Summary:
In August 2017, the U.S. Food and Drug Administration (FDA) took historic action in granting the first approval of gene therapy to tisagenlecleucel. This landmark step brought CAR T-cell therapy to the commercial space and heralded a new era in managing refractory B-cell malignancies and FDA oversight of gene-modified therapies.
In this issue of Clinical Cancer Research, O’Leary and colleagues discuss clinical considerations leading the U.S. Food and Drug Administration (FDA) to grant regular approval to tisagenlecleucel (Kymriah®, Novartis), a form of CD19-targeted chimeric antigen receptor-modified (CAR) T-cell therapy, for the treatment of children and young adults (CAYA, age ≤25) with B-cell acute lymphoblastic leukemia (B-ALL) that is refractory or in second relapse or beyond.(1) This action marked the first approval of gene-modified cell therapy in the United States and established tisagenlecleucel as first finisher in the race of multiple CAR T-cell products to the commercial space.
Historically, CAYA with relapsed or refractory (R/R) B-ALL following multiple lines of treatment have exhibited poor rates of response to salvage therapy and dismal long-term survival outcomes. Therapy with the CD19-directed bispecific T-cell engager blinatumomab leads to complete response (CR) or CR with incomplete hematologic recovery (CRi) in only a minority of pediatric patients with multiply relapsed or chemotherapy-refractory B-ALL, and responses are often poorly durable.(2) CD19-targeted CAR T-cells, in contrast, couple the specificity of a monoclonal antibody with the effector functions and proliferative capacity of living autologous T-cells. Several single-institution studies investigating CAR T-cell therapy, including tisagenlecleucel (formerly CTL019), in pediatric and adult patients with R/R B-ALL have observed extraordinary rates of CR, frequently minimal residual disease (MRD)-negative, though have additionally described high rates of cytokine release syndrome (CRS) in the setting of brisk CAR T-cell expansion, as well as a spectrum of occasionally severe but generally reversible neurologic toxicities.
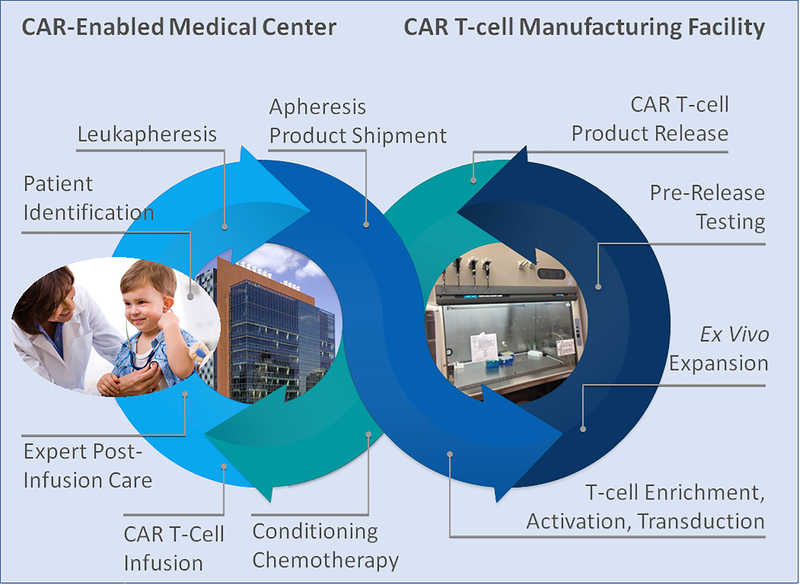
The phase 2 ELIANA trial was a global, multicenter, single-arm study investigating conditioning chemotherapy followed by a single dose of tisagenlecleucel in patients ages 3–21 with R/R B-ALL.(3) In contrast to pilot trials for which cellular products were manufactured locally, in ELIANA, autologous peripheral blood mononuclear cells were collected locally by leukapheresis from enrolled patients and shipped to a manufacturing facility. Apheresis products were then enriched for T-cells and activated with anti-CD3/CD28 antibody-coated beads, transduced with a lentiviral vector encoding the anti-CD19 CAR (itself containing the antigen-specific single-chain fragment variable and cytoplasmic signaling domains of the T-cell receptor [CD3ζ] and a T-cell costimulatory receptor [4–1BB, CD137]), expanded ex vivo, and formulated into a cryopreserved product, which then underwent pre-release evaluation prior to delivery back to the clinical center (Figure 1).
Figure 1:
Workflow for tisagenlecleucel and other CAR T-cell therapies becoming commercially available. Safe and successful delivery of tisagenlecleucel therapy requires considerable institutional engagement (skilled intake team, leukapheresis team, appropriately trained nursing and medical staff, pharmacy staff, and reporting/regulatory compliance), manufacturing facilities equipped to meet commercial product needs, and continued, conscientious FDA oversight.
In evaluating the efficacy of tisagenlecleucel, the FDA considered the patient population planned to receive tisagenlecleucel from the U.S. manufacturing site (n=78), those actually treated with tisagenlecleucel (n=63), and the proportion of patients achieving CR (excluding CRi) and MRD-negativity (40/63, 63%). While median duration of follow-up was quite short (4.8 months, range 1.2–14.1 months), median duration of CR was not reached. These unprecedented rates of deep response in patients with chemotherapy-refractory B-ALL were considered a suitable surrogate of long-term clinical outcomes to determine efficacy, even within the context of a single-arm trial, and furthermore were considered sufficiently compelling evidence of clinical benefit to justify regular rather than accelerated approval (where the latter would require further study to confirm clinical benefit). Of note, the FDA does not consider CRi an established surrogate of clinical benefit. As the composite of CR + CRi (or MRD-negative CR + CRi) is increasingly used as an endpoint in trials of novel therapeutics for B-ALL, the field would benefit from aggregating clinical data to demonstrate whether CRi and MRD-negative CRi are reliable surrogate markers of clinical benefit. More broadly, standards for approval will likely rise as blinatumomab, inotuzumab, and tisagenlecleucel supplant cytotoxic therapy in management of CAYA with high-risk R/R B-ALL.
Tisagenlecleucel’s approval is further notable in light of its toxicity profile; 73% of patients on ELIANA experienced a grade 3–4 tisagenlecleucel-associated event, with 47% of patients developing grade 3–4 cytokine release syndrome (CRS) and requiring intensive care unit (ICU) admission for median 7 days, and 37% requiring treatment with the interleukin-6 receptor antagonist tocilizumab. Additionally, 40% of patients exhibited neurologic toxicity.(3) Perhaps in part due to the expertise of the enrolling centers, only one patient died of non-relapse mortality within 30 days of infusion. The FDA restricted tisagenlecleucel’s approval to patients age ≤25; though broader approval was considered, patients age >21 were excluded from ELIANA. Of further note, CTL019 was associated with high rates of severe CRS, including death, in adults treated in pilot studies.(4) While strategies to prevent severe CRS are under investigation, approving an agent for which half of recipients will require ICU care, even at specialized centers with expertise in CAR T-cell therapy, warrants a rigorous approach to protect patient safety. To this end, the FDA mandated a comprehensive Risk Evaluation and Mitigation Strategies (REMS) program requiring hospitals and their associated clinics to become “CAR-enabled” prior to prescribing tisagenlecleucel; the certification process requires a minimum of two doses of tocilizumab per patient to be available for immediate administration, as well as appropriate training of all staff involved in prescribing, dispending, or administering tisagenlecleucel. The REMS additionally requires Novartis to verify compliance of the participating institutions. “Real-world” data from CAR-enabled institutions and ongoing reporting to the REMS program will help to characterize the risks of severe CRS and neurologic toxicity among the broader population of centers treating patients with commercial tisagenlecleucel (vs. on ELIANA) and may inform future approval considerations.
As noted by O’Leary et al., neither the FDA’s approval nor ELIANA itself establishes how on-label tisagenlecleucel is best used in CAYA with R/R B-ALL. While only 9% of patients proceeded to allogeneic hematopoietic cell transplantation (alloHCT) post-tisagenlecleucel on ELIANA, the majority had undergone prior allogeneic hematopoietic cell transplantation (alloHCT). Risk of CD19-negative relapse is notable even in the presence of persisting CAR T-cells. However, whether first alloHCT, where applicable, should be pursued in tisagenlecleucel responders remains uncertain, and further clinical follow-up will better characterize rates of durable response to tisagenlecleucel in the absence of subsequent alloHCT. Additionally, similar to the observed experience in pediatric patients with blinatumomab,(2) response rates to tisagenlecleucel appear to be higher among patients with lower disease burden at the time of treatment,(3) and several reports have suggested greater toxicity of CD19-targeted CAR T-cell therapy among patients with greater disease burden at time of infusion. Whether “debulking” therapy prior to tisagenlecleucel infusion in patients with ≥50% lymphoblasts in the marrow would optimize clinical outcomes remains uncertain. Future investigation of tisagenlecleucel in patients with persistent MRD in CR1 or beyond would further characterize the risk/benefit profile in patients with less advanced disease.
Tisagenlecleucel’s approval additionally represents a milestone for the FDA in oversight of gene therapy as it does in therapeutic development for B-ALL. The FDA has now granted approval for 3 gene therapy products since 2017: tisagenlecleucel and axicabtagene ciloleucel (CAR T-cell therapy for hematologic malignancies), and voretigene neparvovec (for Leber’s congenital amaurosis). FDA leadership describes increasing comfort overseeing gene therapy development, while limiting the role of the NIH and special advisory boards such as the Recombinant DNA Advisory Committee (RAC).(5) As clinical investigation of gene-modified immune effector cell therapy for hematologic and solid tumors expands, and as genome editing technology is further applied in this setting, the FDA will increasingly need to weigh the above considerations for approval and ongoing safety assessment, and we welcome their commitment to oversee this coming generation of therapies.
Acknowledgments:
American Society of Hematology (M. Geyer); Lymphoma Research Foundation (M. Geyer); NIH/National Center for Advancing Translational Sciences, UL1TR00457 (M. Geyer)
Footnotes
Disclosure of potential conflicts of interest: Geyer: Dava Oncology (honorarium)
References:
- 1.O’Leary MC, Lu X, Huang Y, Lin X, Mahmood I, Przepiorka D, et al. FDA Approval Summary: Tisagenlecleucel for Treatment of Patients with Relapsed or Refractory B-Cell Precursor Acute Lymphoblastic Leukemia. Clinical Cancer Research 2018. [DOI] [PubMed] [Google Scholar]
- 2.von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, et al. Phase I/Phase II Study of Blinatumomab in Pediatric Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia. J Clin Oncol 2016;34(36):4381–9 doi 10.1200/JCO.2016.67.3301. [DOI] [PubMed] [Google Scholar]
- 3.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. The New England journal of medicine 2018;378(5):439–48 doi 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frey NV, Levine BL, Lacey SF, Grupp SA, Maude SL, Schuster SJ, et al. Refractory Cytokine Release Syndrome in Recipients of Chimeric Antigen Receptor (CAR) T Cells. Blood 2014;124(21):2296-. [Google Scholar]
- 5.Collins FS, Gottlieb S. The Next Phase of Human Gene-Therapy Oversight. The New England journal of medicine 2018;379(15):1393–5 doi 10.1056/NEJMp1810628. [DOI] [PMC free article] [PubMed] [Google Scholar]