Abstract
Mutations in pre-mRNA splicing factors are the second most common cause of autosomal dominant retinitis pigmentosa, and a major cause of vision loss [1–3]. The development of gene augmentation therapy for disease caused by mutations in PRPF31 necessitates defining pre-treatment characteristics and disease progression of patients with PRPF31-related retinitis pigmentosa. We show rates of visual field area decline of 6.9% per year and 30-Hz flicker cone response of −9.2% per year, which are both similar to observed rates for retinitis pigmentosa. We hypothesize that RNA splicing factor retinitis pigmentosa will be amenable to treatment by AAV-mediated gene therapy, and that understanding the clinical progression rates of PRPF31 retinitis pigmentosa will help with the design of gene therapy clinical trials.
Keywords: Inherited retinal degeneration, retinitis pigmentosa, RNA splicing factor
Introduction
Mutations in PRPF31 are one of the most common causes of autosomal dominant retinitis pigmentosa. In the eyeGENE Network, this gene was found to account for 2.5% of autosomal dominant retinitis pigmentosa [3]. Most PRPF31 pathogenic mutations are single-base changes or deletions that lead to premature termination codons and nonsense mediated mRNA decay [4]. This suggests pathogenic mutations cause disease through haploinsufficiency [4, 5]. In support of this hypothesis, levels of expression of the remaining wild-type allele of PRPF31 might be a contributing factor to the severity of retinitis pigmentosa [5, 6]. As PRPF31 mutations cause retinitis pigmentosa by haploinsufficiency, it is a good candidate for AAV-based gene augmentation therapy.
It has been demonstrated the retinal pigment epithelium (RPE) is the primary cell type affected in RNA splicing factor retinitis pigmentosa [7]. This finding in combination with the known mutation mechanism of disease to be haploinsufficiency makes it an ideal candidate for gene augmentation therapy. To move forward with this goal, we first needed to define the pre-treatment clinical characteristics of PRPF31-related retinitis pigmentosa. To do this, we specifically focused on parameters including central visual acuity, visual field area, and 30-Hz cone flicker ERG amplitude. We chose to focus on the 30-Hz flicker stimulus as it has been reported to show less variability than the light-adapted single flash [8]. Rates of decline of visual field area decline are −6.9% per year and 30-Hz cone flicker are −9.2% a year. There was heterogeneity in the rate of change in visual field area and 30-Hz amplitude, which may be due to a combination of genetic modifiers, allelic heterogeneity, and inter-visit variability. Future studies will determine if newer imaging studies such as fundus autofluorescence and the optical coherence tomography are more sensitive for following disease progression in clinical trials.
Methods
This study was approved by the Institutional Review Board of the Massachusetts Eye and Ear Infirmary and Harvard Medical School. From a database of approximately 8000 patients with retinitis pigmentosa, 26 patients with a pathogenic mutation in PRPF31 were identified using molecular genetics.
Best corrected Snellen visual acuities, Goldmann kinetic visual field areas (V4e white test light), and dark adapted full-field ERG amplitudes to 30-Hz flashes were measured. The lower normal for visual field area is 11,399 deg2 and the lower normal for 30-Hz ERG amplitude is 50 μV. Statistical analysis was performed with JMP, version 10.0. Visual acuity measurements were converted to decimal values and ERG amplitudes were transformed to natural logarithms. The range of normal values for the ERG amplitudes to 30-Hz flashes was 50–125 μV. To determine longitudinal exponential decay rates, regression analysis was performed with JMP, version 10.0. Data was censored to reduce ceiling and floor effects.
Results
Retinal findings in patients with PRPF31-related retinitis pigmentosa were noted to include typical areas of degeneration of the retinal pigment epithelium with retinitis pigmentosa-associated bone spicules. Pallor of the optic nerve and attenuation of the vessels was often observed. Rates of ERG recordings and visual field area progression vary. With the rapid advance of ocular gene therapies, we characterized the rates of progression of ERG 30-Hz flicker amplitudes and visual field area.
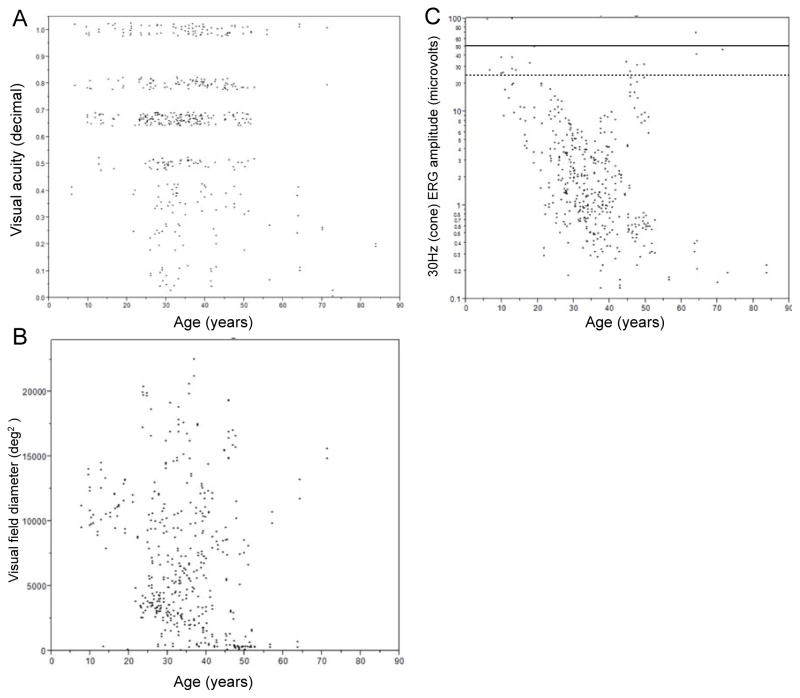
Figure 1 shows the mean annual exponential rates of change for visual acuity, visual field area, and 30-Hz ERG amplitude. The mean rate of change for visual acuity was −2.0% (p<0.05) (Figure 1A). Thus, given the slow rate of change, it is unlikely to be a useful parameter for a short-term clinical trial for PRPF31 retinitis pigmentosa. It is of interest to note that visual acuity is a sensitive parameter for the RPE65-related Leber Congenital Amaurosis clinical trial in which vision is profoundly affected from an early age [9].
Figure 1.
Vision plotted as a function of age among patients with PRPF31-associated retinitis pigmentosa. Visual acuity (A) and visual field diameter (deg2) (B) are plotted for every patient visit for each eye individually. Visual acuity has been converted to decimal notation. Lower normal is 11,399 deg2 for visual field area with a V4e light stimulus. 30-Hz flicker ERG amplitudes (C) by age among patients with PRPF31-associated retinitis pigmentosa. The 30-Hz (cone) amplitudes are plotted for every patient visit for each eye individually. The solid horizontal line represents the lower limit of normal for 30-Hz amplitude (50 μV) and the dashed horizontal line represents half the lower limit of normal (25 μV).
The mean rate of change of visual field area using the Goldmann visual field with a V4e stimulus (16mm2) was −6.9% during the period of observation (p<0.001) (Figure 1B). This rate is similar to the reported observed rate of change of 5% to 15% per year for patients with retinitis pigmentosa [10]. It is worth noting though there was variability in the rate of visual field progression in our study. While this may be due to genetic heterogeneity, it has also been reported that visual field testing in patients with retinitis pigmentosa has less reproducibility than in unaffected individuals [11].
The mean rate of change for ERG amplitude to 30-Hz flashes was −9.2% (p<0.001) (Figure 1C). This is equivalent to approximately a loss of 50% of function every 7 years. Variability was observed in the rate of progression between patients with PRPF31– related retinitis pigmentosa. Part of this variability may be due to inter-visit variability of ERG responses. In 32 patients with a clinical diagnosis of retinitis pigmentosa, a reduction of 35% of the 30-Hz flicker stimulus amplitude was found to be significant [12]. In 40 unaffected subjects, a 52% amplitude reduction in the 31-Hz stimulus was significant, indicating a high amount of test-retest variation [13]. Inter-visit variability increases in patients with retinitis pigmentosa as the amplitude decreases compared to controls [8]. This suggests that given the rates of full field ERG amplitude decline, this may also not be an optimal parameter for detecting change in a PRPF31 gene therapy clinical trial where only a small area of the retina is treated.
Recent research shows that optical coherence tomography may help determine the progression of retinitis pigmentosa with a higher degree of sensitivity than conventional methods. In particular, decreases in the thickness of the outer nuclear layer and outer segment have been reported to provide a quantitative measurement of the progression in retinitis pigmentosa [14]. The inner segment ellipsoid zone is a higher reflective structure, and where it disappears, indicates loss of the outer segment. The mean rate of decline in the ellipsoid zone width is 7% for patients with X-linked retinitis pigmentosa [14], which is similar to the rates of change for visual field area and ERG amplitude observed in our cohort. Of interest, there was low repeat variability (0.08° test-retest difference or 3.6%) [14]. The low test-retest variability of the ellipsoid zone width suggests that it may be a useful measurement for future studies of retinal gene therapy. Limitations of using the ellipsoid zone width include advanced retinitis pigmentosa when the ellipsoid zone is lost as well as early retinitis pigmentosa when it is preserved in the central 30° [15].
Fundus autofluorescence using wide-field imaging has recently been shown to correlate with Goldmann perimetry. This suggests it may be a useful measure for monitoring response to a therapy in a clinical trial for retinitis pigmentosa. Fundus hypo-autofluorescence correlates with RPE degeneration and provides an objective measurement as to the rate of disease progression. A recent study showed visual field testing in patients with retinitis pigmentosa has a high degree of variability, as it is a subjective test dependent on both the patient and tester’s attention and cooperation [16]. Further, in advanced retinitis pigmentosa, the optokinetic reflex often reorients the eyes to the visual field target, which may increase the visual field size [16]. Thus, given that fundus autofluorescence has a high degree of correlation to the visual field using Goldmann perimetry, ongoing studies will determine the rate of autofluorescence loss in patients with PRPF31–related retinitis pigmentosa. The other benefit to using fundus autofluorescence in an ocular gene therapy clinical trial is that it can be determined whether the treated area of retina is specifically rescued from further degeneration. In the phase I/II choroideremia clinical trial, the area of fundus autofluorescence was similar at baseline and 6 months after treatment.[17]. Thus in long term clinical trial results, the rate of decline in fundus autofluorescence can be compared to the untreated eye, providing an estimate of disease progression.
Conclusion
While visual field area and ERG 30-Hz amplitude may be used to follow progression in patients with PRPF31–related retinitis pigmentosa, we observed heterogeneity in disease progression in this cohort of patients. This may be due to allelic heterogeneity, genetic modifiers, and inter-test variability. Recent advances in imaging including measuring the ellipsoid band width with optical coherence tomography and fundus autofluorescence may help decrease inter-test variability compared to conventional methods including full-field ERG and Goldmann perimetry. Ongoing analysis of these parameters in the cohort of patients with PRPF31–related retinitis pigmentosa will help further define the pre-treatment characteristic of this cohort, and may give insight into the design of optimal outcome measurements for ocular gene therapy clinical trials.
References
- 1.Daiger SP, Bowne SJ, Sullivan LS. Perspective on genes and mutations causing retinitis pigmentosa. Arch Ophthalmol. 2007;125(2):151–8. doi: 10.1001/archopht.125.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368(9549):1795–809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan LS, et al. Prevalence of mutations in eyeGENE probands with a diagnosis of autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2013;54(9):6255–61. doi: 10.1167/iovs.13-12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rio Frio T, et al. Premature termination codons in PRPF31 cause retinitis pigmentosa via haploinsufficiency due to nonsense-mediated mRNA decay. J Clin Invest. 2008;118(4):1519–31. doi: 10.1172/JCI34211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vithana EN, et al. Expression of PRPF31 mRNA in patients with autosomal dominant retinitis pigmentosa: a molecular clue for incomplete penetrance? Invest Ophthalmol Vis Sci. 2003;44(10):4204–9. doi: 10.1167/iovs.03-0253. [DOI] [PubMed] [Google Scholar]
- 6.McGee TL, et al. Evidence that the penetrance of mutations at the RP11 locus causing dominant retinitis pigmentosa is influenced by a gene linked to the homologous RP11 allele. Am J Hum Genet. 1997;61(5):1059–66. doi: 10.1086/301614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farkas MH, et al. Mutations in Pre-mRNA Processing Factors 3, 8, and 31 Cause Dysfunction of the Retinal Pigment Epithelium. Am J Pathol. 2014 doi: 10.1016/j.ajpath.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fishman GA, et al. Short-term inter-visit variability of erg amplitudes in normal subjects and patients with retinitis pigmentosa. Retina. 2005;25(8):1014–21. doi: 10.1097/00006982-200512000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Maguire AM, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358(21):2240–8. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seiple W, et al. Test-retest reliability of the multifocal electroretinogram and humphrey visual fields in patients with retinitis pigmentosa. Doc Ophthalmol. 2004;109(3):255–72. doi: 10.1007/s10633-005-0567-0. [DOI] [PubMed] [Google Scholar]
- 11.Ogura S, et al. Wide-field fundus autofluorescence imaging to evaluate retinal function in patients with retinitis pigmentosa. Am J Ophthalmol. 2014;158(5):1093–8. doi: 10.1016/j.ajo.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Berson EL, et al. Natural course of retinitis pigmentosa over a three-year interval. Am J Ophthalmol. 1985;99(3):240–51. doi: 10.1016/0002-9394(85)90351-4. [DOI] [PubMed] [Google Scholar]
- 13.Grover S, et al. Variability of full-field electroretinogram responses in subjects without diffuse photoreceptor cell disease. Ophthalmology. 2003;110(6):1159–63. doi: 10.1016/S0161-6420(03)00253-7. [DOI] [PubMed] [Google Scholar]
- 14.Birch DG, et al. Spectral-domain optical coherence tomography measures of outer segment layer progression in patients with X-linked retinitis pigmentosa. JAMA Ophthalmol. 2013;131(9):1143–50. doi: 10.1001/jamaophthalmol.2013.4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramachandran R, et al. A Comparison of Methods for Tracking Progression in X-Linked Retinitis Pigmentosa Using Frequency Domain OCT. Transl Vis Sci Technol. 2013;2(7):5. doi: 10.1167/tvst.2.7.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer MD, et al. A new method to monitor visual field defects caused by photoreceptor degeneration by quantitative optical coherence tomography. Invest Ophthalmol Vis Sci. 2008;49(8):3617–21. doi: 10.1167/iovs.08-2003. [DOI] [PubMed] [Google Scholar]
- 17.MacLaren RE, et al. Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial. Lancet. 2014;383(9923):1129–37. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]