Abstract
Background:
Accurate symptom assessment remains challenging in teen populations. Little is known of usual symptom/response patterns, and self-reported paper diaries have traditionally low compliance rates. Therefore, we used concurrent digital voice-diaries to capture daily asthma experiences.
Objective:
(1) To qualitatively explore usual symptom patterns and self-management responses; and (2) to quantitatively explore relationships between symptom severity and sentiment scores (a marker of emotional response to events).
Methods:
Fourteen minority and non-minority teenagers (age 13–17) with controlled (50%) and uncontrolled asthma used digital recorders to report about their asthma once daily over 14 days. Dairy entries were coded for symptom frequency, severity, type, and self-management responses, while sentiment analysis was used to evaluate the emotional valence of diary entries and to explore whether increased symptom levels correlated with greater negative sentiment.
Results:
Symptom frequency and severity recorded in voice-diaries was much higher than teens indicated at baseline, and was discordant with clinical assessments of asthma control. Of 175 entries, teens had symptoms 69.1% of days (121/175) and severe symptoms on one-third of these. Atypical symptoms (coughing, throat-clearing) were reported twice as often as traditional symptoms (wheezing, chest tightness), and often not recognized as asthma, but rather attributed to being “sick” (25.6% of symptom days). Teens frequently minimized symptoms, used rescue and controller medication inconsistently, and resorted to alternative strategies to manage symptoms. Sentiment was not significantly correlated with assessed control (β=0.14, p=0.28), but for teens reporting severe symptoms, sentiment scores decreased by 0.31 relative to teens without symptoms (P=0.006).
Conclusions & Clinical Relevance:
Teens may minimize symptoms and have greater symptom frequency and severity than is recognized by themselves or providers. Screening for specific symptoms including coughing, throat-clearing, and respiratory illness may be needed to identify those experiencing burden from asthma.
Introduction
Accurate symptom assessment remains an ongoing challenge for clinical asthma management. In most healthcare settings, diagnosis of asthma severity and control is largely reliant on self-reported symptom levels [1]. When patients do not report symptoms accurately, clinical assessment is hindered, and providers may not prescribe sufficient controller therapy. Inadequate treatment translates to uncontrolled disease, which is associated with poor health outcomes, decreased quality of life, and negative psychological states (e.g. depression and anxiety) [2, 3].
Teenagers are particularly at risk in this respect, as they often overlook and under-report symptoms and have poorer asthma control than older and younger individuals [4, 5]. Compared to individuals with better asthma management, they are sick more often, less physically active, and experience higher rates of anxiety and depression [6]. Thus, there is a clear need to improve symptom recognition in this population as a means to achieving better outcomes [7]. Most interventions targeting self-monitoring have met with little or indeterminate success, which is often attributed to adolescent cognitive immaturity, forgetfulness, or lack of interest [8–10]. Yet, it may be that inability to modify teen’s self-monitoring practices is partially attributable to insufficient understanding of the root causes of their behavior [11]. Developing a better understanding of existing symptom patterns and how these are perceived by teens may therefore be a necessary step in addressing this important clinical issue.
Thus, as part of a broader qualitative study (Teens Experiences of Asthma Study) [12, 13] we used voice-diaries to prospectively explore asthma symptoms, self-management responses, and emotional perceptions of asthma-related events. Specifically, we asked teens to report on their symptoms using daily voice-dairies, as a means to both track symptom patterns and provide insight regarding perceptions of events and responses. The objective of this exploratory study was to: (1) qualitatively and quantitatively evaluate self-reported symptom patterns and responses to symptoms; and (2) assess whether the emotional valence of narratives (an established marker of affective state and situational emotional response) corresponded with symptom levels [14]. Because lower sentiment scores typically correlate with more negative experiences [15], it was hypothesized that as asthma symptoms increased sentiment scores would correspondingly decrease.
Methods
Participants, setting, sample.
Teens (n=14) living in upstate NY were recruited from the community, prior study participants, Pulmonary Department, and Emergency Department (ED, using word-of-mouth referrals, study lists, and daily clinic rosters. Participants were contacted by phone (community, prior study) or in person during healthcare visits (Pulmonary Department, ED). Participants were purposefully sampled for equal representation of minority/non-minority racial status and controlled/uncontrolled asthma. Final sample size was determined by data saturation, and further sampling was stopped when no new information was elicited during face-to-face interviews in the parent study [12]. Eligible teens were: 13–17 years old, English-speaking, with persistent asthma by EPR-3 criteria [16]. Individuals with potentially confounding cardiac or respiratory co-morbidities were proposed for exclusion, however none of the teens had these co-morbidities.
Data collection.
Data collection occurred over the course of 1 year, from May 2014 to March 2015, in participant’s homes. Baseline asthma information (retrospective symptom frequency, medications, clinical assessment of severity and control), and demographic data (age, gender, race/ethnicity, income, insurance) were collected via brief structured survey. Teens were given small hand-held digital recorders, and asked to describe their daily asthma experiences and self-management behaviors daily for 14 days. They were encouraged to talk about anything relating to asthma that was important to them, but were also provided with diary guidelines that included prompts such as “Tell me about your asthma symptoms; Describe as much as you can; What do you think or feel about it?; What did you do or not do to handle your asthma? Tell me about the medications you are taking; What made you decide to take medication?” [12] Daily reminders were sent via text-message to each teen (or their parent), excluding two participants whose family did not have reliable cell-phone access. Most participants were newly acquainted with the researcher (Caucasian, female); two were marginally acquainted via prior community events. Participants received $30 upon pick-up of the recorder, regardless of number of entries they recorded.
Data analysis.
Fourteen voice-diaries (180 total entries) were collected and transcribed. Mixed qualitative and quantitative analytic techniques were used. First, we used qualitative content analysis, where each entry was specifically coded for: (a) presence or absence of symptoms, (b) severity of symptoms, (c) types of symptoms reported, and (d) types of responses to symptoms [17, 18]. As with traditional paper diaries, verbal diary entries that had any evidence of active (same-day) symptoms were coded as a symptom positive day (mild/moderate or severe). Frequencies (e.g. number of symptom positive days; number of days with specific symptoms) and percentages (number of symptom positive days/total number of days) were calculated. As defined by the Cleveland Clinic, mild to moderate symptoms included chest-tightness, wheezing, shortness of breath, coughing and repetitive throat clearing; Severe asthma symptoms included wheezing with inspiration and expiration, coughing that does not stop, inability to catch breath, chest pain or pressure, trouble talking in sentences, inability to exhale fully, feelings of anxiety/panic, or symptoms persisting throughout the day [19].
Sentiment analysis.
Sentiment analysis techniques were used to explore the emotional valence of diary entries, to better understand how teens felt about the symptoms and experiences they reported [14]. Sentiment analysis is the use of machine-learning methods to characterize the sentiment content of text, often as positive or negative. Complete diary entries (including all words) were used for the analysis. Emotional valence (a quantitative indicator of affective/emotional state) was determined by quantifying the use of negative/positive and neutral emotion words and phrases in an individual diary entry [20]. The Text Analytics API for sentiment analysis (Microsoft Cognitive Services) was used for the analysis, which for each diary entry returned a value in the interval [0,1], with 0 being the most negative and 1 the most positive. Higher proportionate use of negative words (e.g. bad, dislike, difficult, hard) would typically result in a lower sentiment score, whereas positive words (e.g. like, happy, good) would result in a higher score; a score of 0.5 is essentially neutral or objective. In practice, Text Analytics sentiment analysis employs a model trained on text already labeled for sentiment; this is a more flexible approach than simpler dictionary-based sentiment analysis, because it more effectively accounts for the context of words, e.g. like or just as filler words. Sentiment score summary statistics were compiled (median and IQR, mean ± standard deviation) for each diary entry, and linear mixed-effects modeling was used to determine whether sentiment changed over time, or based on age, race, gender, symptom severity, and asthma control.
Results
Demographics
Average age for teens was 14.79 years (SD 1.48), with racial composition being White (42.9%), Black (28.6%), Multiracial (21.4%), and Hispanic/Latino (7.14%). Average household income was $60,000 (SD $39,170; range $10,000 to >$100,000), with half of families meeting low-income thresholds and having public insurance [21]. Clinical classification of baseline asthma severity was mild (21.4%), moderate (57.2%), and severe persistent (21.4%). Classification of asthma control based on self-reported symptom levels was 50% well controlled, 21.4% not-well-controlled, and 28.6% very poorly controlled, based on EPR-3 criteria (e.g. daytime symptoms, nocturnal wake up, activity limitations, and rescue medication use) [1]. By self-perception, half of the teens felt they had good asthma control, four felt it was acceptable, and only three (21.4%) felt their asthma was uncontrolled.
Diary Results
A total of 180 diary entries were recorded by 14 teens. Teens completed an average of 12.9 recordings out of 14 solicited (92.3% completion rate). Most participants (11/14; 78.6%) completed all 14 days. The two youngest African-American teenagers, who did not receive reminder text-messages due to lack of cellphone service, completed the fewest (3 to 5 entries). Some teens (4/14; 28.6%) spontaneously recorded additional days while waiting for the diary to be picked up (range 1–4 extra). On five days, recording was initiated but nothing recorded (i.e. background noise), leaving 175 entries with substantive data for analysis (97.2%).
Length of individual diary entries ranged from a few seconds to eight minutes (mean: 114 seconds; SD 114 seconds). Figure 1 shows the length of individual recordings in lines of transcription. There was no observable pattern of quality or quantity attributable to age, sex, ethnicity, severity, or control. Most teens recorded detailed descriptions of their daily asthma experiences, including type, frequency, and severity of symptoms, triggering events, situational assessments, self-management responses, and personal thoughts and feelings. For example:
T13 (13y/F): Today is the first day that I’m off my prednisone and I feel short of breath. … I’m like trapped…it hurts, [and] in gym I constantly had to go off and take my inhaler, but I did it in the locker so nobody would see me. … I feel uncomfortable taking [prednisone] because I know it’s a steroid…but when I was on the prednisone I was safe … I took the Flovent today, but it didn’t really help. So I kinda got frustrated… its changed the way I think of everything. Because now I realize that I need the prednisone more than the Flovent….I can rely on it more [and] I was able to do more things without worrying about having asthma attacks.
Figure 1.
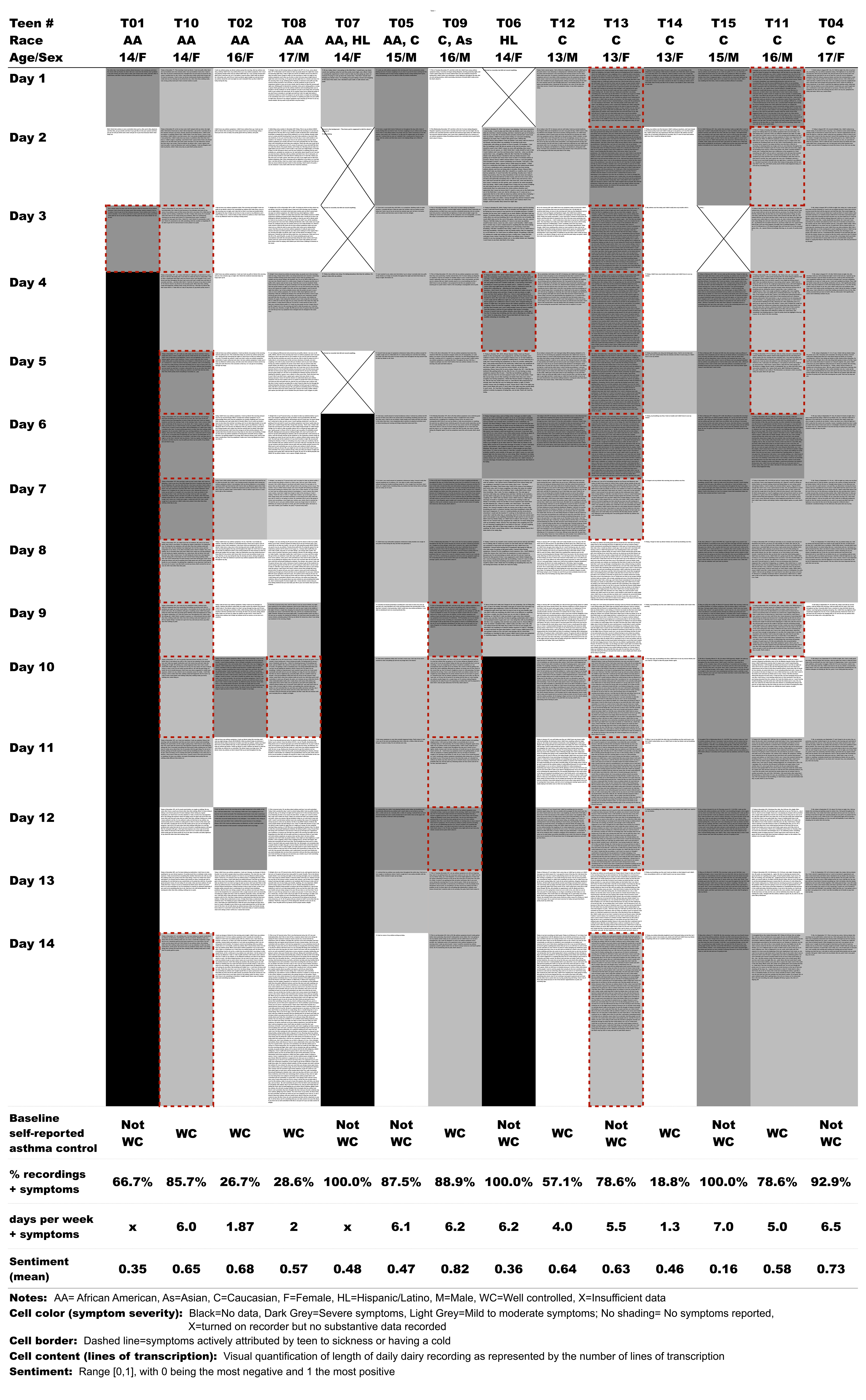
Graphical representation of’ voice dairy data displaying symptom days, symptom severity, sick days, asthma control, sentiment score, and length of diary recording by teen
Symptoms
Baseline retrospective assessment of asthma control (EPR-3) did not corresponded well with the frequency of symptoms reported prospectively in daily diaries (Figure 1). A total of 121 of 175 diary days were found to be symptom positive (69.1%). Of these, 81 (65.9%) were mild to moderate while the other 40 (33.1%) classified as severe. On 23 of the 121 symptom positive days (19%), teens reported that they had no symptoms despite clear evidence of audible wheezing or coughing on recording (9/14 teens). Symptom patterns and severity for the two-week diary period are graphically displayed in Figure 1 by cell color, with mild to moderate symptom days in light grey and severe symptom days in dark grey. The following quotes illustrate mild/moderate versus severe symptoms:
T14 (13y/F): Today, my asthma was okay…It’s just in gym when I was exercising it was getting a little heavier to breathe … it feels like someone is sitting on my um chest and I can’t breathe (mild-moderate symptoms)
T15 (15y/M): …when I woke up my asthma was very bad. … I took my inhaler and I was lightheaded right from the beginning …I came upstairs and I had to sit down, … all like fuzzy and stuff and feeling like I was sucking through a straw for air (clears throat) (severe symptoms).
Symptom misperception was commonly noted in diary entries. As seen in Table 1, this fell into two categories: minimizing versus not recognizing. Minimizing was evident when teens stated they had symptoms but indicated it wasn’t important or “not too bad” (11/14 teens on 51/121 symptom positive days; 42.1%), whereas not recognizing occurred when teens stated that they had no asthma symptoms despite clear evidence of active symptoms (23/121 symptom positive days; 19%). This is seen in the following excerpts:
T09 (16y/M): If I breathe out I can hear wheezing … but the symptoms aren’t too bad (minimizing)
T10 (14y/F): Nothing really new, um I have a small like congestion cough but …I’m not really worried and I don’t think it’s my asthma either…I haven’t had any problems lately…so I’m pretty good. (not recognizing)
Table 1.
Categories of Symptom Misperception
| Minimizing | Not recognizing |
|---|---|
| T02: I was short of breath and it was hard for me to catch my breath. … I took my Albuterol, so my symptoms didn’t get that bad… Right now, at this moment, my asthmas doing pretty well. I can, I can catch my breath. |
T04: I didn’t really have any chest tightness today … I came up the stairs to grab something from my room and I got to the top of the stairs and I felt pretty winded. |
| T05: I have had a successful day with little or no symptoms. Nothing really to make mention. I actually did feel a little bit under the weather with my asthma earlier today but I had a cup of tea and I do feel a lot better |
T05: I haven’t had any type of symptoms whatsoever today with my asthma except for a slight cough… haven’t had to take my inhaler at all |
| T06: It wasn’t that bad. Um I didn’t wheeze, it was just shortage of breath and trying to catch my breath back |
T06: I didn’t have any symptoms at all … my lungs are working a lot better than they were when I (coughs) was having symptoms… like I said, I’ve been coughing a lot. |
| T09: I’ve been coughing periodically throughout the day, … I’ve noticed where I’ll be like talking to someone and then I’ll have to like take a break from like even mid-sentence and just like clear my throat. And um I notice now that if I breathe out I can hear wheezing like … Um the symptoms aren’t too bad |
T09: My asthma symptoms have been fine, I didn’t notice any asthma symptoms or anything like that. … coughed up phlegm but I don’t believe that was asthma related |
| T10: I didn’t have really any real big problems. … I’m getting tired really quick and- and it’s hard- getting harder for me to breathe. I’m getting like a tightness in my chest … I was like that’s normal you know, that’s normal for me |
T10: I had to like clear my throat continuously but I thought that was just kind of normal for me….Nothing really new …I have a small like congestion cough but …I’m not really worried about that and I don’t think it’s my asthma either. |
| T11: today was a pretty quiet, normal day asthma-wise. …I took a nap on our couch with our cat … when I woke up um, my throat was tight … so I went and took a shower … and I also took a puff of my rescue inhaler |
T11: I mean it wasn’t like asthma symptoms …it was just the cough…Asthma is good ((coughs)) |
| T12: On the walk, I didn’t get to the point where it was wheezing …it was just like I can feel my chest start to get tight and then I would stop so I didn’t let it get horrible or bad at all um I just slowed down … I think today was pretty good. |
T12: I didn’t have any symptoms and um I didn’t get to the point where I think I needed to take it so I didn’t take it and I felt good ((coughing)) excuse me. |
| T13: So today um my asthma wasn’t as bad as it usually is… but I’m still kinda short of breath,… I’m still kind of like calming down, like resting a little. |
T13: So today um my asthma was really good actually ((coughs)) and I didn’t take the Albuterol. It’s just now I have a cough … but it’s not really my asthma. |
| T15: I spent the night at my cousin’s … I woke up the next morning at 7 o’ clock and this is on a weekend, like I don’t usually wake up at 7 o’ clock. And I had to take their nebulizer …then we went sledding and my asthma is kind of messed up from going up and down those big hills. I took my inhaler on the way home and then when I got home ..and after dinner and my asthma was a little bad |
T14: Today, my asthma was okay. It’s just in gym when I was exercising it was getting a little heavier to breathe … Um, it feels like someone is sitting on my um chest and I can’t breathe and get a breath in. |
In their diaries, teens mentioned both present and prior symptoms, and often referred to the same symptoms repetitively within a single entry. Therefore, symptom frequency was quantified as both the total instances each symptom was mentioned (present, prior, and repeated) and total number of days a given symptom was actively present. For example:
T02 (16y/F): If I have asthma symptoms I try to acknowledge them early. My symptoms are usually coughing and wheezing, and I get really short of breath. (prior symptoms)
T05 (15y/M): I woke up with asthma symptoms this morning and I had a smoky feeling and now I’ve been coughing … it’s been consistent and I keep coughing. (active symptoms; two instances of coughing mentioned on one day)
While all teens talked generally about having symptoms and trouble breathing (e.g. “hard to breathe,” “breathing problem”), coughing was mentioned more than twice as often as any other specific symptom (n=102; 11/14 teens). Other common symptoms, by order of frequency (Table 2) were throat clearing (n=64; 8/14 teens) chest tightness (n=50; 9/14 teens), wheezing (n=39; 8/14 teens), shortness-of-breath (n=36; 9/14 teens), tiredness (n=15; 4/14 teens), and chest pain (n=11; 4/14 teens). Notably, nearly half of teens (6/14; 42.8%) did not relate coughing to asthma, but attributed it to having a cold (25.6% of symptom days; 17.7% of all days). As one girl explained:
T06 (14y/F): [I’m] coughing ‘cause I’m sick…just like having a cold…I feel like I’m doing okay breathing, just the cold is affecting me a little bit. (Audible wheezing on recording)
T01 (14y/F): For a whole week I’ve been sick but the doctors think it’s my asthma
Table 2.
Asthma symptom frequencies
| # Instances mentioned |
# Days present |
# Teens reporting symptom (n=14) |
% Symptom positive days (n=121) |
% All days (n=175) |
|
|---|---|---|---|---|---|
| Cough | 102 | 43 | 11 | 34.7 | 24.6 |
| Clearing throat | 64 | 41 | 8 | 33.9 | 23.4 |
| Hard to breathe | 68 | 38 | 14 | 31.4 | 21.7 |
| Chest tightness | 50 | 33 | 9 | 27.3 | 18.9 |
| Short of breath | 36 | 26 | 9 | 21.5 | 14.9 |
| Wheeze | 39 | 22 | 8 | 18.2 | 12.6 |
| Tired | 15 | 15 | 4 | 12.4 | 8.6 |
| Chest pain | 11 | 11 | 4 | 9.1 | 6.3 |
Symptom management behaviors
Controller medication.
Ten of the 14 teens (all with persistent asthma) reported taking controller medications (80/175 days; 45.7% of the time). Two teens were not prescribed controller medications; two were prescribed controller medications but never mentioned them.
Rescue medication.
Teens used rescue medication less than half of the days on which they reported having symptoms (52/121; 43.0%). Some did not take rescue medication even when symptoms were severe (15/40 severe symptom days; 37.5%). Participants indicated they did not use their inhaler at times because symptoms were not bad enough (27/121 days; 21.3%), or they did not have an accessible inhaler (11/121 days; 9.1%). Symptoms were triggered or exacerbated by exercise on 34.7% of symptom days (42/121), but few teens preemptively took rescue medication (9 instances).
Other.
Alternative symptom management strategies included taking cold or allergy medications (28/121 days), activity reduction (21/121 days), getting a drink (17/121 days), using breathing control (7/121 days), and taking a shower (5/121). Avoidance of triggers (e.g. cold air) was also mentioned (22/175 days).
Sentiment Analysis
Overall, the sentiment (i.e. the positive/negative emotional tone of the diary entries) of individual teen participants was stable, and did not increase or decrease significantly over the two weeks of study participation. Average sentiment for all diary entries was near neutral (0.578; SD 0.46), indicating neither strong negative nor positive emotional valence. All teens reported some level of asthma symptoms, from 1.3 up to 7 days per week. However, sentiment was not significantly correlated with baseline EPR-3 clinical asthma control (β=0.14, p=0.28). As hypothesized, teens who reported severe symptoms produced diary entries whose sentiment was significantly more negative than entries of teens who reported no symptoms (Table 3; 40 severe symptom days vs. 52 symptom-free days; β=−0.31, p=0.006). However, there was no statistically significant difference in sentiment between participants with mild/moderate symptoms and participants without symptoms (81 vs 52 days respectively; β= −0.09, p=0.34). There was also no significant difference in average sentiment (all days with or without symptoms) between teens who reported severe symptoms and those reporting only mild to moderate symptoms. As shown in Table 3, there were no other significant differences in sentiment by age, gender, race, or sex.
Table 3.
Linear mixed-effects model to showing change in sentiment over time in teen asthma diaries
|
Dependent variable: Sentiment |
|
|---|---|
| Day | −0.012 (−0.027, 0.004) |
| Age | 0.041 (−0.039, 0.120) |
| Race: African−Amer. | −0.096 (−0.383, 0.192) |
| Race: other | 0.050 (−0.204, 0.305) |
| Gender: male | −0.084 (−0.328, 0.159) |
| Asthma control: well−controlled | 0.144 (−0.103, 0.390) |
| Symptoms: mild/moderate | −0.087 (−0.263, 0.089) |
| Symptoms: severe | −0.311** (−0.529, −0.093) |
| Constant | 0.131 (−1.022, 1.283) |
| Observations | 175 |
| Log Likelihood | −119.882 |
| Akaike Inf. Crit. | 261.764 |
| Bayesian Inf. Crit. | 296.577 |
Notes: p<0.05
p<0.01
p<0.001
Discussion
This is the first study to use longitudinal voice diaries to explore patterns of symptoms and self-management responses in teens with asthma. Most teens in our sample perceived themselves as having controlled asthma despite regular and sometimes severe symptoms. In conjunction with prior research, this suggests that teens may not only misrepresent how often symptoms occur [22], but also how severe symptoms are. Our data suggest that symptom misperception may be a key factor in under-reporting [4, 23], with two conceptually distinct underlying reasons: minimizing (i.e. aware, but believing it not too bad), and not recognizing (either perceptually unaware or not recognizing a symptom as being due to asthma). While teens sometimes failed to recognize symptoms (e.g. coughing), they more often downplayed the experience. Thus, an important step in changing reporting patterns may be modifying awareness of the range of asthma symptoms and addressing beliefs about the potential importance of these symptoms [10, 24, 25].
In particular, our data highlight the need to screen for coughing and respiratory illness as a possible sign of uncontrolled asthma. Coughing and throat clearing (a cough variant) were the most commonly overlooked asthma symptoms in this sample [26, 27]. In fact, coughing was mentioned more than classically recognized asthma symptoms (e.g. tightness and wheezing). We further found that teens identified “being sick” a disproportionately high percentage of days (17%), which has not been previously noted in the literature. These rates of illness are markedly in excess of what would be expected. In general, incidence of the common cold ranges from 5 to 7 episodes per year (mean duration 7 days) in preschool children and decreasing to 2–3 episodes per year at maturity [28, 29]. Thus a teenager might be expected to experience 3–4 episodes a year, and cold symptoms less than 8% of the time. While it is impossible to determine retrospectively whether the high frequency of cough and cold symptoms was indeed due to asthma, illness, allergic rhinitis or otherwise, the discrepancy between self-reported versus expected rates of illness raises suspicions that excessive reports of coughing/illness might be attributable to asthma, either as a result of symptom misperception or true increased frequency of illness stemming from uncontrolled disease. In either case, increasing the dose of controller medication in response to recurrent illness episodes is likely to promote better asthma control, and further research on illness patterns in this population may be warranted.
It is also worth noting that while all teens in this study had persistent asthma, they reported using controller medication less than half of days, and rescue medication less than half of instances where they had symptoms. Due to the unstructured nature of the dairies, it is unclear whether teens were not using medication the remainder of the time, or whether they did not feel it was important enough to mention. Either explanation is concerning, as necessity beliefs and personal priorities are a basis for self-management behaviors [30–32]. Clinicians may want to consider asking about patterns of medication use and reasons for using or not using medications when attempting to address issues of medication adherence.
We believe this is the first study to examine sentiment in relation to asthma control and symptom severity. In our small sample, narratives about asthma were generally neutral to marginally positive. As expected, there was a highly significant correlation between negative sentiment and severe symptoms, however this was not the case for mild or moderate symptoms. This is somewhat surprising, and raises the question as to whether teens are not bothered by milder symptoms (i.e. becoming used to symptoms), or whether our sample size was too small to detect more subtle variations in sentiment related to milder symptoms. These exploratory findings should be interpreted with caution, but may point to sentiment analysis as another possible means for evaluating high levels of symptom severity. Given that prior research has shown strong correlation between sentiment scores and anxiety/depression [33, 34], as well as transient negative experiences [35], it may be worthwhile to further explore the relationship between symptom perception, sentiment, and general affective state.
This is also the first study to use voice-diaries to capture asthma-related experiences over time. Written symptom diaries and journals have been used for decades as a means of estimating symptom levels for both epidemiologic and instrument validation purposes [36–39]. Albeit useful in reducing recall bias, paper diary methods are limited by absolute reliance on an individual’s ability to recognize and accurately report symptoms [38]. Yet, if teens fail to recognize symptoms, they may not be able to accurately quantifying them using this method [12, 27, 40]. Additionally, paper diaries have traditionally low compliance rates (e.g. 30%), whereas the two-week voice-diary completion rate in this study was greater than 92% [41]. Other advantages of voice-diary technique included ability to capture symptoms the individual was not aware of (e.g. coughing, colds), along with a wealth of fine detail (audible symptoms, descriptions of daily events) which served to paint an illuminating picture of the quality and quantity of symptoms, underlying thinking, perceptual patterns, and self-management responses to situations. Lastly, because of the largely unconstrained format, individuals were able to prioritize what was most important to them, thereby offering a far more personalized picture. This type of knowledge may be useful in developing future strategies for self-management interventions. Thus, voice-diaries have potential to add needed qualitative insight to traditionally quantitative symptom monitoring methods, however, may be more time and resource intensive to analyze.
Limitations and future research.
Generalizability of findings from this study are limited by the small sample size and non-random sampling strategies, as well as the unstructured nature of diary recordings. It is possible that participants did not report all symptoms or medication use, and frequency of both these items may therefore be higher than indicated by our data. Despite these limitations, we believe the high completion rate (>92%) and multiple data collection points over time greatly enhance the credibility of these findings. Further research may be useful to explore patterns of medication use, beliefs about asthma medication, patterns of respiratory illness and coughing in teens with asthma, and the broader utility of voice-diary methods for gathering data on symptom patterns and self-management approaches. Study replication with a larger sample of teens with diverse background and correlation of perceived/unrecognized symptoms and narrative sentiment with other key outcomes (e.g. FEV1, nitric oxide, or validated measures of asthma control and quality of life) would help to establish generalizability and validity of findings.
Conclusion
Teens appear to have higher symptom frequency than they report, and may be unaware of the severity of their asthma. This may be due to failure to recognize or minimizing the importance of symptoms. Careful assessment and education regarding specific symptoms may be necessary. Exploring emotional perceptions of asthma and asking about atypical symptoms such as coughing, respiratory illness, and throat symptoms should be considered during routine assessments to help identify those who may be experiencing physical and psychological burden from asthma.
Acknowledgements:
Research reported in this publication was supported by the National Institute of Nursing Research of the National Institutes of Health (F31NR014952) and partially supported by Sigma Theta Tau, Epsilon Xi. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors also wish to thank the teens and parents who participated in this study.
This study was supported by the National Institute of Nursing Research of the National Institutes of Health (F31NR014952) and Sigma Theta Tau Nursing Honor Society. The study was reviewed by the University of Rochester Institutional Review Board and age-appropriate assent/consent obtained.
Footnotes
Conflict of Interest
The authors have no conflict of interest to declare.
References
- 1.National Heart Lung and Blood Institues [NHLBI]. Asthma care quick reference Bethesda, Md.: National Asthma Education and Prevention Program; 2011. [Google Scholar]
- 2.Burkhart PV, Svavarsdottir EK, Rayens MK, Oakley MG, Orlygsdottir B. Adolescents with asthma: predictors of quality of life. JAN 2009;65(4):860–6. [DOI] [PubMed] [Google Scholar]
- 3.Bruzzese JM, Reigada LC, Lamm A, Wang J, Li M, Zandieh SO, et al. Association of Youth and Caregiver Anxiety and Asthma Care Among Urban Young Adolescents. Acad Pediatr 2016;16(8):792–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhee H, Belyea MJ, Halterman JS. Adolescents’ perception of asthma symptoms and health care utilization. J Pediatr Health Care 2011;25(2):105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhee H, Belyea MJ, Sterling M, Bocko MF. Evaluating the Validity of an Automated Device for Asthma Monitoring for Adolescents: Correlational Design. J Med Internet Res 2015;17(10):e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butz A, Kub J, Donithan M, James NT, Thompson RE, Bellin M, et al. Influence of caregiver and provider communication on symptom days and medication use for inner-city children with asthma. J Asthma 2010;47(4):478–85. [DOI] [PubMed] [Google Scholar]
- 7.Krishnan JA, Lemanske RF Jr., Canino GJ, Elward KS, Kattan M, Matsui EC, et al. Asthma outcomes: symptoms. J Allergy Clin Immunol 2012;129(3 Suppl): S124–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welsh EJ, Carr R. Pulse oximeters to self monitor oxygen saturation levels as part of a personalised asthma action plan for people with asthma. Cochrane Database Syst Rev 2015(9):CD011584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kew KM, Cates CJ. Home telemonitoring and remote feedback between clinic visits for asthma. Cochrane Database Syst Rev 2016(8):CD011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holley S, Walker D, Knibb R, Latter S, Liossi C, Mitchell F, et al. Barriers and facilitators to self-management of asthma in adolescents: An interview study to inform development of a novel intervention. Clin Exp Allergy 2018;[ahead of press]. [DOI] [PubMed]
- 11.Britto MT, Byczkowski TL, Hesse EA, Munafo JK, Vockell ALB, Yi MS. Overestimation of impairment-related asthma control by adolescents. J Pediatr 2011;158(6):1028–30. [DOI] [PubMed] [Google Scholar]
- 12.Mammen JR, Rhee H, Norton SA, Butz AM. Perceptions and experiences underlying self-management and reporting of symptoms in teens with asthma. J Asthma 2017;54(2):143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mammen JR, Rhee H, Norton SA, Butz AM, Halterman JS, Arcoleo KS. An Integrated Operational Definition and Conceptual Model of Asthma Self-Management in Teens. J Asthma (in press). [DOI] [PMC free article] [PubMed]
- 14.Liu B Sentiment Analysis and Opinion Mining. Synthesis Lectures on Human Language Technologies [Internet] 2012; 5(1):[1–167 pp.]. Available from: 10.2200/S00416ED1V01Y201204HLT016. [DOI] [Google Scholar]
- 15.Fung CK, Moore MM, Karcher NR, Kerns JG, Martin EA. Emotional word usage in groups at risk for schizophrenia-spectrum disorders: An objective investigation of attention to emotion. Psychiatry Res 2017;252:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Heart Lung and Blood Institues [NHLBI]. Expert Panel Report 3: Guidelines for the diagnosis and management of asthma Bethesda, Md.: National Asthma Education and Prevention Program; 2007. [Google Scholar]
- 17.Saldaña J The Coding Manual for Qualitative Researchers 2nd ed. Washington, DC: Sage; 2013. [Google Scholar]
- 18.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15(9):1277–88. [DOI] [PubMed] [Google Scholar]
- 19.Cleveland Clinic. Asthma Symptoms Cleveland, OH2017. Available from: https://my.clevelandclinic.org/health/articles/symptoms-of-asthma.
- 20.Indurkhya N, Damerau F. Handbook of Natural Language Processing 2nd ed. Hebrich R, Graepel T, editors. Cambridge, UK: Microsoft Research Ltd.; 2010. [Google Scholar]
- 21.US Department of Housing and Urban Development [US DoHUD]. Uniform Act or URA - FY 2011 Low Income Limits State of NY 2015. Available from: https://www.huduser.gov/datasets/ura/ura15/FY2015_URA_ny.pdf.
- 22.Rhee H, Belyea M, Mammen J. Visual analogue scale (VAS) as a monitoring tool for daily changes in asthma symptoms in adolescents: a prospective study. Allergy Asthma Clin Immunol 2017;13:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee H, Belyea MJ, Elward KS. Patterns of asthma control perception in adolescents: associations with psychosocial functioning. J Asthma 2008;45(7):600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becker M The health belief model and personal health behavior. Health Educ Quart 1977;2(326). [Google Scholar]
- 25.Sofianou A, Martynenko M, Wolf MS, Wisnivesky JP, Krauskopf K, Wilson EA, et al. Asthma beliefs are associated with medication adherence in older asthmatics. J Gen Internal Med 2013;28(1):67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhee H, Fairbanks E, Butz A. Symptoms, feelings, activities and medication use in adolescents with uncontrolled asthma: lessons learned from asthma diaries. J Pediatr Nurs 2013;29(1):39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhee H, Allen J, Mammen J, Swift M. Mobile phone-based asthma self-management aid for adolescents (mASMAA): a feasibility study. Patient Prefer Adher 2014;8:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sexton D, McClain M. The common cold in adults: diagnosis and clinical features Waltham, MA: UpToDate; [cited 2017]. Available from: https://www.uptodate.com. [Google Scholar]
- 29.Prasad AS, Beck FW, Bao B, Snell D, Fitzgerald JT. Duration and severity of symptoms and levels of plasma interleukin-1 receptor antagonist, soluble tumor necrosis factor receptor, and adhesion molecules in patients with common cold treated with zinc acetate. J Infect Dis 2008;197(6):795–802. [DOI] [PubMed] [Google Scholar]
- 30.Holley S, Morris R, Knibb R, Latter S, Liossi C, Mitchell F, et al. Barriers and facilitators to asthma self-management in adolescents: A systematic review of qualitative and quantitative studies. Pediatr Pulmonol 2017;52(4):430–42. [DOI] [PubMed] [Google Scholar]
- 31.Horne R, Chapman SC, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the Necessity-Concerns Framework. PLoS One 2013;8(12):e80633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koster ES, Philbert D, Winters NA, Bouvy ML. Adolescents’ inhaled corticosteroid adherence: the importance of treatment perceptions and medication knowledge. J Asthma 2015;52(4):431–6. [DOI] [PubMed] [Google Scholar]
- 33.Sood A, Hooda M, Dhir S, Bhatia M. An initiative to identify depression using sentiment analysis: a machine learning approach. Indian J Science Technol 2018;11(4):1–6. [Google Scholar]
- 34.Park M, Cha C, Cha M. Depressive moods of users portrayed in twitter ACM; Beijing, China: 2012. [Google Scholar]
- 35.Mammen J, Elson M, Java J, Beck C, Beran D, Biglan K, et al. Patient and Physician Perceptions of Virtual Visits for Parkinson’s Disease: A Qualitative Study. Telemed J eHealth 2017. [DOI] [PubMed]
- 36.Juniper EF. Asthma Control Questionnaire: background, administration and analysis. In: QOL Technologies, editor. 2004.
- 37.Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the Asthma Quality of Life Questionnaire. Chest 1999;115(5):1265–70. [DOI] [PubMed] [Google Scholar]
- 38.Reznik M, Sharif I, Ozuah PO. Classifying asthma severity: prospective symptom diary or retrospective symptom recall? J Adolesc Health 2005;36(6):537–8. [DOI] [PubMed] [Google Scholar]
- 39.Globe G, Wiklund I, Lin J, Chen WH, Martin M, Mattera MS, et al. Psychometric Properties of the Asthma Symptom Diary (ASD), a Diary for Use in Clinical Trials of Persistent Asthma. J Allergy Clin Immunol Pract 2016;4(1):60–6 e4. [DOI] [PubMed] [Google Scholar]
- 40.Mulvaney SA, Ho YX, Cala CM, Chen Q, Nian H, Patterson BL, et al. Assessing adolescent asthma symptoms and adherence using mobile phones. J Med Internet Res 2013;15(7):e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient compliance with paper and electronic diaries. Control Clin Trials 2003;24(2):182–99. [DOI] [PubMed] [Google Scholar]


