Abstract
Background
Huntington’s disease (HD) is a rare, progressive neurodegenerative disease. Currently, there is no cure for the disease, but treatment may alleviate HD symptoms. In recent years, several exercise training interventions have been conducted in HD patients. In the current article, we review previous studies investigating targeted exercise training interventions in HD patients.
Methods
We performed a literature search using the PubMed, Scopus, Web of Science, and Google Scholar databases on exercise training interventions in HD patients. Six publications fulfilled the criteria and were included in the review.
Results
Exercise training resulted in beneficial effects on cardiovascular and mitochondrial function. Training effects on cognition, motor function, and body composition were less congruent, but a positive effect seems likely. Health-related quality of life during the training interventions was stable. Most studies reported no related adverse events in response to training.
Discussion
Exercise training seems to be safe and feasible in HD patients. However, current knowledge is mainly based on short, small-scale studies and it cannot be transferred to all HD patients. Therefore, longer-term interventions with larger HD patient cohorts are necessary to draw firm conclusions about the potentially positive effects of exercise training in HD patients.
Keywords: Resistance training, endurance training, cardiovascular function, motor function, cognition, body composition
Background
Huntington’s disease (HD) is a rare, inherited neurodegenerative disorder. Clinical symptoms of HD encompass motor and non-motor domains. Motor, cognitive, psychological, and behavioral functions may be progressively impaired before the diagnosis of HD is made,1,2 and result in limitations of daily activities.3 In later disease stages, patients are dependent on caregivers. To date, there is still no causative treatment for HD. Most treatment approaches intend to alleviate HD symptoms, but none of them has a long-term disease-modifying effect.4,5 Accordingly, the aim of symptomatic treatments is to maintain quality of life and independency for as long as possible.
Multiple approaches were introduced to cope with the diversity of HD symptoms, encompassing physical, cognitive, and drug therapy. Physical therapy was integrated in the treatment process decades ago in order to attenuate impairments in motor function,6,7 and focused on task-specific exercises to imitate and improve activities of daily living. Studies with physical therapy interventions showed an improvement in specific tasks or at least an attenuation of the natural disease progress for these tasks.8–16
Based on the close relationship between some physical therapy exercises and resistance or endurance exercise, interest was aroused in whether the last two training modalities might have an effect in HD patients. However, several years ago, the potential benefits of exercise interventions were clouded by suspected disadvantages. On the one hand, it was known that a passive lifestyle might lead to an earlier age at symptom onset in HD patients,17 and that exercise exerts a positive effect in other neurodegenerative diseases, e.g. amyotrophic lateral sclerosis and Parkinson disease.18,19 On the other hand, a case study postulated a relationship between excessive endurance training and acute myopathy with rhabdomyolysis in a semi-professional marathon runner with premanifest HD.20 In conjunction with skeletal muscle mitochondrial impairments in HD patients,21–24 a negative effect of excessive training on skeletal muscle energy metabolism was suspected. Nonetheless, based on the promising results of the first studies and an overwhelming proportion of studies showing a positive effect of exercise in the HD mouse model (for a review see Mo et al.25), several studies investigated the effects of structured exercise training in HD patients within the last 5 years.
In this article, we review and summarize the findings of previous studies assessing the effects of targeted exercise training interventions in HD patients. This review refers only to studies applying structured endurance and/or resistance training programs with quantitative outcome data in human patients. Readers interested in the effects of physical therapy and multidisciplinary rehabilitation interventions in HD are referred to a recent review by Fritz et al.26
Methods
We searched the PubMed, Scopus, Web of Science, and Google Scholar databases using variant names of the disease, i.e. “Huntington disease” or “Huntington’s disease”, in combination with exercise intervention-related terms, i.e. “exercise”, “training”, “endurance”, “resistance training”, “resistance exercise”, “strength training”, and “strength exercise”. In addition, the reference lists of included articles were screened for further candidate publications. The search was restricted to articles in the English language involving human HD patients. In the current review, we included scientific literature on structured exercise intervention programs incorporating targeted endurance and/or resistance training. Exercise training is defined as a structured, purposefully planned activity that can be progressed by means of volume and/or intensity changes. Quantitative data on the effects of training were necessary for inclusion in the review. We conducted the search in October 2018 and all publications up to the date of the search were screened.
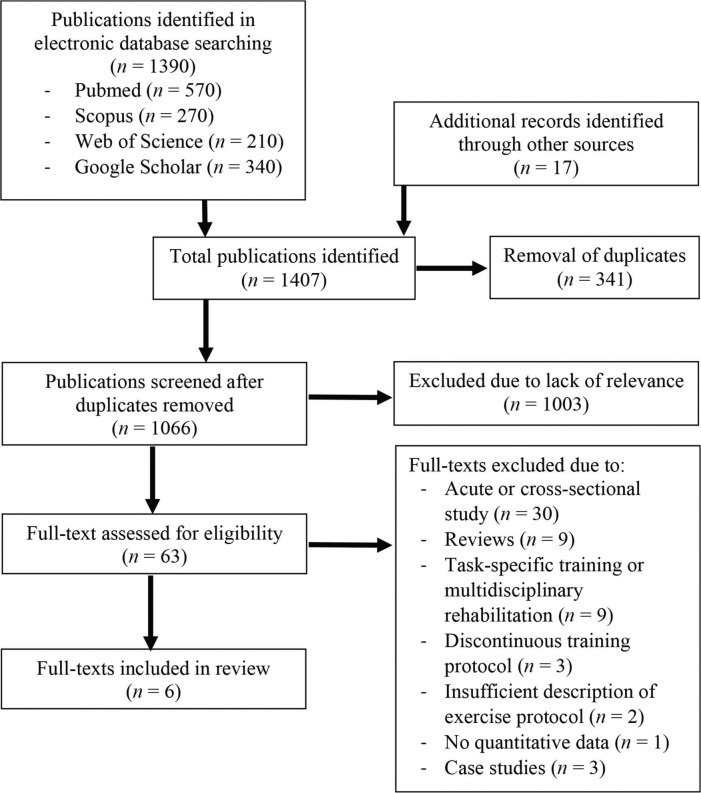
The search identified 1,407 candidate publications (Fig. 1). After removal of duplicates (n = 341), 1,066 candidate publications remained. Afterwards, we screened the abstracts of all articles to check if they fell in to the scope of this review. Based on the available abstracts, 1,003 articles were excluded because of lack of relevance. The full texts of the remaining 63 articles were screened for eligibility. Fifty-seven full-text articles were excluded for the following reasons: acute or cross-sectional study (n = 30), reviews (n = 9), task-specific training (n = 9), discontinuous training protocol (n = 3), insufficient description of exercise protocol (n = 2), no quantitative data (n = 1), and case studies (n = 3). Finally, six publications were included in the review, two of which were from our own research group (Table 1).
Figure 1. Flow Chart of the Study Selection Process.
Table 1. Characteristics of the Endurance and Resistance Training Interventions in Huntington’s Disease Patients.
| Duration | Frequency | ET | RT | |||||
|---|---|---|---|---|---|---|---|---|
| ET Intervention | ET Duration | ET Intensity | RT Intervention | RT Duration | RT Intensity | |||
| Busse et al.27 | 12 weeks | 1×/week (c) 2×/week (h) | Cycling CLT (c) walking (h) | 20–30 min (c) 10–30 min (h) | 55–75% of predicted HRmax (c) Borg value: 3–4 (h) | Leg press, leg extension, lat pulldown, hamstring curl, calf raises | 40 min (c) | 2×8–12 repetitions 60–70% 1RM, progressive |
| Khalil et al.28 | 8 weeks | 3×/week | Gradual progressive walk program | 30 min | Light subjective exercise intensity | Circuit resistance training and strengthening exercises | n.r. | Gradually increase number of repetitions and increase difficulty of exercises |
| Dawes et al.29 | 12 weeks | 1×/week (c) 2×/week (h) | Cycling CLT (c) walking (h) | 20–30 min (c) 10–30 min (h) | 55–75% of predicted HRmax (c)Quickly but still able to talk (h) | Leg press, leg extension, lat pulldown, hamstring curl, calf raises | n.r. | 2×8–12 repetitions, progressive |
| Quinn et al.30 | 12 weeks | 3×/week | Cycling CLT | up to 30 min | 65-85% of age predicted HRmax | Chair stand, seated wood chop, plank by wall, chair lunge | 10–15 min | 2×10–15 repetitions, progressive |
| Frese et al.31 | 26 weeks | 3×/week | Cycling CLT and/or HIIT | 30–50 min | 65% VO2peak (CLT) 90–95% HRpeak/70–85% VO2peak (HIIT) | – | – | – |
| Mueller et al.32 | 26 weeks | 3×/week | Cycling CLT and/or HIIT | 30–50 min | 65% VO2peak (CLT) 90–95% HRpeak/70–85% VO2peak (HIIT) | – | – | – |
Abbreviations: 1RM, One-repetition Maximum; (c), Supervised Session at Clinic; (h), Session at Home; CLT, Constant-load Training; ET, Endurance Training; HIIT, High-intensity Interval Training; HR, Heart Rate; min, Minutes; n.r., Not Reported; RT, Resistance Training.
Results
Study characteristics
The training group size in the included studies consisted of nine to 16 HD patients who completed the interventions. The average age of the participants was in all studies between 53 and 55 years with reported standard deviation (SD) between 7 and 13 years. In two publications, only male HD patients were included in the analyses. In three studies, the proportion of male and female HD patients was similar, while in a further study, sex of the patients was not reported. The duration of the training interventions ranged between 8 and 26 weeks (Table 1). Frequency of training could be divided into two categories (Table 1). On the one hand, patients were supervised during every training session and had three training sessions per week (four studies).28,30–32 On the other hand, patients had only one supervised training session per week. In addition, patients were advised to exercise at home twice a week (two studies).27,29 Training protocols differed largely between the studies. Most of the training protocols (four studies)27–30 included endurance and resistance exercises, while in our two publications,31,32 the results of an exclusive endurance training intervention were presented. In the last two publications, endurance training consisted of periods of constant-load moderate-intensity and high-intensity interval protocols, as well as the combination of these modalities. In the two studies with supervised and unsupervised training sessions, the supervised sessions consisted of constant-load low-/moderate-intensity endurance training for 10–30 minutes and whole-body resistance training, mainly targeting major muscle groups.27,29 Home-based training consisted of walking at a low exercise intensity. In a further study, patients exercised three times a week at home with an exercise DVD, including a whole-body resistance training program.28 In addition, they were advised to go for a gradual progressive walk at light subjective exercise intensity. Over all the studies, training intensity for the supervised constant-load endurance exercise sessions was between 55% and 85% of (age-predicted) maximum heart rate for the constant-load session. In the high-intensity training protocol, the target heart rate was 90–95% of predetermined peak heart rate. Training intensity for the resistance training interventions was 60–70% of one-repetition maximum,27 not reported,28 or was defined by sets and repetitions,29,30 with the remark that patients were encouraged to exceed these numbers to ensure training progression (Table 1).
Adverse events
Information about adverse events in response to the training intervention was reported in all included publications (Table 2). Five studies reported no related adverse events in the intervention group.27–29,31,32 Quinn et al.30 reported two adverse events in the intervention group. Namely, recurrence of back pain (n = 1) and Wolf–Parkinson–White syndrome (n = 1) were observed, which resulted in withdrawal of the patients. In the same study, two serious adverse events were reported in the control group (i.e. attempted suicide possibly related to the study and a suicide unlikely to be related to the study).
Table 2. Outcomes of Training Intervention Studies in Huntington’s Disease Patients.
| AE | Cognition | Motor Function | Quality of Life | Cardiovascular Function | Body Composition | Further Variables | |
|---|---|---|---|---|---|---|---|
| Busse et al.27 | No related AE | → UHDRS cognitive score | → UHDRS motor score, self-selected + fast gait speed, 6-min walk test, CSTS, Romberg No falls |
↑ SF-36 mental component score → SF-36 other |
→ HR at submaximal power | – | – |
| Khalil et al.28 | No related AE | – | ↑ UHDRS motor score, gait speed, BBS, CSTS, PPT No falls |
→ SF-36 | – | – | – |
| Dawes et al.29 | No AE | – | – | – | → BP + HR at submaximal power | ↑ total body mass | – |
| Quinn et al.30 | 2 AE intervention, 2 SAE control | → category + verbal fluency, Stroop tests, TMT | ↑ UHDRS motor score → 15 rep chair stand, 3-min walk test |
→ EQ-5D-3L | ↑ predicted VO2peak | → total body mass | – |
| Frese et al.31 | No AE | → DRS-2, HVLT-R, verbal category fluency, Stroop tests, TMT, SDMT | → UHDRS motor score | – | ↑ VO2peak, Ppeak, Tlim | → total lean mass ↓ fat mass | – |
| Mueller et al.32 | No AE | – | – | – | – | – | ↑ CS activity, complex III + V activity, SCCR↓ relative complex I + II activity↑ PETF, L−, PCI, P, ETS, COX |
Abbreviations: →, Stable/Not Significantly Altered; ↑, Improvement; ↓, Reduction; AE, Adverse Event; BBS, Berg Balance Scale; BP, Blood Pressure; COX, Respiratory Capacity of Complex IV; CS, Citrate Synthase; CSTS, Chair Stand Test; DRS-2, Mattis Dementia Rating Scale; EQ-5D-3L, Three-level Version of the EuroQol Five-dimensional Questionnaire; ET, Endurance Training; ETS, Electron Transport System Capacity; HR, Heart Rate; HVLT-R, Hopkins Verbal Learning Test-Revised; L−, lactate; P, oxidative phosphorylation capacity; PCI, Respiratory Capacity of Complex I; PETF, Fatty Acid Oxidative Capacity; Ppeak, Peak Cycling Power; PPT, Physical Performance Test; SAE, Serious Adverse Event; SCCR, Succinate Cytochrome c Reductase; SDMT, Symbol Digit Modalities; SF-36, Short Form (36) Health Survey; Tlim, Cycling Time-to-Exhaustion; TMT, Trail Making Trials; UHDRS, Unified Huntington's Disease Rating Scale; VO2peak, Peak Oxygen Uptake.
Effects on motor function
Four studies reported the Unified Huntington’s Disease Rating Scale (UHDRS) motor score or a modified UHDRS motor score before and after the training intervention (Table 2).27,28,30,31 Two studies reported lower motor scores after the intervention (−2.3 in 8 weeks and −2 in 12 weeks), with significant group × time interactions, compared with alterations in a HD control group.28,30 Two studies reported statistically not altered values (+3.9 in 12 weeks and +0.8 in 26 weeks).27,31 In all four studies, information on the natural disease progression of motor scores was also included. In one of our publications, natural disease progression of the UHDRS motor score was assessed in the 26 weeks before onset of the training intervention in the same HD patients.31 The UHDRS motor score increased significantly in this first inactive period by an average of 7.4 points, while the increase was almost completely attenuated (+0.8) during the training intervention. In the further three studies, data of a HD control group were reported. In all these studies, motor scores remained within ±3 points during the observation period.27,28,30
Effects on mobility
During the training intervention, self-selected gait speed was stable in one study27 and improved in another study.28 Performance during the 3-minute (one study)30 and 6-minute (one study)27 walk tests were not altered in response to the training interventions. Repeated chair stand test performance was not altered in two studies,27,30 but the 30-second chair stand test performance was improved in another study.28 One study reported an improved result in the physical performance test after the intervention.28
Effects on balance/postural stability and falls
Balance assessments indicated an improved (Berg Balance Scale)28 or not altered (Romberg test)27 outcome. Two studies reported that no falls occurred in the intervention group during the study period,27,28 while a slightly lower number of falls was reported in the intervention group than in the control group in a third study.30
Effects on cognition
Three studies assessed cognitive variables before and after the training intervention (Table 2).27,30,31 In most of the assessed cognitive variables, stable values or no significant alterations could be detected during the training interventions. In these studies, the UHDRS cognitive score and/or the Hopkins Verbal Learning Test-Revised (HVLT-R), verbal and category fluency, Stroop/color word interference test, trail making trials, and symbol digit modalities tests were conducted.
Effects on cardiovascular function
In one of our publications with exclusive endurance training, improvements in VO2peak, peak power, and time-to-exhaustion at 85% of peak power were observed (Table 2).31 Another study reported an increase in predicted VO2max following resistance and endurance training.30 In two further studies, heart rate at a submaximal cycling power was not altered during the training intervention.27,29 One of these later studies also reported stable blood pressures at a submaximal cycling power.29
Effects on body composition
In one study, body composition analysis was performed by dual-energy X-ray absorptiometry (Table 2).31 In this study, there were no alterations in total body mass and lean mass, while fat mass was significantly reduced after a 6-month endurance training intervention.31 In two further studies, total body mass was reported. In one study, patients exhibited a significant increase in total body mass,29 and in the other study, body mass remained constant in the intervention group.30
Effects on mitochondrial function
In one of our own publications, absolute values for citrate synthase, complex III, complex V, and succinate cytochrome c reductase activities, measured spectrophotometrically, increased significantly during the intervention period (Table 2).32 In contrast, relative complex I and II activities decreased similarly in HD patients and healthy control participants. Mitochondrial respiration measurements in a subgroup of patients revealed significantly improved mass-specific fatty acid oxidative capacity, respiratory capacity of complex I, oxidative phosphorylation capacity, electron transport system capacity, and respiratory capacity of complex IV in response to the training intervention, while mitochondria-specific respiratory capacities remained constant.
Effects on quality of life measures
In three studies, quality of life was assessed by the Short Form (36) Health Survey (SF-36) or by the three-level version of the EuroQol five-dimensional questionnaire (EQ-5D-3L; Table 2).27,28,30 Quality of life was stable during the training interventions in two studies.28,30 In one study, the mental component of the SF-36 was improved after the training intervention, while the other components of the SF-36 remained stable.27 The control groups in these studies did not exhibit significant worsening of quality of life measures.
Discussion
In our current review, we included six studies on the effects of structured and targeted exercise training in HD patients. A multitude of outcome variables were investigated in these studies, including variables on safety, motor function, cognition, cardiovascular and mitochondrial function, body composition, and quality of life. Most of the studies revealed improved or stable values for the investigated variables during the intervention period.
Safety of the intervention is a main priority when implementing a new treatment approach in HD patients. All the reviewed articles included information about adverse events during the training intervention. Except from one study,30 no training-related adverse events were reported. Since the reported concomitant conditions were aggravated during the training interventions, both patients were withdrawn from the study.30 Based on the data available, endurance and resistance training seem to be safe in HD patients. Nonetheless, in HD, any accelerated worsening of any measured variable might also represent a safety issue. The comparisons between the alterations of measured variables during the training interventions and worsening during natural disease progression will be discussed within every subsequent topic.
Skeletal muscle atrophy is supposed to be a primary health risk in HD patients.33 However, several cross-sectional studies in early- and mid-stage HD patients could not detect significant differences in fat-free mass or lean mass in comparison to age-matched healthy controls.31,34–36 These studies highlighted that observed alterations in muscle mass in HD patients rely on a multitude of factors, e.g. physical activity and nutrition. In theory, the increased skeletal muscle turnover in response to physical activity and training might represent a risk for HD patients prone to muscle atrophy. In contrast, an increased skeletal muscle mass in response to resistance training would represent a promising option to alleviate this condition. Four of the included studies did incorporate resistance exercises in the training program.27–30 Unfortunately, none of these studies determined body composition. Two studies reported total body mass before and after the training intervention. Total body mass was not altered in one study30 and was significantly increased (∼3 kg) in the other study.29 However, information about the composition of these body mass alterations is lacking. In a further study on multidisciplinary rehabilitation in HD patients, incorporating resistance training in the exercise intervention, a significant increase in whole-body fat-free mass was reported.37 Based on the available results, exercise training seems not to accelerate skeletal muscle atrophy in HD patients. On the contrary, resistance training might even have the potential to increase body mass in early- and mid-stage HD patients. Further investigations of structured resistance training in HD patients with exact body composition measurements are desirable. In addition, we would like to point out that every training intervention should be accompanied by nutrition counseling to prevent a loss of skeletal muscle mass due to inadequate energy and protein intake.
In addition to skeletal muscle atrophy, skeletal muscle mitochondrial impairments are another constraint of peripheral tissue in HD patients.21–24,38 The results of one of our own publications indicated that mitochondrial content was increased in response to endurance training, while mitochondrial quality remained stable.32 This result emphasized that mitochondrial biogenesis is physiologically possible in early- and mid-stage HD patients. Besides the increased mitochondrial content, we also observed a rise in the overall capillary-to-fiber ratio during endurance training. The baseline values and the increase in capillarization were similar to the one measured in healthy control participants, indicating that capillarization and angiogenesis are well maintained in early- and mid-stage HD patients compared with that in healthy controls.
Our aforementioned results implicate that oxygen exchange between blood and muscle fibers and oxygen utilization of skeletal muscle fibers are improved after endurance training. This represents one explanation for the reported improvement in (predicted) VO2peak in two studies. Otherwise, no study has measured cardiac output before and after the training intervention, whereby information on cardiac adaptations remain unknown. Two studies that measured heart rate at a submaximal cycling power question the occurrence of ventricular hypertrophy in their patients.27,29 In both studies, the heart rate at a similar cycling power was not altered before and after the training intervention. Provided that significant ventricular hypertrophy was present, a reduction in heart rate at an identical cycling intensity would be expected. However, in both studies, no further cardiovascular variables were reported, whereby it remains incomprehensible whether the specific training protocols resulted in any cardiovascular adaptation. Epidemiological studies on the immediate causes of death in HD indicated that cardiovascular diseases are the second leading cause behind pneumonia.39–41 Since it is well known that physical activity and cardiovascular fitness lead to a reduction in cardiovascular mortality in the general public,42,43 a similar effect might be present in HD patients. However, two recent reviews have summarized incidences of cardiac (autonomic) dysfunctions in HD patients and highlight a potential cardiac risk.44,45 Hence, further data on cardiovascular adaptations following exercise training interventions in HD patients are required to elucidate health-related cardiovascular benefits and to examine the effects on potential cardiac dysfunctions.
In most included studies, exercise training had a positive effect on motor function. A quantification of the positive effect on the UHDRS motor score is difficult due to the variability in the progression of UHDRS motor scores in the control groups. Data in three studies provide an inconsistent outcome regarding the natural disease progression of the UHDRS motor score.27,28,30 In one of our publications, the natural disease progression of the UHDRS motor score was markedly attenuated during the intervention phase.31 The value attained for the natural disease progression in our own study was markedly higher than the reported longitudinal change in the UHDRS motor score in larger cohort studies (3.2±8.4 in 6 months46 or 4.7 per year47). The explanation for this latter comparison might be based on the high interindividual variation in UHDRS motor score progression.48,49 For example, due to the low sample sizes in all training intervention studies, no allocation of HD patients according to motor subtypes was feasible. Further assessed motor functional variables, namely gait speed, walk tests, and chair stand tests, exhibited stable values during the training interventions.27,30 Only in the study by Khalil et al.28 were significant improvements in the Berg Balance Scale, gait speed, 30-second chair sit to stand test, and physical performance test reported in relation to the HD control group. However, for all these variables the significant improvement depicted mainly a catch-up effect to the better baseline values of the control group. Based on current results of exercise training on motor function, natural disease progression of HD patients might be attenuated by the interventions. Whether this attenuation has a functional benefit on activities of daily living remains unknown. To gain further insights into the functional benefit of training on activities of daily living, prolonged interventions are necessary.
Over all studies, most assessed cognitive variables were not significantly altered during the training interventions. In comparison to the natural disease progression for cognitive variables,50–53 it remains ambiguous whether the training interventions highlight a stabilization of values or slowing of the natural disease progression. In several large-scale investigations, significant decreases in some, but not all, cognitive test variables were detected after 12 months of observation, while these alterations were consolidated after 24 and/or 36 months.50–52 Since the duration of training interventions in the included studies ranged from 8 to 26 weeks, the length of the observation period, but also the relatively small size of intervention cohorts, might have been insufficient to detect significant alterations in cognitive variables. This argument is supported by the non-altered cognitive variables in our 6-month natural disease observation period,31 and by the statistically not significant alterations in the HD control groups in other studies.27,30 Significant improvements in some cognitive variables were previously reported in studies on multidisciplinary rehabilitation in HD patients.37,54 In one of these studies, significant improvements were positively correlated with the change in gray matter volume in the dorsolateral prefrontal cortex.54 This latter result might even point to preserved neuroplasticity in HD patients.
Self-reported quality of life seems not to be influenced by exercise training in HD patients, nor was it changed during the observation phases in control groups. However, the results might be explained by the low sensitivity of the questionnaires. While it is well known that quality of life is inversely associated with increasing stages of the disease55–58 and increased symptoms are associated with lower quality of life,59,60 less is known about the magnitude of longitudinal changes in quality of life.61 Indeed, there is currently no health-related quality of live measure recommended to measure change of severity over time.62
Limitations of previous studies and future perspectives
The included studies have several limitations that might compromise the outcome of these studies and, thereby, affect the interpretation in this review. First, training intensity for endurance exercise programs was sometimes very low. While training intensity in the supervised training session was moderate to high, home-based endurance training consisted mainly of walking exercise. Since supervised sessions were only conducted once per week and home-based walking twice per week, the overall intensity of these endurance training protocols might not have been sufficient to achieve a significant training effect. Furthermore, patients in the mid- or late-stage of the disease might not be able to achieve a sufficiently high training stimulus due to motor and cognitive impairment. Therefore, exercise training might be a valid strategy to modulate disease symptoms only during early- to mid-stages of HD. Second, in most studies incorporating resistance exercise, important information about resistance exercise determinants63 is missing. These deficits complicate the interpretation of resistance training effects. We recommend that all relevant exercise determinants are reported for resistance and endurance training programs in future studies. Furthermore, it remains to be elucidated which training volume, intensity, and modality best modulates disease symptoms. Third, only one study included exact measurements of body composition. Based on the potential risk that a training intervention might accelerate skeletal muscle atrophy, training intervention studies should incorporate exact body composition measurements and nutrition counseling. Fourth, individual progress adumbrates that adaptation to the training stimulus underlies large interindividual variability. Therefore, studies with larger cohorts might shed more light on the individual alterations in variables. Previous training programs were adapted to restrictions of patients and targeted to facilitate exercise participation (e.g. home-based exercise or supported exercise at a clinic to assure comfortable training sessions for the patients), but were not individualized to enhance training adaptation. Moreover, the effect of disease severity and stage of disease on adaptation to training remains scarce. Fifth, most studies documented the intake of medication. Since it is well known that several active pharmaceutical ingredients interact with training adaptation processes, study outcomes might have been altered by drug intake. However, current data are insufficient to draw conclusions about these influences.
Conclusions
Based on the six included studies in this review, exercise training seems to be a safe and feasible treatment approach in HD patients. These studies on exercise training interventions in HD patients point to a beneficial effect of training on cardiovascular and mitochondrial function. Training effects on cognition, motor function, and body composition are less congruent, but overall evidence seems to support a possible positive effect. However, our conclusions are based on small-scale studies and cannot be transferred to all HD patients. To shed more light on the effects of exercise training on the aforementioned variables, longer-term intervention studies with larger HD cohorts are desirable. For reasons of safety, it is recommended that all exercise training interventions are accompanied by frequent assessments to detect any accelerated worsening of symptoms.
Footnotes
Funding: None.
Financial Disclosures: This work was supported by grants from the Swiss National Science Foundation (320030_135539) and the Jacques and Gloria Gossweiler Foundation.
Conflicts of Interest: The authors report no conflicts of interest.
Ethics Statement: Not applicable for this category of article.
References
- 1.Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, et al. Detection of Huntington’s disease decades before diagnosis: the Predict-HD study. J Neurol Neurosurg Psychiatry. 2008;79:874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paulsen JS, Miller AC, Hayes T, Shaw E. Cognitive and behavioral changes in Huntington disease before diagnosis. In: Feigin AS, Anderson KE, . Amsterdam, The Netherlands: Elsevier Science BV; 2017. pp. p 69–91. editors. Huntington disease. (Handbook of Clinical Neurology; vol. 144) [DOI] [PubMed] [Google Scholar]
- 3.Roos RA. Huntington’s disease: a clinical review. Orph J Rare Dis. 2010;5:40. doi: 10.1186/1750-1172-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dayalu P, Albin RL. Huntington disease. Pathogenesis and treatment. Neurol Clin. 2015;33:101–114. doi: 10.1016/j.ncl.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Dickey AS, La Spada AR. Therapy development in Huntington disease: from current strategies to emerging opportunities. Am J Med Genet. 2018;176:842–861. doi: 10.1002/ajmg.a.38494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lavers A. An account of a weekly activity group with Huntington’s chorea patients on a ling stay ward. Occup Ther. 1981;44:387–392. doi: 10.1177/030802268104401209. [DOI] [Google Scholar]
- 7.Peacock IW. A physical therapy program for Huntington’s disease patients. Clinic Manag Phys Ther. 1987;7:22–23. [Google Scholar]
- 8.Bohlen S, Ekwall C, Hellström K, Vesterlin H, Björnefur M, Wiklund L, et al. Physical therapy in Huntington’s disease—toward objective assessments? Eur J Neurol. 2013;20:389–393. doi: 10.1111/j.1468-1331.2012.03760.x. [DOI] [PubMed] [Google Scholar]
- 9.Ciancarelli I, Tozzi Ciancarelli MG, Carolei A. Effectiveness of intensive neurorehabilitation in patients with Huntington’s disease. Eur J Phys Rehabil Med. 2013;49:189–195. [PubMed] [Google Scholar]
- 10.Ciancarelli I, De Amicis D, Di Massimo C, Sandrini G, Pistarini C, Carolei A, et al. Influence of intensive multifunctional neurorehabilitation on neuronal oxidative damage in patients with Huntington’s disease. Funct Neurol. 2015;30:47–52. [PMC free article] [PubMed] [Google Scholar]
- 11.Mirek E, Filip M, Banaszkiewicz K, Rudzińska M, Szymura J, Pasiuta S, et al. The effects of physiotherapy with PNF concept on gait and balance of patients with Huntington’s disease – pilot study. Neurol Neurochir Pol. 2015;49:354–357. doi: 10.1016/j.pjnns.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Mirek E, Filip M, Chwała W. The influence of motor ability rehabilitation on temporal-spatial parameters of gait in Huntington's disease patients on the basis of a three-dimensional motion analysis system: An experimental trial. Neurol Neurochir Pol. 2018;49:354–357. doi: 10.1016/j.pjnns.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Piira A, van Walsem MR, Mikalsen G, Nilsen KH, Knutsen S, Frich JC. Effects of a one year intensive multidisciplinary rehabilitation program for patients with Huntington’s disease: a prospective intervention study. PLoS Curr. 2013;5 doi: 10.1371/currents.hd.9504af71e0d1f87830c25c394be47027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piira A, van Walsem MR, Mikalsen G, Øie L, Frich JC, Knutsen S. Effects of a two-year intensive multidisciplinary rehabilitation program for patients with Huntington’s disease: a prospective intervention study. Version 2. PLoS Curr. 2015;6 doi: 10.1371/currents.hd.2c56ceef7f9f8e239a59ecf2d94cddac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn L, Debono K, Dawes H, Rosser AE, Nemeth AH, Rickards H, et al. Task-specific training in Huntington disease: a randomized controlled feasibility trial. Phys Ther. 2014;94:1555–1568. doi: 10.2522/ptj.20140123. [DOI] [PubMed] [Google Scholar]
- 16.Zinzi P, Salmaso D, De Grandis R, Graziani G, Maceroni S, Bentivoglio A, et al. Effects of an intensive rehabilitation programme on patients with Huntington’s disease: a pilot study. Clin Rehabil. 2007;21:603–613. doi: 10.1177/0269215507075495. [DOI] [PubMed] [Google Scholar]
- 17.Trembath MK, Horton ZA, Tippett L, Collins VR, Churchyard A, Roxburgh R. A retrospective study of the impact of lifestyle on age at onset of Huntington disease. Mov Disord. 2010;25:1444–1450. doi: 10.1002/mds.23108. [DOI] [PubMed] [Google Scholar]
- 18.Lui AJ, Byl NN. A systematic review of the effect of moderate intensity exercise on function and disease progression in amyotrophic lateral sclerosis. J Neurol Phys Ther. 2009;33:68–87. doi: 10.1097/NPT.0b013e31819912d0. [DOI] [PubMed] [Google Scholar]
- 19.Speelman AD, van de Warrenbrug BP, van Nimwegen M, Petzinger GM, Munneke M, Bloem BR. How might physical activity benefit patients with Parkinson disease? Nat Rev Neurol. 2011;7:528–534. doi: 10.1038/nrneurol.2011.107. [DOI] [PubMed] [Google Scholar]
- 20.Kosinski CM, Schlangen C, Gellerich FN, Gizatullina Z, Deschauer M, Schiefer J, et al. Myopathy as a first symptom of Huntington’s disease in a marathon runner. Mov Disord. 2007;22:1637–1640. doi: 10.1002/mds.21550. [DOI] [PubMed] [Google Scholar]
- 21.Arenas J, Campos Y, Ribacoba R, Martín Pharm MA, Rubio JC, Ablanedo P, et al. Complex I defect in muscle from patients with Huntington's disease. Ann Neurol. 1998;43:397–400. doi: 10.1002/ana.410430321. [DOI] [PubMed] [Google Scholar]
- 22.Ciammola A, Sassone J, Alberti L, Meola G, Mancinelli E, Russo MA, et al. Increased apoptosis, Huntingtin inclusions and altered differentiation in muscle cell cultures from Huntington’s disease subjects. Cell Death Differ. 2006;13:2068–2078. doi: 10.1038/sj.cdd.4401967. [DOI] [PubMed] [Google Scholar]
- 23.Lodi R, Schapira AH, Manners D, Styles P, Wood NW, Taylor DJ, et al. Abnormal in vivo skeletal muscle energy metabolism in Huntington's disease and dentatorubropallidoluysian atrophy. Ann Neurol. 2000;48:72–76. doi: 10.1002/1531-8249(200007)48:1<72::AID-ANA11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 24.Saft C, Zange J, Andrich J, Müller K, Lindenberg K, Landwehrmeyer B, et al. Mitochondrial impairment in patients and asymptomatic mutation carriers of Huntington’s disease. Mov Disord. 2005;20:674–679. doi: 10.1002/mds.20373. [DOI] [PubMed] [Google Scholar]
- 25.Mo C, Hannan AJ, Renoir T. Environmental factors as modulators of neurodegeneration: insights from gene-environment interactions in Huntington’s disease. Neurosci Biobehav Rev. 2015;52:178–192. doi: 10.1016/j.neubiorev.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Fritz NE, Rao AK, Kegelmayer D. Physical therapy and exercise interventions in Huntington’s disease: a mixed methods systematic review. J Huntington Dis. 2017;6:217–235. doi: 10.3233/JHD-170260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Busse M, Quinn L, Debono K, Karen J, Johnathan C, Rebecca P, et al. A randomized feasibility study of a 12-week community-based exercise program for people with Huntington’s disease. J Neurol Phys Ther. 2013;37:149–158. doi: 10.1097/NPT.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 28.Khalil H, Quinn L, van Deursen R, Dawes H, Playle R, Rosser A, et al. What effect does a structured home-based exercise programme have on people with Huntington’s disease? A randomized, controlled pilot study. Clin Rehabil. 2013;27:646–658. doi: 10.1177/0269215512473762. [DOI] [PubMed] [Google Scholar]
- 29.Dawes H, Collett J, Debono K, Quinn L, Jones K, Kelson MJ, et al. Exercise testing and training in people with Huntington’s disease. Clin Rehabil. 2015;29:196–206. doi: 10.1177/0269215514540921. [DOI] [PubMed] [Google Scholar]
- 30.Quinn L, Hamana K, Kelson M, Dawes H, Collett J, Townson J, et al. A randomized, controlled trial of a multi-modal exercise intervention in Huntington’s disease. Parkinsonism Relat Disord. 2016;31:46–52. doi: 10.1016/j.parkreldis.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 31.Frese S, Petersen JA, Ligon-Auer M, Mueller SM, Mihaylova V, Gehrig SM, et al. Exercise effects in Huntington disease. J Neurol. 2017;264:32–39. doi: 10.1007/s00415-016-8310-1. [DOI] [PubMed] [Google Scholar]
- 32.Mueller SM, Gehrig SM, Petersen JA, Frese S, Mihaylova V, Ligon-Auer M, et al. Effects of endurance training on skeletal muscle mitochondrial function in Huntington disease patients. Orph J Rare Dis. 2017;12:184. doi: 10.1186/s13023-017-0740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zielonka D, Piotrowska I, Marcinkowski JT, Mielcarek M. Skeletal muscle pathology in Huntington’s disease. Front Physiol. 2014;5:380. doi: 10.3389/fphys.2014.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cubo E, Rivadeneyra J, Gil-Polo C, Armesto D, Mateos A, Mariscal-Pérez N. Body composition analysis as an indirect marker of skeletal muscle mass in Huntington’s disease. J Neurol Sci. 2015;358:335–338. doi: 10.1016/j.jns.2015.09.351. [DOI] [PubMed] [Google Scholar]
- 35.Pratley RE, Salbe AD, Ravussin E, Caviness JN. Higher sedentary energy expenditure in patients with Huntington’s disease. Ann Neurol. 2000;47:64–70. doi: 10.1002/1531-8249(200001)47:1<64::AID-ANA11>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 36.Süssmuth SD, Müller VM, Geitner C, Landwehrmeyer GB, Iff S, Gemperli A, et al. Fat-free mass and its predictors in Huntington’s disease. J Neurol. 2015;262:1533–1540. doi: 10.1007/s00415-015-7753-0. [DOI] [PubMed] [Google Scholar]
- 37.Thompson JA, Cruickshank TM, Penailillo LE, Lee JW, Newton RU, Barker RA, et al. The effects of multidisciplinary rehabilitation in patients with early-to-middle-stage Huntington’s disease: a pilot study. Eur J Neurol. 2013;20:1325–1329. doi: 10.1111/ene.12053. [DOI] [PubMed] [Google Scholar]
- 38.Gehrig SM, Petersen JA, Frese S, Mueller SM, Mihaylova V, Ligon-Auer M, et al. Skeletal muscle characteristics and mitochondrial function in Huntington’s disease patients. Mov Disord. 2017;32:1258–1259. doi: 10.1002/mds.27031. [DOI] [PubMed] [Google Scholar]
- 39.Lanska DJ, Lavine L, Lanska MJ, Schoenberg BS. Huntington’s disease mortality in the United States. Neurology. 1988;38:769–772. doi: 10.1212/WNL.38.5.769. [DOI] [PubMed] [Google Scholar]
- 40.Solberg OK, Filkukova P, Frich JC, Billaud Feragen KJ. Age at death and causes of death in patients with Huntington disease in Norway in 1986-2015. J Hunt Dis. 2018;7:77–86. doi: 10.3233/JHD-170270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorensen SA, Fenger K. Causes of death in patients with Huntington’s disease and in unaffected first degree relatives. J Med Genet. 1992;29:911–914. doi: 10.1136/jmg.29.12.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fogelholm M. Physical activity, fitness and fatness: relations to mortality, morbidity and disease risk factors. A systematic review. Obes Rev. 2010;11:202–221. doi: 10.1111/j.1467-789X.2009.00653.x. [DOI] [PubMed] [Google Scholar]
- 43.Nocon M, Hiemann T, Müller-Riemenschneider F, Thalau F, Roll S, Willich SN. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil. 2008;15:239–246. doi: 10.1097/HJR.0b013e3282f55e09. [DOI] [PubMed] [Google Scholar]
- 44.Abildtrup M, Shattock M. Cardiac dysautonomia in Huntington’s disease. J Hunt Dis. 2013;2:251–261. doi: 10.3233/JHD-130054. [DOI] [PubMed] [Google Scholar]
- 45.Zielonka D, Piotrowska I, Mielcarek M. Cardiac dysfunction in Huntington’s disease. Exp Clin Cardiol. 2014;20:2547–2554. [Google Scholar]
- 46.Huntington Study Group Unified Huntington’s disease rating scale: reliability and consistency. Mov Disord. 1996;11:136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 47.Meyer C, Landwehrmeyer B, Schwenke C, Doble A, Orth M, Ludolph AC, et al. Rate of change in early Huntington’s disease: a clinicometric analysis. Mov Disord. 2012;27:118–124. doi: 10.1002/mds.23847. [DOI] [PubMed] [Google Scholar]
- 48.Hart EP, Marinus J, Burgunder JM, Bentivoglio AR, Craufurd D, Reilmann R, et al. Better global and cognitive functioning in choreatic versus hypokinetic-rigid Huntington’s disease. Mov Disord. 2013;28:1142–1145. doi: 10.1002/mds.25422. [DOI] [PubMed] [Google Scholar]
- 49.Jacobs M, Hart EP, van Zwet EW. Progression of motor subtypes in Huntington’s disease: a 6-year follow-up study. J Neurol. 2016;263:2080–2085. doi: 10.1007/s00415-016-8233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snowden J, Craufurd D, Griffiths H, Thompson J, Neary D. Longitudinal evaluation of cognitive disorder in Huntington’s disease. J Int Neuropsychol Soc. 2001;7:33–44. doi: 10.1017/S1355617701711046. [DOI] [PubMed] [Google Scholar]
- 51.Stout JC, Jones R, Labuschagne I, O'Regan AM, Say MJ, Dumas EM, et al. Evaluation of longitudinal 12 and 24 month cognitive outcomes in premanifest and early Huntington’s disease. J Neurol Neurosurg Psychiatry. 2012;83:687–694. doi: 10.1136/jnnp-2011-301940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tabrizi SJ, Reilmann R, Roos RA, Durr A, Leavitt B, Owen G, et al. Potential endpoints for clinical trials in premanifest and early Huntington’s disease in the TRACK-HD study: analysis of 24 month observational data. Lancet Neurol. 2012;11:42–53. doi: 10.1016/S1474-4422(11)70263-0. [DOI] [PubMed] [Google Scholar]
- 53.Tabrizi SJ, Scahill RI, Owen G, Durr A, Leavitt BR, Roos PR, et al. Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington’s disease in the TRACK-HD study: analysis of 36-month observational data. Lancet Neurol. 2013;12:637–649. doi: 10.1016/S1474-4422(13)70088-7. [DOI] [PubMed] [Google Scholar]
- 54.Cruickshank TM, Thompson JA, Dominguez JF, Reyes AP, Bynevelt M, Georgiou-Karistianis N, et al. The effect of multidisciplinary rehabilitation on brain structure and cognition in Huntington’s disease: an exploratory study. Brain Behav. 2015;5:e00312. doi: 10.1002/brb3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carlozzi NE, Ready RE, Frank S, Cella D, Hahn EA, Goodnight SM, et al. Patient-reported outcomes in Huntington’s disease: quality of life in neurological disorders (Neuro-QoL) and Huntington’s disease health-related quality of life (HDQLIFE) physical function measures. Mov Disord. 2017;32:1096–1102. doi: 10.1002/mds.27046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carlozzi NE, Schilling SG, Lai JS, Paulsen JS, Hahn EA, Perlmutter JS, et al. HDQLIFE: development and assessment of health-related quality of life in Huntington disease (HD) Qual Life Res. 2016;25:2441–2455. doi: 10.1007/s11136-016-1386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Read J, Jones R, Owen G. Quality of life in Huntington’s disease: a comparative study investigating the impact for those with pre-manifest and early manifest disease, and their partners. J Huntingtons Dis. 2013;2:159–175. doi: 10.3233/JHD-130051. [DOI] [PubMed] [Google Scholar]
- 58.Van Walsem MR, Howe EI, Ruud GA, Frich JC, Andelic N. Health-related quality of life and unmet healthcare needs in Huntington’s disease. Health Qual Life Outcomes. 2017;15:6. doi: 10.1186/s12955-016-0575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ready RE, Mathews M, Leserman A, Paulsen JS. Patient and caregiver quality of life in Huntington’s disease. Mov Disord. 2008;23:721–726. doi: 10.1002/mds.21920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thorley EM, Iyer RG, Wicks P, Curran C, Gandhi SK, Abler V, et al. Understanding how chorea affects health-related quality of life in Huntington disease: an online survey of patients and caregivers in the United States. Patient. 2018;11:547–559. doi: 10.1007/s40271-018-0312-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boileau NR, Stout JC, Paulsen JS, Cella D, McCormack MK, Nance MA, et al. Reliability and validity of the HD-PRO-TriadTM, a health-related quality of life measure designed to assess the symptom triad of Huntington’s disease. J Huntingtons Dis. 2017;6:201–215. doi: 10.3233/JHD-170238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mestre TA, Carlozzi NE, Ho AK, Burgunder JM, Walker F, Davis AM, et al. Quality of life in Huntington’s disease: critique and recommendations for measures assessing patient health-related quality of life and caregiver quality of life. Mov Disord. 2018;33:742–749. doi: 10.1002/mds.27317. [DOI] [PubMed] [Google Scholar]
- 63.Toigo M, Boutellier U. New fundamental resistance exercise determinants of molecular and cellular muscle adaptations. Eur J Appl Physiol. 2006;97:643–663. doi: 10.1007/s00421-006-0238-1. [DOI] [PubMed] [Google Scholar]