstock for sweet orange because of favorable growth characteristics, such as strong growth, strong root system, high survival rate, cold resistance, drought resistance and resistance to pests and diseases. Pi is an essential macronutrient for plant growth and development, and the CsGRAS gene family is involved in responding to Pi deficiency. For example, AtSHR and AtSCR genes have been functionally characterized in Arabidopsis under Pi deficiency59. It was reported that the transcriptional level of multiple transporters and some transcription factors, such as GRAS, NAP, CCAAT-binding and GATA TFs, changed under P-deficiency in Pt33.
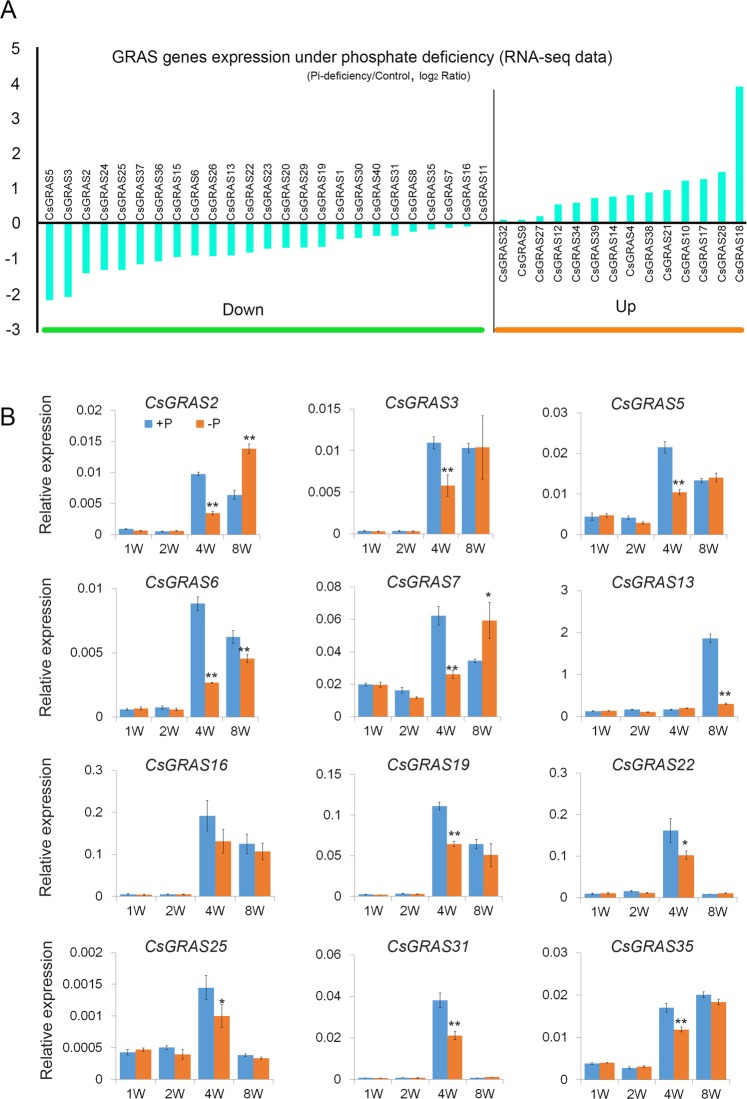
In this study, we investigate the expression patterns of CsGRAS genes in response to Pi deficiency by RNA-seq data analysis in P. trifoliata and quantitative real-time PCR. Firstly, expression data for all 39 CsGRAS genes under Pi deficiency treatment for 4 weeks in the root of P. trifoliata were downloaded from the report33. Log2-transformed expression values were used to create the histogram (Fig. 7A), and expression changes of more than two-fold were considered significant under Pi deficiency treatment. The CsGRAS genes were divided into two groups according to changes in expression levels under Pi deficiency: the ‘up-regulated’ group consisted of 14 CsGRAS genes that were upregulated by more than one-fold (on a log2 scale); 25 CsGRAS genes made up the ‘down-regulated’ group containing genes that were downregulated by more than one-fold (on a log2 scale) (Fig. 7A). The results revealed that 7 CsGRAS genes (CsGRAS5, CsGRAS3, CsGRAS2, CsGRAS24, CsGRAS25, CsGRAS37 and CsGRAS36) showed significantly decreased abundance, especially CsGRAS5 and CsGRAS3 which were reduced more than four-fold, while 4 CsGRAS genes (CsGRAS10, CsGRAS17, CsGRAS28 and CsGRAS18) were notably up regulated under Pi deficiency. In particular, the highest up-regulated gene was CsGRAS18, which increased more than six-fold. Intriguingly, the significantly up regulated genes CsGRAS10, CsGRAS18 and CsGRAS28, all belonged to the DELLA subfamily.
Figure 7.
The GRAS genes expression under Pi-deficiency in P. trifoliata. (A) The value was RNA-seq (RPKM) which deal with the Pi-deficiency data divided by the control data, and was transformed by log2 fold change. (B) Expression level of 12 CsGRAS genes under Pi-deficiency treatment at 1W, 2W, 4W and 8W, with the Citrus actin gene as internal control Error bars denotes the standard deviation calculated from three independent experiments, statistical significance were analyzed by Student’s t-test (∗∗p < 0.01, *p < 0.05).
It was found that Pi deficiency promoted the accumulation of a DELLA protein (RGA) and caused a reduction of bioactive GA and attenuation of GA metabolism in root cells of Arabidopsis60. In our study, four CsGRAS genes (CsGRAS10, CsGRAS17, CsGRAS28 and CsGRAS18), were significantly up-regulated under Pi deficient conditions in RNA-seq data (Fig. 7A), and three of these genes (CsGRAS10, CsGRAS18 and CsGRAS28) belonged to the DELLA subfamily. However, CsGRAS8 and CsGRAS26, homologous to RGA and GAI, were down-regulated (Fig. 7A), indicating that these members of the DELLA subfamily may be involved in DELLA-GA signaling under Pi deficiency.
Also, CsGRAS3 and CsGRAS2, CsGRAS24 and CsGRAS25 were part of AtSCR subfamily respectively (Fig. 2). In Arabidopsis, AtSCR and AtSHR proteins play a key role in root radial patterning52. PDR2 (PHOSPHATE DEFICIENCY RESPONSE 2), encoding the P5-type ATPase, was reported to restrict AtSHR movement and maintain AtSCR level during Pi deprivation59. CsGRAS2, which is homologous to AtSHR, showed decreased expression levels under Pi-deficiency suggesting that CsGRAS2 may be involved in Pi deficiency response in the root of woody plants (Fig. 7A).
These results appeared consistent with earlier studies in Arabidopsis and crops, which demonstrated that Pi deficiency affected root development by reducing growth of primary roots while increasing the number and length of lateral roots and root hairs34.
We then examined the transcriptional level of 35 CsGRAS genes under Pi deficient conditions lasting one week, two weeks, four weeks and eight weeks (Figs 7B and S3). We found that The CsGRAS genes revealed time-dependent differences in expression (Figs 7B and S3), demonstrating that plants response to Pi deficiency is a complex process. The CsGRAS genes participating in regulating plant response to phosphorous deficiency, functioned in multiple pathways. Most CsGRAS genes showed lower expression level with Pi starvation treatment in the first two weeks than that at the fourth and eighth week, especially at the fourth week, such as CsGRAS9, CsGRAS14, CsGRAS22 and CsGRAS31 (Figs 7B and S3). In addition, the expression level of CsGRAS2, CsGRAS3, CsGRAS5, CsGRAS6, CsGRAS7, CsGRAS13, CsGRAS16, CsGRAS19, CsGRAS22, CsGRAS25, CsGRAS31 and CsGRAS35 decreased with Pi starvation treatment at the fourth week (Fig. 7B), which was consistent with the RNA-seq data analysis (Fig. 7A). Moreover, CsGRAS2, CsGRAS27, CsGRAS32 and CsGRAS33 showed reduced transcriptional abundance at fourth week under Pi deficiency, while an increased transcriptional abundance at the eighth week (Figs 7B and S3). Clear increasement was detected of CsGRAS23 at the fourth week under Pi starvation treatment, which was inconsistent with the RNA-seq data (Figs 7A and S3). And some genes showed obvious response until eighth-week Pi starvation treatment, such as CsGRAS4, CsGRAS13 and CsGRAS15 (S3). In conclusion, the expression of CsGRAS genes exhibited a time-dependent response to Pi deficiency in trifoliate roots.
Expression profiles of CsGRAS genes in response to GA3 and NaCl treatment
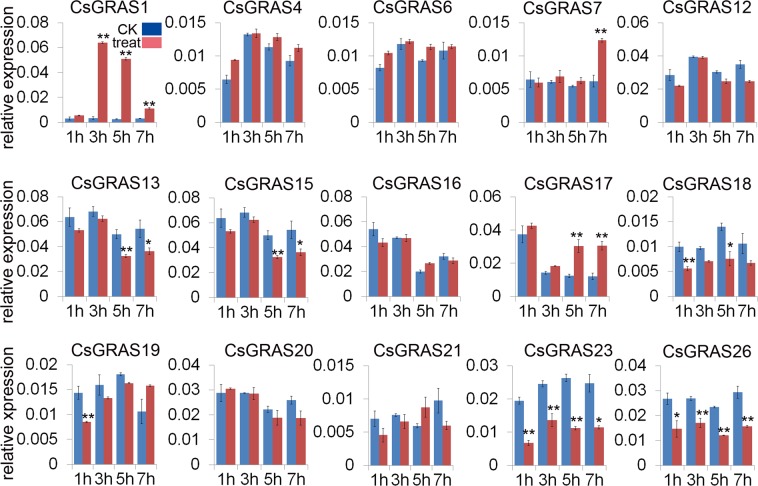
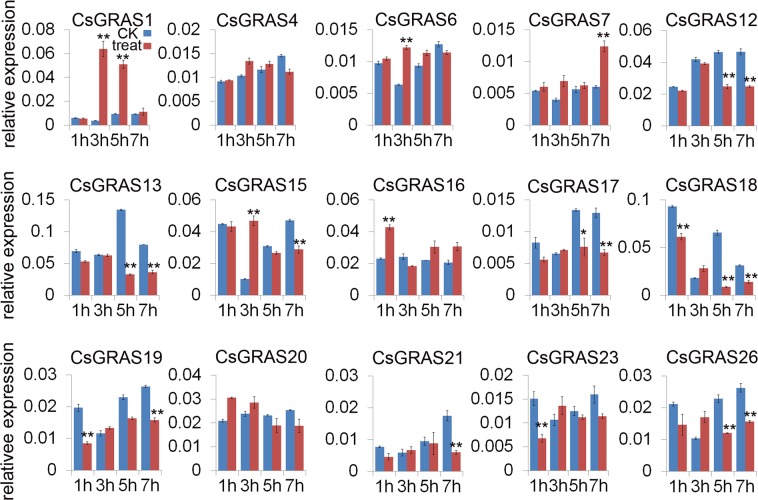
GA3 plays a critical role in plant growth and development, and GRAS proteins in Arabidopsis and rice participate in GA responses16–19. To investigate CsGRAS gene expression in response to GA treatment, qRT-PCR was performed to analyze changes in the expression levels of 15 CsGRAS genes in GA treatment at 1 h, 3 h, 5 h and 7 h, with water treatment as control. Three genes (CsGRAS1, CsGRAS 7 and CsGRAS 17) were up-regulated at 3 h, 7 h and 5 h during GA treatment, respectively, whereas CsGRAS18, CsGRAS19, CsGRAS23 and CsGRAS26 were all down-regulated at 1 h, CsGRAS13 and CsGRAS15 showed lower expression level at 5 h and 7 h under GA treatment (Fig. 8). Moreover the promoters of CsGRAS1, CsGRAS13, CsGRAS15, CsGRAS23 and CsGRAS26 have the response element to gibberellin (Table S3), indicating that certain CsGRAS genes could be involved in the GA signaling pathway.
Figure 8.
Differential expression detected for 15 CsGRAS genes response to GA treatment at 1 h, 3 h, 5 h, and 7 h (marked treat in red), with water treatment as control (marked CK in blue). The relative expression of selected GRAS genes under 100 mM NaCl was determined by qRT-PCR. The Citrus actin gene was used as internal control. Error bars denotes the standard deviation calculated from three independent experiments, statistical significance were analyzed by Student’s t-test (**p < 0.01, *p < 0.05).
Previous studies showed that GRAS proteins function in abiotic stress40,41, therefore we checked the transcriptional level of CsGRAS genes in response to NaCl treatment. CsGRAS1 showed increased expression level at 3 h, and CsGRAS7 at 7 h, similar to their response to GA treatment (Fig. 9). CsGRAS6 and CsGRAS15 were up-regulated at 3 h, while CsGRAS16 was upregulated at 1 h (Fig. 9). Interestingly, CsGRAS18, CsGRAS23 and CsGRAS26 were down-regulated in response to NaCl treatment, similar to their expression changes under GA treatment (Fig. 9). CsGRAS genes (CsGRAS 12, 13, 17, 19 and 21) also showed lower transcriptional level than control at different NaCl treatment times, in comparison CsGRAS17 showed higher expression levels under GA treatment (Fig. 9). Collectedly, more CsGRAS genes were affected by NaCl treatment than by GA treatment. Some genes may play roles in both NaCl and GA responses, while some genes may show opposite roles under different treatments.
Figure 9.
Differential expression detected for 15 CsGRAS genes response to NaCl treatment at 1 h, 3 h, 5 h, and 7 h (marked treat in red), with water treatment as control (marked CK in blue). The relative expression of selected GRAS genes under 100 mg/L GA3 was determined by qRT-PCR. The Citrus actin gene was used as internal control. Error bars denotes the standard deviation calculated from three independent experiments, statistical significance were analyzed by Student’s t-test (**p < 0.01, *p < 0.05).
Many GRAS members have been found to be involved in GA signal regulation in Arabidopsis, rice and other species. RGA, GAI, RGL1, RGL2 and RGL3 are negative regulators of the GA signal in Arabidopsis27. Rice GRAS protein, SLR1, is known to be involved in GA signaling55, and CIGR1 and CIGR2, belonging to the rice GRAS family, are rapidly induced by exogenous gibberellins39. Moreover, SlGRAS24, target of tomato miR171, showed high sequence identity with AtSCL647, which is involved in GA and auxin signaling61. CsGRAS18 and CsGRAS26, which are members of DELLA subfamily, showed lower transcriptional level than control at 1 h, 3 h, 5 h and 7 h under GA3 treatment (Fig. 8). In addition, CsGRAS7 and CsGRAS17, which are in the same subfamily as OsCIGR1 and OsCIGR2, could be induced by GA3 at 7 h and 5 h respectively (Fig. 8). Therefore, the CsGRAS proteins may play similar roles as other proteins in the same subfamily. All the genes affected by GA3 were up-regulated or down-regulated under 100 mM NaCl treatment, implying that these genes could be involved in both GA signaling and stress response.
Conservation of GRAS subfamily in different species
To investigate the distribution and conservation of the GRAS gene subfamilies, we statistically analyzed gene identities among six species. Using Arabidopsis as reference, we chose 9 GRAS genes in Arabidopsis and two genes from rice and C. sinensis as a query to blast the most homologous genes in the other 5 species.
From peptide analysis of 6 species (Populus trichocarpa, Oryza sativa, Physcometrella patens, Selaginella moellendorffii, A. thaliana, and C. sinensis) (Fig. S4), AtPAT1 and AtSCR seemed the most conserved of the GRAS subfamilies, related to fundamental functions, such as phytochrome signaling, root radial patterning and maintenance of QC identity. We discovered genes orthologous to AtPAT1 among the 5 species and orthologous to AtSCR among 4 species (Fig. S4). The proteins of these two subfamilies shared high identity and contained extremely conserved fragments, indicating that they originated from one ancestor, with minimal change through evolution. AtSCL3, DELLA, DLT, AtSHR and LISCL subfamilies were highly conserved among the six species, especially among the dicotyledonous Arabidopsis, Citrus and Populus (Fig. S4). AtLAS & At4/7 were highly conserved among Arabidopsis, Citrus and Populus, and moderately conserved among the other three species (Fig. S4). However, there was no AtHAM subfamily in P. patens, S. moellendorffii, and O. sativa, but which conserved in C. sinensis and Populus. Os19 subfamily was discovered in C. sinensis, O. sativa and Populus, and was absent in the other three species. The CsGRAS34 subfamily of C. sinensis also existed in Populus, but was absent in the other four species. This indicates the CsGRAS34 subfamily may exist specifically in woody plants.
Comparison of the subfamilies from 6 species revealed subfamily conservation between species (Fig. S4). Certain subfamilies were species specific. Os19 only existed in rice, C. sinensis and Populus. While CsGRAS34 existed only in C. sinensis and populus, indicating that these subfamilies originated simultaneously with species differentiation in evolution. On the other hand, the subfamily conservation analysis confirmed high homology between citrus and populus.
Conclusion
In conclusion, a total of 50 CsGRAS genes were identified and characterized in the sweet orange genome, and were phylogenetically classified into 11 distinct subfamilies. We found segmental duplications and tandem duplication had contributed to the expansion of CsGRAS family. Moreover, some CsGRAS genes appeared to be differentially expressed in different tissues, and expression levels of some CsGRAS genes were influenced by phosphorus deficiency, salt stress, and GA3 treatment. These data will provide the basis for understanding evolutionary history and the developmental roles of CsGRAS proteins in sweet orange, and may be helpful for future exploration of the biological functions of CsGRAS genes. These findings will also serve as a resource for identifying genes that improve citrus growth under stress conditions and enable potential breeding and genetic improvements for agriculture.
Materials and Methods
Identification and Chromosomal Locations of GRAS genes in C. sinensis
To identify all GRAS genes in orange (C. sinensis), all annotated proteins were downloaded from C. sinensis annotation project database of Huazhong Agricultural University (http://citrus.hzau.edu.cn/orange/) and the phytozome Citrus sinensis v1.1 database (http://phytozome.jgi.doe.gov/pz/portal.html). The Hidden Markov Model (HMM) profile of the GRAS domain (PF03514) downloaded from Protein family (Pfam) (http://pfam.sanger.ac.uk/) was used for identification of the GRAS genes from the downloaded database of orange genome using HMMER3.0. All output genes with default (<1.0) E-value were collected and the online software SMART (http://smart.embl-heidelberg/) was used to confirm the integrity of the GRAS domain with E-value < 0.1, and the incorrectly predicted genes were rejected. Finally, the non-redundant and confident genes were gathered and assigned as orange GRAS genes. The physical positions of CsGRAS genes were obtained from Citrus sinensis annotation project database of Huazhong Agricultural University and the phytozome Citrus sinensis v1.1 Citrus sinensis database. Motif location of GRAS protein in C. sinensis was discovered by online program MEME (http://meme-suite.org/tools/meme).
Multiple sequence alignment and phylogenetic analysis of GRAS genes in C. sinensis combined with rice and Arabidopsis
Multiple sequence alignment was executed by ClustalX 2.0 program and GeneDoc. Phylogenetic trees were constructed using MEGA 6.0 by the Neighbor-Joining (NJ) methods and the bootstrap test carried out with 1000 iterations.
Putative cis-elements in the promoter regions
The 1500 bp upstream sequences from the translation start codon of all of the CsGRAS genes were obtained from Citrus sinensis annotation project database of Huazhong Agricultural University (http://citrus.hzau.edu.cn/orange/). The putative stress or hormone responsive cis-acting regulatory elements in these sequences were predicted using the PlantCARE web server http://bioinformatics.psb.ugent.be/webtools/plantcare/html/)62,63 and then to identify the putative cis-acting regulatory elements.
Expression profiles of GRAS gene family in C. sinensis by RNA-seq analysis
Expression profile data of CsGRAS gene family in 4 tissues for sweet orange were extracted from C. sinensis annotation project database of Huazhong Agricultural University (http://citrus.hzau.edu.cn/orange/)1. Three independent samples and libraries sequenced for each of the tissues were used. The data was calculated by reads per kilobase per million mapped reads (RPKM) as transcript abundance and the RPKM values were transformed in log10 fold change. The heat map generation and cluster analyses were performed using R v3.3.0. To validate the accuracy of the RNA-seq data, three independent samples for each tissue (leaf, callus, flower, and fruit) were collected, then the RNA was extracted and the expression levels of some selective CsGRAS genes in sweet orange tissues by qRT-PCR were investigated.
RNA extraction and real-time quantitative PCR analysis
Total RNA was extracted using TransZol Reagent (TransGen Biotech, China) according to manufacturer instructions. RNA integrity was verified by 1% agar gel electrophoresis and the RNA concentration was measured using NanoDrop (2000, USA). First-stand cDNA was synthesized from 4 μg total RNA using the TransScript® One-step gDNA Removal and cDNA Synthesis SuperMix Kit (TransGen Biotech, China) following manufacturer protocols. Real-Time quantitative PCR was carried out on an Applied Biosystems®QuantStudioTM 7 Flex Real-Time PCR System (life technologies, USA) using 2 × HSYBR qPCR Mix (With ROX II) (ZOMANBIO, China). Each reaction was performed in a 10 μl volume containing 5 μl 2 × HSYBR qPCR Mix, 0.4 μl template DNA (1–10 ng cDNA), 0.2 μl each primer (10 μM), and finally adding the RNase-free water to give a total volume of 10 μl. The PCR amplification cycle was as follows: 95 °C for 10 min, 40 cycles at 95 °C for 15 s, and 60 °C for 1 min. Melting curve analysis was executed for verifying the specificity of primer with the following program: 95 °C for 15 s, 60 °C for 1 min, 95 °C for 15 s, 60 °C for 15 s. All quantitative Real-Time PCR experiments were conducted in three biological replicates. Relative fold differences were calculated based on the comparative Ct method using the 2−△△Ct method with ACTIN2 as the internal reference gene. All the primers (Table S4) for qRT-PCR were designed based on the reference sequence obtained from the C. sinensis Annotation Project (http://citrus.hzau.edu.cn/orange/).
PPI network of GRAS protein in C. sinensis
PPI (protein-protein interact) network of GRAS protein in C. sinensis was built. Orthologous-based and domain-based methods were employed to predict PPI network in C. sinensis annotation project database of Huazhong Agricultural University (http://citrus.hzau.edu.cn/orange/), which was shown by Cytoscape 3.3.0.
Plant Growth and Treatment with phosphorus deficiency in P. triforniata
A common rootstock, P. triforniata (L) Raf (Pt), was used in this study. Pt seeds were sown in plastic pots filled with vermiculite as previously described. Twenty days later, uniform seedlings were transplanted into sand culture. Pt seedlings in sand pots were grown in a chamber with a 14 h light period at 23–28 °C and a 10 h dark period at 18–20 °C. Five Pt seedlings per sand pot were irrigated with 200 ml Hoagland nutrient solution. Two-month-old seedlings were used for Pi starvation treatment. The control samples (+P) were irrigated with Hoagland nutrient solutions containing 1 mM P, whereas P starvation samples (−P) were irrigated with Hoagland nutrient solutions containing 1 μM P33. Root samples were collected at 0, 1, 2, 4, 8, or 10 weeks after (−P) irrigation treatment. Each root sample was prepared from three pots with each containing five seedlings. Three root samples/per treatment were collected for each time point from three independent experiments33.
Expression of GRAS genes in Pt under phosphorus deficiency via analysis RNA-seq profiles
We selected expression profile data of GRAS genes from the transcriptome sequencing using Illumina HiSeqTM2000 under Pi deficiency treatment for 4 weeks33. The data was calculated by reads per kilobase per million mapped reads (RPKM) as transcript abundance and the RPKM values were transformed in log2 fold change values in Microsoft excel 2013. To validate the accuracy of the RNA-seq data, we collected roots of Pt under 0, 1, 2, 4, 8, or 10 weeks after (-P) irrigation treatment and examined the expression of selected CsGRAS genes, which exhibited up- or down-regulation by more than two-fold, based on qRT-PCR.
Plant growth and treatments with GA3 and NaCl in sweet orange
One-month-old sweet orange plants, grown in greenhouse at 28 °C, with 16-h light/8-h dark photoperiod, were used to examine the CsGRAS gene expression level under treatments. Uniform and healthy plants were selected from the plants and inserted in flasks containing 100 mg/L GA3 or 100 mM NaCl respectively, with distilled water as control. Three independent samples were collected at 1, 3, 5, and 7 h after treatment, then frozen immediately in liquid nitrogen and stored at −80 °C until using for RNA extraction.
Supplementary information
Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 31672112), the Fundamental Research Funds for the Central Universities (2662018PY099) and the Start-up Foundation of Hubei University of Medicine (2017QDJZR26). We thank Dr. Simon Moore (Department of Biosciences, Durham University) for improving academic writing and English language. We thank Prof. Qiang Xu (College of Horticulture and Forest Sciences, Huazhong Agricultural University), Dr. Zhiyong Pan (College of Horticulture and Forest Sciences, Huazhong Agricultural University) and Prof. Ton Bisseling (Wageningen University) for helpful discussion.
Author Contributions
H. Zhang and C. Chen conceived and designed the experiments. H. Zhang, L. Mi, L. Xu, C. Yu and C. Li performed experiments and recorded data. H. Zhang, L. Mi, L. Xu, C. Yu and C. Chen participated in the analyses of data and wrote the paper. All authors discussed the results and context of the manuscript. C. Chen supervised the project.
Data Availability
All the data reported in our manuscript is available and can be found in the article and supplemental materials.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-38185-z.
References
- 1.Xu Q, et al. The draft genome of sweet orange (Citrus sinensis) Nature Genetics. 2013;45:59–66. doi: 10.1038/ng.2472. [DOI] [PubMed] [Google Scholar]
- 2.Yuan Y, et al. Overexpression of VaPAT1, a GRAS transcription factor from Vitis amurensis, confers abiotic stress tolerance in Arabidopsis. Plant Cell Reports. 2016;35:655–666. doi: 10.1007/s00299-015-1910-x. [DOI] [PubMed] [Google Scholar]
- 3.Jiao WB, et al. Genome-wide characterization and expression analysis of genetic variants in sweet orange. Plant Journal. 2013;75:954–964. doi: 10.1111/tpj.12254. [DOI] [PubMed] [Google Scholar]
- 4.Bolle C. The role of GRAS proteins in plant signal transduction and development. Planta. 2004;218:683–692. doi: 10.1007/s00425-004-1203-z. [DOI] [PubMed] [Google Scholar]
- 5.Sun X, et al. A functionally required unfoldome from the plant kingdom: intrinsically disordered N-terminal domains of GRAS proteins are involved in molecular recognition during plant development. Plant Molecular Biology. 2011;77:205–223. doi: 10.1007/s11103-011-9803-z. [DOI] [PubMed] [Google Scholar]
- 6.Sun X, Jones WT, Rikkerink EH. GRAS proteins: the versatile roles of intrinsically disordered proteins in plant signalling. Biochemical Journal. 2012;442:1–12. doi: 10.1042/BJ20111766. [DOI] [PubMed] [Google Scholar]
- 7.Di Laurenzio L, et al. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell. 1996;86:423–433. doi: 10.1016/S0092-8674(00)80115-4. [DOI] [PubMed] [Google Scholar]
- 8.Sabatini S, Heidstra R, Wildwater M, Scheres B. SCARECROW is involved in positioning the stem cell niche in the Arabidopsis root meristem. Genes & Development. 2003;17:354–358. doi: 10.1101/gad.252503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helariutta Y, et al. The SHORT-ROOT gene controls radial patterning of the Arabidopsis root through radial signaling. Cell. 2000;101:555–567. doi: 10.1016/S0092-8674(00)80865-X. [DOI] [PubMed] [Google Scholar]
- 10.Long Y, et al. SCARECROW-LIKE23 and SCARECROW jointly specify endodermal cell fate but distinctly control SHORT-ROOT movement. Plant Journal. 2015;84:773–784. doi: 10.1111/tpj.13038. [DOI] [PubMed] [Google Scholar]
- 11.Schumacher K, Schmitt T, Rossberg M, Schmitz G, Theres K. The Lateral suppressor (Ls) gene of tomato encodes a new member of the VHIID protein family. Proc Natl Acad Sci. 1999;96:290–295. doi: 10.1073/pnas.96.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greb T, et al. Molecular analysis of the LATERAL SUPPRESSOR gene in Arabidopsis reveals a conserved control mechanism for axillary meristem formation. Genes & Development. 2003;17:1175–1187. doi: 10.1101/gad.260703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, et al. Control of tillering in rice. Nature. 2003;422:618–621. doi: 10.1038/nature01518. [DOI] [PubMed] [Google Scholar]
- 14.Zhang B, et al. Novel function of a putative MOC1 ortholog associated with spikelet number per spike in common wheat. Sci Rep. 2015;5:12211. doi: 10.1038/s41598-018-22275-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stuurman J, Jaggi F, Kuhlemeier C. Shoot meristem maintenance is controlled by a GRAS-gene mediated signal from differentiating cells. Genes & Development. 2002;16:2213–2218. doi: 10.1101/gad.230702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morohashi K, Minami M, Takase H, Hotta Y, Hiratsuka K. Isolation and characterization of a novel GRAS gene that regulates meiosis-associated gene expression. Journal of Biological Chemestry. 2003;278:20865–20873. doi: 10.1074/jbc.M301712200. [DOI] [PubMed] [Google Scholar]
- 17.Peng J, et al. TheArabidopsis GAI gene defines a signaling pathway that negatively regulates gibberellin responses. Genes & Development. 1997;11:3194–3205. doi: 10.1101/gad.11.23.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silverstone AL, Ciampaglio CN, Sun T. The Arabidopsis RGA gene encodes a transcriptional regulator repressing the gibberellin signal transduction pathway. Plant Cell. 1998;10:155–169. doi: 10.1105/tpc.10.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen CK, Chang C. Arabidopsis RGL1 encodes a negative regulator of gibberellin responses. Plant Cell. 2002;14:87–100. doi: 10.1105/tpc.010325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piskurewicz U, et al. The gibberellic acid signaling repressor RGL2 inhibits Arabidopsis seed germination by stimulating abscisic acid synthesis and ABI5 activity. Plant Cell. 2008;20:2729–2745. doi: 10.1105/tpc.108.061515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roy S, Chattopadhyay T, Maiti MK. Overexpression of Rice in Rice and Tobacco Modulates Gibberellic Acid-Dependent Responses. Crop Science. 2015;55:2201. doi: 10.2135/cropsci2014.11.0776. [DOI] [Google Scholar]
- 22.Zhang ZL, et al. Scarecrow-like 3 promotes gibberellin signaling by antagonizing master growth repressor DELLA in Arabidopsis. Proc Natl Acad Sci. 2011;108:2160–2165. doi: 10.1073/pnas.1012232108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wild M, et al. The Arabidopsis DELLA RGA-LIKE3 is a direct target of MYC2 and modulates jasmonate signaling responses. Plant Cell. 2012;24:3307–3319. doi: 10.1105/tpc.112.101428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong H, et al. DWARF AND LOW-TILLERING, a new member of the GRAS family, plays positive roles in brassinosteroid signaling in rice. Plant Journal. 2009;58:803–816. doi: 10.1111/j.1365-313X.2009.03825.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen L, et al. OsGRAS19 may be a novel component involved in the brassinosteroid signaling pathway in rice. Molecular Plant. 2013;6:988–991. doi: 10.1093/mp/sst027. [DOI] [PubMed] [Google Scholar]
- 26.Smit P, et al. NSP1 of the GRAS protein family is essential for rhizobial Nod factor-induced transcription. Science. 2005;308:1789–1791. doi: 10.1126/science.1111025. [DOI] [PubMed] [Google Scholar]
- 27.Kalo P, et al. Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators. Science. 2005;308:1786–1789. doi: 10.1126/science.1110951. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch S, et al. GRAS proteins form a DNA binding complex to induce gene expression during nodulation signaling in Medicago truncatula. Plant Cell. 2009;21:545–557. doi: 10.1105/tpc.108.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu W, et al. Strigolactone biosynthesis in Medicago truncatula and rice requires the symbiotic GRAS-type transcription factors NSP1 and NSP2. Plant Cell. 2011;23:3853–3865. doi: 10.1105/tpc.111.089771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Zeijl A, et al. The strigolactone biosynthesis gene DWARF27 is co-opted in rhizobium symbiosis. BMC Plant Biology. 2015;15:260. doi: 10.1186/s12870-015-0651-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holford ICR. Soil phosphorus: its measurement, and its uptake by plants. Australian Journal of Soil Research. 1997;35:227. doi: 10.1071/S96047. [DOI] [Google Scholar]
- 32.Williamson LC, Ribrioux SPCP, Fitter AH, Leyser HMO. Phosphate Availability Regulates Root System Architecture in Arabidopsis. Plant Physiology. 2001;126:875–882. doi: 10.1104/pp.126.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian H, De Smet I, Ding Z. Shaping a root system: regulating lateral versus primary root growth. Trends in Plant Science. 2014;19:426–431. doi: 10.1016/j.tplants.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Bai F, et al. Transcriptome responses to phosphate deficiency in Poncirus trifoliata (L.) Raf. Acta Physiologiae Plantarum. 2014;36:3207–3215. doi: 10.1007/s11738-014-1687-5. [DOI] [Google Scholar]
- 35.Ruiz Herrera LF, Shane MW, Lopez-Bucio J. Nutritional regulation of root development. Wiley Interdisciplinary Reviews Developmental Biology. 2015;4:431–443. doi: 10.1002/wdev.183. [DOI] [PubMed] [Google Scholar]
- 36.Bolle C, Koncz C, Chua NH. PAT1, a new member of the GRAS family, is involved in phytochrome A signal transduction. Genes & Development. 2000;14:1269–1278. [PMC free article] [PubMed] [Google Scholar]
- 37.Torres-Galea P, Hirtreiter B, Bolle C. Two GRAS proteins, SCARECROW-LIKE21 and PHYTOCHROME A SIGNAL TRANSDUCTION1, function cooperatively in phytochrome A signal transduction. Plant Physiology. 2013;161:291–304. doi: 10.1104/pp.112.206607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torres-Galea P, Huang LF, Chua NH, Bolle C. The GRAS protein SCL13 is a positive regulator of phytochrome-dependent red light signaling, but can also modulate phytochrome A responses. Molecular Genetics and Genomics. 2006;276:13–30. doi: 10.1007/s00438-006-0123-y. [DOI] [PubMed] [Google Scholar]
- 39.Fode B, Siemsen T, Thurow C, Weigelm R, Gatz C. The Arabidopsis GRAS protein SCL14 interacts with class II TGA transcription factors and is essential for the activation of stress-inducible promoters. Plant Cell. 2008;20:3122–3135. doi: 10.1105/tpc.108.058974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Day RB, Shibuya N, Minami E. Identification and characterization of two new members of the GRAS gene family in rice responsive to N-acetylchitooligosaccharide elicitor. Biochimica et Biophysica Acta. 2003;1625:261–268. doi: 10.1016/S0167-4781(02)00626-7. [DOI] [PubMed] [Google Scholar]
- 41.Xu K, et al. OsGRAS23, a rice GRAS transcription factor gene, is involved in drought stress response through regulating expression of stress-responsive genes. BMC Plant Biology. 2015;15:141. doi: 10.1186/s12870-015-0532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma HS, Liang D, Shuai P, Xia XL, Yin WL. The salt- and drought-inducible poplar GRAS protein SCL7 confers salt and drought tolerance in Arabidopsis thaliana. Journal of Experimental Botany. 2010;61:4011–4019. doi: 10.1093/jxb/erq217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tian C, Wan P, Sun S, Li J, Chen M. Genome-wide analysis of the GRAS gene family in rice and Arabidopsis. Plant Molecular Biology. 2004;54:519–532. doi: 10.1023/B:PLAN.0000038256.89809.57. [DOI] [PubMed] [Google Scholar]
- 44.Lee MH, et al. Large-scale analysis of the GRAS gene family in Arabidopsis thaliana. Plant Molecular Biology. 2008;67:659–670. doi: 10.1007/s11103-008-9345-1. [DOI] [PubMed] [Google Scholar]
- 45.Song XM, et al. Genome-wide analysis of the GRAS gene family in Chinese cabbage (Brassica rapa ssp. pekinensis) Genomics. 2014;103:135–146. doi: 10.1016/j.ygeno.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Liu X, Widmer A. Genome-wide comparative analysis of the GRAS gene family in Populus, Arabidopsis and Rice. Plant Molecular Biology Reporter. 2014;32:1129–1145. doi: 10.1007/s11105-014-0721-5. [DOI] [Google Scholar]
- 47.Abarca D, et al. The GRAS gene family in pine: transcript expression patterns associated with the maturation-related decline of competence to form adventitious roots. BMC Plant Biology. 2014;14:354. doi: 10.1186/s12870-014-0354-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu J, Wang T, Xu Z, Sun L, Zhang Q. Genome-wide analysis of the GRAS gene family in Prunus mume. Molecular Genetics and Genomics. 2015;290:303–317. doi: 10.1007/s00438-014-0918-1. [DOI] [PubMed] [Google Scholar]
- 49.Huang W, Xian Z, Kang X, Tang N, Li Z. Genome-wide identification, phylogeny and expression analysis of GRAS gene family in tomato. BMC Plant Biology. 2015;15:209. doi: 10.1186/s12870-015-0590-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen YQ, et al. Homology-based analysis of the GRAS gene family in tobacco. Genetics and Molecular Research. 2015;14:15188–15200. doi: 10.4238/2015.November.25.7. [DOI] [PubMed] [Google Scholar]
- 51.Sun X, et al. A characterization of grapevine of GRAS domain transcription factor gene family. Functional & Integrative Genomics. 2016;16:347–363. doi: 10.1007/s10142-016-0479-y. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, et al. Genome-wide identification and expression analysis of GRAS family transcription factors in tea plant (Camellia sinensis) Sci Rep. 2018;8:3949. doi: 10.1038/s41598-018-22275-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patthy L. Intron-dependent evolution: preferred types of exons and introns. FEBS Letters. 1987;214:1–7. doi: 10.1016/0014-5793(87)80002-9. [DOI] [PubMed] [Google Scholar]
- 54.Cui H, et al. An evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science. 2007;316:421–425. doi: 10.1126/science.1139531. [DOI] [PubMed] [Google Scholar]
- 55.Wu S, et al. A plausible mechanism, based upon Short-Root movement, for regulating the number of cortex cell layers in roots. Proc Natl Acad Sci. 2014;111:16184–16189. doi: 10.1073/pnas.1407371111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ikeda A, et al. slender rice, a constitutive gibberellin response mutant, is caused by a null mutation of the SLR1 gene, an ortholog of the height-regulating gene GAI/RGA/RHT/D8. Plant Cell. 2001;13:999–1010. doi: 10.1105/tpc.13.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreno-Risueno MA, et al. Transcriptional control of tissue formation throughout root development. Science. 2015;350:426–430. doi: 10.1126/science.aad1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miguel A, Milhinhos A, Novak O, Jones B, Miguel CM. The SHORT-ROOT-like gene PtSHR2B is involved in Populus phellogen activity. Journal of Experimental Botany. 2016;67:1545–1555. doi: 10.1093/jxb/erv547. [DOI] [PubMed] [Google Scholar]
- 59.Ouyang Y, Huang X, Lu Z, Yao J. Genomic survey, expression profile and co-expression network analysis of OsWD40 family in rice. BMC Genomics. 2012;13:100. doi: 10.1186/1471-2164-13-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ticconi CA, et al. ER-resident proteins PDR2 and LPR1 mediate the developmental response of root meristems to phosphate availability. Proc Natl Acad Sci. 2009;106:14174–14179. doi: 10.1073/pnas.0901778106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang C, Gao X, Liao L, Harberd NP, Fu X. Phosphate Starvation Root Architecture and Anthocyanin Accumulation Responses Are Modulated by the Gibberellin-DELLA Signaling Pathway in Arabidopsis. Plant Physiology. 2007;145:1460–1470. doi: 10.1104/pp.107.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang W, et al. Overexpression of a tomato miR171 target gene SlGRAS24 impacts multiple agronomical traits via regulating gibberellin and auxin homeostasis. Plant Biotechnology Journal. 2017;15:472–488. doi: 10.1111/pbi.12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lescot M, et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Research. 2002;30:325–7. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data reported in our manuscript is available and can be found in the article and supplemental materials.