Abstract
INTRODUCTION:
This study examines the role of educational attainment, an indicator of cognitive reserve, on transitions in later life between cognitive states (normal MMSE, mild MMSE impairment, severe MMSE impairment) and death.
METHODS:
Analysis of six international longitudinal studies was performed using a coordinated approach. Multistate survival models were used to estimate the transition patterns via different cognitive states. Life expectancies were estimated.
RESULTS:
Across most studies, a higher level of education was associated with a lower risk of transitioning from normal MMSE to mild MMSE impairment but was not associated with other transitions. Those with higher levels of education and socioeconomic status had longer non-impaired life expectancies.
DISCUSSION:
This study highlights the importance of education in later life and that early life experiences can delay later compromised cognitive health. This study also demonstrates the feasibility and benefit in conducting coordinated analysis across multiple studies to validate findings.
Keywords: cognition, dementia, life expectancy, education, socioeconomic status, multi-state modeling
1. Introduction
Increasing dementia prevalence [1, 2] presents a challenge to communities and governments across the world and emphasizes the imminent need for more research to understand the cognitive aging process and the risks for transitioning from intact functioning to compromised cognitive health, dementia, and finally to death. Such research is urgently needed to inform new initiatives, including interventions.
Cognitive aging refers to a gradual decline in mean levels of cognitive abilities and is generally considered to be an unavoidable consequence of aging-related processes. However, for some individuals, the rate of decline in cognitive functioning (e.g., memory) becomes noticeable and greater than what would be expected with normal aging, though not severe enough to have an impact on activities of daily living, referred to as mild cognitive impairment or MCI [3]. Individuals with MCI are more likely to progress to dementia than the general population; however, some individuals remain MCI and do not progress to dementia whilst others transition to the normal range of cognitive functioning as a result of biologic and/or random variability [3-5].
Several risk factors producing substantial inter-individual differences have been identified for their association with cognitive decline and dementia, including occupational attainment [6-8], education [8-12], and other life experiences [13, 14]. According to a recent study, increases in educational attainment may help explain the decline in dementia prevalence in the United States [15]. Resilience to the progressive neuropathology that is associated with dementia is often referred to as cognitive reserve [16]. In some studies, the magnitude of the cognitive reserve is assumed to be related to educational attainment and cognitive engagement [8, 17-21], although a wide range of risk factors and molecular markers can influence reserve [22, 23]. Several studies suggest that higher education can delay the onset of cognitive decline in individuals prior to the diagnosis of dementia. However, after the diagnosis of dementia, those with higher educational attainment exhibit a steeper rate of decline and lower remaining life expectancy compared to those with fewer years of education [18, 24-26]. Thus, cognitive reserve appears to delay the onset of dementia, but once a critical threshold is reached the progression of dementia in terms of effects on cognitive decline is more rapid which in turn leads to fewer years before death [19].
Evaluation of transitions to pathological cognitive states from normative cognitive aging has rarely been evaluated using population-based longitudinal data [27]. Recent advances in multi-state modeling allow for a better understanding of the role of putative risk factors in transitions between cognitive states. Furthermore, this approach allows for the estimation of overall and non-cognitively impaired life expectancies. The knowledge gained from using this approach will improve our understanding of risk factor models differentiating cognitive aging and progressive dementia, potentially facilitating earlier detection for inclusion in preventative intervention aimed also to improve the quality of life of older adults and their families.
One previous study [28] used multi-state modeling to show that older adults with a higher level of education and more complex occupation were less likely to progress to a state of MCI. However, they were also more likely to transition from dementia to death. We aim to expand and assess the robustness of these findings by undertaking analyses using data from six independent longitudinal studies permitting an opportunity to evaluate whether these results are replicated and examine cross-country generalizability. The objectives of this study are to (1) examine the relationship between education and transitions between different cognitive states (i.e., normal MMSE, mild MMSE impairment, severe MMSE impairment) and death and (2) to estimate life-expectancies for older adults with different levels of education.
2. Methods
2.1. Studies
We used data from 6 longitudinal studies of aging. We briefly describe each of the studies below and present baseline characteristics from each study in Table 1. Only respondents with valid data on the MMSE at baseline, information on education and SES, and two known states were included in the analysis. Death status was retrieved from death registers where respondents were living (e.g. Swedish Causes of Death Register).
Table 1.
Population characteristics at baseline for each study.
| Studies | ||||||
|---|---|---|---|---|---|---|
| Variables | Octo-Twin | LASA | Whitehall | H70 | LBC1921 | MAP |
| Age (SD) | 83.53 (3.11) | 70.7 (8.5) | 63.3 (2.1) | 73.11 (3.02) | 79.12 (0.57) | 79.8(7.5) |
| Sex – n (%) | ||||||
| Male | 231 (66.7) | 1284 (50.0) | 896 (69.0) | 407 (40.3) | 237 (41.8) | 391(26.0) |
| Female | 463 (33.3) | 1286 (50.0) | 403 (31.0) | 604 (59.7) | 330 (58.2) | 1,111(74.0) |
| SES – n (%) | ||||||
| Low | 320 (48.7) | 813 (31.6) | 251 (19.3) | 407 (44.0) | 19 (4.6) | 346 (23.9) |
| Medium | 253 (38.5) | 762 (29.6) | 499 (39.4) | 386 (41.7) | 216 (51.8) | 656 (45.3) |
| High | 84 (12.8) | 995 (38.7) | 549 (42.3) | 133 (14.4) | 182 (43.6) | 447 (30.8) |
| Education – n (%) | ||||||
| 0-9 years | 576 (83.0) | 1626 (63.3) | 69 (5.3) | 821 (81.3) | 210 (38.5) | 67(4.5) |
| 10-11 years | 33 (4.8) | 582 (22.6) | 280 (21.6) | 129 (12.8) | 182 (33.3) | 57(3.8) |
| >11 years | 85 (12.2) | 362 (14.1) | 950 (73.1) | 60 (5.9) | 154 (28.2) | 1,376(91.6) |
| MMSE – n (%) | ||||||
| No impairment | 397 (57.2) | 1792 (69.7) | 958 (73.7) | 821 (81.2) | 472 (86.4) | 1,159(77.2) |
| Mild impairment | 146 (21.0) | 634 (24.7) | 329 (25.3) | 130 (12.9) | 70 (12.8) | 230(15.3) |
| Moderate to severe | 151 (21.8) | 144 (5.6) | 12 (0.9) | 60 (5.9) | 4 (0.7) | 110(7.3) |
| Number of deaths | 662 | 1925 | 110 | 270 | 842 | |
Note. MMSE = mini mental state examination. SES = socioeconomic status.
2.1.1. Origins of Variance in the Oldest-Old (OCTO-Twin).
The OCTO-Twin study included dizygotic (DZ) and monozygotic (MZ) twin pairs aged 80 years of age and older [29, 30]. The sample was selected from older adults in the population-based Swedish Twin Registry [31]. Five cycles of longitudinal data were collected at two year intervals. The initial sample consisted of 702 respondents (351 same-sex pairs) with some missing on MMSE. The final analysis included 694 respondents.
2.1.2. Longitudinal Aging Study Amsterdam (LASA).
LASA is an ongoing study on the functioning of older adults in the Netherlands. Details on the sampling and data collection of LASA have been published elsewhere [32, 33]. A nationally representative survey was conducted in 1992/93 among 3107 respondents between the ages of 55 and 85. Follow-up measurements are collected approximately every 3 years. For the current study, we used data from seven LASA measurement waves from 1992/93 to 2011/12. The final sample consisted of 2,570 respondents (4 missing on MMSE & 533 missing on income).
2.1.3. H70.
The H70 studies started in the early 1970s to study health and health-related conditions in an older population in Gothenburg, Sweden. The H70 study has been described in detail previously [34, 35]. In this analysis, we used data from the cohort born 1930 (n= 1250), examined at ages 70, 75 and 79 years. We included participants with valid data on the MMSE and no missing values for covariates (educational level, socioeconomic status). The final sample consisted of 913 respondents.
2.1.4. LBC1921.
The Lothian Birth Cohort 1921 (LBC1921) was derived from the respondents of the Scottish Mental Survey 1932 (SMS1932), which had been carried out by the Scottish Council for Research in Education [36]. The survey was designed to test the intelligence in all children born in 1921. The initial survey comprised 87,498 children [37]. Recruitment of the LBC1921 respondents started in 1999. The final sample for this report included 548 (550 LBC1921 participants but only 548 had valid MMSE at wave 1) respondents (316 women), most of whom has taken part in the SMS1932, and who were followed up at mean ages of 79, 83, 87, 90 and 92 years. Details of the selection process are provided elsewhere [38]. Data included here are from the five follow-up waves.
2.1.5. The Memory and Aging Project (MAP).
MAP is a longitudinal study begun in 1997 with ongoing enrollment [39, 40]. Participant recruitment was focused on retirement communities in northeastern Illinois. The sample size at the first wave consisted of 1,852 respondents. The data was collected on annual basis. The subject pool was updated with new respondents at each wave. The data from 19 waves have been used in the analysis. The final sample consisted of 1,449 respondents.
2.1.6. Whitehall.
The Whitehall II (WII) Study is a longitudinal cohort study on 10,308 respondents recruited from twenty British Civil Service departments in 1985. At the time of recruitment, the respondents were aged 35 to 55. Since the first measurement wave in 1985/1986, every two to five years, data has been collected. Data was used from the fifth (1997/99), seventh (2003/04), and ninth (2008/09) measurement waves, since data on cognitive state was only collected during these waves. The final sample consisted of 1396 respondents (8464 were missing on baseline MMSE, 306 died before the fifth measurement wave, & 142 were missing on education or did not have two known states).
2.2. Measures
2.2.1. Cognitive assessment.
The Mini-Mental State Examination (MMSE) was used to assess global cognitive functioning of respondents at each time point [41]. Following Marioni and colleague’s procedure, we classified study participants as normal MMSE (27<=MMSE<=30), mild MMSE impairment (23<=MMSE<=26) and severe MMSE impairment (MMSE<=22) [28]. The MMSE was not used to determine clinical diagnosis but as suggestive of MCI and dementia.
2.2.2. Covariates.
We were interested in the effects of years of age, education, sex, and socioeconomic status (SES) on the transitions between the cognitive states and on life expectancies. Sex was coded as male participant = 1 and female participant = 0. There were minor differences in the original SES variables across the studies. In OCTO-twin and H70 participants were asked “What has been your main occupation for most of your working life?” (coded into manual, non-manual, and intermediate/professional occupations). In MAP, participants’ income at age 40 was used as a proxy for SES. In LASA, SES was indicated by net monthly household income at baseline. In LBC1921, the SES variable asked for the highest occupation achieved across the lifetime. In Whitehall, SES was coded as clerical/support, administrative, and professional/executives. For all studies, SES was recoded as low (−1), medium (0), and high (1). The original education variable also differed slightly between studies but was first recoded as 0 to 9 years = low, 10 to 11 years = medium, and 12 years and over = high. Given that the main objective of this paper focused on the role of educational attainment, education was further re-coded as using binary indicators, medium vs. low and high vs. low, with low education defined as the reference group. This allowed for the comparison between low vs. high and low vs. medium educational attainment on the transitions.
2.3. Statistical Analysis
A coordinated analysis approach was used to emphasize the importance of replication and comparison. This approach implies running parallel analysis across independent studies using the same analytical approach and variables. In addition to the analysis at the individual study level, a meta-analytic approach was used to combine the results, assigning more weight to studies with larger sample sizes.
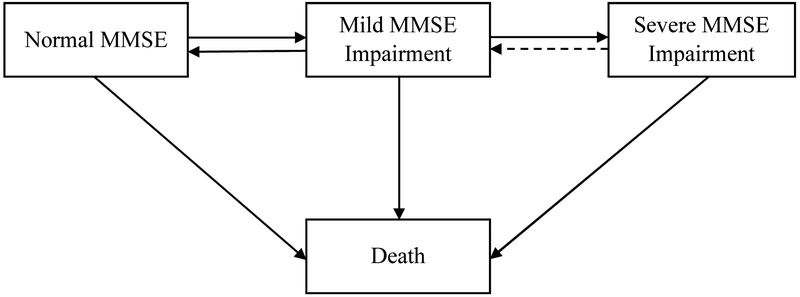
Multi-state modeling (MSM) was used to assess the transitioning of older adults through the different cognitive states. MSM allows us to simultaneously model transitions between four states and examine the role of risk factors on all transitions simultaneously whereas Cox regression only allows the modelling of one transition. A four-state model was used with State 1 defined as normal MMSE (suggests normal cognitive functioning), State 2 as mild MMSE impairment (suggests mild cognitive impairment), State 3 as severe MMSE impairment (suggests dementia), and State 4 defined as death (See Figure 1 for a pictorial representation of the four-state model). Given that misclassification of states was assumed to be present, a model for the misclassification was included for all studies except for LBC1921, H70 and Whitehall which had too few cases transitioning from State 3 to State 2 to reliably include the misclassification model [42]. In this model, individuals were not allowed to transition from State 3 to State 2 without it being a result of a misclassification of individuals being diagnosed as having dementia even though they did not have dementia or because of variability. The estimated misclassification probabilities were low across studies (OCTO-Twin = .16; LASA = .10; and MAP = .09). Interval censoring was used to consider individuals with missing states between two known states and right censoring was used when an individual’s last state was missing but they were known to still be alive. Age of death was used to identify the death state beyond the last wave of data when the individual was known to still be alive at the last data collection wave but a known date of death occurred after the study was completed.
Fig. 1.
Four-state model for cognitive functioning. Normal MMSE = state 1, mild MMSE impairment = state 2, severe MMSE impairment = state 3, death = state 4. The solid line illustrates observed transitions modeled over time. The dotted line represents the observed model which is assumed to be a misclassification of the true state. Only studies with observed backward transitions from state 3 to state 2 included misclassification. The models are adjusted for age, sex, socioeconomic status, and education. Abbreviation: MMSE, Mini-Mental State Examination.
Note. Four-state model for cognitive functioning. Normal MMSE = State 1, Mild MMSE impairment = State 2, Severe MMSE impairment = State 3, Death = State 4. The solid line illustrates observed transitions modelled over time. The dotted line represents the observed model which is assumed to be a misclassification of the true state. Only studies with observed backward transitions from state 3 to state 2 included misclassification. The models are adjusted for age, sex, socioeconomic status, and education.
Age was included as a covariate on all transitions modeled; SES was included on transitions from state 1 to state 2, and state 2 to state 3; sex and education were included on all transitions except for the backward transition from state 2 to state 1. Sex, education, and SES were excluded from the backward transition given that fewer cases transition from state 2 to state 1 making it difficult to model all covariates on this transition. For the same reason, SES was also excluded from the transitions to state 4. Additional parameters were fixed in some studies when too few cases transition.
The MSM package for R [43, 44] was used to estimate the multi-state survival models and ELECT (Estimating Life Expectancies (LEs) in Continuous Time, Version 0.2) [45] for R was used to estimate LEs. ELECT fits a multinomial regression model for state prevalence to estimate total and marginal LE. Total and non-cognitively impaired life expectancies and years spent as cognitively impaired were estimated to examine the role of covariates on life expectancy and on time remaining free of impairment. LEs were estimated for individuals ages 80 and 85 years. These ages were selected as they aligned best with the age ranges from each study (e.g., Octo-Twin only includes older adults 80 years of age and older). We chose not to compute LEs for the Whitehall data, since the age range of the respondents in the Whitehall data, at the last wave, was 64 to 80 years. Computing LEs would therefore force us to extrapolate beyond the range of the data, lowering the reliability of the estimates.
3. Results
3.1. Effect of covariates
Effects of covariates on transitions between the different states are presented in Table 2. The results include hazards ratios and 95% confidence intervals. A visual depiction of the pooled results (meta-analysis results) illustrating the hazard ratios (95% CIs) for the effect of age and education (high versus low) on each transition are presented in Figure 2 and Figure 3, respectively. Forest plots for the effect of age and education (high versus low) on each transition are included in Appendix A.
Table 2.
Hazard ratio and 95% confidence intervals for the effect of covariates on transitions of older adults through the different states of cognitive functioning.
| Octo-Twin (N=694) |
LASA (N=2570) |
Whitehall (N=1396) |
H70 (N=898) |
LBC1921 (N=550) |
MAP (N=1449) |
|
|---|---|---|---|---|---|---|
| Transition | Hazard ratios (95% CIs) | |||||
| Age | ||||||
| State 1 - State 2 | 1.12 (1.06, 1.17)* | 1.05 (1.04, 1.06)* | 1.02 (0.95, 1.09) | 1.09 (0.98, 1.20) | 1.14 (1.05, 1.24)* | 1.08 (1.07, 1.09)* |
| State 1 - State 4 | 1.16 (1.09, 1.23)* | 1.09 (1.07, 1.10)* | 1.14 (1.06, 1.22)* | 1.08 (0.99, 1.18) | 1.18 (1.10, 1.27)* | 1.10 (1.07, 1.13)* |
| State 2 - State 1 | 0.96 (0.88, 1.04) | 0.96 (0.95, 0.97)* | 0.91 (0.86, 0.96)* | 1.16 (1.03, 1.30)* | 0.99 (0.87, 1.12) | 0.98 (0.96, 0.99)* |
| State 2 - State 3 | 1.08 (1.04, 1.13)* | 1.11 (1.09, 1.13)* | 1.15 (0.98, 1.36) | 1.13 (1.01, 1.27)* | 1.22 (0.95, 1.57) | 1.05 (1.02, 1.07)* |
| State 2 - State 4 | 1.11 (0.98, 1.27) | 1.06 (1.03, 1.08)* | 1.12 (0.92, 1.36) | 1.16 (1.00, 1.36)* | 1.19 (0.99, 1.44) | 1.11 (1.04, 1.18)* |
| State 3 - State 4 | 1.05 (1.02, 1.07)* | 1.05 (1.04, 1.07)* | --- | 1.00 (0.92, 1.10) | 1.16 (1.02, 1.31)* | 1.06 (1.03, 1.09)* |
| Sex | ||||||
| State 1 - State 2 | 1.45 (1.07, 1.94)* | 1.45 (1.26, 1.67)* | 0.91 (0.62, 1.34) | 1.02 (0.70, 1.49) | 1.12 (0.63, 1.99) | 1.36 (1.17, 1.58)* |
| State 1 - State 4 | 1.45 (0.92, 2.29) | 1.80 (1.44, 2.24)* | 1.00 (0.61, 1.65) | 1.98 (1.03, 3.82)* | 0.51 (0.26, 0.99)* | 1.44 (0.94, 2.20) |
| State 2 - State 3 | 1.42 (1.02, 1.97)* | 1.04 (0.77, 1.39) | 2.72 (0.68, 10.91) | 1.21 (0.66, 2.22) | 2.30 (0.54, 9.79) | 0.87 (0.65, 1.17) |
| State 2 - State 4 | 0.61 (0.08, 4.86) | 1.95 (1.33, 2.84)* | --- | 2.89 (0.97, 8.66) | 1.25 (0.24, 6.60) | 1.74 (0.94, 3.25) |
| State 3 - State 4 | 1.60 (1.25, 2.03)* | 1.27 (1.02, 1.58)* | --- | 1.50 (0.85, 2.65) | 1.05 (0.47, 2.37) | 1.30 (0.98, 1.72) |
| Medium vs Low Education | ||||||
| State 1 - State 2 | 0.46 (0.22, 0.96)* | 0.53 (0.45, 0.63)* | 0.51 (0.26, 1.03) | 0.88 (0.53, 1.46) | 0.70 (0.40, 1.22) | 0.50 (0.30, 0.83)* |
| State 1 - State 4 | 1.94 (1.09, 3.43)* | 0.94 (0.73, 1.20) | --- | 0.94 (0.30, 2.93) | --- | 1.31 (0.26, 6.66) |
| State 2 - State 3 | 1.39 (0.65, 2.96) | 0.92 (0.62, 1.36) | 1.20 (0.21, 6.72) | 1.83 (0.85, 3.91) | 0.87 (0.27, 2.83) | 2.39 (0.99, 5.80) |
| State 2 - State 4 | --- | 1.04 (0.66, 1.64) | --- | 0.43 (0.03, 5.55) | --- | 0.84 (0.04,15.75) |
| State 3 - State 4 | 0.87 (0.48, 1.59) | 1.33 (0.97, 1.83) | --- | 1.12 (0.52, 2.39) | --- | 1.22 (0.54, 2.75) |
| High vs Low Education | ||||||
| State 1 - State 2 | 0.48 (0.25, 0.90)* | 0.40 (0.32, 0.50)* | 0.48 (0.26, 0.91)* | 0.95 (0.57, 1.58) | 0.68 (0.37, 1.26) | 0.40 (0.29, 0.54)* |
| State 1 - State 4 | 1.44 (0.82, 2.51) | 0.92 (0.70, 1.20) | --- | 1.11 (0.49, 2.50) | --- | 0.82 (0.21, 3.15) |
| State 2 - State 3 | 1.42 (0.65, 3.12) | 1.09 (0.67, 1.77) | 0.33 (0.06, 1.84) | 1.55 (0.66, 3.66) | 0.39 (0.09, 1.80) | 1.33 (0.66, 2.66) |
| State 2 - State 4 | --- | 0.97 (0.51, 1.84) | --- | 0.36 (0.04, 3.19) | --- | 1.13 (0.25, 4.97) |
| State 3 - State 4 | 1.56 (0.84, 2.91) | 1.15 (0.76, 1.72) | --- | 0.80 (0.32, 1.98) | --- | 0.77 (0.43, 1.39) |
| Socioeconomic Status | ||||||
| State 1 - State 2 | 0.94 (0.77, 1.15) | 0.79 (0.73, 0.86)* | 0.95 (0.74, 1.21) | 0.69 (0.51, 0.92)* | 0.93 (0.61, 1.44) | 0.90 (0.82, 0.99)* |
| State 2 - State 3 | 0.88 (0.70, 1.11) | 0.84 (0.72, 1.00)* | 0.90 (0.45, 1.76) | 0.70 (0.40, 1.22) | 0.69 (0.29, 1.64) | 0.95 (0.79, 1.13) |
Note. State 1 = No cognitive impairment; State 2 = Mild cognitive impairment; State 3 = Dementia, State 4 = Mortality.
significant hazard ratio.
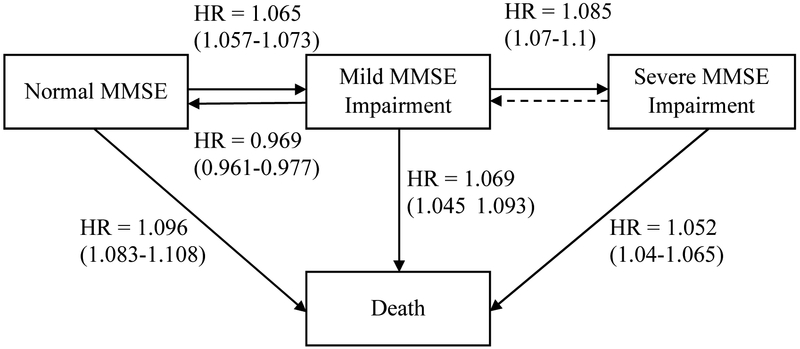
Fig. 2.
Four-state model which illustrates the effect of age on cognitive functioning including pooled HRs (95% confidence intervals). The dotted line represents the observed model which is assumed to be a misclassification of the true state. Abbreviations: HR, hazard ratio; MMSE, Mini-Mental State Examination.
Note. Four-state model which illustrates the effect of age on cognitive functioning including pooled HRs (95% confidence intervals). HR = hazard ratio. The dotted line represents the observed model which is assumed to be a misclassification of the true state.
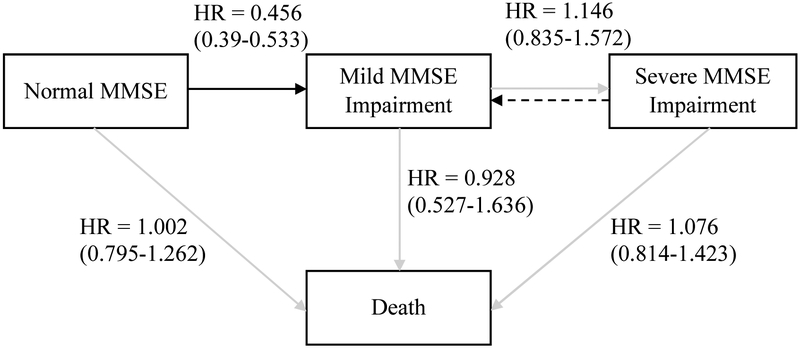
Fig. 3.
Four-state model which illustrates the effect of high versus low education on cognitive functioning including pooled HRs (95% confidence intervals). The dotted line represents the observed model which is assumed to be a misclassification of the true state. Abbreviations: HR, hazard ratio; MMSE, Mini-Mental State Examination.
Note. Four-state model which illustrates the effect of high versus low education on cognitive functioning including pooled HRs (95% confidence intervals). HR = hazard ratio. The dotted line represents the observed model which is assumed to be a misclassification of the true state.
3.1.1. Education.
Having a high level of education versus low was associated with lower risk of transitioning from normal MMSE to mild MMSE impairment in four studies and having a medium versus low level of education was associated with lower risk of transitioning from normal MMSE to mild MMSE impairment in three studies. Having a medium level of education versus low was associated with higher risk of transitioning from normal MMSE to death in Octo-Twin. Aggregated hazard ratios (i.e. meta-analysis results) were significant only for transitions from normal MMSE to mild MMSE impairment and only when comparing high versus low levels of educational impairment (HR=.456, CI=.39 - .53). See Figure 3.
3.1.2. Chronological Age.
Increasing age was associated with higher risk of transitioning from normal MMSE to death across all studies. Four studies suggested that increasing age was associated with higher risk of transitioning from normal MMSE to mild MMSE impairment. Four studies suggested that increasing age was also associated with higher risk of transitioning from mild MMSE impairment to severe MMSE impairment. In LASA, H70, and MAP increasing age was further associated with higher risk of transitioning from mild MMSE impairment to death. All studies except H70 suggested that increasing age was associated with higher risk of transitioning from severe MMSE impairment to death. With regards to the reverse transition, in LASA, Whitehall, and MAP results suggested that increasing age was associated with a decreasing likelihood of transitioning from mild MMSE impairment to normal MMSE whereas in H70, increasing age was associated with an increasing likelihood of transitioning from mild MMSE impairment to normal MMSE. Aggregated hazard ratios (i.e. meta-analysis results) illustrate the importance of age when transitioning from normal to mild MMSE impairment (HR=1.065, CI=1.057-1.073) and back (HR=0.969, CI=0.961-0.977), mild to severe MMSE impairment (HR=1.085, CI=1.07-1.1), normal MMSE to death (HR=1.096, CI=1.083-1.108), mild MMSE to death (HR=1.069, CI=1.045-1.093), and severe MMSE to death (HR=1.052, CI=1.04-1.065). See Figure 2.
3.1.3. Sex.
Being a male participant was associated with an increased risk of transitioning from normal MMSE to mild MMSE impairment in three studies, from normal MMSE to death in three studies, from mild MMSE impairment to severe MMSE impairment in Octo-Twin, from mild MMSE impairment to death in LASA, and from severe MMSE impairment to death in two studies.
3.1.4. Socioeconomic status (SES).
Information on SES was only included on transitions from normal MMSE to mild MMSE impairment and mild MMSE impairment to severe MMSE impairment. A higher level of SES was associated with a decreased likelihood of transitioning from normal MMSE to mild MMSE impairment in LASA, H70, and MAP and was associated with a decreased likelihood of transitioning from mild MMSE impairment to severe MMSE impairment in LASA.
3.2. Overall Life Expectancy – SES, Education and Sex
See Table 3 for the life expectancies and 95% CIs for 80 and 85-year-old male and female participants with high, medium, and low levels of SES and education levels. An 80-year-old male participant with a high SES and education level had remaining life expectancy of 5.80 years in OCTO-Twin, 6.80 years in LASA, 6.99 years in MAP, 7.41 years in LBC1921, and 9.98 years in H70. In two studies, individuals with similar characteristics but with low SES and education had longer life expectancies (6.35 years & 7.56 years, respectively), but in the other 3 studies, their life expectancies were shorter (6.78 years, 6.05 years, & 7.73 years).
Table 3.
Life expectancies for male and female participants of different ages with different education and SES levels
| Octo-Twin (N=694) | LASA (N=2570) | H70 (N=913) | LBC1921 (N=550) | MAP (N=1449) | |
|---|---|---|---|---|---|
| Life expectancies in years (9i5% CIs)a |
|||||
| Age 80 | |||||
| Male, Educ(H), SES(H) | 5.80 (4.59,6.51) | 6.80 (6.12,7.33) | 9.98 (3.81,11.72) | 7.41 (6.51,7.89) | 6.99(4.16,8.62) |
| Male, Educ(M), SES(M) | 5.69 (4.12,6.97) | 6.33 (5.82,6.82) | 9.41 (4.21,13.49) | 7.26 (6.29,7.62) | 8.88(8.21,9.51) |
| Male, Educ(L), SES(L) | 6.35 (5.60,6.79) | 6.05 (5.67,6.40) | 7.73 (3.76,11.45) | 6.78 (5.46,7.34) | 7.56(4.97,9.24) |
| Female, Educ(H), SES(H) | 7.39(5.88,8.73) | 9.45 (8.52,10.29) | 14.49 (7.19,18.31) | 8.69 (6.68,9.49) | 8.35(5.93,9.93) |
| Female, Educ(M), SES(M) | 7.34(5.76,9.08) | 8.77 (8.00,9.41) | 13.72 (6.83,17.58) | 8.31 (6.51,8.66) | 10.76(10.21,11.21) |
| Female, Educ(L), SES(L) | 8.20(7.54,8.68) | 8.19 (7.80,8.60) | 12.17 (6.25,16.35) | 7.11 (5.62,8.74) | 9.09(6.53,10.74) |
| Age 85 | |||||
| Male, Educ(H), SES(H) | 3.43 (2.59,4.09) | 4.84 (4.28,5.39) | 7.98 (2.57,12.77) | 4.47 (3.68,5.08) | 4.83(2.91,6.24) |
| Male, Educ(M), SES(M) | 3.59 (2.59,4.63) | 4.48 (4.03,4.87) | 7.36 (2.41,12.20) | 4.34 (3.24,4.70) | 6.22(5.63,6.74) |
| Male, Educ(L), SES(L) | 4.05 (3.50,4.44) | 4.40 (4.04,4.72) | 6.24 (1.44,12.55) | 3.97 (3.12,4.56) | 5.30(3.27,6.63) |
| Female, Educ(H), SES(H) | 4.59 (3.77,5.58) | 6.81 (5.97,7.57) | 11.64 (4.74,15.33) | 5.31 (3.20,5.75) | 5.88(3.68,7.06) |
| Female, Educ(M), SES(M) | 4.87 (3.34,6.42) | 6.28 (5.67,6.81) | 10.89 (5.18,15.85) | 5.02 (2.79,5.36) | 7.76(7.21,8.22) |
| Female, Educ(L), SES(L) | 5.44 (5.02,5.71) | 5.99 (5.65,6.39) | 9.84 (3.92,15.27) | 4.17 (2.66,4.94) | 6.53(4.60,7.91) |
| Non-cognitively impaired life expectancy in years (95% CIs)b |
|||||
| Age 80 | |||||
| Male, Educ(H), SES(H) | 4.21(3.29,5.12) | 4.67 (3.85,5.38) | 8.45 (3.81,11.72) | 5.71 (3.90,6.34) | 4.30(2.74,5.44) |
| Male, Educ(M), SES(M) | 3.70(2.53,5.00) | 3.28 (2.95,3.62) | 7.58 (3.97,12.04) | 5.49 (4.34,6.49) | 5.49(5.01,5.94) |
| Male, Educ(L), SES(L) | 3.35(2.85,3.88) | 1.93 (1.70,2.19) | 5.58 (2.80,8.47) | 4.19 (2.18,5.36) | 3.11(2.11,3.94) |
| Female, Educ(H), SES(H) | 5.39(4.39,6.31) | 6.00 (5.31,6.64) | 12.06 (5.33,16.15) | 6.68 (4.33,7.50) | 5.35(3.76,6.65) |
| Female, Educ(M), SES(M) | 4.79(3.78,5.95) | 4.88 (4.34,5.32) | 10.41 (4.91,15.25) | 6.23 (3.70,7.02) | 6.86(6.42,7.20) |
| Female, Educ(L), SES(L) | 4.44(7.61,8.75) | 2.93 (2.65,3.19) | 8.07 (3.51,12.50) | 4.25 (2.43,5.72) | 4.05(2.89,4.95) |
| Age 85 | |||||
| Male, Educ(H), SES(H) | 2.10(1.42,2.61) | 2.47 (1.99,2.88) | 6.44 (1.96,10.05) | 3.14 (1.10,3.79) | 2.56(1.44,3.43) |
| Male, Educ(M), SES(M) | 1.80(1.16,2.37) | 1.96 (1.58,2.28) | 5.77 (2.59,9.32) | 2.98 (0.96,3.62) | 3.34(2.96,3.64) |
| Male, Educ(L), SES(L) | 1.62(1.23,1.86) | 1.11 (0.90,1.31) | 4.13 (1.50,6.51) | 2.13 (0.77,3.38) | 1.73(1.06,2.22) |
| Female, Educ(H), SES(H) | 2.80(2.10,3.57) | 3.76 (3.07,4.39) | 9.53 (3.32,13.42) | 3.79 (1.16,4.95) | 3.29(2.12,4.15) |
| Female, Educ(M), SES(M) | 2.43(1.57,3.10) | 3.01 (2.49,3.48) | 8.19 (3.15,13.24) | 3.48 (0.84,4.25) | 4.33(3.90,4.65) |
| Female, Educ(L), SES(L) | 2.24(1.91,2.56) | 1.73 (1.42,1.99) | 6.23 (2.20,10.76) | 2.20 (0.40,3.47) | 2.35(1.60,2.98) |
Note.
Total LE in years.
LE in state 1 irrespective of where you are at a given age.
Across all studies, female participants had longer life expectancies than men. An 80-year-old female participant with high SES and education level had a remaining life expectancy of 7.39 years in OCTO-Twin, 9.45 years in LASA, 8.35 years in MAP, 8.69 years in LBC1921, and 14.49 years in H70. Two studies showed longer and three shorter remaining life expectancy for female participants with low SES and education. An 80-year-old female participant with low SES and education level had remaining life expectancy of 8.20 years in OCTO-Twin, 8.19 years in LASA, 9.09 years in MAP, 7.11 years in LBC1921, and 12.17 years in H70. Similar LEs were estimated for age 85, except that remaining LEs were 1.65 to 3 years shorter (See Table 3).
3.3. Non-cognitively Impaired Life Expectancy – SES, Education and Sex
An 80-year-old male participant with high SES and education level had a remaining non-impaired life expectancy of 4.21 years in OCTO-Twin, 4.67 years in LASA, 4.30 years in MAP, 5.71 years in LBC1921, and 8.85 years in H70. In all studies, individuals with low SES and education level had shorter non-impaired life expectancies. That is, an 80-year-old male participant with low SES and education level had a non-cognitively impaired life expectancy of 3.35 years in OCTO-Twin, 1.93 years in LASA, 3.11 years in MAP, 4.19 years in LBC1921, and 5.58 years in H70 (See Table 3).
Across all studies, female participants had longer non-cognitively impaired life expectancies. An 80-year-old female participant with high SES and education level had a non-cognitively impaired remaining life expectancy of 5.39 years in OCTO-Twin, 6.00 years in LASA, 5.35 years in MAP, 6.68 years in LBC1921, and 12.06 years in H70. All studies showed shorter non-cognitively impaired life expectancy for those with low SES and education. That is, an 80-year-old female participant with low SES and education had a non-cognitively impaired life expectancy of 4.44 years in OCTO-Twin, 2.93 years in LASA, 4.05 years in MAP, 4.25 years in LBC1921, and 8.07 years in H70. Similar non-cognitively impaired LEs were computed at age 85 except that LEs were between 1.2 to 3.38 years shorter (See Table 3).
3.4. Time spent with cognitive Impairment – SES, Education and Sex
An 80-year-old male participant with high SES and education was expected to be cognitively impaired for 1.59 years in OCTO-Twin, 2.13 years in LASA, 2.69 years in MAP, 1.70 years in LBC1921, and 1.53 years in H70. In all studies except H70, males with low SES and education spend more years as cognitively impaired. That is, an 80-year-old male participant with low SES and education was expected to be cognitively impaired for 3 years in OCTO-Twin, 4.12 years in LASA, 4.45 years in MAP, 2.59 years in LBC1921, and 1.49 years in H70.
An 80-year-old female participant with high SES and education was expected to be cognitively impaired for 2 years in OCTO-Twin, 3.45 years in LASA, 3 years in MAP, 2.01 years in LBC1921, and 2.43 years in H70. In all studies, females with low SES and education spent more years as cognitively impaired. That is, an 80-year-old female participant with low SES and education was expected to be cognitively impaired for 3.75 years in OCTO-Twin, 5.26 years in LASA, 5.04 years in MAP, 2.94 years in LBC1921, and 4.10 years in H70.
Discussion
Using multi-state survival modeling in six international longitudinal datasets, our study analyzed transitions of older adults over cognitive health states and provides insight into the cognitive reserve hypothesis and the importance of education and SES for later risk of mild MMSE impairment (suggesting MCI), severe MMSE impairment (suggesting dementia) and survival. Education and SES delayed the progression to mild MMSE impairment (suggesting MCI), which supports findings from a previous study which used the same analytical approach [28]. The finding that education was not related to any of the later transitions suggests that education may be more protective against cognitive decline in the earlier stages of the disease progression and in non-pathologic cognitive aging but less so for the later stages. Other studies, using different analytical approaches, have also found similar results [12, 25, 46, 47].
Our finding on non-cognitively impaired life expectancies and years spent as cognitively impaired was more consistent with previous studies showing that higher education and SES level is related to longer non-cognitively impaired life expectancy and fewer years as cognitively impaired. This aligns with studies suggesting that education and SES help to delay the progression of cognitive decline to MCI, but that once decline reaches a certain critical threshold, the progression to death is accelerated compared to those with lower education and SES [19].
Results regarding the importance of education and SES for late life survival were mixed with some studies showing greater life expectancy for individuals with higher education and SES and others showing lower life expectancy for individuals with higher education and SES. The mixed results are likely due to characteristics of each study (e.g. average age and age range). For example, OCTO-Twin is a sample of twins, all aged 80 years of age or older at baseline which makes it important to consider the impact of mortality-related selection (i.e., healthy survivor effect, left truncation).
Cognitive aging research focuses largely on describing age-related patterns of change. In this paper, we examined the effect of chronological age on progressive decline in cognitive states and found that aging is associated with an increased risk of transitioning from normal cognitive aging to impaired cognition and death. Whereas education is important as a risk factor in the earlier stages of the disease progression, age is important at all stages of the disease progression.
The six longitudinal studies included encompass different cohorts with heterogenous distributions in education level and MMSE scores. One limitation is that this can have an impact on the comparison of results across studies since those more likely to be cognitively impaired are also likely to be born in earlier cohorts and more likely to have lower levels of education. Despite this limitation, there is a need for more coordinated approaches [48, 49] making the comparison across 6 longitudinal studies a clear advantage of the present study. The inclusion of several studies allowed replication of the same analysis and presentation of results across studies, allowing evaluation of the generalizability of our results. Our knowledge of each study allowed us to better understand and explain the differences found across studies. For example, the limited effect of age found with the Whitehall data compared to the other studies is likely a reflection of the younger age range of the participants. Even though comparisons across studies are possible, identical replication across studies was not feasible due to features of the data. For example, some studies had too few backward transitions to be able to include these in every model. Still, our results are fairly consistent suggesting some generalizability across birth cohorts, national differences in education and SES, and contextual settings.
This study used the MMSE to characterise the cognitive states and for the estimation of transitions. A known limitation of the MMSE includes the ceiling effect. Also, as educational attainment is often used as a proxy for prior cognitive functioning, it is possible that the association between education and the transition from no cognitive impairment to MCI is simply capturing changes that occur with normal aging [50]. That is, individuals with lower educational attainment and presumably lower prior cognitive abilities also have lower later abilities. The use of clinical guidelines for the diagnosis of MCI and dementia would have been preferred [51], but this information was not available in most of the studies. Therefore, the MMSE was not used to determine clinical diagnosis. Rather it was used as an indication of the level of MMSE impairment and as suggestive of MCI and dementia. Moreover, we used the MMSE because we wanted to compare and expand on the work of Marioni and colleagues [28]. In order to examine the comparability of results using clinical diagnosis of dementia rather than MMSE, we re-ran the model using clinical diagnosis of dementia available in the Octo-Twin data. We found similar results for the role of education on cognitive transitions. Having a high or medium level of education versus low was associated with a lower risk of transitioning from normal MMSE to mild MMSE impairment [(HR for medium versus low education = 0.47 (95% CI, 0.22 - 0.98); HR for high versus low education 0.48 (95% CI, 0.25 - 0.90)]; and having a medium level of education versus low was associated with higher risk of transitioning from normal MMSE to death [HR = 2.03 (95% CI, 1.11 - 3.72)]. Another limitation of this study is that we did not examine whether respondents with high educational attainment are more likely to be in the normal category versus whether respondents with high educational attainment are less likely to transition to an impaired state during follow-up.
Another limitation of this study is that we were unable to include biomarkers and genetic markers as risk factors given that these were not available in the longitudinal studies we used. Further research should examine the role of these risk factors on transitions between cognitive states and death. Given that the Octo-Twin data consists of twins, accounting for the violation of the assumption of independence between observations would have been preferable. However, given that we assumed the effect to be negligible and that we were running the same models across six studies, we decided not to include random effects in this paper.
Our results demonstrated that some individuals transitioned from a state of impaired MMSE (indicative of possible MCI) back to a state of healthy cognition. The overlap in symptoms with other conditions such as depression and other neurodegenerative diseases is one likely explanation for the recovery from MCI and for the misclassification of individuals with dementia. Our findings align with previous research which also found reversion from MCI to normal cognitive functioning for some individuals [52-55]. However, more research is needed using clinical guidelines for the diagnosis of MCI to better understand these transitions. By using clinical guidelines, we would be able to more accurately differentiate those individuals with MCI due to dementia from those unlikely to progress in dementia and identify predictors of these.
This is the first study to examine change in cognitive states over time across six longitudinal studies using a coordinated analysis approach. The overall consistency of results across studies provides strong support for the role of higher education and SES on non-cognitively impaired life expectancy, while still highlighting study specific characteristics that are important in the interpretation of results. Besides furthering our understanding of the relationship of education and SES with changes in cognitive states, it also highlighted the feasibility and benefits of conducting coordinated analysis across multiple longitudinal studies.
Highlights.
There are benefits to coordinated analysis across multiple longitudinal studies.
Education and SES delays the progression to MCI.
Higher education and SES increases non-cognitively impaired life expectancy.
Research in Context.
Systematic review: The authors conducted a literature review by searching relevant research databases. Studies suggest that higher education and more complex occupation can delay the onset of cognitive decline in individuals prior to the diagnosis of dementia. Relevant citations are cited.
Interpretation: Our study analyzed transitions of older adults over cognitive health states and provides insight into the cognitive reserve hypothesis and the importance of education and SES for delaying the progression to MCI and increasing non-cognitively impaired life expectancy. Other studies, using different analytical approaches, have also found similar results.
Future directions: This study used the MMSE to characterise the cognitive states. Further research should explore the use of clinical guidelines to identify MCI and dementia status, to examine whether differences in results might be found. Future research should also explore the importance of participation in social, physical, and cognitive activities on transitioning through the cognitive states and mortality.
Acknowledgments
Funding:
Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number P01AG043362, Integrative Analysis of Longitudinal Studies of Aging and Dementia. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The OCTO-Twin study was funded by the National Institute on Aging at the National Institutes of Health (Grant number AG08861), The Swedish Council for Working Life and Social Research, The Adlerbertska Foundation, The Hjalmar Svensson Foundation, The Knut and Alice Wallenberg Foundation, The Wenner-Gren Foundations, and The Wilhelm and Martina Lundgrens Foundation, and the Swedish Brain Power Consortium. The Lothian Birth Cohort 1921 (LBC1921) was supported by grants from the Biotechnology and Biological Sciences Research Council (BBSRC: Wave 1: SR176), Chief Scientist Office (CSO: Wave 3: CYB/4/505; Wave 4: ETM/55) and the Medical Research Council (MRC: Wave 5: R42550). Ethics permission for the Lothian Birth Cohort 1921 (LBC1921) was obtained from the Lothian Research Ethics Committee (Wave 1: LREC/1998/4/183; Wave 2: LREC/2003/7/23; Wave 3: LREC1702/98/4/183) and the Scotland A Research Ethics Committee (Wave 4: 10/S1103/6; Wave 5: 10/MRE00/87). The Longitudinal Aging Study Amsterdam (LASA) is largely supported by a grant from the Netherlands Ministry of Health, Welfare and Sports, Directorate of Long-Term Care. The Whitehall II study is supported by grants from the US National Institutes on Aging (R01AG013196; R01AG034454), the UK Medical Research Council (MRC K013351), and British Heart Foundation (RG/13/2/30098). The H70-studies was supported by The Swedish Research Council (11267, 825-2007-7462, 825-2012-5041, 2013-8717, 2015-0283), Swedish Research Council for Health, Working Life and Welfare (no 2001-2849, 2005-0762, 2008-1210, 2001-2835, AGECAP 2013-2300, 2013-2496, Epilife 2006-1506), Alzheimer's Association (IIRG-03-6168), Eivind och Elsa K:son Sylvans stiftelse, Stiftelsen Söderström-Königska Sjukhemmet, Stiftelsen för Gamla Tjänarinnor, Handlaren Hjalmar Svenssons Forskningsfond, Sahlgrenska University Hospital (ALF), Fredrik and Rosa von Malmborgs Foundation for Brain Research, Swedish Brain Power and Riksbankens Jubileumsfond (P14-0824:1). Rush Alzheimer's Disease Center (RADC) is supported by grants from the National Institute on Aging: R01AG17917, R01AG15819, R01AG22018, R01AG24480, R01AG24871, R01AG26916, R01AG33678, R01AG34374, R21AG30765, K23AG23040, the Illinois Department of Public Health, the Elsie Heller Brain Bank Endowment Fund, and the Robert C. Borwell Chair of Neurological Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. The Lancet. 2005;366:2112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].International WHOaAsD. Dementia a public health priority. Geneva: Geneva: : World Health Organization, 2012; 2012. [Google Scholar]
- [3].Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–8. [DOI] [PubMed] [Google Scholar]
- [4].Visser PJ, Kester A, Jolles J, Verhey F. Ten-year risk of dementia in subjects with mild cognitive impairment. Neurology. 2006;67:1201–7. [DOI] [PubMed] [Google Scholar]
- [5].Geda YE. Mild Cognitive Impairment in Older Adults. Current psychiatry reports. 2012;14:320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Qiu C, Karp A, von Strauss E, Winblad B, Fratiglioni L, Bellander T. Lifetime principal occupation and risk of Alzheimer's disease in the Kungsholmen project. American journal of industrial medicine. 2003;43:204–11. [DOI] [PubMed] [Google Scholar]
- [7].Wajman JR, Bertolucci PHFF. Intellectual demand and formal education as: cognitive protection factors in Alzheimer's disease. Dementia & Neuropsychologia. 2010;4:320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of Education and Occupation on the Incidence of Alzheimer's Disease. JAMA. 1994;271:1004–10. [PubMed] [Google Scholar]
- [9].Scarmeas N, Zarahn E, Anderson KE, Honig LS, Park A, Hilton J, et al. Cognitive Reserve–Mediated Modulation of Positron Emission Tomographic Activations During Memory Tasks in Alzheimer Disease. Archives of Neurology. 2004;61:73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Allegri RT FE; Krupitzki H; Serrano CM; Dillon C; Sarasola D; et al. Role of cognitive reserve in progression from mild cognitive impairment to dementia. Dementia & Neuropsychologia. 2010;4:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brayne C, Ince PG, Keage HA, McKeith IG, Matthews FE, Polvikoski T, et al. Education, the brain and dementia: neuroprotection or compensation? Brain : a journal of neurology. 2010;133:2210–6. [DOI] [PubMed] [Google Scholar]
- [12].Terrera GM, Minett T, Brayne C, Matthews FE. Education associated with a delayed onset of terminal decline. Age Ageing. 2014;43:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fabrigoule C, Letenneur L, Dartigues JF, Zarrouk M, Commenges D, Barberger-Gateau P. Social and leisure activities and risk of dementia: a prospective longitudinal study. J Am Geriatr Soc. 1995;43:485–90. [DOI] [PubMed] [Google Scholar]
- [14].Wilson RS, Boyle PA, Yu L, Barnes LL, Schneider JA, Bennett DA. Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology. 2013;81:314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Langa KM, Larson EB, Crimmins EM, Faul JD, Levine DA, Kabeto MU, et al. A Comparison of the Prevalence of Dementia in the United States in 2000 and 2012. JAMA internal medicine. 2017;177:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stern Y What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society : JINS. 2002;8:448–60. [PubMed] [Google Scholar]
- [17].Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends in Cognitive Sciences. 2013;17:502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stern Y, Albert S, Tang MX, Tsai WY. Rate of memory decline in AD is related to education and occupation: cognitive reserve? Neurology. 1999;53:1942. [DOI] [PubMed] [Google Scholar]
- [19].Stern Y Cognitive reserve in ageing and Alzheimer's disease. Lancet neurology. 2012;11:1006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychological Medicine. 2006;36:441–54. [DOI] [PubMed] [Google Scholar]
- [21].Xu W, Yu JT, Tan MS, Tan L. Cognitive reserve and Alzheimer's disease. Molecular neurobiology. 2015;51:187–208. [DOI] [PubMed] [Google Scholar]
- [22].Yu L, Boyle PA, Segawa E, Leurgans S, Schneider JA, Wilson RS, et al. Residual decline in cognition after adjustment for common neuropathologic conditions. Neuropsychology. 2015;29:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Buchman AS, Yu L, Boyle PA, Schneider JA, De Jager PL, Bennett DA. Higher brain BDNF gene expression is associated with slower cognitive decline in older adults. Neurology. 2016;86:735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Stern Y, Tang MX, Denaro J, Mayeux R. Increased risk of mortality in alzheimer's disease patients with more advanced educational and occupational attainment. Annals of Neurology. 1995;37:590–5. [DOI] [PubMed] [Google Scholar]
- [25].Hall CB, Derby C, Levalley A, Katz MJ, Verghese J, Lipton RB. Education delays accelerated decline on a memory test in persons who develop dementia. Neurology. 2007;69:1657. [DOI] [PubMed] [Google Scholar]
- [26].Meng X, D’Arcy C. Education and Dementia in the Context of the Cognitive Reserve Hypothesis: A Systematic Review with Meta-Analyses and Qualitative Analyses. PLOS ONE. 2012;7:e38268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yu L, Boyle PA, Leurgans S, Schneider JA, Kryscio RJ, Wilson RS, et al. Effect of common neuropathologies on progression of late life cognitive impairment. Neurobiology of aging. 2015;36:2225–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Marioni RE, Valenzuela MJ, van den Hout A, Brayne C, Matthews FE. Active cognitive lifestyle is associated with positive cognitive health transitions and compression of morbidity from age sixty-five. PLoS One. 2012;7:e50940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Johansson B, Hofer SM, Allaire JC, Maldonado-Molina M, Piccinin AM, Sea Berg. Change in memory and cognitive functioning in the oldest-old: The effects of proximity to death in genetically related individuals over a six-year period. Psychology and Aging. 2004;19:145–56. [DOI] [PubMed] [Google Scholar]
- [30].McClearn GE, Johansson B, Berg S, Pedersen NL, Ahern F, Petrill SA, et al. Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science. 1997;276:1560–3. [DOI] [PubMed] [Google Scholar]
- [31].Cederlöf R, Lorich U. The Swedish Twin Registry In: Nance WE, Allen G, Parisi P, editors. Twin research: Biology and epidemiology. New York, NY: Alan R. Riss; 1978. p. 189–95. [PubMed] [Google Scholar]
- [32].Hoogendijk E, Deeg D, Poppelaars J, van der Horst M, Broese van Groenou M, Comijs H, et al. The Longitudinal Aging Study Amsterdam: cohort update 2016 and major findings. European Journal of Epidemiology. 2016;31:927–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Huisman M, Poppelaars J, van der Horst M, Beekman AT, Brug J, van Tilburg TG, et al. Cohort profile: The Longitudinal Aging Study Amsterdam. International Journal of Epidemiology. 2011;40:868–76. [DOI] [PubMed] [Google Scholar]
- [34].Sigström R, Skoog I, Karlsson B, Nilsson J, Östling S. Nine-year follow-up of specific phobia in a population sample of older people Depression and Anxiety. 2016;33:339–46. [DOI] [PubMed] [Google Scholar]
- [35].Skoog I Psychiatric epidemiology of old age: the H70 study – the NAPE Lecture 2003. Acta Psychiatrica Scandinavica. 2004;109:4–18. [DOI] [PubMed] [Google Scholar]
- [36].Scottish Council for Research in Education. The intelligence of Scottish children: A National Survey of an age-group. London: University of London Press; 1933. [Google Scholar]
- [37].Deary IJ, Whalley LJ, Starr JM. A lifetime of intelligence : follow-up studies of the Scottish mental surveys of 1932 and 1947. Washington, D.C.2009. [Google Scholar]
- [38].Deary IJ, Gow AJ, Pattie A, Starr JM. Cohort Profile: The Lothian Birth Cohorts of 1921 and 1936. International Journal of Epidemiology. 2012. [DOI] [PubMed] [Google Scholar]
- [39].Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–75. [DOI] [PubMed] [Google Scholar]
- [40].Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Current Alzheimer Research. 2012;9:646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- [42].van den Hout A Multi-State Survival Models for Interval-Censored Data. Boca Raton, FL: Taylor & Francis Group; 2017. [Google Scholar]
- [43].Jackson C Multi-State Models for Panel Data: The msm Package for R. Journal of Statistical Software. 2011;38:28. [Google Scholar]
- [44].Jackson C Multi-State Models for Panel Data: The msm Package for R. Version 1.6.4. 2016. p. 57. [Google Scholar]
- [45].Van den Hout A ELECT: Estimation of life expectancies using continuous-time multi-state survival models ELECT version 0.2. Vignette: ed2016. [DOI] [PubMed] [Google Scholar]
- [46].Yu L, Boyle P, Wilson RS, Segawa E, Leurgans S, De Jager PL, et al. A random change point model for cognitive decline in Alzheimer’s disease and mild cognitive impairment. Neuroepidemiology. 2012;39:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Education modifies the association of amyloid but not tangles with cognitive function. Neurology. 2005;65:953–5. [DOI] [PubMed] [Google Scholar]
- [48].Khachaturian AS, Meranus DH, Kukull WA, Khachaturian ZS. Big data, aging, and dementia: Pathways for international harmonization on data sharing. 2013. p. S61–S2. [DOI] [PubMed] [Google Scholar]
- [49].Hofer SM, Piccinin AM. Integrative Data Analysis Through Coordination of Measurement and Analysis Protocol Across Independent Longitudinal Studies. Psychological Methods. 2009;14:150–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Dykiert D, Der G, Starr JM, Deary IJ. Why is Mini-Mental state examination performance correlated with estimated premorbid cognitive ability? Psychological Medicine. 2016;46:2647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia: The Journal of the Alzheimer's Association. 2011;7:270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gifford KA, Liu D, Lu Z, Tripodis Y, Cantwell NG, Palmisano J, et al. The source of cognitive complaints predicts diagnostic conversion differentially among nondemented older adults. Alzheimer's & Dementia: The Journal of the Alzheimer's Association. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Han JW, Kim TH, Lee SB, Park JH, Lee JJ, Huh Y, et al. Predictive validity and diagnostic stability of mild cognitive impairment subtypes. Alzheimer's & Dementia: The Journal of the Alzheimer's Association. 2012;8:553–9. [DOI] [PubMed] [Google Scholar]
- [54].Canevelli M, Grande G, Lacorte E, Quarchioni E, Cesari M, Mariani C, et al. Spontaneous Reversion of Mild Cognitive Impairment to Normal Cognition: A Systematic Review of Literature and Meta-Analysis. Journal of the American Medical Directors Association. 2016;17:943–8. [DOI] [PubMed] [Google Scholar]
- [55].Malek-Ahmadi M Reversion From Mild Cognitive Impairment to Normal Cognition: A Meta-Analysis. Alzheimer disease and associated disorders. 2016;30:324. [DOI] [PubMed] [Google Scholar]