Abstract
Melioidosis is caused by the gram-negative bacillus Burkholderia pseudomallei, endemic to northern Australia and Southeast Asia. We present a patient who traveled to Mexico, returned to the United States, and developed progressive manifestations of melioidosis, culminating as central nervous system disease. Standard therapy was contraindicated, and a prolonged intensive phase was employed.
Keywords: melioidosis, Burkholderia pseudomallei, CNS, Mexico, travel
CASE REPORT
A 60-year-old woman presented to a hospital in Rochester, New York, with fever and cough, 1 week after a 9-day stay in Cabo San Lucas, Baja California Sur, Mexico, in late August to early September 2017. There, she experienced construction near her residence and heavy rainfall, overlapping with the arrival of Tropical Storm Lidia. Her history was notable for progressive multiple myeloma (diagnosed 13 years previously, recently treated with carfilzomib, lenalidomide, and dexamethasone), pulmonary embolism (on warfarin), penicillin allergy (anaphylaxis), and a travel history additionally notable for 2 Caribbean cruises in 2009 and 2010. Chest x-ray revealed an infiltrate, she was treated with cefpodoxime and doxycycline for community-acquired pneumonia, her symptoms resolved, and she was discharged after 4 days.
Two weeks after discharge, she presented with fever and meningismus. Lumbar puncture (LP) was delayed until reversal of anticoagulation. Following antibiotic initiation, LP was completed and revealed 831 nucleated cells/uL (87% neutrophils), 118 mg/dL protein, and 28 mg/dL glucose. All cerebrospinal fluid (CSF) microbiological tests returned negative. Magnetic resonance imaging (MRI) of the head was unremarkable. Due to persistent fevers, she was transitioned to meropenem and trimethoprim-sulfamethoxazole (TMP-SMX) and clinically improved. She was discharged after 9 days in the hospital, and she completed 3 weeks of meropenem and 2 weeks of TMP-SMX at home. TMP-SMX was stopped early due to severe drug-induced thrombocytopenia to a nadir of 18th ou/uL, which resolved shortly after cessation.
Two weeks after antibiotic cessation, she presented with new-onset generalized tonic-clonic seizure and fever. She was started on meropenem, vancomycin, and acyclovir. LP was completed after antibiotic initiation and revealed 7 nucleated cells/uL, 56 mg/dL protein, and 51 mg/dL glucose. CSF microbiologic tests were again negative. MRI of the head revealed left sphenoid wing osteomyelitis, left frontal pachymeningitis, and extra-axial empyema with an intraparenchymal component and surrounding cerebritis (Figure 1A, B). She underwent nasopharyngoscopy and left sphenoid bone biopsy with negative cultures. A dural tissue biopsy was performed, and the culture showed 1+ nonlactose fermenting gram-negative bacilli, identified by the New York State Public Health laboratory, Wadsworth Center, as Burkholderia pseudomallei. After a 23-day hospitalization, her symptoms resolved, and she returned home on meropenem with addition of doxycycline.
Figure 1.
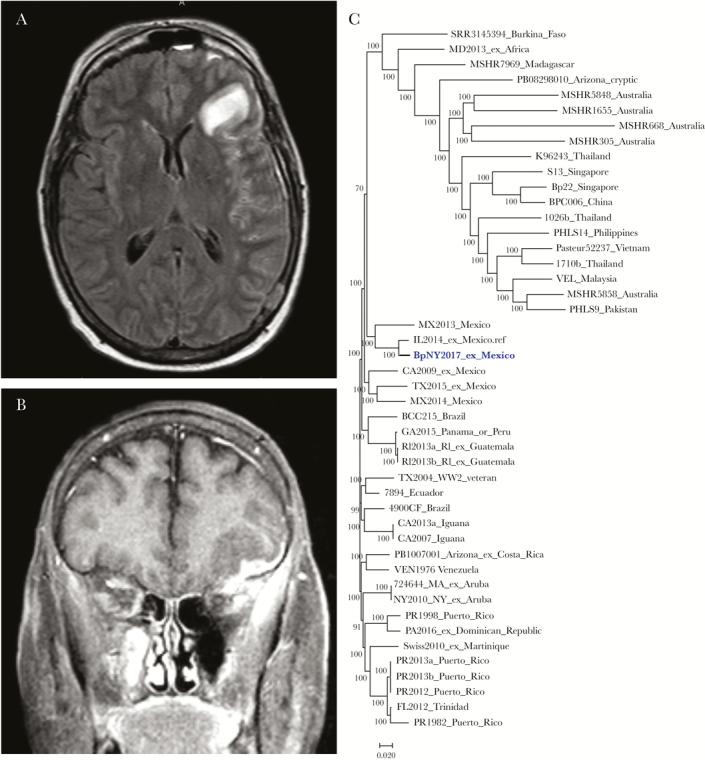
A, Axial T2-weighted magnetic resonance imaging (MRI) head with fluid-attenuated inversion recovery (FLAIR) sequencing. B, Coronal T1-weighted MRI head. C, Dendrogram. The isolate from the patient is indicated as BpNY2017. The term “ex” is to delineate the location where isolates were believed to have been acquired, based on travel history.
DISCUSSION
Melioidosis is an infection caused by B. pseudomallei, a nonlactose fermenting gram-negative bacillus with a characteristic “safety pin” appearance on gram stain. It is found in soil and groundwater in endemic areas, namely northern Australia and Southeast Asia [1]. Local and travel-related cases are increasingly reported in the Western hemisphere, including the Caribbean, Mexico, and Central and South America [2–5].
B. pseudomallei may be transmitted from the environment via percutaneous inoculation, inhalation, or ingestion. Subsequent infection may be asymptomatic (evidenced by high seropositivity in endemic regions), latent (4% of cases in the Darwin Prospective Study, with reactivation described years after exposure), chronic, or acute [6]. Manifestations of acute melioidosis vary and partially reflect the mode of transmission. Acute disease ranges from limited cutaneous involvement to pneumonia, sepsis, and central nervous system (CNS) disease [6]. In the Darwin Prospective Study, pneumonia was the primary focus of infection in 51% of cases, and incidence of pneumonia was correlated with rainfall [6]. The majority of cases occur in the rainy season, and intensity of rainfall was an independent predictor of fatal outcome [7]. CNS disease only accounted for 3% of melioidosis cases in northern Australia and is rarely seen in other geographic regions [6]. B. pseudomallei has a predilection for the frontal lobe and brainstem and may manifest as stroke, meningitis, encephalitis, or abscess [8]. In a case series of 12 patients with CNS melioidosis, 3 (25%) patients died and only 3 (25%) patients had a complete recovery [8].
Diagnosis requires a positive culture, which can be difficult to obtain. In some instances, <50% of melioidosis cases will have a positive blood culture; thus culture growth may require a targeted sample [9]. Rapid detection of B. pseudomallei by polymerase chain reaction is available through public health laboratories in the United States but is not readily available in clinical laboratories. Additionally, definitive characterization can take up to a week and may be problematic, as some biochemical assays can misidentify B. pseudomallei as other bacteria [10]. Recognition of a typical susceptibility pattern (resistance to aminoglycosides and polymyxins; susceptibility to amoxicillin-clavulanic acid) may aid in identification while awaiting definitive testing [11].
Treatment is complicated by antibiotic resistance, slow response to appropriate antibiotics, and, when indicated, the necessity of adequate CNS penetration. To avoid treatment failure, a 2-step approach is recommended. Initial intensive therapy traditionally consists of 10–14 days of ceftazidime, meropenem, or imipenem, with or without the addition of TMP-SMX [11–13]. Initial intensive therapy is followed by at least 3 months of eradication therapy with TMP-SMX monotherapy [11–13]. Relapses occur almost exclusively in patients not compliant with eradication therapy, and little evidence exists for the efficacy of regimens using other antibiotics as monotherapy for eradication [13]. Doxycycline has been used as an adjunct to TMP-SMX in eradication therapy, but when used alone, it has been associated with a higher rate of relapse [13].
Our case is unique in (1) the geographic location in which the patient was infected, (2) the progression and extent of disease, and (3) the need for an alternative treatment regimen. The patient was probably infected in Cabo San Lucas, Baja California Sur, Mexico, via inhalation of contaminated water or dust, supported by her experience of heavy rainfall and construction during her stay, strengthening the association of travel-related melioidosis to environmental events within the Western hemisphere.
The patient developed pneumonia within a week of exposure, and B. pseudomallei was likely partially treated with the doxycycline component of her antibiotic regimen targeted at community-acquired pneumonia. Her disease then progressed from primary pneumonia to meningitis, ultimately culminating as sphenoid osteomyelitis, pachymeningitis, extra-axial empyema, and cerebritis. The extent of her CNS disease has rarely been described outside of northern Australia.
The patient’s severe thrombocytopenia after 2 weeks of TMP-SMX introduced a therapeutic dilemma, as little evidence exists for eradication therapy with other antibiotics [13]. No consensus guidelines exist to guide duration of parenteral therapy for CNS melioidosis in the absence of TMP-SMX eradication, but case reports have suggested the effectiveness of a prolonged initial intensive regimen of 6 to 8 weeks [14]. Thus, we elected for a prolonged intensive therapy with a carbapenem and added doxycycline for augmentation. After approximately 5 weeks of treatment, susceptibility testing performed at the Centers for Disease Control and Prevention (CDC) revealed a lower minimum inhibitory concentration for imipenem, which was then substituted for meropenem. Since then, serial head MRIs of the patient have shown interval improvement but persistent enhancement of the left dural tissue and left frontal white matter. The patient has received a total of 21 weeks of carbapenem-based treatment overall, with a plan to continue holding chemotherapy, retry TMP-SMX in place of doxycycline, and continue imipenem-based prolonged intensive therapy beyond radiographic resolution.
A comprehensive laboratory investigation by the New York State Department of Health and the CDC was completed on the isolate, BpNY2017. Multilocus sequence typing (MLST) characterized the isolate as ST1459, which is seen in examples from South America [15]. Higher-resolution characterization with single nucleotide polymorphism (SNP) analysis of the draft whole-genome sequence (accession number: SRP145120) indicates that BpNY2017 is within the population of isolates with origins in the Western hemisphere [3]. It is also similar to but not clonal with IL2014, an isolate with ST92 from an Illinois resident who traveled to Cabo San Lucas, Baja California Sur, Mexico, during a hurricane in 2014 and returned with melioidosis pneumonia [2]. A dendrogram based on the whole-genome SNP analysis in Parsnp (Harvest 1.3) illustrates the relatedness of the 2 isolates in Figure 1C.
CONCLUSIONS
This case presentation further confirms the presence of B. pseudomallei in Mexico. Melioidosis should be a consideration in returning travelers from Mexico, if presenting with pneumonia or CNS infection refractory to routine antibiotic regimens, particularly from hurricane-prone areas.
Acknowledgments
We thank the New York State Department of Health and the Centers for Disease Control and Prevention for their help in this patient’s diagnosis and investigation. We thank Sandra Boyd at the CDC for performing antimicrobial susceptibility testing.
Financial support. None to declare.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Disclaimers. This publication made use of the Burkholderia pseudomallei MLST website (http://pubmlst.org/bpseudomallei/) hosted by the University of Oxford. The development of this site was funded by the Wellcome Trust. The conclusions, findings, and opinions expressed by the authors contributing to this journal do not necessarily reflect the official position of the US Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors’ affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
References
- 1. Currie BJ, Dance DA, Cheng AC. The global distribution of Burkholderia pseudomallei and melioidosis: an update. Trans R Soc Trop Med Hyg 2008; 102(Suppl 1):S1–4. [DOI] [PubMed] [Google Scholar]
- 2. Cheng JW, Hayden MK, Singh K, et al. Burkholderia pseudomallei infection in US traveler returning from Mexico, 2014. Emerg Infect Dis 2015; 21:1884–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gee JE, Gulvik CA, Elrod MG, et al. Phylogeography of Burkholderia pseudomallei Isolates, Western hemisphere. Emerg Infect Dis 2017; 23:1133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rolim D, Lima R, Ribeiro A, et al. Melioidosis in South America. Trop Med Infect Dis 2018; 3:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanchez-Villamil J, Torres A. Melioidosis in Mexico, Central America, and the Caribbean. Trop Med Infect Dis 2018; 3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Currie BJ, Ward L, Cheng AC. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis 2010; 4:e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Currie BJ, Jacups SP. Intensity of rainfall and severity of melioidosis, Australia. Emerg Infect Dis 2003; 9:1538–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Currie BJ, Fisher DA, Howard DM, Burrow JN. Neurological melioidosis. Acta Trop 2000; 74:145–51. [DOI] [PubMed] [Google Scholar]
- 9. Wuthiekanun V, Limmathurotsakul D, Wongsuvan G, et al. Quantitation of B. Pseudomallei in clinical samples. Am J Trop Med Hyg 2007; 77:812–3. [PubMed] [Google Scholar]
- 10. Novak RT, Glass MB, Gee JE, et al. Development and evaluation of a real-time PCR assay targeting the type III secretion system of Burkholderia pseudomallei. J Clin Microbiol 2006; 44:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. N Engl J Med 2012; 367:1035–44. [DOI] [PubMed] [Google Scholar]
- 12. Limmathurotsakul D, Peacock SJ. Melioidosis: a clinical overview. Br Med Bull 2011; 99:125–39. [DOI] [PubMed] [Google Scholar]
- 13. Lipsitz R, Garges S, Aurigemma R, et al. Workshop on treatment of and postexposure prophylaxis for Burkholderia pseudomallei and B. mallei infection, 2010. Emerg Infect Dis 2012; 18:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pitman MC, Luck T, Marshall CS, et al. Intravenous therapy duration and outcomes in melioidosis: a new treatment paradigm. Plos Negl Trop Dis 2015; 9:e0003586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Godoy D, Randle G, Simpson AJ, et al. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. J Clin Microbiol 2003; 41:2068–79. [DOI] [PMC free article] [PubMed] [Google Scholar]


