Abstract
The hepatitis C virus (HCV) represents a substantial threat to human health worldwide. The virus expresses a dual-function protein, NS3 having both protease and RNA helicase activities that are essential for productive viral replication and sustained infections. While viral protease and polymerase inhibitors have shown great successes in treating chronic HCV infections, drugs that specifically target the helicase activity have not advanced. A robust and quantitative 96-well plate-based fluorescent DNA unwinding assay was used to screen a class of indole thio-barbituric acid (ITBA) analogs using the full-length, recombinant HCV NS3, and identified three naphthoyl-containing analogs that efficiently inhibited NS3 helicase activity in a dose-dependent manner, with observed IC50 values of 21–24 µM. Standard gel electrophoresis helicase assays using radiolabeled duplex DNA and RNA NS3 substrates confirmed the inhibition of NS3 unwinding activity. Subsequent anisotropy measurements demonstrated that the candidate compounds did not disrupt NS3 binding to nucleic acids. Additionally, the rate of ATP hydrolysis and the protease activity were also not affected by the inhibitors. Thus, these results indicate that the three ITBA analogs containing N-naphthoyl moieties are the foundation of a potential series of small molecules capable of inhibiting NS3 activity via a novel interaction with the helicase domain that prevents the productive unwinding of nucleic acid substrates, and may represent the basis for a new class of therapeutic agents with the potential to aid in the treatment and eradication of hepatitis C virus.
Keywords: helicase, Hepatitis C virus, nonstructural protein 3, thio-barbituric acid.
Graphical Abstract
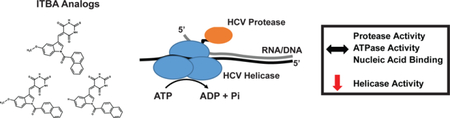
Hepatitis C virus (HCV) is a world-wide health concern as it affects an estimated 71 million individuals1 with at least 2.7 million people in the US living with chronic viral infection.2 Untreated, HCV can lead to hepatic fibrosis, cirrhosis and hepatocellular carcinoma.3 Powerful new therapies have been developed to combat viral infection, but with continued heavy use and the error-prone nature of viral replication4, resistant viruses will continue to appear in the population. Without a viable vaccine, low cost new drugs specifically targeting critical viral components are needed to alleviate the health risks to infected individuals, and provide opportunities to continue the therapeutic successes into the future.
The HCV genome encodes several non-structural enzymes and accessory proteins as well as structural coat and envelope proteins for virion assembly and productive infections. Translation of the genome results in a single polyprotein that is proteolytically processed by host peptidases and a virally encoded protease into functional proteins.5 In addition, replication requires the helicase activity of NS3 to unwind secondary structures in the nascent transcript, and NS5B, a functional RNA-dependent polymerase.6 Interestingly, NS3 is a dual-function viral protein with an N-terminal protease domain involved in viral processing, and a C-terminal helicase domain that is classified as a member of the superfamily 2 (SF2) DExH/D-box helicases.7, 8 NS3 has an affinity for single-stranded RNA and DNA, and is able to unidirectionally translocate in the 3’ to 5’ direction along the nucleic acid strand to displace nucleic acid binding proteins9 that may impede NS5B polymerase progression as well as unwinding secondary structures and duplex RNA that form in the large viral genome.
Recent therapeutic successes with compounds specifically targeting the HCV RNA polymerase (NS5B), RNA binding protein (NS5A) and N-terminal protease (NS3-protease), either as single drug regimens or in a combinational cocktail,10, 11 demonstrate that severely disabling the viral replication complex without toxic damage to hepatocyte function leads to viral eradication in the patient. Despite its essential function during HCV replication, however, few successes have been reported targeting the NS3 helicase activity, perhaps owing to the conserved structure of NS3 helicase domain and the essential nature of endogenous helicase activity in a multitude of cellular processes. Effective direct-acting antiviral agents specifically inhibiting the NS3 helicase activity without significant off-target effects have been particularly difficult to identify.
Penthala et al 12 described the rapid synthesis scheme for this series of N-aroyl-substituted indole thio-barbituric acid (ITBA) analogs (Figure 1A) by refluxing a selection of N-aroylindole-3-carboxaldehyde precursor molecules with 2-thiobarbituric acid in methanol. The resulting 5((1-aroyl-1H-indol-3-yl)methylene)-2-thioxodihydropyrimidine-4,6(1H,5H)-dione analogs exhibited low micromolar potency as anti-neoplastic agents against a large library of cancer cells 12. Subsequently, Coggins et al 13 and Zafar et al 14 used the ITBA library in search of inhibitors of the stress-response human DNA polymerase η. Their structure-activity data suggested that the ITBA analogs, particularly those containing an indolic N-naphthoyl substituent, bound with micromolar affinities in and around the nucleic acid binding site, and inhibited the polymerase with selectivity over other cellular polymerases 13. The encouraging results from these studies with a nucleic acid binding protein led us to use the ITBA library in search of compounds that would specifically interact with the helicase domain of HCV NS3 and provide the basis for a new class of HCV therapeutic compounds.
Figure 1.
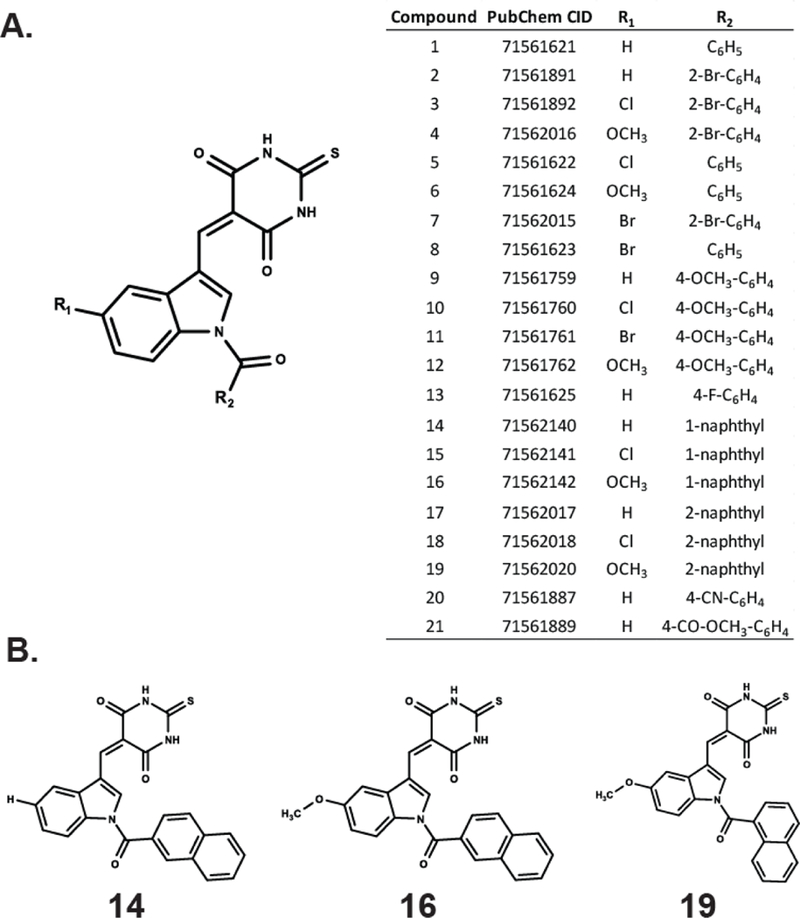
Small library of indole thio-barbituric acid (ITBA) analogs for screening as HCV NS3 inhibitors. A) Chemical scaffold, PubChem CID numbers and substituent groups used to construct the library. B) The structural formulas of three N-naphthoyl-substituted indole thio-barbituric acid analogs identified in this study.
Initially, we employed a real-time FAM-based quantitative assay as a first-pass screen of the ITBA library to determine the extent of compound-mediated inhibition of helicase catalyzed DNA unwinding (See Supplemental Data and Figure S1). In the presence of saturating amounts of ATP and Mg2+, recombinant full-length NS3 efficiently unwound the duplex DNA substrate as measured by the increase in FAM fluorescence. Of the twenty-one ITBA analogs screened, three (Analogs 14, 16 and 19 (Figure 1B)) inhibited NS3 helicase activity at 30 µM concentration (Figure 2), and were selected for further study. Interestingly, all three analogs contained an N-1-naphthoyl or N-2-naphthoyl substituent consistent with enhanced DNA polymerase η inhibition through suspected interactions with nucleic acid binding motifs.14 The interaction of the ITBA analogs with NS3 also seem to favor the 5-methoxyindole moiety (analogs 16 and 19), although analog 14 had no 5-substituent in the indole ring, yet exhibited significant inhibition of helicase activity. However, the presence of a 5-chloroindole moiety (analogs 15 and 18) exhibited poor inhibition, suggesting an important role for the 5-indole substituent in NS3 binding. Using the fluorescent DNA unwinding assay, we also obtained an IC50 value in the range of 21–24 µM for the ITBA analogs (Table 1) suggesting that analogs 14, 16 and 19 were reasonably potent NS3 helicase inhibitors worthy of further study.
Figure 2.
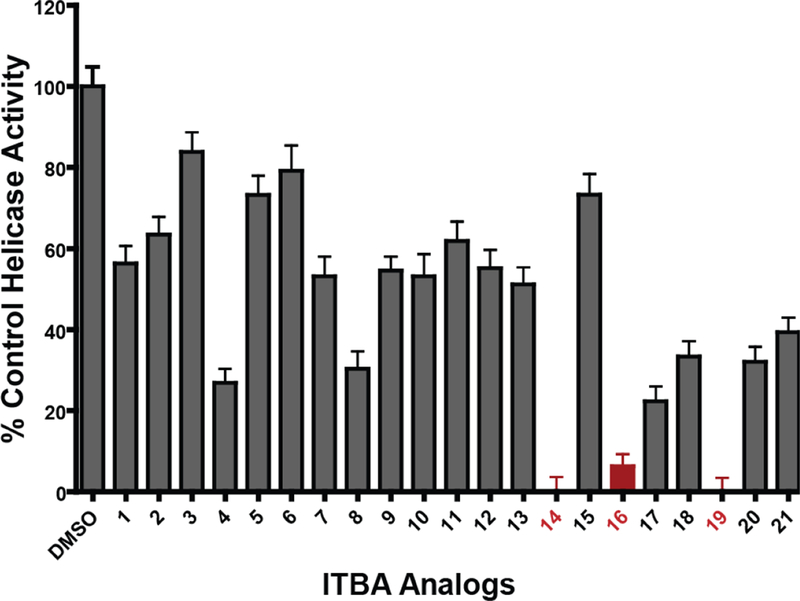
Inhibition of recombinant NS3 helicase activity using fluorescent substrates in the presence of 30 µM of the ITBA analogs solubilized in 5% DMSO. The DNA unwinding activity of recombinant full-length NS3 was measured using a fluorescently labeled duplex-containing DNA substrate in the presence of ATP and Mg2+. The NS3 unwinding rates in the presence of the inhibitors were normalized to the DMSO-containing control samples. Red bars indicate the candidate inhibitors selected for further study. Bars represent the mean ± standard deviations of triplicate measurements.
Table 1:
The IC50 for NS3 DNA Unwinding Activity for candidate ITBA analogs
| Helicase Activity |
|
|---|---|
| IC50 (µM) | |
| DMSO | - |
| 14 | 21.6 ± 1.9 |
| 16 | 21.4 ± 2.4 |
| 19 | 23.5 ± 1.8 |
To investigate the effects of analogs 14, 16 and 19 on the DNA unwinding activity of NS3 more rigorously, we utilized a polyacrylamide gel-based unwinding assay in the presence of 25 µM of the inhibitors. Employing a radiolabeled duplex DNA substrate in the presence of excess ATP, enzyme activity was measured as the amount of the substrate that was unwound by the NS3 helicase at the designated time points, plotted and fit to a single exponential (Figure 3). The resulting reduction in the rate of duplex DNA unwinding closely agreed with our real-time assay indicating that the presence of the inhibitor reduced NS3 DNA unwinding activity. These results provide strong evidence that the ITBA analogs form specific interactions with the enzyme to reduce the ability of NS3 to unwind DNA substrates. Thus, using recombinant HCV NS3, we were able to screen multiple potential inhibitory compounds using a 96-well plate real-time assay to identify potential candidate compounds as NS3 helicase inhibitors and confirm their activity with additional helicase activity measurements.
Figure 3.
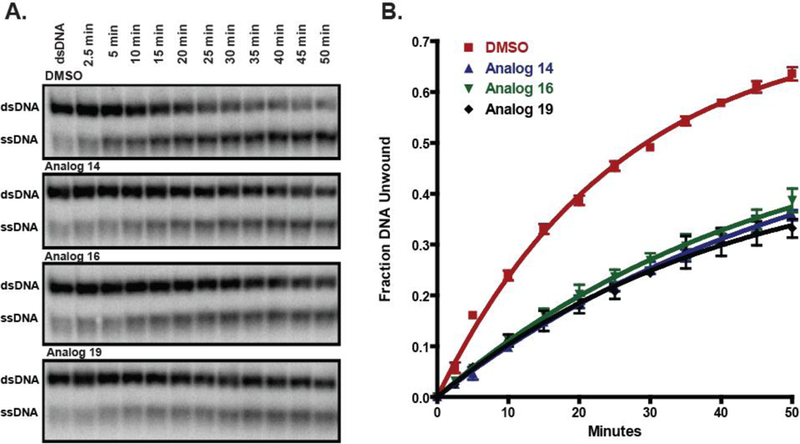
ITBA analogs inhibit in vitro NS3 DNA unwinding activity. A) Unwinding of 2 nM of radiolabeled duplex DNA by 100 nM of recombinant NS3 in the presence of 25 µM of the indicated ITBA analogs. Representative gels from triplicate measurements are shown. B) Quantitation of the radioactivity for time points of triplicate experiments were averaged and fit to a single exponential to calculate the following DNA unwinding rate constants: DMSO control (red squares): 0.039 ± 0.002 min−1, Analog 14 (blue triangles): 0.018 ± 0.001 min−1, Analog 16 (green inverted triangles): 0.021 ± 0.002 min−1, and Analog 19 (black diamonds): 0.024 ± 0.002 min-1.
Since RNA is the natural substrate for NS3 during viral replication, we also investigated RNA duplex unwinding in the same polyacrylamide-based unwinding assay utilizing a 38 bp duplex RNA (Figure 4). Our RNA substrate was susceptible to degradation resulting in a faster migrating RNA duplex species that melted upon heating (Figure 4, compare the first lane to the last lane). Since NS3 does not bind and unwind duplex RNA lacking a single-stranded 3’ tail,23 we considered only the loss of dsRNA and the appearance of the ssRNA in our evaluation of the RNA helicase activity. Curiously, the ITBA analogs had less of an effect on the rate of RNA unwinding than DNA unwinding, perhaps owing to the different length of the nucleic acid duplex substrates. Although the rate of product formation was similar under the conditions of our experiments, we observed a 50% reduction in the quantity of NS3-mediated product with all three compounds supporting the hypothesis that the binding of the inhibitors to the full-length NS3 protein has a significant and specific effect on both the DNA-and RNA-dependent helicase activities.
Figure 4:
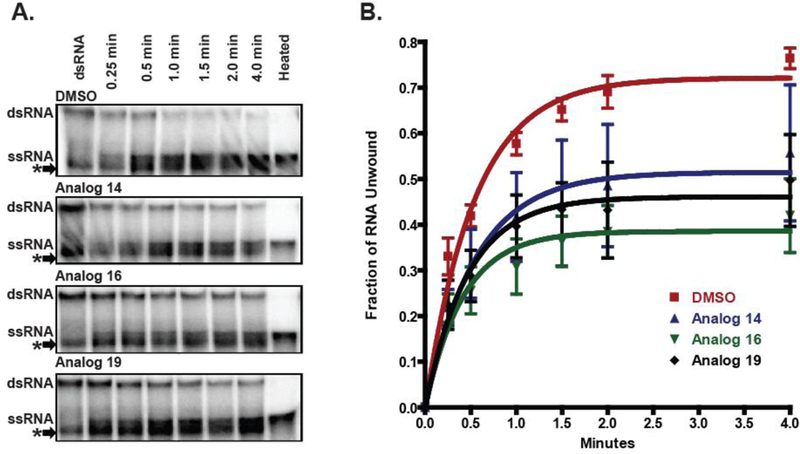
ITBA analogs inhibit in vitro NS3 RNA unwinding activity. A) Unwinding of 2 nM of radiolabeled duplex RNA by 500 nM of recombinant NS3 in the presence of 25 µM of the indicated ITBA analogs. A contaminating duplex RNA species indicated by an asterisk (*) was disregarded in the evaluation of the helicase activity (see text and Supplemental data). Representative gels from triplicate measurements. B) Quantitation of the radioactivity for time points of triplicate experiments were averaged and fit to a single exponential to calculate the following RNA unwinding rate constants: DMSO control (red squares): 1.84 ± 0.19 min−1, Analog 14 (blue triangles): 1.81 ± 0.77 min−1, Analog 16 (green inverted triangles): 2.38 ± 0.72 min−1, and Analog 19 (black diamonds): 2.14 ± 0.69 min-1.
Multiple reports have described HCV NS3 helicase inhibitors that disrupt or modify the nucleic acid binding region of the protein to alter the viral replicative capacity.15–19 To determine if the mode of inhibition includes the disruption of protein-nucleic acid interactions, we used fluorescence polarization to measure the anisotropy of the NS3-DNA complex to determine the equilibrium dissociation constant (KD) in the presence of analogs 14, 16 and 19. Our results indicated that the KD for the NS3 binding to ssDNA was unaffected by 25 µM of the ITBA analogs (Figure 5) indicating that the ITBA analogs do not interfere with nucleic acid binding to the helicase.
Figure 5:
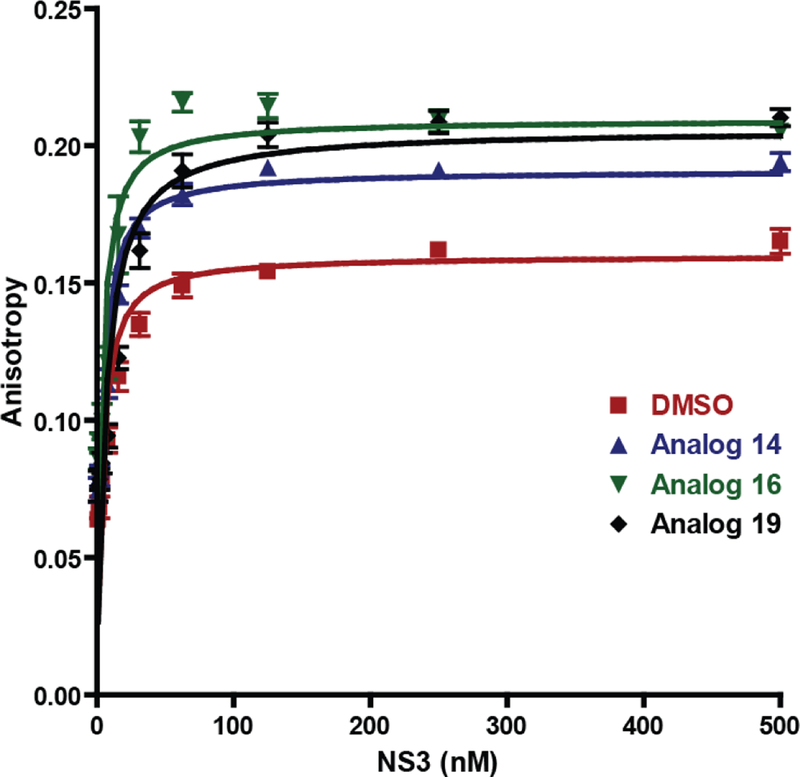
ITBA analogs do not alter the interaction of HCV NS3 with nucleic acids. Binding of increasing concentrations of full-length NS3 to fluorescein-labelled T20 oligomer was measured by fluorescence polarization in the presence of 1 nM DNA and 25 µM of the indicated ITBA analogs. The anisotropy of the NS3-ssDNA complex was calculated and fit to a quadratic function to obtain the protein-ssDNA dissociation constants: DMSO control (red squares): KD = 3.3 ± 0.2 nM, Analog 14 (blue triangles): KD = 3.1 ± 0.2 nM, Analog 16 (green inverted triangles): KD = 2.8 ± 0.4 nM, and Analog 19 (black diamonds): KD = 5.7 ± 0.6 nM.
The interaction of the NS3 protease with the helicase domain is important for NS3 activity,20 therefore we measured the effect of the ITBA analogs on the protease activity of recombinant NS3–4A, an isolate with full protease activity using an EDANS/DABCYL fluorescent peptide substrate. While 10 µM telepravir, a well-characterized NS3 protease inhibitor,10 reduced the protease activity by 85%, the ITBA analogs 14, 16 and 19 had no effect (Figure 6) on the protease activity of the protein. Thus the inhibition of NS3 helicase activity does not involve interference with the active site of the protease or its proteolytic activity, per se.
Figure 6:
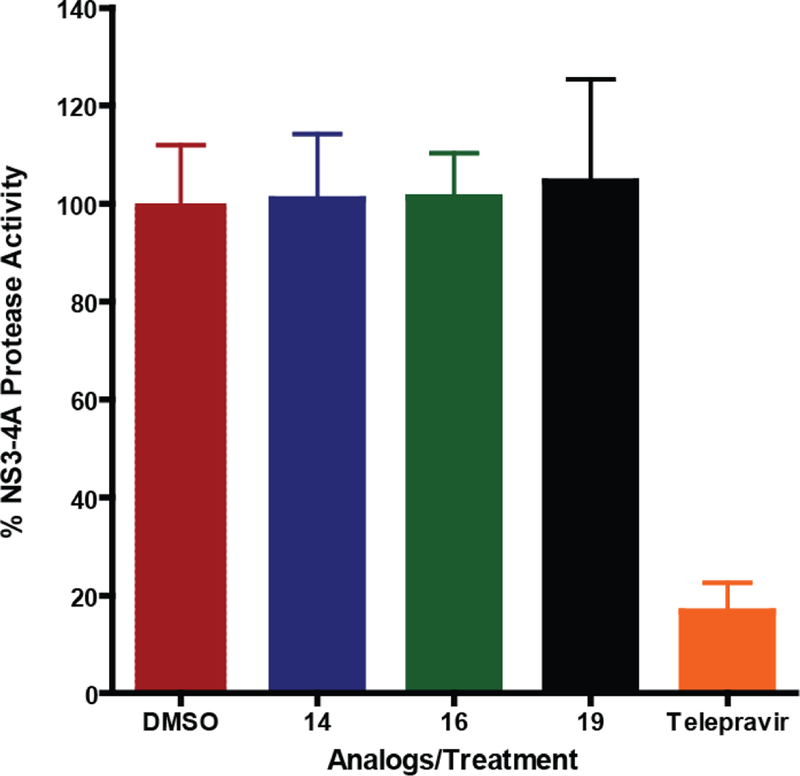
ITBA analogs do not inhibit the protease activity of HCV NS3–4A. The protease activity of NS3–4A was measured with the internally quenched substrate Ac-Asp-Glu-Asp-EDANS-Glu-Glu-Abu-L-Lactoyl-Ser-Lys DABCYL-NH2 that fluoresces after cleavage by NS3–4A. ITBA analogs were examined at 25 µM in the reactions. The NS3–4A inhibitor Telepravir (10 µM) was used as the positive control reducing protease activity by 85%. Bars represent the mean ± standard deviation of normalized protease activities from triplicate measurements.
Finally, since NS3 utilizes the energy from the hydrolysis of ATP to unwind nucleic acid duplexes, the RNA-stimulated ATPase activities of NS3 at sub-saturating and saturating concentrations of ATP were measured using a coupled spectrophotometric assay. The maximal rate of ATP hydrolysis with excess polyU RNA (75 µM nt) and ATP (5 mM) was unaffected by 25 µM of ITBA analogs 14, 16 or 19, unlike the inhibition of the helicase activity with similar ATP concentrations (Figure 7). At sub-saturating ATP concentrations, the presence of the inhibitors had a small effect on the ATPase rate and the calculated KM indicating that at low concentrations of ATP, the ITBA analogs may affect the association of the ATP in the active site. Based on the conditions in our nucleic acid unwinding assays, we contend that the observed increase in the KM would have a minor contribution to the inhibition of the helicase activity. Taken together, our results suggest that the ITBA analogs have a unique binding site affecting NS3 activity that does not interfere with nucleic acid binding, protease activity or ATPase activity.
Figure 7:
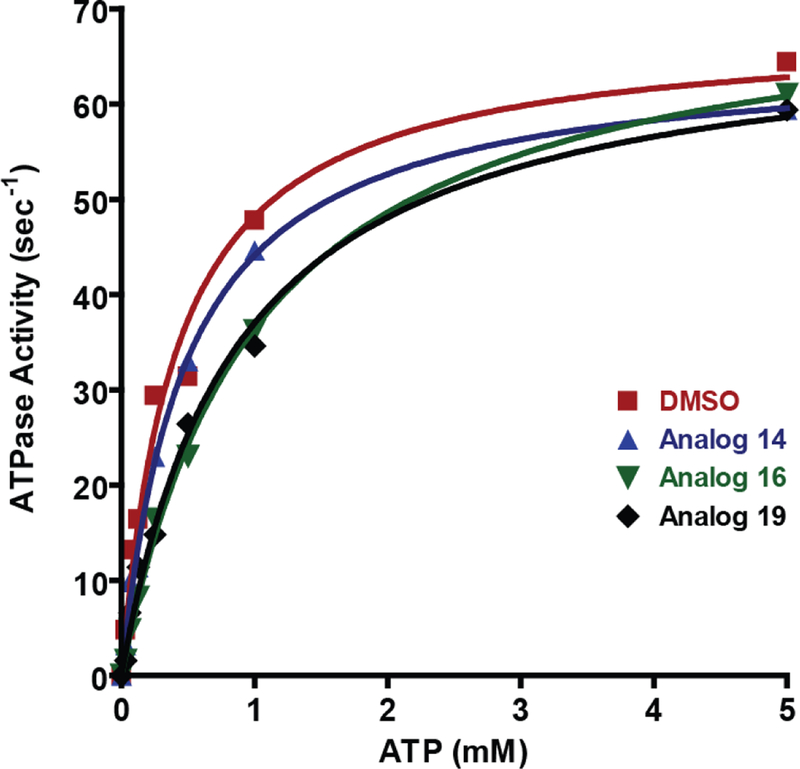
ITBA analogs do not alter the HCV NS3 RNA-stimulated ATPase activity. ATPase activity of 50 nM full-length NS3 was measured in the presence of saturating amounts of RNA (75 µM (nucleotides) polyU) and 25 µM of the indicated ITBA analogs. The ATP concentration was varied from 0–5 mM, and the kinetic constants kcat and KM calculated using the Michaelis-Menton equation: DMSO control (red squares): KM = 0.41 mM, kcat = 68.0 s−1, Analog 14 (blue triangles): KM = 0.48 mM, kcat = 65.3 s−1, Analog 16 (green inverted triangles): KM = 1.00 mM, kcat = 73.0 s−1 and Analog 19 (black diamonds): KM = 0.86 mM, kcat = 68.7 s-1.
In conclusion, we evaluated a series of 5((1-aroyl-1H-indol-3-yl)methylene)-2-thioxodihydropyrimidine-4,6(1H,5H)-dione analogs as HCV NS3 helicase activity inhibitors using a rapid plate-based fluorescent assay, and confirmed the ability of analogs 14, 16 and 19 to inhibit the nucleic acid unwinding ability of NS3 with an IC50 of approximately 21 µM without directly affecting nucleic acid binding, ATP hydrolysis or the protease activity. These small compounds are unique, since their apparent mode of action does not seem to involve the disruption of nucleic acid binding as reported for other helicase inhibitors.15–19 Full-length NS3 containing both the helicase and the protease domains has been shown to require conformational changes21, 22 in order to efficiently unwind duplex RNA. The unique binding properties of the naphthoyl moiety in the ITBA analogs 14, 16, and 19 to NS3 may involve a specific disruption of the conformational changes leading to greatly reduced helicase activity. The discovery of these three candidate molecules that inhibit viral helicase activity via a novel mechanism is an exciting start for this new class of compounds. Further refinements in the drug-likeness, water solubility and efficacy of these compounds will allow for a strong foundation to build the next-generation of HCV NS3 therapeutics.
Supplementary Material
Highlights.
Hepatitis C Virus NS3 helicase activity is inhibited by N-naphthoyl-containing indole thiobarbituric acid analogs
Both RNA and DNA-dependent unwinding activities of NS3 helicase are inhibited.
Nucleic acid binding, protease and ATPase activities are not inhibited by the compounds.
Acknowledgements
This work was supported by the National Institutes of Health, Grant Nos. R35 GM122601, R01 GM098922 (K.D.R), and Grant No. AG012411 (P.A.C.).
Abbreviations
- NS3
nonstructural protein 3
- HCV
hepatitis C virus
- ITBA
indole thio-barbituric acid
- ssDNA
single-stranded DNA
- dsDNA
Double-stranded DNA
- DMSO
dimethylsulfoxide
- FAM
6-carboxyfluoroscein
Footnotes
Declarations of Interests: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.H.C.V.C. Polaris Observatory, Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study, Lancet Gastroenterol Hepatol, 2017, 2, 161–176. doi: 10.1016/S2468-1253(16)30181-9. [DOI] [PubMed] [Google Scholar]
- 2.Denniston MM; Jiles RB; Drobeniuc J; Klevens RM; Ward JW; McQuillan GM;Holmberg SD, Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010, Ann. Intern. Med, 2014, 160, 293–300. doi: 10.7326/M13-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manns MP; Buti M; Gane E; Pawlotsky JM; Razavi H; Terrault N; Younossi Z, Hepatitis C virus infection, Nat Rev Dis Primers, 2017, 3, 17006. doi: 10.1038/nrdp.2017.6. [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro RM; Li H; Wang S; Stoddard MB; Learn GH; Korber BT; Bhattacharya T; Guedj J; Parrish EH; Hahn BH; Shaw GM; Perelson AS, Quantifying the diversification of hepatitis C virus (HCV) during primary infection: estimates of the in vivo mutation rate, PLoS Pathog, 2012, 8, e1002881. doi: 10.1371/journal.ppat.1002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hijikata M; Mizushima H; Akagi T; Mori S; Kakiuchi N; Kato N; Tanaka T; Kimura K; Shimotohno K, Two distinct proteinase activities required for the processing of a putative nonstructural precursor protein of hepatitis C virus, J. Virol, 1993, 67, 4665–4675. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam AM; Frick DN, Hepatitis C virus subgenomic replicon requires an active NS3 RNA helicase, J. Virol, 2006, 80, 404–411. doi: 10.1128/JVI.80.1.404-411.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frick DN, HCV Helicase: Structure, Function, and Inhibition, in: Tan SL (Ed.) Hepatitis C Viruses: Genomes and Molecular Biology, Norfolk (UK), 2006. [Google Scholar]
- 8.Byrd AK; Raney KD, Superfamily 2 helicases, Front Biosci (Landmark Ed), 2012, 17, 2070–2088. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matlock DL; Yeruva L; Byrd AK; Mackintosh SG; Langston C; Brown C; Cameron CE; Fischer CJ; Raney KD, Investigation of translocation DNA unwinding, and protein displacement by NS3h, the helicase domain from the hepatitis C virus helicase, Biochemistry, 2010, 49, 2097–2109. doi: 10.1021/bi901977k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das D; Pandya M, Recent Advancement of Direct-acting Antiviral Agents (DAAs) in Hepatitis C Therapy, Mini Rev. Med. Chem, 2018, 18, 584–596. doi: 10.2174/1389557517666170913111930. [DOI] [PubMed] [Google Scholar]
- 11.Vermehren J; Park JS; Jacobson IM; Zeuzem S, Challenges and perspectives of direct antivirals for the treatment of hepatitis C virus infection, J. Hepatol, 2018, doi: 10.1016/j.jhep.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Penthala NR; Ponugoti PR; Kasam V; Crooks PA, 5-((1-Aroyl-1H-indol-3-yl)methylene)-2-thioxodihydropyrimidine-4,6(1H,5H)-diones as potential anticancer agents with anti-inflammatory properties, Bioorg. Med. Chem. Lett, 2013, 23, 1442–1446. doi: 10.1016/j.bmcl.2012.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coggins GE; Maddukuri L; Penthala NR; Hartman JH; Eddy S; Ketkar A; Crooks PA; Eoff RL, N-Aroyl indole thiobarbituric acids as inhibitors of DNA repair and replication stress response polymerases, ACS Chem. Biol, 2013, 8, 1722–1729. doi: 10.1021/cb400305r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zafar MK; Maddukuri L; Ketkar A; Penthala NR; Reed MR; Eddy S; Crooks PA; Eoff RL, A Small-Molecule Inhibitor of Human DNA Polymerase eta Potentiates the Effects of Cisplatin in Tumor Cells, Biochemistry, 2018, 57, 1262–1273. doi: 10.1021/acs.biochem.7b01176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang N; Sun C; Zhang L; Liu J; Song F, Identification and Analysis of Novel Inhibitors against NS3 Helicase and NS5B RNA-Dependent RNA Polymerase from Hepatitis C Virus 1b (Con1), Front. Microbiol, 2017, 8, 2153. doi: 10.3389/fmicb.2017.02153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S; Yoon KD; Lee M; Cho Y; Choi G; Jang H; Kim B; Jung DH; Oh JG; Kim GW; Oh JW; Jeong YJ; Kwon HJ; Bae SK; Min DH; Windisch MP; Heo TH; Lee C, Identification of a resveratrol tetramer as a potent inhibitor of hepatitis C virus helicase, Br. J. Pharmacol, 2016, 173, 191–211. doi: 10.1111/bph.13358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherjee S; Hanson AM; Shadrick WR; Ndjomou J; Sweeney NL; Hernandez JJ; Bartczak D; Li K; Frankowski KJ; Heck JA; Arnold LA; Schoenen FJ; Frick DN, Identification and analysis of hepatitis C virus NS3 helicase inhibitors using nucleic acid binding assays, Nucleic Acids Res, 2012, 40, 8607–8621. doi: 10.1093/nar/gks623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furuta A; Tsubuki M; Endoh M; Miyamoto T; Tanaka J; Salam KA; Akimitsu N; Tani H; Yamashita A; Moriishi K; Nakakoshi M; Sekiguchi Y; Tsuneda S; Noda N, Identification of Hydroxyanthraquinones as Novel Inhibitors of Hepatitis C Virus NS3 Helicase, Int. J. Mol. Sci, 2015, 16, 18439–18453. doi: 10.3390/ijms160818439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherjee S; Weiner WS; Schroeder CE; Simpson DS; Hanson AM; Sweeney NL; Marvin RK; Ndjomou J; Kolli R; Isailovic D; Schoenen FJ; Frick DN, Ebselen inhibits hepatitis C virus NS3 helicase binding to nucleic acid and prevents viral replication, ACS Chem. Biol, 2014, 9, 2393–2403. doi: 10.1021/cb500512z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aydin C; Mukherjee S; Hanson AM; Frick DN; Schiffer CA, The interdomain interface in bifunctional enzyme protein 3/4A (NS3/4A) regulates protease and helicase activities, Protein Sci, 2013, 22, 1786–1798. doi: 10.1002/pro.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding SC; Kohlway AS; Pyle AM, Unmasking the active helicase conformation of nonstructural protein 3 from hepatitis C virus, J. Virol, 2011, 85, 4343–4353. doi: 10.1128/JVI.02130-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Appleby TC; Anderson R; Fedorova O; Pyle AM; Wang R; Liu X; Brendza KM; Somoza JR, Visualizing ATP-dependent RNA translocation by the NS3 helicase from HCV, J. Mol. Biol, 2011, 405, 1139–1153. doi: 10.1016/j.jmb.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pang PS; Jankowsky E; Planet PJ; Pyle AM, The hepatitis C viral NS3 protein is a processive DNA helicase with cofactor enhanced RNA unwinding. EMBO J, 2002, 21, 1168–1176. doi: 10.1093/emboj/21.5.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


