Abstract
Effective administration of traditional cytotoxic chemotherapy is often limited by off-target toxicities. This clinical dilemma is epitomized by cisplatin, a platinating agent that has potent antineoplastic activity due to its affinity for DNA and other intracellular nucleophiles. Despite its efficacy against many adult-onset and pediatric malignancies, cisplatin elicits multiple off-target toxicities that can not only severely impact a patient’s quality of life, but also lead to dose reductions or the selection of alternative therapies that can ultimately affect outcomes. Without an effective therapeutic measure by which to successfully mitigate many of these symptoms, there have been attempts to identify a priori those individuals who are more susceptible to developing these sequelae through studies of genetic and nongenetic risk factors. Older age is associated with cisplatin induced ototoxicity, neurotoxicity and nephrotoxicity. Traditional genome-wide association studies have identified single nucleotide polymorphisms in ACYP2 and WFS1 associated with cisplatin-induced hearing loss. However, validating associations between specific genotypes and cisplatin-induced toxicities with enough stringency to warrant clinical application remains challenging. This review summarizes the current state of knowledge with regard to specific adverse sequelae following cisplatin-based therapy with a focus on ototoxicity, neurotoxicity, nephrotoxicity, myelosuppression and nausea/emesis. We discuss variables (genetic and nongenetic) contributing to these detrimental toxicities, and currently available means to prevent or treat their occurrence.
Introduction
Cisplatin and the platinating agents represent one of the most widely used and successful groups of cytotoxic drugs worldwide. Each year, more than 5.8 million patients are diagnosed with cancers for which first-line therapy potentially includes platinating agents (colon, rectum, cervix, endometrium, bladder, stomach, head and neck, lung, esophagus, pancreas, osteosarcoma, ovary, testis, and childhood cancers) (1). Although cisplatin elicits potent antineoplastic activity through the formation of DNA crosslinks (2), the agent also triggers several severe off-target toxicities (Table 1), some of which affect patients acutely and resolve after treatment, and some which display little reversibility (2, 3). Although not the focus of this review, mechanisms by which cisplatin elicits these toxicities are listed in Table 1. For a more comprehensive description, refer to previous reviews for ototoxicity (4–7); neurotoxicity (8, 9); nephrotoxicity (10–12); myelosuppression (13); and nausea/emesis (14, 15).
Table 1.
Frequently reported cisplatin-induced adverse sequelae.
| Toxicity | Acute and Chronic Complications | Frequency (%) | Pathophysiology |
|---|---|---|---|
| Nausea/Emesis | • Therapy-related anxiety • Anticipatory chemotherapy-induced nausea/vomiting • Fatigue and weakness • Weight loss, dehydration and loss of appetite • Bone fractures and tears to the throat |
Acute symptoms occur in > 90% of patients when antiemetic prophylaxis is not administered (14). Delayed symptoms occur in 60–90% of patients under the same conditions (77). | Perturbs the lining of the gastrointestinal tract, which subsequently promotes Ca2+-dependent exocytic release of serotonin from enterochromaffin cells (15). Successful binding of serotonin to its receptors on the vagal afferent neurons activates the chemoreceptor trigger zone and vomiting center that promote the initiation of the emetogenic response. |
| Ototoxicity | • Irreversible sensorineural hearing loss • Tinnitus |
Some degree of hearing loss occurs in 75–80% of patients (5, 23), while 13–18% develop severe-to-profound hearing loss
(4–6). Symptoms of tinnitus occur in approximately 40% of patients (6), with 13–22% experiencing severe tinnitus (6, 8). |
Increases reactive oxygen species concentrations and depletes antioxidants used for detoxification (90–92). Cisplatin also activates big conductance potassium channels in spiral ligament fibrocytes (SLFs), which adversely disrupt the electrochemical gradient within cells and trigger apoptosis (93). The cochlea retains cisplatin for months to years after treatment, with accumulation being particularly high in the stria vascularis, the SLF-containing region vital for the maintenance of endolymph ionic composition (94). Platinum-DNA adducts have been detected in the hair cells of the cochlea and the marginal cells of the stria vascularis. Since these cells do not proliferate, platination of mitochondrial DNA (mtDNA) is considered responsible for toxicity (4, 9). |
| Neurotoxicity (Peripheral Neuropathy) | • Persistent peripheral sensory neuropathy • Reduced physical activity and weight gain |
Peripheral sensory neuropathy occurs in 36–38% of patients. (48, 49). Almost all patients who receive a cumulative dose of 500–600 mg/m2 experience nerve damage (33). | Accumulates in the dorsal root ganglia of the spinal cord and peripheral neurons via passive diffusion or metal transporters. Forms DNA adducts and inhibits DNA repair pathways that ultimately induce p53-mediated apoptosis via Bax activation (95). The affinity of cisplatin for mtDNA may help explain the clinical quandary of coasting, as the absence of the nucleotide excision repair (NER) in the mitochondria could potentiate a gradual attrition of mitochondria, subsequently reducing ATP levels that neurons require to maintain their functional activity (96). |
| Myelosuppression | • Cytopenias • Residual bone marrow injury and reduction in hematopoietic stem cell reserves • Potential susceptibility to secondary leukemia |
Between 25–30% of patients develop symptoms of myelosuppression, while 5–6% (66) are diagnosed with severe myelosuppression. | Elicits both genotoxic effects and oxidative stress to deplete blood counts(68). Patients may also develop residual bone marrow injury in which there is a sustained reduction in hematopoietic stem cell reserves due to self-renewal impairment (69, 70). |
| Nephrotoxicity | • Acute kidney injury • Hypomagnesemia • Permanent kidney damage |
Acute kidney injury occurs in 20–30% of patients (12). Hypomagnesemia manifests in 40–100% of patients. | Active transport through Oct2 and Ctr1 (97, 98) induces toxicity primarily in the renal proximal tubules (11). Mechanisms of damage include endoplasmic reticulum stress and mitochondrial dysfunction leading to oxidative stress, as well as inflammation mediated by tumor necrosis factor and other chemokines (11, 12). |
Due to improved survival rates, most notably in testicular cancer and in pediatric malignancies, there are a significant number of survivors living with these severe adverse sequelae that affect quality of life. The non-uniformity of these toxicities in patient populations has been the subject of much research in efforts to circumvent their occurrence. Figure 1 provides an overview of nongenetic risk factors contributing to cisplatin-induced toxicities. Of particular interest is the association between older age and an increased susceptibility to several cisplatin-induced toxicities. Although the exact mechanisms of this association have not been explicitly studied, it is known that drug clearance can decrease with age, particularly when elimination is mediated by renal clearance (16). Since cisplatin is eliminated predominantly through the kidney, and is also known to be highly nephrotoxic, the agent ultimately reduces the ability for platinum to be excreted from the body (10), thereby increasing the likelihood of developing cisplatin-induced toxicities. Further, it is not surprising that older adults are associated with cisplatin-induced ototoxicity and neurotoxicity because these individuals often experience age-related hearing loss/tinnitus (17, 18) and paresthesias/neuropathies (19, 20), and the addition of cisplatin will likely exacerbate symptoms.
Figure 1.
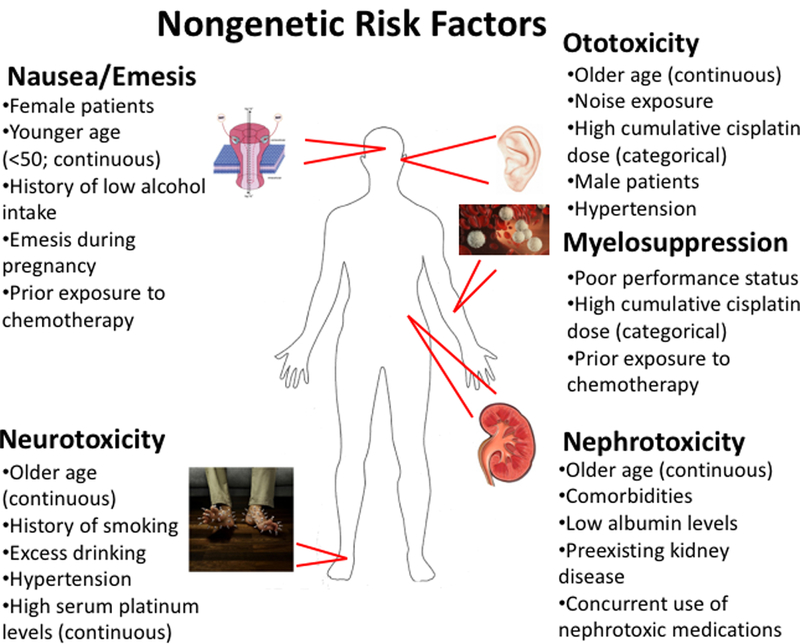
Nongenetic risk factors that may predispose patients to developing adverse events following cisplatin-based therapy. Where relevant, risk factors are denoted as being either continuous or categorical variables based on how they were examined for association with the given toxicity.
Variability in patient response can also be explained in part by pharmacogenomics, which aims to provide the foundation for genetically guided treatment regimens that maximize efficacy and minimize toxicity. Initially developed from candidate gene approaches, advances in genomic sequencing technologies over the past decade have enabled agnostic genome-wide analyses of patient populations characterized for specific drug response phenotypes. Thus, pharmacogenomics has elucidated genetic variability as a key determinant in both therapeutic benefit and potential toxicities likely to be experienced during cisplatin-based chemotherapy. One of the challenges in pharmacogenomics is that most cancers are treated with a multi-drug regimen making it difficult to ascertain the genetic variants associated with a specific chemotherapeutic toxicity. This is the case for cisplatin, however some toxicities (i.e. ototoxicity and nephrotoxicity) are primarily due to cisplatin; therefore the genetic variants identified are most likely associated with cisplatin exposure. However, understanding both the functional significance and clinical application of these findings remains elusive. Therefore, this review will highlight nongenetic and genetic risk factors contributing to cisplatin-induced toxicities, and provide recent data on novel therapeutic strategies by which to reduce adverse effects.
Ototoxicity
Cisplatin is associated with irreversible, bilateral sensorineural hearing loss that occurs at a much higher rate than other ototoxic drugs. Reports indicate that up to 75–80% of patients may experience some degree of hearing loss and 13–18% may develop severe-to-profound hearing loss (21–23). In addition, approximately 40% of cisplatin-treated patients (23) experience some degree of tinnitus, which occurs at a significantly higher rate than either the general population (15%; (24)), or in comparable cancer patients not given cisplatin-based chemotherapy (12%; (25)). The frequency of severe tinnitus is also markedly increased in cisplatin-treated patients (13–22%; (23, 25)) with one study noting that 42% of patients report tinnitus as a major symptom after dose-intensive cisplatin chemotherapy (25). In contrast, severe tinnitus occurs in only 1–2% of the general population (24). Further, cancer survivors with hearing loss, tinnitus, and neuropathy are more likely to report poorer quality of life than those with neuropathy only (26). Another investigation reported worse perceived stress among cancer survivors with tinnitus (27).
Although nongenetic risk factors for cisplatin-associated ototoxicity (CAO) have been identified (Figure 1), previous studies have focused almost exclusively on hearing loss susceptibility. Further, there have been conflicting results with regard to the importance of noise exposure and cumulative cisplatin dose on hearing loss (23, 28, 29). However, in pediatric cancer patients, males appear to be more susceptible to cisplatin-induced hearing loss than females (p=0.005; (29)). Hypertension has also been identified as a potential risk factor for hearing loss in testicular cancer patients, with the association remaining significant when controlling for age and cisplatin dose (p=0.0066; (23)).
Recently, the FDA granted sodium thiosulfate (STS) “fast-track designation” to prevent cisplatin-related hearing loss in pediatric patients diagnosed with hepatoblastoma based on the results of a clinical trial of 109 pediatric hepatoblastoma patients in which 20 g/m2 STS was administered intravenously 6 hours after the discontinuation of cisplatin for four preoperative and two postoperative courses. Not only did STS treatment reduce grade 1 or higher hearing loss incidence by 48% (18 of 55 children (33%) in the cisplatin-STS group experienced hearing loss compared to 29 of 46 (63%) in the cisplatin-alone group; relative risk, 0.52; 95% confidence interval [CI]: 0.33 to 0.81; p=0.002), but cisplatin-STS conferred overall and event-free survival rates comparable to those who did not receive the protective agent (30). However, of the 16 serious adverse reactions experienced by patients, 8 were likely attributed to STS, including two grade 3 infections, two grade 3 neutropenias, one grade 3 anemia leading to transfusion, and two tumor progressions. In addition, the otoprotective effect of STS was associated with a large sodium load that must be considered in planning therapy. The investigators also cautioned that, despite the use of prophylactic antiemetic agents, STS remained emetogenic. These adverse events are in accord with prior experiences that note the frequency and severity of STS toxicities (31), and the agent must be administered with considerable caution. Although STS has demonstrated potential in the pediatric setting among a small subgroup of cancer patients, there has yet to be a large, multi-institutional study in adult patients, thereby limiting the potential applicability of the agent. Consequently, there remains no FDA approved agent to reduce ototoxicity for the vast majority of patients who receive cisplatin.
A number of pharmacogenetics studies have been conducted to identify CAO risk-conferring genotypes (summarized in Supplementary Table 1). In a study (32) that utilized a platform containing primarily single nucleotide polymorphisms (SNPs) in metabolizing genes, genetic variants in TPMT (rs12201199) and COMT (rs9332377) were identified that prompted the FDA to revise their label recommendations in 2012 for pediatric patients given cisplatin. However, the modification was rescinded in 2015 due to conflicting evidence of association between TPMT genetic variants and cisplatin-induced hearing loss provided by two replication studies and a meta-analysis (33–35). The lack of reproducibility in pharmacogenomic studies related to cisplatin is likely due to genetic heterogeneity as well as heterogeneity in treatment protocols and population substructures, small sample sizes, and the use of cranial radiation in combination with cisplatin which could substantially increase the likelihood of CAO due to its ototoxic effects (36). Specimen type, handling, sequencing method, gene calling, as well as the method of assessment of the particular toxicity being studied could also contribute to the lack of reproducibility. This points to the importance of replication of these pharmacogenomic studies.
Technological advances have enabled agnostic, genome-wide study designs to identify contributing SNPs associated with a selected trait. Contrary to candidate gene studies, such alleles are not limited by a priori hypotheses of loci that generally reside in exonic genomic regions. If fact, the majority of disease-associated variants identified from genome-wide association studies (GWAS) reside in intergenic regions associated with transcriptional regulatory mechanisms including expression quantitative trait loci (eQTL) known to influence gene expression (37, 38). Chemotherapeutic drug susceptibility-associated SNPs, including those for cisplatin-induced cytotoxicity, are more likely to be eQTLs and be associated with the expression levels of multiple genes (39).
The first GWAS of CAO in 238 pediatric brain tumor patients identified an association with a genetic variant in ACYP2 (rs1872328, hazard ratio (HR)=4.5, 95% CI 2.63–7.69, p=3.9 × 10−8), and results were replicated in a second cohort of 68 pediatric patients (40). Further, increased ACYP2 expression highly correlated with cisplatin sensitivity in lymphoblastoid cell lines in vitro (p=6.5 × 10−5), but the genotype at the SNP rs1872328 position was not associated with cisplatin sensitivity in vitro, nor was it related to expression of ACYP2 and other genes 300 kb within this index SNP. Nevertheless, three studies have replicated this association with cisplatin-induced hearing loss in 156 osteosarcoma patients (41), 149 pediatric cancer patients (42) and 229 testicular cancer patients (43). ACYP2 encodes for an enzyme that catalyzes phosphate hydrolysis in membrane pumps, most notably the Ca2+/Mg2+ ATPase from the sarcoplasmic reticulum of skeletal muscle (44). Importantly, ACYP2 is expressed in the cochlea for ATP-dependent Ca2+ signaling that is critical for hair cell development and has been directly implicated in hair cell damage (45, 46), providing a rationale for its association with CAO.
The first GWAS of CAO in adult-onset cancer in 511 testicular cancer survivors identified a genome-wide significant SNP (rs62283056; p=1.4 × 10−8) in the first intron of Mendelian deafness gene WFS1 (wolframin ER transmembrane glycoprotein) (47). This finding was replicated in a Canadian study of 229 testicular cancer patients when evaluating the same phenotype, i.e., the geometric mean of hearing thresholds at 4–12 kHz (p=5.67 × 10−3, OR = 3.2), although it was not replicated using a phenotype of audiologist-defined hearing loss (43). This difference in statistical significance based on the definition of hearing loss is important to note because it indicates that the same genotype can having varying levels of statistical association with a phenotype of interest based on how the trait is defined by the investigators. Nevertheless, the SNP is an eQTL for WFS1 based on the Genotype-Tissue Expression (GTEx) project, with the risk (and minor) allele being associated with lower gene expression in several human tissues. Using an independent cohort from BioVU (a large, de-identified DNA biobank linked to a clinical data warehouse), WFS1 was associated with ICD-9 derived codes for hearing loss. In a meta-analysis of this GWAS and the GWAS that initially identified ACYP2 (40), rs62283056 in WFS1 remained the top signal. However, the meta-analysis did not support the ACYP2 variant rs1872328 as being significantly associated with adult-onset cancer CAO. Thus, functional validation studies of both WFS1 and ACYP2 using experimental methods are warranted.
Neurotoxicity
Cisplatin-based therapy is associated with peripheral neuropathy (manifested as tingling, numbness, weakness, or burning pain) that occurs in about 36–38% of patients (48, 49). Predominantly affecting sensory nerves, cisplatin-induced peripheral neuropathy (CisIPN) has been described as a dose-dependent phenomenon, as most cases do not occur until a threshold cumulative dose of 300 mg/m2 is reached (50), and almost all patients receiving a cumulative dose of 500–600 mg/m2 have objective evidence of nerve damage (51). In addition, patients may also experience coasting, which is a persistent worsening of symptoms several months after treatment completion (52). The severity of neurotoxicity may also be correlated with serum platinum levels, as shown by Sprauten et al. (53) who demonstrated that long term serum platinum levels are significantly associated with the severity of neurotoxicity 5 to 20 years after cisplatin treatment, and the relationship remains significant after adjustment for initial cisplatin dose.
Depending on neuropathy severity, patients can experience a significant reduction in overall quality of life, with a strong negative correlation between CisIPN and self-reported health (OR = 0.56; p=2.6 × 10−9) demonstrated in 680 cisplatin-treated testicular cancer survivors (48). There was also a strong negative correlation of CisIPN with physical activity (OR=0.72; p=0.02), and a strong positive correlation with weight gain since therapy (OR per Δkg/m2=1.05; p=0.004). Since this investigation was cross-sectional, causal inferences could not be made, and a longitudinal design would help determine whether neuropathy deters from physical activity, and thus promotes weight gain. In a multivariate model, variables significantly related to cisplatin-induced neuropathy included age at diagnosis (OR/yr=1.06, p=2 × 10−9), smoking (OR=1.54, p=0.004), excess drinking (OR=1.83, p=0.007), and hypertension (OR=1.61, p=0.03) (Figure 1). Currently, there are no effective treatments to prevent or reduce the severity of neuropathy induced by cisplatin or other antineoplastic agents, but duloxetine is moderately recommended for associated pain (54).
Within the few studies that have investigated genetic susceptibility to long-term cisplatin neurotoxicity, there have been several reported associations involving glutathione-S-transferases (GSTs), in particular, GSTP1 (55, 56) as well as XPC and ERCC1 (57). None of these were found to be significant when evaluated through a GWAS ((48); Supplementary Table 2).
In contrast to hearing loss (a quantitative phenotype), peripheral neuropathy is either physician-graded or patient-reported using questionnaires. Using the validated EORTC QLQ-CIPN20 questionnaire with 680 cisplatin-treated testicular cancer survivors, there were no genome-wide significant associations (48). However, using PrediXcan (58), a gene-based computational method that uses reference transcriptome (genotype-gene expression) data to generate models to ‘impute’ gene expression levels from genotype data and associate the predicted gene expression with phenotypes of interest, lower expression of RPRD1B was identified as significantly associated with CisIPN. An evaluation of 18,620 genotyped patients from BioVU demonstrated a relationship between RPRD1B gene expression and polyneuropathy due to drugs. RPRD1B is of particular interest because defects in its expression or knockdown have been shown to inhibit DNA repair mechanisms that resolve cisplatin-induced lesions (59). Further, RPRD1B knockdown in human breast carcinoma cells potentiates cisplatin sensitivity (60). As illustrated in this example, the advantage of PrediXcan analysis is that it substantially reduces multiple corrections in comparison to SNP-based GWAS, while also providing a directionality of effect between gene expression and phenotype.
Nephrotoxicity
The kidneys are particularly susceptible to toxicity since cisplatin is eliminated predominantly through renal clearance (11). Consequently, impaired renal function is found in approximately 25–35% of patients after a single cisplatin dose (61). In spite of preventive measures (i.e., intense intravenous hydration during cisplatin administration), successive treatment courses can potentiate a progressive nephrotoxicity that can lead to permanent damage (12). Further, cisplatin induces acute kidney injury in approximately 20–30% of patients, while hypomagnesemia manifests in 40–100% (12).
In addition to known risk factors for cisplatin-induced renal toxicity such as older age, comorbidities, low albumin levels, preexisting kidney disease, and concurrent use of nephrotoxic medications (Figure 1) (12), an increasing number of studies have investigated the importance of genetic contributions, albeit only in candidate gene studies (Supplementary Table 2). A SNP in ERCC1 (8092C>A/rs3212986) has been shown to be significantly associated with a reduced risk of cisplatin-induced nephrotoxicity in two separate candidate gene studies (62, 63), as well as rs1051740 in EPHX1 (64). In addition, two cation transporters vital for cisplatin renal uptake (OCT2/SLC22A2 and CTR1/SLC31A1; Table 1) have SNPs associated with renoprotection and maintenance of estimated glomular filtration rate (rs596881 (OCT2); rs12686377 and rs7851395 (CTR1; (65).
Myelosuppression
As do many antineoplastic agents, cisplatin can profoundly impact hematopoiesis. Myelosuppression occurs in 25–30% of patients, particularly when cisplatin doses exceed 50 mg/m2 (66), and severe myelosuppression develops in approximately 5–6% (13). Cisplatin-based therapy often has a disproportionate effect on erythrocyte production in comparison with other blood cells, resulting in a cumulative, clinically significant anemia (67). The increase in oxidative stress that induces other cisplatin-induced adverse sequelae (Table 1) also appears to contribute to bone marrow toxicity (68). While acute myelosuppression is an immediate clinical concern, many patients receiving chemotherapy and/or radiotherapy also develop residual bone marrow injury, as evidenced by a sustained reduction in hematopoietic stem cell reserves that can potentiate long-term hematological complications (69, 70). Indeed, two studies have shown statistically significant associations between cumulative cisplatin dose and the subsequent development of leukemia (71, 72). In addition, patients who have a poor performance status and have had prior chemotherapy exposure are at increased risk of hematological complications (Figure 1) (73, 74).
Although the analysis of genetic contributions to cisplatin-induced myelosuppression has been limited, a GWAS was undertaken in non-small cell lung carcinoma (NSCLC) patients of Han Chinese descent. Two SNPs (rs13014982 and rs9909179) exhibited associations with myelosuppression in the discovery and replication sets, but did not reach genome-wide significance in the discovery set. Nevertheless, these SNPs retained plausible associations in the subsequent meta-analysis of both patient cohorts (rs13014982: p=1.36 × 10−5; rs9909179: p=0.001 (75). rs13014982 is located in a gene desert at 2q24.3 (within 500 kb of FIGN), limiting its potential genetic significance, but rs9909179 was determined via GTEx to be a plausible eQTL for HS3ST3A1 in blood (p=0.03), an enzyme involved in heparan sulfate biosynthesis, which may be important in hematopoiesis (76).
Nausea/Emesis
Nausea and emesis are frequently cited as among the most feared complications of chemotherapy (14, 77). Although the development of antiemetics has reduced the incidence of these toxicities, many patients still experience either acute (within 24 hours), or delayed nausea and/or emesis. This is epitomized by cisplatin treatment, as doses of 50 mg/m2 or more induce acute nausea and vomiting in > 90% of patients not administered antiemetic prophylaxis (14), with 60–90% experiencing delayed nausea/emesis (77). Women have a higher susceptibility to developing emesis following cisplatin treatment than men, as shown in two separate studies in NSCLC patients (78, 79). Other risk factors for chemotherapy-induced nausea and vomiting include younger age, history of low alcohol intake, experience of emesis during pregnancy, impaired quality of life, and prior chemotherapy exposure (Figure 1) (77, 80).
Variation in the susceptibility of patients experiencing emesis following cisplatin administration based on genetic ancestry has been noted by Khrunin et al. (81) in which Yakuts (North Asians) had a borderline statistically significant difference in developing severe emesis compared to Russians of Eastern European descent (38% vs 25%; p=0.061). Importantly, severe emesis in Yakuts was independently associated with two polymorphisms in the CYP2E1 gene, but was only associated with the GSTT1-null genotype in Eastern European Russians.
Trends in Relevant Pharmacogenomic Studies
Although analyses of genetic predisposition to cisplatin-induced toxicities are relatively novel with most studies published within the last decade, several important trends have emerged that may guide future investigations. Of the 36 genetic studies analyzed in the present review, an overwhelming majority investigated ototoxicity (n=26), with neurotoxicity the next most common toxicity (n=5). Thus, ototoxicity was the only toxicity to have genetic associations investigated for validity through multiple independent replication studies, and many analyses failed to confirm previously identified SNPs (Supplementary Table 1). Although ototoxicity is a prominent cisplatin-related adverse event, other toxicities also occur in a relatively high proportion of patients (Table 1) and can result in the administration of doses that are sub-optimal for antineoplastic efficacy. Further, most investigations have relied on a candidate gene approach, and only four GWAS have been performed (40, 47, 59, 75). GWAS have the potential to identify causal SNPs in genes agnostically, but require large cohorts of patients treated with the same regimen and uniformly phenotyped for toxicity.
In addition to the disproportionate number of studies that have used candidate gene approaches to probe cisplatin-induced toxicities, few investigations have evaluated these adverse sequelae in cohorts not predominantly/exclusively of European ancestry. This observation mirrors the lack of ancestral diversity represented in GWAS of chemotherapeutic toxicities despite known differences in allele frequencies and effect sizes among individuals of differing ancestries (82). One reason for this is that several GWAS were performed in testicular cancer survivors, a disease that disproportionately affects white males (83). As such, genetic variants that are associated with varying levels of cisplatin sensitivity in European-based studies may not be relevant in patients of other genetic ancestries, thereby promoting a gap in health disparities. Although these slight genetic variations may appear to be subtle nuances among heterogeneous patient populations, finding causal associations of adverse sequelae is a hallmark paradigm of precision medicine, and may eventually enable treatment regimens and doses to be tailored specifically to the individual patient to maximize treatment efficacy while limiting toxicities.
Future Directions
Based on the analysis of previous pharmacogenomic studies of cisplatin-induced toxicities, it is apparent that investigators should expand the search of relevant genetic variants beyond ototoxicity and patient populations of European ancestry. Further, the lack of reproducibility found in candidate gene studies of ototoxicity underscores the importance of genome-wide studies with large cohorts of uniformly treated patients to comprehensively examine the entire genome for potential associations with other cisplatin-induced toxicities. Regardless of the in silico approach used to identify genetic variants of potential interest, it is paramount that associations are functionally validated in vitro and/or in vivo (84). Through this critical step, the biological significance of the identified genetic variants can be definitively ascertained. Moreover, physiological validation of the genetic architecture underlying different cisplatin-induced toxicities may potentiate the discovery of novel drug targets that can mitigate the adverse effects, thereby reducing its overall morbidity. These mechanistically based therapeutic strategies may ultimately be leveraged to identify novel drug targets that can reduce selected toxicities without inhibiting antineoplastic efficacy.
The Platinum Study examines the long-term effects of cisplatin treatment in cured testicular cancer survivors to comprehensively evaluate the toxicities associated with cisplatin (1). Since testicular cancer generally affects men of European descent, it is inherently limited in its ability to examine cisplatin toxicities in different genetic ancestries. Current plausible alternative cohorts include the St. Jude LIFE Study and the multi-institutional Childhood Cancer Survivor Study, initiatives designed to examine the long-term effects of radiotherapy and chemotherapy in pediatric cancer survivors (85, 86). However, in both endeavors, only a small subset of patients received cisplatin. Therefore, the development of patient cohorts of varying genetic backgrounds and cancer diagnoses is required to fully characterize the genetic architecture of cisplatin-induced toxicities.
Once viable predictive biomarkers of cisplatin-induced toxicities have been established, additional preclinical and clinical studies will be required to determine how to optimally apply this information to minimize cisplatin-induced toxicities while maintaining therapeutic efficacy. In addition to establishing a priori which patients may likely require either dose reductions or alternative therapy, these patients may also serve as an ideal cohort to examine novel platinum analogs with a reduced toxicity profile (87), provided comparable antineoplastic efficacy has been established.
Conclusion
Cisplatin-induced toxicities are numerous and high in frequency, making identification of patients likely to experience adverse events critical for optimizing clinical care. Understanding non-genetic risk factors for off-target toxicities is informative for physicians because this knowledge can be used to educate patients on their likelihood of experiencing adverse events during and after cisplatin-based therapy. Since cisplatin is a highly used antineoplastic agent, such information is relevant for the treatment of multiple adult-onset and pediatric malignancies. Further, it is important to emphasize that a growing number of patients treated with cisplatin-based chemotherapy are being cured of their disease (i.e. hepatoblastoma, HPV+ oropharyngeal cancer, medulloblastoma, osteosarcoma, and testicular cancer). Cisplatin is also finding use in the neoadjuvant setting for multiple tumor types, particularly bladder cancer (88, 89), indicating that there will be an increasing number of patients exposed to cisplatin who will live many years after their initial cancer diagnosis. Consequently, understanding the underlying basis for cisplatin toxicity has an emerging role in the management of this growing patient population. Given that many patients’ tumors and germline DNA are now being sequenced, understanding genetic predisposition to cisplatin toxicity will provide a basis for a personalized medicine approach to managing its toxicity.
Although cisplatin-induced toxicities have been well-characterized, the importance of genetic variation in the occurrence of adverse reactions is only now becoming appreciated through modern pharmacogenomic approaches. Nevertheless, a diversification of studies in regards to toxicity types and patient cohorts is needed, with greater emphasis on implementing genome-wide analyses followed by independent replication and functional validation. Only then can these associations be considered plausible biomarkers of cisplatin-induced toxicity that can be harnessed to tailor treatment regimens to individual patients.
Supplementary Material
Acknowledgments
This review was supported in part by National Cancer Institute grants RO1CA036401 (subaward to ME Dolan) and CA213466 (subaward to ME Dolan), R01 CA 157823 (LB Travis; subaward to ME Dolan), University of Chicago Women’s Board (ME Dolan), and the University of Chicago Comprehensive Cancer Center P30 CA14599 (M Le Beau).
Abbreviations:
- CAO:
cisplatin-associated ototoxicity
- eQTL:
expression quantitative trait loci
- GTEx:
Genotype-Tissue Expression
- GWAS:
genome-wide association study
- NSCLC:
non-small cell lung carcinoma
- SNP:
single nucleotide polymorphism
- STS:
sodium thiosulfate
Footnotes
Conflict of interest/Disclosure Statement: No potential conflicts of interest were disclosed.
References
- 1.Travis LB, Fossa SD, Sesso HD, Frisina RD, Herrmann DN, Beard CJ, et al. Chemotherapy-induced peripheral neurotoxicity and ototoxicity: new paradigms for translational genomics. J Natl Cancer Inst. 2014;106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartmann JT, Lipp HP. Toxicity of platinum compounds. Expert Opin Pharmacother. 2003;4:889–901. [DOI] [PubMed] [Google Scholar]
- 3.Ruggiero A, Trombatore G, Triarico S, Arena R, Ferrara P, Scalzone M, et al. Platinum compounds in children with cancer: toxicity and clinical management. Anticancer Drugs. 2013;24:1007–19. [DOI] [PubMed] [Google Scholar]
- 4.Rybak LP. Mechanisms of cisplatin ototoxicity and progress in otoprotection. Curr Opin Otolaryngol Head Neck Surg. 2007;15:364–9. [DOI] [PubMed] [Google Scholar]
- 5.Rybak LP, Mukherjea D, Jajoo S, Ramkumar V. Cisplatin ototoxicity and protection: clinical and experimental studies. Tohoku J Exp Med. 2009;219:177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callejo A, Sedo-Cabezon L, Juan ID, Llorens J. Cisplatin-Induced Ototoxicity: Effects, Mechanisms and Protection Strategies. Toxics. 2015;3:268–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paken J, Govender CD, Pillay M, Sewram V. Cisplatin-Associated Ototoxicity: A Review for the Health Professional. J Toxicol. 2016;2016:1809394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avan A, Postma TJ, Ceresa C, Avan A, Cavaletti G, Giovannetti E, et al. Platinum-induced neurotoxicity and preventive strategies: past, present, and future. Oncologist. 2015;20:411–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanat O, Ertas H, Caner B. Platinum-induced neurotoxicity: A review of possible mechanisms. World J Clin Oncol. 2017;8:329–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of Cisplatin nephrotoxicity. Toxins (Basel). 2010;2:2490–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.dos Santos NA, Carvalho Rodrigues MA, Martins NM, dos Santos AC. Cisplatin-induced nephrotoxicity and targets of nephroprotection: an update. Arch Toxicol. 2012;86:1233–50. [DOI] [PubMed] [Google Scholar]
- 12.Manohar S, Leung N. Cisplatin nephrotoxicity: a review of the literature. J Nephrol. 2018;31:15–25. [DOI] [PubMed] [Google Scholar]
- 13.Go RS, Adjei AA. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol. 1999;17:409–22. [DOI] [PubMed] [Google Scholar]
- 14.Schnell FM. Chemotherapy-induced nausea and vomiting: the importance of acute antiemetic control. Oncologist. 2003;8:187–98. [DOI] [PubMed] [Google Scholar]
- 15.Ranganath P, Einhorn L, Albany C. Management of Chemotherapy Induced Nausea and Vomiting in Patients on Multiday Cisplatin Based Combination Chemotherapy. Biomed Res Int. 2015;2015:943618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangoni AA, Jackson SH. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamasoba T, Lin FR, Someya S, Kashio A, Sakamoto T, Kondo K. Current concepts in age-related hearing loss: epidemiology and mechanistic pathways. Hear Res. 2013;303:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatt JM, Lin HW, Bhattacharyya N. Prevalence, Severity, Exposures, and Treatment Patterns of Tinnitus in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142:959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mold JW, Vesely SK, Keyl BA, Schenk JB, Roberts M. The prevalence, predictors, and consequences of peripheral sensory neuropathy in older patients. J Am Board Fam Pract. 2004;17:309–18. [DOI] [PubMed] [Google Scholar]
- 20.Popescu S, Timar B, Baderca F, Simu M, Diaconu L, Velea I, et al. Age as an independent factor for the development of neuropathy in diabetic patients. Clin Interv Aging. 2016;11:313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knight KR, Chen L, Freyer D, Aplenc R, Bancroft M, Bliss B, et al. Group-Wide, Prospective Study of Ototoxicity Assessment in Children Receiving Cisplatin Chemotherapy (ACCL05C1): A Report From the Children’s Oncology Group. J Clin Oncol. 2017;35:440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukherjea D, Rybak LP. Pharmacogenomics of cisplatin-induced ototoxicity. Pharmacogenomics. 2011;12:1039–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frisina RD, Wheeler HE, Fossa SD, Kerns SL, Fung C, Sesso HD, et al. Comprehensive Audiometric Analysis of Hearing Impairment and Tinnitus After Cisplatin-Based Chemotherapy in Survivors of Adult-Onset Cancer. J Clin Oncol. 2016;34:2712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vona B, Nanda I, Shehata-Dieler W, Haaf T. Genetics of Tinnitus: Still in its Infancy. Front Neurosci. 2017;11:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brydoy M, Oldenburg J, Klepp O, Bremnes RM, Wist EA, Wentzel-Larsen T, et al. Observational study of prevalence of long-term Raynaud-like phenomena and neurological side effects in testicular cancer survivors. J Natl Cancer Inst. 2009;101:1682–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miaskowski C, Paul SM, Mastick J, Schumacher M, Conley YP, Smoot B, et al. Hearing loss and tinnitus in survivors with chemotherapy-induced neuropathy. Eur J Oncol Nurs. 2018;32:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miaskowski C, Paul SM, Mastick J, Abrams G, Topp K, Smoot B, et al. Associations Between Perceived Stress and Chemotherapy-Induced Peripheral Neuropathy and Otoxicity in Adult Cancer Survivors. J Pain Symptom Manage. 2018;56:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bokemeyer C, Berger CC, Hartmann JT, Kollmannsberger C, Schmoll HJ, Kuczyk MA, et al. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer. 1998;77:1355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yancey A, Harris MS, Egbelakin A, Gilbert J, Pisoni DB, Renbarger J. Risk factors for cisplatin-associated ototoxicity in pediatric oncology patients. Pediatr Blood Cancer. 2012;59:144–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brock PR, Maibach R, Childs M, Rajput K, Roebuck D, Sullivan MJ, et al. Sodium Thiosulfate for Protection from Cisplatin-Induced Hearing Loss. N Engl J Med. 2018;378:2376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.FDA Center for Drug Evaluation and Research. FDA Label for Sodium Thiosulfate Injection. https://wwwaccessdatafdagov/drugsatfda_docs/nda/2012/203923Orig1s000Lblpdf. 4/19/2018.
- 32.Ross CJ, Katzov-Eckert H, Dube MP, Brooks B, Rassekh SR, Barhdadi A, et al. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat Genet. 2009;41:1345–9. [DOI] [PubMed] [Google Scholar]
- 33.Yang JJ, Lim JY, Huang J, Bass J, Wu J, Wang C, et al. The role of inherited TPMT and COMT genetic variation in cisplatin-induced ototoxicity in children with cancer. Clin Pharmacol Ther. 2013;94:252–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pussegoda K, Ross CJ, Visscher H, Yazdanpanah M, Brooks B, Rassekh SR, et al. Replication of TPMT and ABCC3 genetic variants highly associated with cisplatin-induced hearing loss in children. Clin Pharmacol Ther. 2013;94:243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hagleitner MM, Coenen MJ, Patino-Garcia A, de Bont ES, Gonzalez-Neira A, Vos HI, et al. Influence of genetic variants in TPMT and COMT associated with cisplatin induced hearing loss in patients with cancer: two new cohorts and a meta-analysis reveal significant heterogeneity between cohorts. PLoS One. 2014;9:e115869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ratain MJ, Cox NJ, Henderson TO. Challenges in interpreting the evidence for genetic predictors of ototoxicity. Clin Pharmacol Ther. 2013;94:631–5. [DOI] [PubMed] [Google Scholar]
- 37.Nicolae DL, Gamazon E, Zhang W, Duan S, Dolan ME, Cox NJ. Trait-associated SNPs are more likely to be eQTLs: annotation to enhance discovery from GWAS. PLoS Genet. 2010;6:e1000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albert FW, Kruglyak L. The role of regulatory variation in complex traits and disease. Nat Rev Genet. 2015;16:197–212. [DOI] [PubMed] [Google Scholar]
- 39.Gamazon ER, Huang RS, Cox NJ, Dolan ME. Chemotherapeutic drug susceptibility associated SNPs are enriched in expression quantitative trait loci. Proc Natl Acad Sci U S A. 2010;107:9287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu H, Robinson GW, Huang J, Lim JY, Zhang H, Bass JK, et al. Common variants in ACYP2 influence susceptibility to cisplatin-induced hearing loss. Nat Genet. 2015;47:263–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vos HI, Guchelaar HJ, Gelderblom H, de Bont ES, Kremer LC, Naber AM, et al. Replication of a genetic variant in ACYP2 associated with cisplatin-induced hearing loss in patients with osteosarcoma. Pharmacogenet Genomics. 2016;26:243–7. [DOI] [PubMed] [Google Scholar]
- 42.Thiesen S, Yin P, Jorgensen AL, Zhang JE, Manzo V, McEvoy L, et al. TPMT, COMT and ACYP2 genetic variants in paediatric cancer patients with cisplatin-induced ototoxicity. Pharmacogenet Genomics. 2017;27:213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drogemoller BI, Brooks B, Critchley C, Monzon JG, Wright GEB, Liu G, et al. Further Investigation of the Role of ACYP2 and WFS1 Pharmacogenomic Variants in the Development of Cisplatin-Induced Ototoxicity in Testicular Cancer Patients. Clin Cancer Res. 2018;24:1866–71. [DOI] [PubMed] [Google Scholar]
- 44.Zhang F, Zhang Y, Deng Z, Xu P, Zhang X, Jin T, et al. Genetic variants in the acylphosphatase 2 gene and the risk of breast cancer in a Han Chinese population. Oncotarget. 2016;7:86704–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuchs PA. A ‘calcium capacitor’ shapes cholinergic inhibition of cochlear hair cells. J Physiol. 2014;592:3393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas AJ, Hailey DW, Stawicki TM, Wu P, Coffin AB, Rubel EW, et al. Functional mechanotransduction is required for cisplatin-induced hair cell death in the zebrafish lateral line. J Neurosci. 2013;33:4405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wheeler HE, Gamazon ER, Frisina RD, Perez-Cervantes C, El Charif O, Mapes B, et al. Variants in WFS1 and Other Mendelian Deafness Genes Are Associated with Cisplatin-Associated Ototoxicity. Clin Cancer Res. 2017;23:3325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dolan ME, El Charif O, Wheeler HE, Gamazon ER, Ardeshir-Rouhani-Fard S, Monahan P, et al. Clinical and Genome-Wide Analysis of Cisplatin-Induced Peripheral Neuropathy in Survivors of Adult-Onset Cancer. Clin Cancer Res. 2017;23:5757–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 2014;155:2461–70. [DOI] [PubMed] [Google Scholar]
- 50.Amptoulach S, Tsavaris N. Neurotoxicity caused by the treatment with platinum analogues. Chemother Res Pract. 2011;2011:843019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jongen JL, Broijl A, Sonneveld P. Chemotherapy-induced peripheral neuropathies in hematological malignancies. J Neurooncol. 2015;121:229–37. [DOI] [PubMed] [Google Scholar]
- 52.Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63:419–37. [DOI] [PubMed] [Google Scholar]
- 53.Sprauten M, Darrah TH, Peterson DR, Campbell ME, Hannigan RE, Cvancarova M, et al. Impact of long-term serum platinum concentrations on neuro- and ototoxicity in Cisplatin-treated survivors of testicular cancer. J Clin Oncol. 2012;30:300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32:1941–67. [DOI] [PubMed] [Google Scholar]
- 55.Oldenburg J, Kraggerud SM, Brydoy M, Cvancarova M, Lothe RA, Fossa SD. Association between long-term neuro-toxicities in testicular cancer survivors and polymorphisms in glutathione-s-transferase-P1 and -M1, a retrospective cross sectional study. J Transl Med. 2007;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goekkurt E, Al-Batran SE, Hartmann JT, Mogck U, Schuch G, Kramer M, et al. Pharmacogenetic analyses of a phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil and leucovorin plus either oxaliplatin or cisplatin: a study of the arbeitsgemeinschaft internistische onkologie. J Clin Oncol. 2009;27:2863–73. [DOI] [PubMed] [Google Scholar]
- 57.Lamba JK, Fridley BL, Ghosh TM, Yu Q, Mehta G, Gupta P. Genetic variation in platinating agent and taxane pathway genes as predictors of outcome and toxicity in advanced non-small-cell lung cancer. Pharmacogenomics. 2014;15:1565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gamazon ER, Wheeler HE, Shah KP, Mozaffari SV, Aquino-Michaels K, Carroll RJ, et al. A gene-based association method for mapping traits using reference transcriptome data. Nat Genet. 2015;47:1091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sawant A, Kothandapani A, Zhitkovich A, Sobol RW, Patrick SM. Role of mismatch repair proteins in the processing of cisplatin interstrand cross-links. DNA Repair (Amst). 2015;35:126–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morales JC, Richard P, Rommel A, Fattah FJ, Motea EA, Patidar PL, et al. Kub5-Hera, the human Rtt103 homolog, plays dual functional roles in transcription termination and DNA repair. Nucleic Acids Res. 2014;42:4996–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Han X, Yue J, Chesney RW. Functional TauT protects against acute kidney injury. J Am Soc Nephrol. 2009;20:1323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khrunin AV, Moisseev A, Gorbunova V, Limborska S. Genetic polymorphisms and the efficacy and toxicity of cisplatin-based chemotherapy in ovarian cancer patients. Pharmacogenomics J. 2010;10:54–61. [DOI] [PubMed] [Google Scholar]
- 63.Tzvetkov MV, Behrens G, O’Brien VP, Hohloch K, Brockmoller J, Benohr P. Pharmacogenetic analyses of cisplatin-induced nephrotoxicity indicate a renoprotective effect of ERCC1 polymorphisms. Pharmacogenomics. 2011;12:1417–27. [DOI] [PubMed] [Google Scholar]
- 64.Khrunin AV, Khokhrin DV, Moisseev AA, Gorbunova VA, Limborska SA. Pharmacogenomic assessment of cisplatin-based chemotherapy outcomes in ovarian cancer. Pharmacogenomics. 2014;15:329–37. [DOI] [PubMed] [Google Scholar]
- 65.Chang C, Hu Y, Hogan SL, Mercke N, Gomez M, O’Bryant C, et al. Pharmacogenomic Variants May Influence the Urinary Excretion of Novel Kidney Injury Biomarkers in Patients Receiving Cisplatin. Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prestayko AW, D’Aoust JC, Issell BF, Crooke ST. Cisplatin (cis-diamminedichloroplatinum II). Cancer Treat Rev. 1979;6:17–39. [DOI] [PubMed] [Google Scholar]
- 67.Wood PA, Hrushesky WJ. Cisplatin-associated anemia: an erythropoietin deficiency syndrome. J Clin Invest. 1995;95:1650–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Basu A, Ghosh P, Bhattacharjee A, Patra AR, Bhattacharya S. Prevention of myelosuppression and genotoxicity induced by cisplatin in murine bone marrow cells: effect of an organovanadium compound vanadium(III)-l-cysteine. Mutagenesis. 2015;30:509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Y, Probin V, Zhou D. Cancer therapy-induced residual bone marrow injury-Mechanisms of induction and implication for therapy. Curr Cancer Ther Rev. 2006;2:271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Georgiou KR, Foster BK, Xian CJ. Damage and recovery of the bone marrow microenvironment induced by cancer chemotherapy - potential regulatory role of chemokine CXCL12/receptor CXCR4 signalling. Curr Mol Med. 2010;10:440–53. [DOI] [PubMed] [Google Scholar]
- 71.Travis LB, Holowaty EJ, Bergfeldt K, Lynch CF, Kohler BA, Wiklund T, et al. Risk of leukemia after platinum-based chemotherapy for ovarian cancer. N Engl J Med. 1999;340:351–7. [DOI] [PubMed] [Google Scholar]
- 72.Travis LB, Andersson M, Gospodarowicz M, van Leeuwen FE, Bergfeldt K, Lynch CF, et al. Treatment-associated leukemia following testicular cancer. J Natl Cancer Inst. 2000;92:1165–71. [DOI] [PubMed] [Google Scholar]
- 73.Ouyang Z, Peng D, Dhakal DP. Risk factors for hematological toxicity of chemotherapy for bone and soft tissue sarcoma. Oncol Lett. 2013;5:1736–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kogo M, Watahiki M, Sunaga T, Kaneko K, Yoneyama K, Imawari M, et al. Analysis of the risk factors for myelosuppression after chemoradiotherapy involving 5-fluorouracil and platinum for patients with esophageal cancer. Hepatogastroenterology. 2011;58:802–8. [PubMed] [Google Scholar]
- 75.Cao S, Wang S, Ma H, Tang S, Sun C, Dai J, et al. Genome-wide association study of myelosuppression in non-small-cell lung cancer patients with platinum-based chemotherapy. Pharmacogenomics J. 2016;16:41–6. [DOI] [PubMed] [Google Scholar]
- 76.Holley RJ, Pickford CE, Rushton G, Lacaud G, Gallagher JT, Kouskoff V, et al. Influencing hematopoietic differentiation of mouse embryonic stem cells using soluble heparin and heparan sulfate saccharides. J Biol Chem. 2011;286:6241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jordan K, Sippel C, Schmoll HJ. Guidelines for antiemetic treatment of chemotherapy-induced nausea and vomiting: past, present, and future recommendations. Oncologist. 2007;12:1143–50. [DOI] [PubMed] [Google Scholar]
- 78.Wheatley-Price P, Le Maitre A, Ding K, Leighl N, Hirsh V, Seymour L, et al. The influence of sex on efficacy, adverse events, quality of life, and delivery of treatment in National Cancer Institute of Canada Clinical Trials Group non-small cell lung cancer chemotherapy trials. J Thorac Oncol. 2010;5:640–8. [DOI] [PubMed] [Google Scholar]
- 79.Wheatley-Price P, Blackhall F, Lee SM, Ma C, Ashcroft L, Jitlal M, et al. The influence of sex and histology on outcomes in non-small-cell lung cancer: a pooled analysis of five randomized trials. Ann Oncol. 2010;21:2023–8. [DOI] [PubMed] [Google Scholar]
- 80.Sekine I, Segawa Y, Kubota K, Saeki T. Risk factors of chemotherapy-induced nausea and vomiting: index for personalized antiemetic prophylaxis. Cancer Sci. 2013;104:711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khrunin A, Ivanova F, Moisseev A, Khokhrin D, Sleptsova Y, Gorbunova V, et al. Pharmacogenomics of cisplatin-based chemotherapy in ovarian cancer patients of different ethnic origins. Pharmacogenomics. 2012;13:171–8. [DOI] [PubMed] [Google Scholar]
- 82.Mapes B, El Charif O, Al-Sawwaf S, Dolan ME. Genome-Wide Association Studies of Chemotherapeutic Toxicities: Genomics of Inequality. Clin Cancer Res. 2017;23:4010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ghazarian AA, Trabert B, Graubard BI, Schwartz SM, Altekruse SF, McGlynn KA. Incidence of testicular germ cell tumors among US men by census region. Cancer. 2015;121:4181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gallagher MD, Chen-Plotkin AS. The Post-GWAS Era: From Association to Function. Am J Hum Genet. 2018;102:717–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krull KR, Brinkman TM, Li C, Armstrong GT, Ness KK, Srivastava DK, et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: a report from the St Jude lifetime cohort study. J Clin Oncol. 2013;31:4407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robison LL, Armstrong GT, Boice JD, Chow EJ, Davies SM, Donaldson SS, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sarkar A Novel platinum compounds and nanoparticles as anticancer agents. Pharm Pat Anal. 2018;7:33–46. [DOI] [PubMed] [Google Scholar]
- 88.Dash A, Pettus JAt, Herr HW, Bochner BH, G Dalbagni, Donat SM, et al. A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: a retrospective experience. Cancer. 2008;113:2471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yin M, Joshi M, Meijer RP, Glantz M, Holder S, Harvey HA, et al. Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Two-Step Meta-Analysis. Oncologist. 2016;21:708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clerici WJ, DiMartino DL, Prasad MR. Direct effects of reactive oxygen species on cochlear outer hair cell shape in vitro. Hear Res. 1995;84:30–40. [DOI] [PubMed] [Google Scholar]
- 91.Kopke RD, Liu W, Gabaizadeh R, Jacono A, Feghali J, Spray D, et al. Use of organotypic cultures of Corti’s organ to study the protective effects of antioxidant molecules on cisplatin-induced damage of auditory hair cells. Am J Otol. 1997;18:559–71. [PubMed] [Google Scholar]
- 92.Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–72. [DOI] [PubMed] [Google Scholar]
- 93.Liang F, Schulte BA, Qu C, Hu W, Shen Z. Inhibition of the calcium- and voltage-dependent big conductance potassium channel ameliorates cisplatin-induced apoptosis in spiral ligament fibrocytes of the cochlea. Neuroscience. 2005;135:263–71. [DOI] [PubMed] [Google Scholar]
- 94.Breglio AM, Rusheen AE, Shide ED, Fernandez KA, Spielbauer KK, McLachlin KM, et al. Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat Commun. 2017;8:1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McDonald ES, Windebank AJ. Cisplatin-induced apoptosis of DRG neurons involves bax redistribution and cytochrome c release but not fas receptor signaling. Neurobiol Dis. 2002;9:220–33. [DOI] [PubMed] [Google Scholar]
- 96.McDonald ES, Randon KR, Knight A, Windebank AJ. Cisplatin preferentially binds to DNA in dorsal root ganglion neurons in vitro and in vivo: a potential mechanism for neurotoxicity. Neurobiol Dis. 2005;18:305–13. [DOI] [PubMed] [Google Scholar]
- 97.Pabla N, Murphy RF, Liu K, Dong Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am J Physiol Renal Physiol. 2009;296:F505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sprowl JA, Lancaster CS, Pabla N, Hermann E, Kosloske AM, Gibson AA, et al. Cisplatin-induced renal injury is independently mediated by OCT2 and p53. Clin Cancer Res. 2014;20:4026–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


