Abstract
Autologous stem cell transplantation (ASCT) remains a mainstay in the treatment of multiple myeloma (MM). While the procedure is generally safe, toxicities associated with high-dose melphalan conditioning are common and significantly affect patient quality of life. Recently, a propylene glycol-free melphalan formulation (PG-free MEL; Evomela®) was approved by the United States Food and Drug Administration as an ASCT conditioning regimen for MM. PG-free MEL is more soluble and stable than propylene glycol-solubilized melphalan (PG-solubilized MEL; Alkeran®). As such, there is speculation that it could decrease toxicities and increase the efficacy of ASCT. We compared the outcomes of patients conditioned with PG-free MEL (n=216) to PG-solubilized MEL (n=200) at our institution. The baseline characteristics were similar between the two groups. After Day +0, there were no differences in terms of hospitalizations, neutropenic fevers, intravenous granisetron requirement, World Health Organization grade ≥2 oral/esophageal mucositis, intravenous fluid requirement, or narcotic requirement. However, PG-free MEL patients had a higher incidence of diarrhea, which was mostly C. difficile-negative (82% vs. 71%, P=0.015*). Day +100 hematologic responses and progression-free survival after ASCT were comparable. In summary, we demonstrate that switching to PG-free MEL did not significantly reduce short-term complications of ASCT or improve outcomes in MM.
Keywords: autologous stem cell transplant, multiple myeloma, melphalan, evomela
Introduction
Multiple myeloma (MM) is a heterogeneous disease caused by malignant terminally-differentiated plasma cells.1, 2 Novel agents, such as proteasome inhibitors and immunomodulatory drugs, have remarkably changed the treatment landscape of MM in the past two decades.3 Even so, autologous stem cell transplantation (ASCT) remains a mainstay in the therapeutic armamentarium for fit patients under the age of 70.4–8
High-dose melphalan has been used as a conditioning regimen for ASCT in MM since the 1980s.9, 10 Common toxicities after melphalan administration include nausea, vomiting, diarrhea, alopecia, and gastrointestinal mucositis.11, 12 The latter, particularly of the oral/esophageal mucosa, occurs frequently and significantly affects the nutritional status, hydration, and quality of life of patients during ASCT.13, 14 Further, severe oral/esophageal mucositis increases hospitalization time and thereby costs of care.15–17
A propylene glycol-free formulation of melphalan (PG-free MEL; Evomela®) was recently approved by the United States Food and Drug Administration (FDA) as an ASCT conditioning regimen for patients with MM. The primary impetus for this novel formulation was to improve upon the stability and solubility of propylene glycol-solubilized melphalan (PG-solubilized MEL; Alkeran®) by making use of captisol, a modified cyclodextrin solubilizing agent.18, 19 As such, there is speculation that using PG-free MEL as a conditioning regimen for ASCT may decrease toxicities and increase efficacy compared to PG-solubilized MEL.20–24
For example, it was noted that patients treated with PG-free MEL had a relatively low incidence of severe oral mucositis in the Phase IIb trial which lead to its FDA approval.23 However, it is unclear if ASCT outcomes differ significantly between patients treated with either PG-free or PG-solubilized MEL in clinical practice. In pursuit of this question, we compared the outcomes of patients who underwent ASCT for the treatment of MM before and after an institutional switch to PG-free MEL.
Methods
After approval by the Mayo Clinic Institutional Review Board, we reviewed the medical records of 416 consecutive patients who underwent ASCT for the treatment of MM at Mayo Clinic Rochester from October 15, 2015 to October 27, 2017. All patients were diagnosed according to the 2014 International Myeloma Working Group (IMWG) definition of MM.25 The switch from PG-solubilized MEL (Alkeran®; GlaxoSmithKline, Research Triangle Park, NC, USA) to PG-free MEL (Evomela®; Spectrum Pharmaceuticals, Inc., Irvine, CA, USA) occurred at our institution on October 10, 2016. Thus, 200 patients in our study were conditioned with PG-solubilized MEL; 216 were conditioned with PG-free MEL. Patients who had already received an ASCT prior October 15, 2015 were excluded. In addition, for patients who underwent a second ASCT during the study period, only the first ASCT was considered.
All ASCT for MM at Mayo Clinic Rochester is initiated on an outpatient basis, except for patients who lack a caregiver, or for those who have significant comorbidities and/or poor performance status.26 The IMWG Uniform Response Criteria for Multiple Myeloma was used to assess treatment responses before ASCT and on day +100 after ASCT.27 The international staging system (ISS) was used to stage patients at the time of diagnosis.28 High-risk cytogenetics were defined as t(4;14), del(17/17p), t(14;16), and t(14;20).
Oral/esophageal mucositis was graded by K.C.M. using information in the patient medical records according to the World Health Organization (WHO) oral toxicity criteria. Clinical notes were systematically interrogated for documentation of oral ulcers during the physical exam, as well as severe pain resulting in a significant limitation of oral intake; patients with either of these features were graded as ≥2. Moreover, patients with severe esophagitis pain that significantly limited oral intake were graded as ≥2, even if no oral ulcers were documented. Diarrhea clinically attributable to gastrointestinal mucositis was not considered in grading of mucositis due to lack of standardized criteria. However, clinical notes were systematically reviewed for documentation of loose stools/diarrhea as well as treatment with anti-diarrheal agents.
Neutrophil engraftment was defined as an absolute neutrophil count >0.5 × 109/L for 3 consecutive days. Platelet engraftment was defined as platelets >20,000/mL for 3 consecutive days, with the absence of a platelet transfusion in the preceding 7 days. The Mann-Whitney U test and Fisher’s Exact test were used to compared continuous and categorical variables, respectively. Progression-free survival was measured from the date of ASCT until either progression, as defined by the IMWG criteria,27 or death from any cause, as of May 15th, 2018, with patients who remained alive and progression-free censored at the time of last follow-up. The Kaplan-Meier method and log-rank test were used to compare neutrophil and platelet engraftment kinetics, and progression-free survival between the two treatment groups. Median time of follow-up was calculated using the reverse Kaplan-Meier method. Statistical analysis was completed using JMP 13 (SAS Institute Inc., Cary, NC, USA).
Results
Patient Characteristics
Four-hundred and sixteen consecutive patients underwent ASCT for the treatment of MM: 216 patients were conditioned with PG-free MEL, while 200 patients were conditioned with PG-solubilized MEL. The two treatment groups were balanced with respect to age, sex, type of myeloma, ISS stage at the time of diagnosis, and the presence of high-risk cytogenetics at diagnosis detected by fluorescence in situ hybridization (FISH). The types of induction regimen and duration of induction were comparable between the two groups. Moreover, there was a comparable length of time from diagnosis to ASCT, serum creatinine prior to ASCT, and depth of response at the time of ASCT (Table 1). There was no significant difference between the PG-free MEL and PG-solubilized MEL groups in terms of the dosage of melphalan, i.e. full-dose (200 mg/m2) or reduced-dose (e.g. 140 mg/m2)(P=0.10).
Table 1.
Baseline characteristics for patients conditioned with PG-free MEL (Evomela®) and PG-solubilized MEL (Alkeran®).
| PG-free MEL (Evomela®) n=216 |
PG-solubilized MEL (Alkeran®) n=200 |
P | |
|---|---|---|---|
| Age at Diagnosis, median (range) | 61 (30–75) | 60 (27–76) | 0.85 |
| Male, n (%) | 132 (61) | 116 (58) | 0.55 |
| Type of Myeloma, n (%) | 0.10 | ||
| IgG | 119 (55) | 109 (55) | |
| IgA | 46 (21) | 38 (19) | |
| Light chain | 50 (23) | 45 (23) | |
| Other | 1 (1) | 8 (4) | |
| ISS at diagnosis, n (%) | 0.92 | ||
| I | 57 (26) | 56 (28) | |
| II | 61 (28) | 51 (26) | |
| III | 52 (24) | 51 (26) | |
| Missing data | 46 (21) | 42 (21) | |
| FISH cytogenetics, n (%) | 0.45 | ||
| Standard risk | 131 (61) | 132 (66) | |
| High risk | 55 (25) | 47 (24) | |
| Missing data | 30 (14) | 21 (11) | |
| Induction regimen, n (%) | 0.29 | ||
| Triplet* | 138 (64) | 123 (62) | |
| Doublet† | 5 (2) | 12 (6) | |
| Other | 3 (1) | 2 (1) | |
| Multiple regimens prior to ASCT | 70 (32) | 63 (32) | |
| Duration of Induction, (months), median (IQR) | 4 (3–6) | 4 (3.25–6) | 0.34 |
| Age at ASCT, median (range) | 62 (31–75) | 61 (29–77) | 0.76 |
| Time from ASCT from diagnosis, (months), median (IQR) | 6 (5–10) | 6 (5–9) | 0.92 |
| Serum Creatinine prior to ASCT (mg/dL), median (IQR) | 1 (0.8–1.2) | 1 (0.8–1.2) | 0.51 |
| Disease status prior to ASCT, n (%) | 0.31 | ||
| Stable disease or Progression | 21 (10) | 26 (13) | |
| PR | 72 (33) | 77 (39) | |
| VGPR | 83 (38) | 61 (31) | |
| CR | 17 (8) | 11 (6) | |
| sCR | 23 (11) | 25 (13) | |
| Melphalan dose, n (%) | 0.10 | ||
| 200 mg/m2 | 161 (75) | 164 (82) | |
| Reduced-dose (e.g. 140 mg/m2) | 27 (13) | 22 (11) | |
| Other (e.g. +bortezomib) | 28 (13) | 14 (7) | |
Triplet regimens included a proteasome inhibitor (bortezomib, carfilzomib, or ixazomib), dexamethasone, and either an immunomodulatory agent (thalidomide or lenalidomide) or cyclophosphamide.
Doublet regimens included either bortezomib or lenalidomide, and dexamethasone.
Transplant Outcomes
After stem cell infusion (Day +0), there were no significant differences between patients conditioned with PG-free MEL or PG-solubilized MEL in terms of the number of patients who were hospitalized (42% vs. 39%, P=0.49). Of the patients who required hospitalization, the length of time spent in the hospital did not differ between the two groups (median of 6 nights for both, P=0.67). The primary indications for hospitalization are detailed in Table 2, and were comparable. The most common reasons for hospitalization for both groups were neutropenic fever or infection, followed by hypovolemia/fall risk, then severe oral/esophageal mucositis. The time from Day +0 to the final outpatient ASCT dismissal visit, which is contingent on the patients’ functional recovery as assessed by the transplant team, was identical between the PG-free MEL and PG-solubilized MEL groups (20 vs. 20 days, P=0.79).
Table 2.
Time to dismissal and hospitalization characteristics for patients conditioned with PG-free MEL (Evomela®) and PG-solubilized MEL (Alkeran®).
| PG-free MEL (Evomela®) n=216 |
PG-solubilized MEL (Alkeran®) n=200 |
P | |
|---|---|---|---|
| Time to ASCT dismissal visit (days from Day +0), median (IQR) | 20 (18–21) | 20 (18–22) | 0.79 |
| Hospitalization during ASCT, n (%) | 92 (43) | 78 (39) | 0.49 |
| Number of nights in hospital, median (IQR) | 6 (3–10) | 6 (3–9) | 0.67 |
| Primary reason for hospitalization, n(%) | 0.99 | ||
| Infection/Neutropenic fever | 33 (36) | 24 (31) | |
| Hypovolemia/fall risk | 16 (17) | 16 (21) | |
| Severe oral/esophageal mucositis | 14 (15) | 12 (15) | |
| Refractory nausea | 8 (9) | 7 (9) | |
| Lack of caregiver | 7 (8) | 7 (9) | |
| Cardiac arrhythmia | 6 (7) | 6 (8) | |
| Other | 8 (9) | 6 (8) | |
The time to both neutrophil engraftment and platelet engraftment did not differ between the two treatment groups (Figure 1). Packed red blood cell and platelet transfusion utilization was comparable as well. Further, there were no significant differences in the incidence of neutropenic fevers, bacteremia, or peri-engraftment syndrome requiring systemic corticosteroid treatment (Table 3). There was a trend towards a lower incidence of cardiac arrhythmias, including recurrence of underlying atrial fibrillation/flutter as well as new onset atrial fibrillation/flutter/tachyarrhythmias in patients treated with PG-free MEL (5% vs. 9%, P=0.13), but the difference was not significant.
Figure 1.
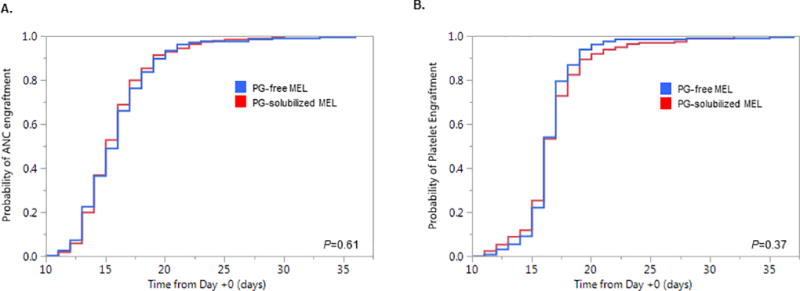
(A) Time to neutrophil (ANC) engraftment from Day +0 compared between PG-free MEL (Evomela®) and PG-solubilized MEL (Alkeran®). (B) Time to platelet engraftment from Day +0 compared between PG-free and PG-solubilized MEL.
Table 3.
ASCT outcomes for patients conditioned with PG-free MEL (Evomela®) and PG-solubilized MEL (Alkeran®).
| PG-free MEL (Evomela®) n=216 |
PG-solubilized MEL (Alkeran®) n=200 |
P | |
|---|---|---|---|
| Neutropenic Fever, n (%) | 133 (62) | 126 (63) | 0.84 |
| w/ Bacteremia | 38 (29) | 37 (29) | 0.80 |
| Periengraftment syndrome, n (%) | 24 (11) | 22 (11) | 1.0 |
| Cardiac arrhythmia, n (%) | 11 (5) | 18 (9) | 0.13 |
| Oral/Esophageal Mucositis (WHO Grade ≥2), n (%) | 52 (24) | 40 (20) | 0.35 |
| Narcotic requirement during ASCT, n (%) | 153 (71) | 144 (72) | 0.83 |
| Oral narcotics (tramadol, oxycodone, etc.) | 108 (50) | 90 (45) | 0.16 |
| Fentanyl patch | 22 (10) | 33 (17) | |
| IV Fentanyl, Hydromorphone, or Morphine | 23 (11) | 21 (11) | |
| IV Fluid requirement (liters), median (IQR) | 7 (3–12) | 6 (3–11) | 0.45 |
| Diarrhea, n (%) | 176 (82) | 142 (71) | 0.015* |
| C. difficile-negative | 167 (95) | 125 (88) | 0.038* |
| C. difficile-positive | 9 (5) | 17 (12) | |
| Anti-diarrheal requirement (amongst patients w/o C. difficile-positive diarrhea), n (%) | 0.003* | ||
| Loperamide alone | 128 (62) | 100 (55) | |
| Loperamide + diphenoxylate/atropine | 29 (14) | 13 (7) | |
| No pharmacologic treatment | 50 (24) | 70 (38) | |
| IV granisetron requirement (days on therapy after Day +0), median (IQR) | 10 (3–13) | 9 (1–13) | 0.48 |
| IV granisetron cumulative dose (mg), median (IQR) | 12.5 (3–20) | 11 (1–18) | 0.36 |
| RBC transfusion requirement (# of units), median (IQR) | 0 (0–1) | 0 (0–1) | 0.79 |
| Platelet transfusion requirement (# of units), median (IQR) | 1 (1–2) | 1 (1–2) | 0.45 |
P< .05
At our institution, patients who develop severe nausea are offered intravenous (IV) granisetron as needed. We compared the IV granisetron requirement between the two groups as a surrogate for the severity of nausea. There were no significant differences in the number of days on IV granisetron treatment (10 vs. 9 days, P=0.48) or cumulative dose of IV granisetron (12.5 vs. 11 mg, P=0.36) between the PG-free MEL and PG-solubilized MEL groups, respectively (Table 3).
The incidence of WHO grade ≥2 oral/esophageal mucositis was similar between the PG-free MEL and PG-solubilized MEL groups (24% vs. 20%, respectively, P=0.35). Interestingly, the PG-free MEL group had a higher incidence of diarrhea (82% vs. 71%, P=0.015) (Table 3). The majority of this was Clostridium difficile-negative, but there was a lower incidence of C. difficile infections in the PG-free MEL group (5% vs. 11%)(P=0.038). A greater number of patients in the PG-free MEL group received anti-diarrheal treatment with either loperamide or a combination of loperamide and atropine/diphenoxylate (when only considering patients without evidence of C. difficile infection). Of these patients, a higher proportion in the PG-free MEL group required dual loperamide plus atropine/diphenoxylate therapy (14% vs. 7%, P=0.003).
The maximum intensity of narcotic requirement, stratified into three tiers (oral narcotics only, fentanyl patches, or intravenous narcotics), was comparable between the two treatment groups. Lastly, IV fluid utilization between the two groups did not significantly differ (Table 3).
Finally, there were no significant differences in Day +100 hematologic responses between the two groups (Figure 2). Although the median follow-up time was relatively short (16.2 months), progression-free survival was comparable between the two treatment groups (Figure 3). By Day +100, one patient in each of the treatment groups was deceased; both patients had extramedullary relapses of their MM.
Figure 2.
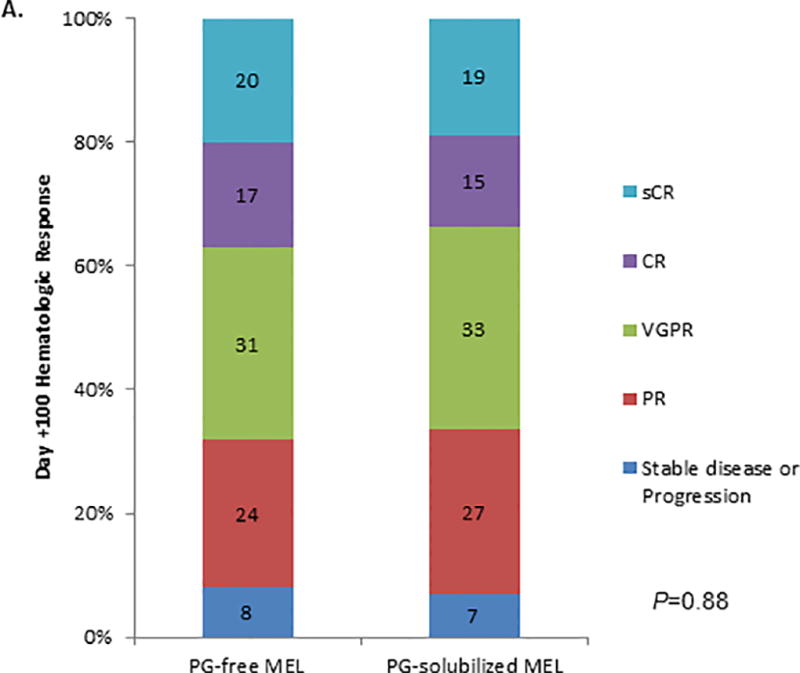
Day +100 hematologic responses for the 216 patients conditioned with PG-free MEL (Evomela®) and 200 patients condition with PG-solubilized MEL (Alkeran®) after ASCT, shown as the proportion of patients achieving each response category.
Figure 3.
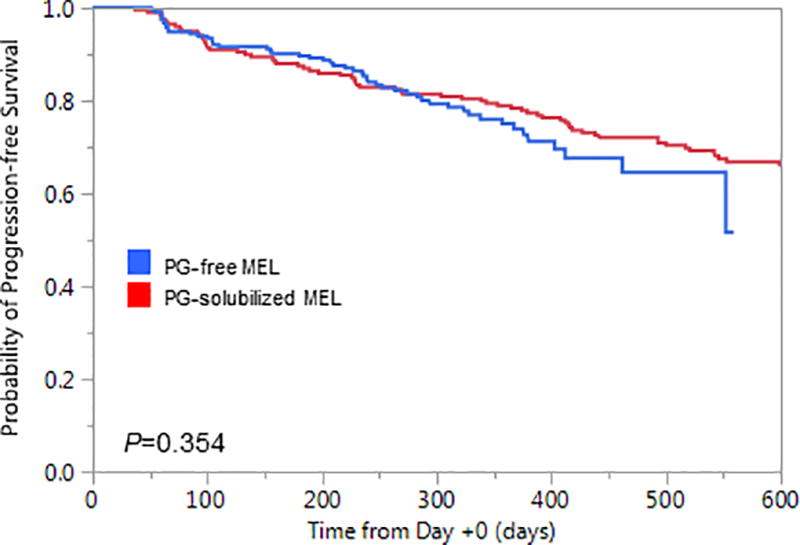
Progression-free survival measured from the date of ASCT (Day +0) for patients conditioned with either PG-free MEL (Evomela®) or PG-solubilized MEL (Alkeran®).
Discussion
High-dose conditioning with melphalan followed by ASCT is integral to the treatment of patients with MM, even in the era of novel agents.7, 8, 29 While ASCT is largely safe,26 morbidity and costs associated with the procedure are significant.30–32 Oral/esophageal mucositis is a major factor affecting patient quality of life during ASCT.13, 14 Given that mucositis is the rate limiting toxicity of melphalan administration,11, 33 it is intuitive that a more stable and soluble formulation, i.e. PG-free MEL, could facilitate less toxicities and greater efficacy.
However, in the present study we find no evidence to suggest improved outcomes in the short-term for patients conditioned with PG-free MEL compared to patients conditioned with PG-solubilized MEL, including the rate of WHO Grade ≥2 oral/esophageal mucositis. Given the outpatient nature of our ASCT practice, we had a relatively high sensitivity to detect complications of ASCT meriting admission to the hospital. Of note, there was no difference in hospitalization rate or the length of stay between the two treatment groups.
While it is known that high-dose melphalan is associated with cardiac toxicities,34, 35 attributing arrhythmias to it per se is difficult in the background of the physiologic stress caused by ASCT; patients often have concomitant electrolyte abnormalities, hypovolemia, and others. We noted a trend towards a lower incidence of both recurrence of underlying atrial fibrillation/flutter and new-onset tachyarrhythmias in patients conditioned with PG-free MEL, but the difference was not statistically significant. Likewise, while high doses of propylene-glycol have been associated with nephrotoxicity,36 it is difficult to ascribe particular toxicities to it in the context of ASCT since patients are volume depleted and have underlying renal impairment due to their MM.
We were unable to quantify the volume or duration of diarrhea during ASCT since the majority of patients were treated entirely in an outpatient setting. Furthermore, we were unable to compare the cumulative dose of anti-diarrheals because most patients used these on an as needed basis outside of the hospital. With these limitations in mind, we found that patients conditioned with PG-free MEL had a higher incidence of C. difficile-negative diarrhea, and significantly more patients required anti-diarrheal therapy with loperamide +/- atropine/diphenoxylate compared to the PG-solubilized MEL group. Also, a greater proportion of patients in the PG-free MEL group required dual anti-diarrheal therapy with loperamide plus atropine/diphenoxylate, suggesting that perhaps there were a greater number of severe cases of C. difficile-negative diarrhea in the PG-free MEL group. On the other hand, the patients conditioned with PG-free MEL had a lower incidence of C. difficile infections. Taken together, it is difficult to attribute these findings to the solubilizing agents, melphalan per se, or inherent limitations in our retrospective study design; there might have been a recent drop in C. difficile as a whole at our institution. Even so, future studies should investigate if conditioning with PG-free MEL affects the gastrointestinal tract in a different manner than PG-solubilized MEL and perhaps leads to more significant diarrhea.
Higher melphalan exposure, measured using area under the concentration vs. time curves (AUC), is associated with improved outcomes after ASCT in MM, but also an increased risk of severe mucositis.37 A recent abstract demonstrated less variability in AUC measurements for PG-free MEL compared to published data with PG-solubilized MEL.38 Further, several recent studies have demonstrated the feasibility of using pharmacokinetic-directed dosing of PG-free MEL to optimize serum melphalan concentrations.24, 39 Future prospective studies should clarify these findings and investigate if this method could be used to improve ASCT outcomes while sparing patients of undue toxicities.
Finally, we provide no evidence to suggest that PG-free MEL improves Day +100 hematologic responses compared to PG-solubilized MEL. This is counter to a prior report from patients treated in the phase IIa trial,20 but not surprisingly that study included almost 10-fold fewer patients than the present analysis. Also, progression-free survival was comparable between the two groups, although longer follow-up will be required to more definitely determine if progression-free and overall survival outcomes differ for patients conditioned with PG-free MEL compared to PG-solubilized MEL.
Conclusions drawn from this analysis are undoubtedly limited because our study was retrospective in nature. However, we did not identify any significant differences in baseline characteristics between the two treatment groups, which each included ≥200 patients. In addition, it is noteworthy that all the patients in the present study underwent ASCT during a two year span from October 2015 to October 2017. Even though significant advancements in the treatment of MM are ongoing, this is unlikely to have confounded our findings in such a short time period. Finally, although we only included MM patients in the present study, PG-free MEL is being used in patients with other plasma cell disorders as well such as light-chain amyloidosis.23 Future studies should assess the impact of PG-free MEL on both short and long-term outcomes in this disease group.
Acknowledgments
S.K.K. is supported in part by US National Cancer Institute grants CA 107476, CA 168762, and CA186781.
Footnotes
Conflicts of Interest
S.K.K. has been a non-paid consultant or advisory board member for AbbVie, Celgene, Janssen, Kite Pharma, Merck, and Takeda and is the Principal Investigator in clinical trials supported by Bristol-Myers Squibb, Celgene, Janssen, Kite Pharma, Roche/Genentech, Sanofi, and Takeda.
Parts of this manuscript were presented in abstract form at the 23rd European Hematology Association Congress in Stockholm, Sweden on June 17, 2018.
References
- 1.Rajkumar SV, Kumar S. Multiple Myeloma: Diagnosis and Treatment. Mayo Clinic proceedings. 2016;91(1):101–119. doi: 10.1016/j.mayocp.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palumbo A, Anderson K. Multiple Myeloma. New England Journal of Medicine. 2011;364(11):1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 3.Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122–1128. doi: 10.1038/leu.2013.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335(2):91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 5.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-Dose Chemotherapy with Hematopoietic Stem-Cell Rescue for Multiple Myeloma. New England Journal of Medicine. 2003;348(19):1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 6.Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, et al. Autologous Transplantation and Maintenance Therapy in Multiple Myeloma. New England Journal of Medicine. 2014;371(10):895–905. doi: 10.1056/NEJMoa1402888. [DOI] [PubMed] [Google Scholar]
- 7.Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. New England Journal of Medicine. 2017;376(14):1311–1320. doi: 10.1056/NEJMoa1611750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahajan S, Tandon N, Kumar S. The evolution of stem-cell transplantation in multiple myeloma. Therapeutic Advances in Hematology. 2018;9(5):123–133. doi: 10.1177/2040620718761776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreau P, Facon T, Attal M, Hulin C, Michallet M, Maloisel F, et al. Comparison of 200 mg/m2 melphalan and 8 Gy total body irradiation plus 140 mg/m2 melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 2002;99(3):731–735. doi: 10.1182/blood.v99.3.731. [DOI] [PubMed] [Google Scholar]
- 10.Bayraktar UD, Bashir Q, Qazilbash M, Champlin RE, Ciurea SO. Fifty Years of Melphalan Use in Hematopoietic Stem Cell Transplantation. Biology of Blood and Marrow Transplantation. 2013;19(3):344–356. doi: 10.1016/j.bbmt.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazarus HM, Herzig RH, Graham-Pole J, Wolff SN, Phillips GL, Strandjord S, et al. Intensive melphalan chemotherapy and cryopreserved autologous bone marrow transplantation for the treatment of refractory cancer. Journal of Clinical Oncology. 1983;1(6):359–367. doi: 10.1200/JCO.1983.1.6.359. [DOI] [PubMed] [Google Scholar]
- 12.Grazziutti ML, Dong L, Miceli MH, Krishna SG, Kiwan E, Syed N, et al. Oral mucositis in myeloma patients undergoing melphalan-based autologous stem cell transplantation: incidence, risk factors and a severity predictive model. Bone Marrow Transplant. 2006;38(7):501–506. doi: 10.1038/sj.bmt.1705471. [DOI] [PubMed] [Google Scholar]
- 13.Seminars in oncology. Elsevier; 2003. The effect of oral mucositis on morbidity and mortality in bone marrow transplant. [DOI] [PubMed] [Google Scholar]
- 14.Fleming S, Harrison SJ, Blombery P, Joyce T, Stokes K, Seymour JF, et al. The Choice of Multiple Myeloma Induction Therapy Affects the Frequency and Severity of Oral Mucositis After Melphalan-Based Autologous Stem Cell Transplantation. Clinical Lymphoma, Myeloma and Leukemia. 2014;14(4):291–296. doi: 10.1016/j.clml.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Vera-Llonch M, Oster G, Ford CM, Lu J, Sonis S. Oral mucositis and outcomes of autologous hematopoietic stem-cell transplantation following high-dose melphalan conditioning for multiple myeloma. The journal of supportive oncology. 2007;5(5):231–235. [PubMed] [Google Scholar]
- 16.Jones JA, Qazilbash MH, Shih YC, Cantor SB, Cooksley CD, Elting LS. In-hospital complications of autologous hematopoietic stem cell transplantation for lymphoid malignancies: clinical and economic outcomes from the Nationwide Inpatient Sample. Cancer. 2008;112(5):1096–1105. doi: 10.1002/cncr.23281. [DOI] [PubMed] [Google Scholar]
- 17.Sonis ST, Oster G, Fuchs H, Bellm L, Bradford WZ, Edelsberg J, et al. Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(8):2201–2205. doi: 10.1200/JCO.2001.19.8.2201. [DOI] [PubMed] [Google Scholar]
- 18.Koltun M, Morizzi J, Katneni K, Charman SA, Shackleford DM, McIntosh MP. Preclinical comparison of intravenous melphalan pharmacokinetics administered in formulations containing either (SBE)7 m-beta-cyclodextrin or a co-solvent system. Biopharmaceutics & drug disposition. 2010;31(8–9):450–454. doi: 10.1002/bdd.725. [DOI] [PubMed] [Google Scholar]
- 19.Singh R, Chen J, Miller T, Bergren M, Mallik R. Solution stability of Captisol-stabilized melphalan (Evomela) versus Propylene glycol-based melphalan hydrochloride injection. Pharmaceutical development and technology. 2016:1–6. doi: 10.1080/10837450.2016.1265557. [DOI] [PubMed] [Google Scholar]
- 20.Aljitawi OS, Ludlow A, Ganguly S, Abhyankar S, Lin T, Pipkin JD, et al. Propylene Glycol-Free Melphalan Induces Higher Remission Rates in Multiple Myeloma Patients Undergoing Autologous Transplantation. Blood. 2012;120(21):4551–4551. [Google Scholar]
- 21.Hari P, Aljitawi OS, Arce-Lara C, Nath R, Callander N, Bhat G, et al. A Phase IIb, Multicenter, Open-Label, Safety, and Efficacy Study of High-Dose, Propylene Glycol-Free Melphalan Hydrochloride for Injection (EVOMELA) for Myeloablative Conditioning in Multiple Myeloma Patients Undergoing Autologous Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015;21(12):2100–2105. doi: 10.1016/j.bbmt.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 22.Aljitawi OS, Ganguly S, Abhyankar SH, Ferree M, Marks R, Pipkin JD, et al. Phase IIa cross-over study of propylene glycol-free melphalan (LGD-353) and alkeran in multiple myeloma autologous transplantation. Bone Marrow Transplant. 2014;49(8):1042–1045. doi: 10.1038/bmt.2014.120. [DOI] [PubMed] [Google Scholar]
- 23.Badar T, Hari P, Chhabra S, Dhakal B, Drobyski WR, Fenske TS, et al. Use of propylene glycol-free melphalan conditioning in light-chain amyloidosis patients undergoing autologous hematopoietic cell transplantation is well tolerated and effective. Bone Marrow Transplantation. 2018 doi: 10.1038/s41409-018-0178-5. e-pub ahead of print 20 April 2018; doi: 10.1038/s41409-018-0178-5. [DOI] [PubMed] [Google Scholar]
- 24.Dhakal B, D'Souza A, Lakshman A, Hamadani M, Chhabra S, Thompson R, et al. Pharmacokinetics of High-Dose Propylene Glycol Free Melphalan in Multiple Myeloma Patients Undergoing Autologous Hematopoietic Cell Transplantation. Biology of Blood and Marrow Transplantation. 2018 doi: 10.1016/j.bbmt.2018.04.028. e-pub ahead of print 8 May 2018. [DOI] [PubMed] [Google Scholar]
- 25.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos M-V, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. The Lancet Oncology. 2014;15(12):e538–e548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 26.Gertz MA, Buadi FK, Hayman SR, Lacy MQ, Dispenzieri A, Dingli D, et al. Safety Outcomes for Autologous Stem Cell Transplant in Multiple Myeloma. Mayo Clinic proceedings. 2018;93(1):56–58. doi: 10.1016/j.mayocp.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23(1):3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Blade J, et al. International staging system for multiple myeloma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(15):3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 29.Kumar SK, Rajkumar SV. The multiple myelomas — current concepts in cytogenetic classification and therapy. Nature Reviews Clinical Oncology. 2018;15(7):409–421. doi: 10.1038/s41571-018-0018-y. [DOI] [PubMed] [Google Scholar]
- 30.Hewan B, JBL, Ryan S, Manju N, Brian M, Aleksandr L, et al. Transplantation related toxicity and mortality in older autologous hematopoietic cell transplantation recipients. American journal of hematology. 2017;92(9):e529–e533. doi: 10.1002/ajh.24814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khera N, Zeliadt SB, Lee SJ. Economics of hematopoietic cell transplantation. Blood. 2012;120(8):1545–1551. doi: 10.1182/blood-2012-05-426783. [DOI] [PubMed] [Google Scholar]
- 32.Chintan P, Shahrukh H, Nandita K, AGM, Angela D, William H, et al. Cost-effectiveness analysis of early vs. late autologous stem cell transplantation in multiple myeloma. Clinical Transplantation. 2014;28(10):1084–1091. doi: 10.1111/ctr.12421. [DOI] [PubMed] [Google Scholar]
- 33.Moreau P, Milpied N, Mahé B, Juge-Morineau N, Rapp MJ, Bataille R, et al. Melphalan 220 mg/m2 followed by peripheral blood stem cell transplantation in 27 patients with advanced multiple myeloma. Bone Marrow Transplantation. 1999;23:1003. doi: 10.1038/sj.bmt.1701763. [DOI] [PubMed] [Google Scholar]
- 34.Feliz V, Saiyad S, Ramarao SM, Khan H, Leonelli F, Guglin M. Melphalan-induced supraventricular tachycardia: incidence and risk factors. Clin Cardiol. 2011;34(6):356–359. doi: 10.1002/clc.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mileshkin LR, Seymour JF, Wolf MM, Gates P, Januszewicz EH, Joyce P, et al. Cardiovascular toxicity is increased, but manageable, during high-dose chemotherapy and autologous peripheral blood stem cell transplantation for patients aged 60 years and older. Leukemia & lymphoma. 2005;46(11):1575–1579. doi: 10.1080/10428190500235884. [DOI] [PubMed] [Google Scholar]
- 36.Zar T, Graeber C, Perazella MA. Recognition, treatment, and prevention of propylene glycol toxicity. Seminars in dialysis. 2007;20(3):217–219. doi: 10.1111/j.1525-139X.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 37.Nath CE, Trotman J, Tiley C, Presgrave P, Joshua D, Kerridge I, et al. High melphalan exposure is associated with improved overall survival in myeloma patients receiving high dose melphalan and autologous transplantation. British journal of clinical pharmacology. 2016;82(1):149–159. doi: 10.1111/bcp.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah GL, Landau H, Sarubbi C, Schofield R, Lin A, Bhatt V, et al. Pharmacokinetics and Toxicities after Evomela; (Propylene Glycol Free Melphalan) with Hematopoietic Stem Cell Transplant (HCT) for Multiple Myeloma (MM), AL Amyloidosis (AL), Lymphoma, Acute Myeloid Leukemia (AML), and Myelodysplastic Syndrome (MDS) Biology of Blood and Marrow Transplantation. 2018;24(3):S79–S80. [Google Scholar]
- 39.Shah GL, Lin A, Schofield R, Sarubbi C, Preston EV, Devlin SM, et al. Feasibility and Toxicity of Pharmacokinetic (PK)-Directed Dosing of Evomela; (propylene glycol free melphalan, PGF-MEL) for Multiple Myeloma (MM) and AL Amyloidosis (AL) Patients Undergoing Autologous Hematopoietic Stem Cell Transplant (AHCT) Biology of Blood and Marrow Transplantation. 2018;24(3):S129–S130. [Google Scholar]


