Abstract
The Women’s Health Initiative studies reported that the menopausal hormone therapy (MHT) regimen containing conjugated equine estrogen (CEE) and medroxyprogesterone acetate increased, whereas CEE alone reduced breast cancer incidence. These observations suggest the possibility that CEE might exert unique actions on breast and also suggest the need to eliminate the progestogen from MHT regimens. A MHT regimen called a tissue selective estrogen complex (TSEC), containing CEE plus baze-doxifene (BZA), to avoid the need for a progestogen, was developed and FDA approved. Our study addressed two questions regarding this TSEC: (i) whether CEE exert effects on breast cancer which differ from those of estradiol (E2) and (ii) whether BZA antagonize the effects of E2 and CEE on breast cancer? Two rodent models (NMU and ACI) were used to compare the effect of CEE with E2 on mammary tumor formation, proliferation and apoptosis. In both the NMU and ACI models, E2 significantly increased tumor incidence and multiplicity whereas in striking contrast CEE did not, even though the estrogenic effects of CEE and E2 on uterine weight were identical. Mechanistically E2 blocked whereas CEE stimulated apoptosis (cleaved caspase-3) in ACI animals and only E2 stimulated proliferation (Ki67). BZA exerted highly potent anti-estrogenic effects on tumors by completely blocking palpable tumor formation. These data suggest that the CEE/BZA TSEC may be a safer, breast-antagonistic, MHT agent for women and might have potential to prevent breast cancer while relieving menopausal symptoms.
Keywords: conjugated equine estrogens, bazedoxifene, estradiol, tissue selective estrogen complex, breast cancer
Menopause is associated with symptoms of hot flashes, night sweats and dyspareunia as well as an increase in bone resorption, osteopenia and osteoporosis.1 Menopausal hormone therapy (MHT) is the most effective treatment for symptoms while for increased bone resorption, MHT as well as bisphosphosphonates and denosumab are effective.1 The most prescribed therapy for menopause in the United States historically was a combination of conjugated equine estrogens (CEE) plus medroxyprogesterone acetate (MPA) but breast cancer risk was a major safety concern with this regimen.2 Randomized, controlled trial data from The Women’s Health Initiative (WHI) study reported a 26% increase (HR 1.26 95% CI 1.00–1.59) in relative risk of breast cancer after 5.2 years of treatment with CEE plus MPA,2 a finding supported by other studies.3–6 Surprisingly, CEE alone did not increase the risk of breast cancer in the WHI study but nonsignificantly reduced the risk of invasive breast cancer (HR 0.77, 95% CI 0.59–1.01) after 6.8 years of intervention7 and significantly (HR 0.79 95% CI 0.65–0.97) reduced risk by 21% at the 6 year follow-up.8
These studies have raised important issues regarding the mechanism whereby CEE alone did not enhance breast cancer risk in the WHI as would be expected with other estrogens and the role of the progestogen in increasing the risk of breast cancer. Over the past few years, safety concerns regarding the risk of breast cancer and heart disease with MHT have stimulated the need to develop safer alternatives. The issues posed above were taken into account in developing a new, innovative, combination treatment which utilizes CEE as the estrogen and a SERM, bazedoxifene (BZA) rather than a progestogen for uterine protection. This combination is considered a tissue selective estrogen complex or TSEC, a newly-coined hormonal class. The rational for combining BZA with CEE was to have BZA exert its anti-estrogen effects on the uterus and breast, but in the appropriate ratio with CEE, maintain the estrogenic effects of CEE on vasomotor, vaginal symptoms and skeletal bone mass maintenance thus eliminating the need for a progestogen.9,10
The strategy of adding a SERM to an estrogen to form a TSEC would appear counterintuitive on initial reflection. However, comprehensive molecular studies have shown that TSECs exert unique cDNA array signatures,11,12 likely due mechanistically to specific alterations of the conformation of the estrogen receptor dimer.13 Such unique signatures translated into pharmacologic profiles which were not predictable and associated with unique clinical properties.
The CEE/BZA combination is the first TSEC approved, with governmental registration by both the FDA and European Union for clinical use in their zones of jurisdiction. Conclusive evidence that BZA would eliminate the need for a progestin was provided by extensive, randomized, controlled, clinical trials which demonstrated no endometrial stimulation or endometrial cancer.14–18 These clinical studies demonstrated beneficial effects of the CEE/BZA TSEC including reduction of hot flashes, treatment of vulvovaginal atrophy and reduction of bone resorption. Safety studies demonstrated no trend toward enhanced incidence of venothrombotic events from that observed with CEE alone and as yet, no signal suggesting adverse cardiac or cerebral effects (albeit with a limited duration of observation).
An important, unresolved issue regarding the BZA/CEE TSEC is its specific effects on normal breast tissue and breast cancer. Clinical trials have not been of sufficient duration to assess this effect and breast cancer incidence in the trials has been minimal (4 out of 1,585 women with 0.45 mg CEE and 20 mg BZA and two cases in the placebo arm of 1,241 women).19 As definitive clinical studies on breast cancer development will require years to complete, we concluded that pre-clinical studies were critically important to address two unanswered questions: (i) does CEE exert effects on breast tumor development, which differ from those of estradiol and (ii) Does BZA completely block the effects of estrogens on breast tumor development? If the blocking effects of BZA on breast parallel those on the uterus, this TSEC might potentially reduce the risk of breast cancer in a manner analogous to the effects of tamoxifen and raloxifene in women.20,21
To preliminarily address these two questions, we had previously studied the effects of CEE and E2 with or without addition of BZA on MCF-7 xenografts in nude mice. Surprisingly, CEE, at doses which markedly stimulated the uterus, did not stimulate growth of MCF-7 tumors whereas E2 caused substantial growth of both.22 These studies suggested that CEE acts as a potent estrogen on uterus but may have SERM-like anti-estrogenic properties on breast cancer. While we considered these preliminary findings intriguing, we reasoned that further studies in other models were necessary to provide evidence of generalizability and potential applicability to women.
Two mammary tumor models were chosen for the current studies: one involved tumor induction with N-nitroso-N-methylurea (NMU) in Sprague-Dawley rats and the other with estradiol in August-Copenhagen-Irish (ACI) rats. The NMU model is a widely used animal model to study the progression, treatment and prevention of breast cancer.23 Extensive, prior studies have demonstrated that approximately 50% of the NMU–induced tumors are hormone dependent.24 The ACI rat model is unique as the only estrogen-induced breast cancer model and its hormone dependence has been demonstrated by prevention with pre-treatment administration of tamoxifen.25,26 Based on these characteristics, both the NMU and ACI models appeared to be ideally suitable for the two categories of studies described in this manuscript: first, the comparison of the effects of CEE with E2 on breast cancer development and uterine stimulation and second, the inhibitory effects of BZA on these estrogen effects on both tissues.
Our findings report that CEE and E2 exert divergent effects on breast cancer development, proliferation and apoptosis while BZA efficaciously inhibits both breast cancer development and uterine stimulation. Taken together, our findings provide evidence of the inhibitory properties of the CEE/BZA on breast cancer development. These data support the possibility that the TSEC, CEE/BZA, might prevent breast cancer in women in a manner similar to tamoxifen and raloxifene while providing symptomatic relief of menopausal symptoms
Material and Methods
Materials
N-methyl-N-nitrosourea ISOPAC was purchased from Sigma Aldrich (St. Louis, MI). N-methyl-N-nitrosourea ISOPAC came in a bottle with 41.2% water and 8.5% acetic acid. On the day of injection, 98 ml sterile saline was added to the bottle to make 1% solution. The pH value of the solution was ~4 measured by a pH strip. The NMU solution bottle was wrapped with aluminum foil and kept on ice. Estradiol (E2) was from Steraloids (Newport, RI). Conjugated equine estrogens (CEE) and bazedoxifene (BZA) were provided by Pfizer. CEE and BZA solutions were prepared in a vehicle of 2% Tween 80 and 0.5% methylcellulose according to the protocol of Peano et al.27 Anti-Ki67 and cleaved caspase-3 anti-bodies were purchased from Abcam (Cambridge, MA) and Cell Signaling Technology (Danvers, MA) respectively.
Carcinogen induced tumor model
All animal studies were carried out in accordance with current National Institutes of Health (NIH) guidelines and protocols were approved by the University of Virginia Animal Care and Use Committee (ACUC). Female Sprague-Dawley rats, aged 5–6 weeks were obtained from Harlan. The rats were housed in solid-bottomed polycarbonate cages bedded with hardwood chips. All rats were fed regular rodent chow and distilled water ad libitum throughout the experiment. At 50 ± 4 days of age, animals were injected intraperitoneally with N-methyl-N-nitrosourea (NMU) at the dose of 50 mg/kg. Twenty days after NMU injection, ovaries were removed from 72 rats and in 10 rats the ovaries remained intact. Ovariectomized animals were divided into seven groups which received the following treatment: cholesterol implant (15 mg, 9.5 mm) as a vehicle control, estradiol implant (3 mg E2 + 12 mg cholesterol, 9.5 mm), CEE 3 mg/kg/day, po, CEE 3 mg/kg + BZA 2 mg/kg, po, CEE 10 mg/kg, po, CEE 10 mg/kg + BZA 13 mg/kg, po, estradiol implant + BZA 13 mg/kg, po. The doses of CEE and BZA in the current studies were determined based on previous studies of our laboratory and others.22,27 Drug doses are adjusted weekly according to body weight. Four rats received no treatment as a control. Preparation of the cholesterol and estradiol implants was as described previously.28
Estradiol-induced tumor model
Sixty-five female August-Copenhagen-Irish (ACI) rats, 5- to 6-week old, were obtained from Harlan. A 23-mm Silastic tubing packed with 27 mg estradiol was inserted subcutaneously one week after arrival. The E2 implant was kept for 90 days, a duration deemed sufficient to initiate tumor development, but not sufficient to allow growth to a palpable size.26 The E2 implants were then removed from the rats which were assigned to CEE plus or minus BZA. The E2 implants were retained in the rats for the E2 and E2 + BZA groups. Drug doses are adjusted weekly according to body weight.
Measurement of plasma estradiol
Two methods were utilized to measure plasma estradiol levels: an ELISA assay previously validated in depth for rodent samples29 and a liquid chromatography-mass spectrometry (LC-MS) assay previously validated in post-menopausal women.30 Estradiol measured by LC-MS included both unconjugated and conjugated estradiol measured after enzymatic cleavage of estradiol sulfate and glucuronide with helix pomatia overnight. The methodology was described in detail previously. The coefficient of variation of this assay at levels measured ranged from 10.1% at levels of 1.5 pg/ml to 2.1% at 75 pg/ml.30
Additional assay validation: As biologic evidence of assay validity, the uterine weight bioassay was utilized to demonstrate a correlation between measured estradiol levels and degree of stimulation of uterine weight.
Monitoring tumor incidence
Tumor formation was monitored by observation and palpation twice a week. Once a tumor was found it would be removed within one week. Tumor multiplicity was recorded. Tumor incidence was calculated and compared by Kaplan-Meier analysis.
Immunohistochemistry
Tumors were excised and fixed in formalin for hematoxylin and eosin stain and immunohistochemical staining for Ki67 and cleaved caspase-3. Paraffin-embedded tissue sections were dewaxed and hydrated. The slides were merged in TE buffer (Tris 10 mM, EDTA 1 mM, pH 9) and autoclaved for 10 min to unmask antigens. Tissue sections were incubated with primary antibody in a moisture chamber at 4°C overnight. Elite VectaStain ABC kit and DAB (Vector Laboratories, Burlingame, CA) were used for the rest steps of IHC following the manufacturer’s instruction. The sections were counter stained by hematoxylin. Ki67 or cleaved caspase-3 positive cells were counted using ImageJ software. Difference between treatments was analyzed by Student’s t test.
Whole-mount analysis
Our previously published methods were used to prepare whole mounts of mammary glands.31
Results
Serum estradiol levels in NMU treated Sprague-Dawley rats
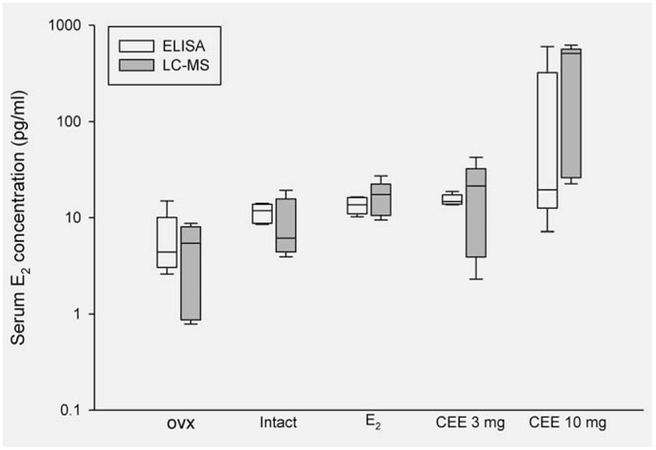
ELISA assays (Fig. 1) demonstrated the expected low levels of E2 in ovx animals (6.1 pg/ml) compared to concentrations in intact animals (11.3 pg/ml) and in ovx animals administered E2 (13 pg/ml). In ovx animals receiving CEE at 3 mg, the resulting metabolism of CEE components to E2 resulted in levels similar (12 pg/ml) to those given E2 and were slightly higher (20 pg/ml) in animals given 10 mg CEE. Measurements with the highly specific LC-MS assay (Fig. 1) provided similar results in the respective groups, providing confidence in results from the ELISA assay. It should be noted that the 10 mg CEE dose was associated with somewhat enhanced but unexplained variability.
Figure 1.
Serum concentrations of estradiol of Sprague-Dawley rats measured by ELISA and LC-MS methods. n = 5 each group. Note that the y-axis is on a logarithmic scale.
Differential effects of CEE and estradiol on mammary tumor development and uterine weight in the NMU model
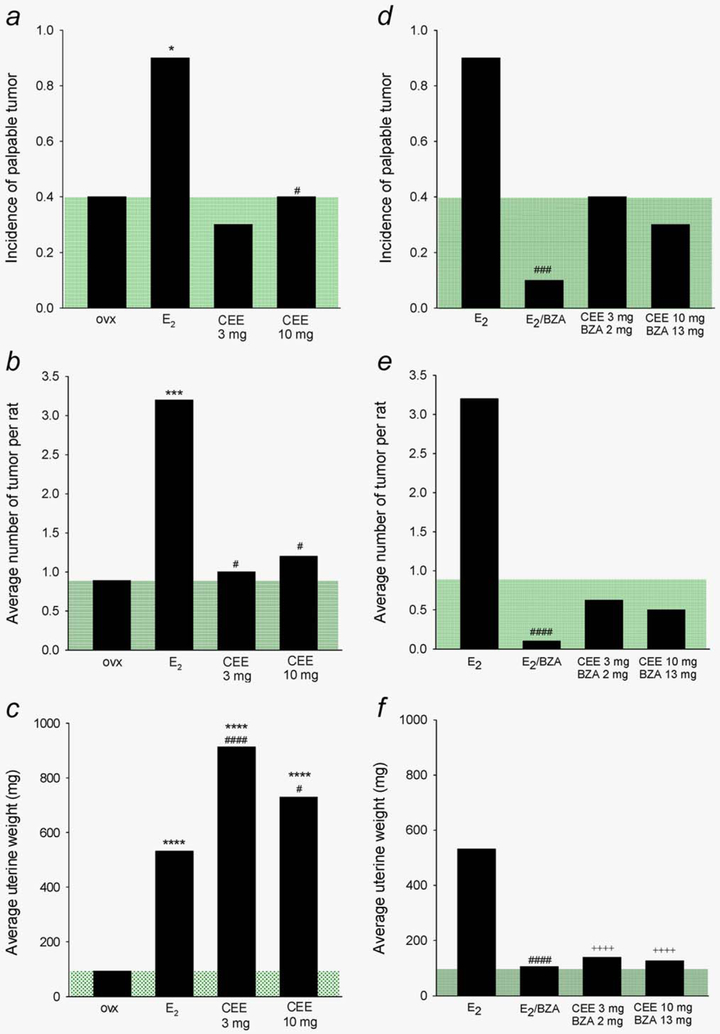
The first question to be addressed in this study concerned the differential effect of CEE and E2 on breast tumor development. These studies used the Sprague-Dawley, NMU carcinogenesis model. As reported in the literature, approximately 50% of NMU induced tumors in these rats develop in the absence of ovarian hormones and are thus hormone-independent while the remainder are hormone-dependent.24 Our data are concordant as 40% of our ovx animals developed tumors in the absence of ovarian hormones during 40 weeks of follow up (Fig. 2a). As evidence of the hormone-dependence of the remaining 50% of tumors, E2 administration to ovx animals resulted in 90% tumor incidence (p = 0.003 vs. ovx). This model then allowed us to assess the effects of CEE in comparison. In marked contrast to the effects of E2, administration of CEE at 3 and 10 mg did not stimulate tumor development over that observed in the OVX group (i.e., above the shaded green area in Fig. 2a).
Figure 2.
Differential effects of E2 and CEE on NMU-induced mammary tumor and uterus and inhibition of estrogenic effect of the estrogens by BZA. (a, d). Tumor incidence; (b, e) Number of tumor per rat; (c, f) Uterine weight. Green shaded areas indicate the levels of ovx rats. Statistical significance levels: *p < 0.05, **p < 0.01, ***p < 0.005, ****p < 0.001 compared to ovx; #p < 0.05, ##p < 0.01, ###p < 0.005, ####p < 0.001 compared to E2; ++++p < 0.001 compared to CEE treatment without BZA.
NMU often induces multiple breast tumors in each animal.32 In quantitating this parameter, we found that E2 treatment also increased the number of tumors developing in each animal (i.e., 3.2 per rat) compared to those in the ovx rats (i.e., 1.0 per rat). In contrast, CEE did not increase tumor number over those in the ovx rats (1.0 and 1.2 vs. 1.0 in ovx animals Fig. 2b).
NMU induced palpable tumors exhibit a wide spectrum of pathology ranging from fibroadenomas, adenomas, to Type I and II papillary carcinomas,33 often with characteristics of each in surrounding breast tissue (Supporting Information Fig. 1). This spectrum of heterogeneity likely represents the stochastic effects of NMU as a carcinogen, a condition observed in other but not all experimental models of breast cancer.34,35 In intact animals, 88.2% of tumors were Type I and II papillary carcinomas versus 47.7% in the E2 treated animals whereas the remainder were palpable benign fibroadenomas and adenomas (Supporting Information Table 1).
We reasoned that the stimulatory effects of E2 on breast tumor development in ovx animals and lack of similar effects of CEE could have occurred as a result of greater estrogen potency of E2. To assess this possibility, we utilized uterine weight as a bioassay of estrogenic effects. Estradiol and CEE at both doses markedly stimulated uterine weight (Fig. 2c) with effects proportional to the plasma levels of estradiol achieved (correlation coefficient of plasma estradiol versus uterine weight R = 0.997 ELISA assay and R = 0.998 mass spec assay, Supporting Information Fig. 2).
Blockade of the estrogenic effect of E2 and CEE by BZA in the NMU model
The second question to be addressed concerned the ability of BZA to block the effects of E2 and CEE on tumor development and on uterine weight. Treatment with BZA (13 mg/kg) significantly blocked E2-induced tumor development (Fig. 2d) as well as reducing the multiplicity of tumors (Fig. 2e) in this model. BZA inhibited both the Type I and II papillary carcinomas as well as adenomas and fibroadenomas, an important finding emphasized in the discussion. As the tumors in the CEE treated groups were considered hormone-independent (i.e., same tumor number as in ovx animals), BZA exerted no significant effects on the incidence nor number of breast tumors in these groups (Fig. 2e).
As evidence of potent antiestrogen effects of BZA on the uterus, this SERM reduced uterine weight in the animals receiving E2 to the levels approaching those in the ovx rats. BZA also effectively blocked the uterine stimulatory effects of both the 3 and 10 mg doses of CEE (Fig. 2f).
Apoptosis and proliferation in NMU model
Careful histologic examination of palpable tumors revealed substantial histologic heterogeneity both within and between tumors and highly variable caspase-3 levels as an index of apoptosis and Ki67 as an index of proliferation. Accordingly, no statistically significant differences in these two parameters were observed when comparing CEE with E2 (data not shown).
Effects of E2 and CEE on benign mammary tissue
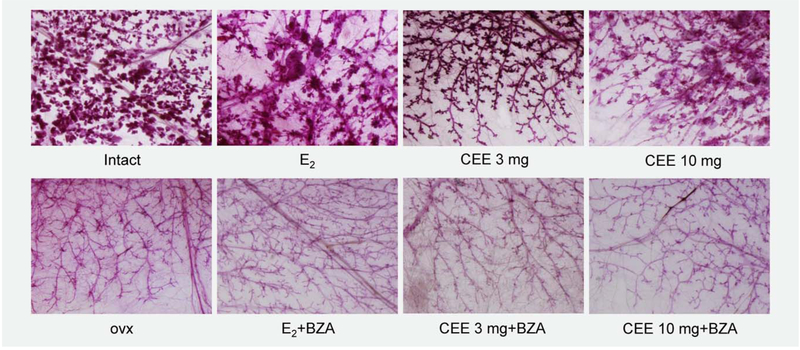
Having shown differential effects of CEE and E2 on breast tumor cancer development, we sought to examine the effects of these hormones on non-tumor containing breast tissue. We considered these experiments pertinent as our prior data in mice demonstrated the striking finding that both CEE and E2 similarly stimulated benign breast duct and alveoleor development in ovariectomized mice but exerted divergent effects on breast cancer.22 As shown in Figure 3, the degree of ductal and alveolar breast development in the ovx animals was minimal when compared that of intact animals. CEE stimulated both alveoli and branching in ovx animals but to a slightly lesser extent than E2. BZA completely blocked these estrogenic effects such that the ductal and alveolar pattern returned to that seen in the ovx animals.
Figure 3.
Mammary gland whole mounts of NMU treated Sprague-Dawley rats. The ducts and alveoli of the mammary gland are fully developed in intact animals. E2 caused ductal enlargement and further proliferation of alveoli. The effect of CEE was similar to that of E2. BZA completely blocked the effects of CEE and E2.
Differential effects of E2 and CEE in the ACI rat model
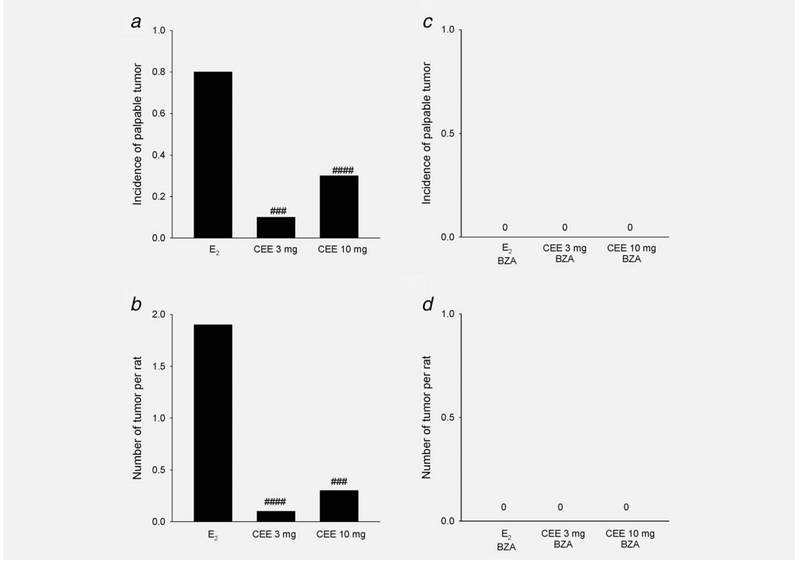
The majority of animals (80%) developed palpable tumors in rats receiving E2 by implant. In contrast, palpable tumors in CEE treated animals were only 10% for the 3 mg/kg group (p < 0.005) and 30% for the 10 mg/kg group (p < 0.001) (Fig. 4a). In addition, the latent period before palpable tumor development was longer in the high dose CEE Group (286 ± 62 days) than that in E2 Group (163 ± 52 days). Average tumor numbers were 1.9/rat in E2 treated rats and markedly lower in CEE treated animals (an average of 0.1 and 0.3 per rat (Fig. 4b).
Figure 4.
Differential effects of E2 and CEE on mammary tumor formation in ACI rats and inhibitory effect of BZA. (a, c) Tumor incidence; (b, d) Number of tumor per rat; ###p < 0.005, ####p < 0.001 compared to E2. The zero designation in the right panels indicates that no palpable tumors were present.
These results indicated that CEE exerted lesser effects on breast tumor development in the ACI rat than did E2. We also demonstrated that BZA completely inhibited E2-induced tumor formation in both groups (Figs. 4c and 4d). These results are concordant with our findings in the MCF-7 and NMU models.
Differential effects of E2 and CEE on cellular proliferation and apoptosis
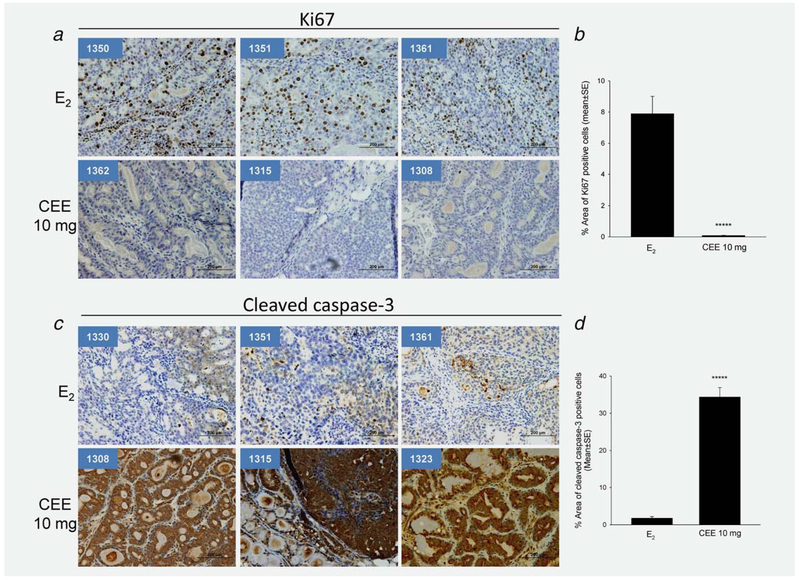
The ACI rat tumors were uniformly malignant and exhibited only a minimal degree of heterogeneity on histologic analysis25 and on Ki67 and cleaved caspase-3 indices. The lack of marker variance facilitated quantitation of the statistical significance of the degree of proliferation (Ki67) and apoptosis (cleaved caspase-3). Seventeen sections of eight tumors from E2-treated ACI rats and 11 sections of five tumors from CEE-treated rats were examined. All tumors from E2 treated rats contained Ki67 positive cells (Fig. 5a and Supporting Information Fig. 3) whereas, in striking contrast, all of five tumors from CEE (10 mg/kg) treated animals were Ki67 negative (Fig. 5a and Supporting Information Fig. 3). Differences in Ki67 expression between E2 and CEE treatments are highly significant (Fig. 5b). With respect to apoptosis in E2-treated tumors, cleaved caspase-3 was negative in most tumors whereas positive staining of cleaved caspase-3 was observed in all CEE-treated tumors (Figs. 5c and 5d and Supporting Information Fig. 4). These results were consistent with our prior findings in the nude mouse model with MCF-7 xenografts and suggested that the induction of apoptosis with a lesser degree of proliferation contributed to low tumor incidence in CEE-treated animals.
Figure 5.
Effects of E2 and CEE on cellular proliferation marker Ki67 and cleaved caspase-3 in mammary tumors from ACI rats. (a) Images of immunohistological staining of Ki67 of three sections from each group. (b) Percentage of area of Ki67 positive cells of all tumor sections examined. *****p = 0.000003. (c) Images of immunohistological staining of cleaved caspase-3 of three sections from each group. Animal ID number is shown in left upper corner of each panel. (b) Percentage of area of cleaved caspase-3 positive cells of all tumor sections examined. *****p = 0.0000003.
Discussion
The first question addressed in this study was whether CEE exerts effects on breast tumors which differ from those of estradiol. Our results clearly demonstrated divergent effects of E2 and CEE on the number of palpable of breast tumors in two rat mammary tumor models and confirmed our prior results in MCF-7 xenografts (Table 1). In the NMU model, E2 increased both the number of animals with tumors and the number of tumors per animal compared to the ovx group. In marked contrast, CEE caused no stimulation over that observed in the ovx animals. In the ACI model, 80% of the E2 treated animals developed palpable tumors but only 10–30% with CEE administration. E2 stimulated proliferation and blocked apoptosis in the ACI rats whereas CEE exerted opposite effects with stimulation of apoptosis but not proliferation. As further evidence of divergence, our previously published studies on MCF-7 xenografts also demonstrated differential effects of CEE and E2 on the expression of PR, c-Myc, AREG and WISP-2.22 In marked contrast to the effect on breast tumors in these and our prior studies, CEE and E2 both potently stimulated uterine weight, effects which were concordant with changes in measured levels of plasma E2.
Table 1.
Differential effect of CEE and E2 in three breast cancer models
| MCF-7 xenografts |
NMU model |
ACI model |
||||
|---|---|---|---|---|---|---|
| Parameters | E2 | CEE | E2 | CEE | E2 | CEE |
| Uterine weight | ↑↑↑ | ↑↑↑ | ↑↑↑ | ↑↑↑ | N/A | N/A |
| Tumor incidence | N/A | N/A | ↑↑↑ | ↔ | ↑↑↑ | ↔ or ↑ |
| # of tumor per animal | N/A | N/A | ↑↑↑ | ↔ | ↑↑↑ | ± |
| Tumor growth | ↑↑↑ | ↔ | N/A | N/A | N/A | N/A |
| Proliferation (Ki67) | ↑↑↑ | ↔ | Variable | Variable | ↑↑↑ | ↓↓↓ |
| Apoptosis | ↓↓↓ | ↑↑↑ | Variable | Variable | ↓↓↓ | ↑↑↑ |
±: equivocal effect.
↔: no effect.
These findings suggest that CEE, a composite of >200 components, of which 50% of the mass is represented by estrogens and also include other small molecules,36 exhibits mixed functional estrogenic activity (SERM like properties) with stimulatory effects on uterus and neutral effects on breast tumor development. While this conclusion is hypothetical, the SERM like properties of CEE might provide an explanation why CEE alone did not increase the risk of breast cancer in the WHI study, and in fact, reduced the risk observed at the 13 year follow-up through its pro-apoptoic properties.8 Other authors, based on the findings of several preclinical studies,37–39 have also suggested that apoptosis might be involved in the reduction of breast cancer with CEE alone in the WHI trial.40
The second question addressed by this study was whether BZA could block the effects of estrogens on breast tumor development. In our rat models, the answer was clearly yes as BZA blocked tumor development in both the E2 treated NMU and ACI animals. Additional evidence was provided by our prior observations that BZA blocked the E2 induced growth of MCF-7 xenografts.22 Considering that CEE exerts no (NMU) or only minimal (ACI) effects on breast tumor development, BZA would be even more likely to block the breast effects of CEE in women. These findings support the concept that the combination of CEE and BZA might prevent breast cancer in a fashion analogous to tamoxifen and raloxifene in menopausal women. It has been well established that women have been reluctant to take tamoxifen for prevention.41 Use of a TSEC would be more acceptable as they would be taking this agent for symptom relief and the prevention of breast cancer would be only a secondary consideration for most women.
NMU in these studies induced palpable tumors which on careful histologic assessment were both benign and malignant, suggesting a stochastic effect to induce a variable number of mutations in tumor tissue.42 The benign lesions likely represent pre-malignant tumor precursors. BZA blocked the formation of these benign lesions as well as the malignant Type I and II papillary carcinomas. Notably, in women receiving tamoxifen for breast cancer prevention, the incidence of benign lesions is significantly reduced.43 Taken together, these data provide further support for the possibility that the TSEC, CEE/BZA might prevent both benign pre-malignant lesions as well as frank malignancy. Definitive randomized controlled trials in women will be necessary to determine if these possibilities will prove correct.
The use of Premarin for menopausal symptoms had been increasing since it was marketed in 1943 until the mid-1970s when an association between estrogen therapy and endometrial cancer was reported.44,45 Since the early 1980s, the use of combined estrogen–progestogen became more common, only hysterectomized women were treated with estrogens alone. The issue that there might be a difference between E2 and CEE on breast cancer risk had not generally been raised until the 13-year follow-up of the WHI was reported. In that manuscript, Manson et al. reported a 21% reduction of breast cancer with CEE alone after 13 years of follow-up.8 This raised the issue of differential effects of CEE and E2 on breast cancer development as several observational studies had previously demonstrated that E2 does increase breast cancer risk if given for a sufficiently long period of time.4
With a comprehensive literature review, we could find no comparisons of E2 and CEE on breast tissue in women. In macaque monkeys, CEE appears to have a diminished stimulatory effect on breast compared to E2.46 We emphasize then that an important gap at the present time is lack of a direct, randomized, head-to-head comparison of E2 alone versus CEE alone in menopausal women. This is critically necessary to provide further evidence of differential effects on breast cancer development in women.
Tumor growth is determined by the balance of proliferation and apoptosis. Our prior studies with a human breast cancer xenograft model have shown an increase of apoptosis in E2 treated tumors22 and the current study demonstrated this in the ACI model. Most striking was the divergent effects in the ACI model with E2 stimulating proliferation (Ki67 as marker) and blocking apoptosis (cleaved caspase-3 as marker) whereas CEE stimulated apoptosis and reduced proliferation.
The SERM-like effect of CEE might occur because that some of the components in CEE are anti-estrogenic. CEE is a mixture of 200 plus compounds (50% by mass being estrogens) many of which do not exist in human body.36 Some of the components may act as tissue selective estrogens.47,48 Although the pharmacological properties of most individual components of CEE are not defined, biological data suggest that combination of these compounds exert SERM-like effects.48 In the NMU rat model, we found that the average uterine weight of high dose CEE-treated rats is lower than that receiving low dose CEE even though the total estradiol levels were higher. These data and those of tumor incidence strongly suggest a SERM-like component of CEE which is more potent as the dose of CEE increases.
A striking finding of our prior study in mice was that CEE stimulated benign breast ductal and end bud development to the same extent as biologically equivalent doses of E2. Our data in the NMU models demonstrated a similar finding with respect to proliferation in benign tissue. It has not previously been considered that a SERM could exert one effect on nontumor components of a tissue and opposite effects on tumors of the same tissue. The fact that CEE stimulates growth of benign breast components but not breast tumors appears to be an example of this phenomenon. Hypothetically, other factors in the tumor tissue could alter the pharmacologic effects of CEE as a SERM. Such a phenomenon has been proposed to explain why tamoxifen is an estrogen on the uterus whereas it acts as an anti-estrogen on breast tissue.
Tamoxifen and raloxifene, when used to prevent breast cancer are likely not blocking de novo tumor initiation but more likely, promotion of occult tumors. Breast cancer growth models suggest that breast cancers require on average about 16 years to grow large enough to be detected clinically. Autopsy studies indicate that approximately 7% of women age 40–70 harbor occult tumors too small to be detected clinically.49,50 The potent anti-estrogenic effect of BZA on breast tumors would suggest that in women receiving the TSEC, CEE/BZA, the overall effect on occult breast cancers would be to slow down their growth and prevent them from reaching the size required for mammographic detection or clinical palpability. Such findings would be interpreted as breast cancer prevention, when in reality, prevention of breast cancer detection is the more likely explanation.
In summary, CEE and E2 appear to exert divergent effects on breast tumor growth and the addition of BZA blocks these effects. Long term clinical trials are now necessary to determine if similar effects occur in women and if this therapy prevents the growth of occult breast tumors.
Supplementary Material
What’s new?
To alleviate menopausal discomforts, doctors frequently prescribe hormone therapy that includes conjugated equine estrogen (CEE) plus medroxyprogesterone acetate (MPA). But this combo ups the risk of breast cancer. Here, the authors investigated the safety of CEE paired with bazedoxifene (BZA), a selective estrogen receptor modulator, in rat models. First, they compared CEE and estradiol. While estradiol blocked apoptosis and increased tumor incidence, CEE stimulated apoptosis and showed no effect on tumor incidence or proliferation. They then showed that BZA blocked tumor development in rats treated with estradiol. Long-term clinical trials will show whether these findings hold in humans as well.
Acknowledgements
We acknowledge the Reproductive Endocrinology Core Lab at the University of Virginia for measurements of serum estradiol levels by ELISA. Dr. Jose Russo provided consultancy with pathology of the NMU-induced mammary tumors.
Grant sponsor: Pfizer
Abbreviations:
- ACI
Augusta-Copenhagen-Irish rat
- BZA
bazedoxifene
- CEE
conjugated equine estrogens
- E2
estradiol
- MHT
menopause hormone therapy
- NMU
N-methyl-N-nitrosourea
- SERM
selective estrogen receptor modulator
- TSEC
tissue selective estrogen complex
Footnotes
Conflict of Interest: Richard J. Santen received grant from the Pfizer Inc. All other authors declare no potential conflicts of interest.
References
- 1.Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2015;100:3975–4011. [DOI] [PubMed] [Google Scholar]
- 2.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321–33. [DOI] [PubMed] [Google Scholar]
- 3.Santen RJ, Allred DC, Ardoin SP, et al. Postmen-opausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab 2010;95:s1–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beral V, Banks E, Reeves G, et al. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 2003;362:1330–27. [DOI] [PubMed] [Google Scholar]
- 5.Chen WY, Manson JE, Hankinson SE, et al. Unopposed estrogen therapy and the risk of invasive breast cancer. Arch Intern Med 2006;166: 1027–32. [DOI] [PubMed] [Google Scholar]
- 6.Reeves GK, Beral V, Green J, et al. Hormonal therapy for menopause and breast-cancer risk by histological type: a cohort study and meta-analysis. Lancet Oncol 2006;7:910–8. [DOI] [PubMed] [Google Scholar]
- 7.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA 2004;291:1701–12. [DOI] [PubMed] [Google Scholar]
- 8.Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the women’s health initiative randomized trials. JAMA 2013;310:1353–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komm BS, Mirkin S, Jenkins SN. Development of conjugated estrogens/bazedoxifene, the first tissue selective estrogen complex (TSEC) for management of menopausal hot flashes and postmenopausal bone loss. Steroids 2014;90:71–81. [DOI] [PubMed] [Google Scholar]
- 10.Mirkin S, Ryan KA, Chandran AB, et al. Bazedoxifene/conjugated estrogens for managing the burden of estrogen deficiency symptoms. Maturitas 2014;77:24–31. [DOI] [PubMed] [Google Scholar]
- 11.Chang KCN, Wang Y, Bodine PVN, et al. Gene expression profiling studies of three SERMs and their conjugated estrogen combinations in human breast cancer cells: insights into the unique antagonistic effects of bazedoxifene on conjugated estrogens. J Steroid Biochem Mol Biol 2010;118: 117–24. [DOI] [PubMed] [Google Scholar]
- 12.Wardell SE, Kazmin D, McDonnell DP. Research resource: transcriptional profiling in a cellular model of breast cancer reveals functional and mechanistic differences between clinically relevant SERM and between SERM/estrogen complexes. Mol Endocrinol 2012;26:1235–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, Han SJ, Smith CL. Cooperative activation of gene expression by agonists and antagonists mediated by estrogen receptor heteroligand dimer complexes. Mol Pharmacol 2013;83:1066–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kagan R, Williams RS, Pan K, et al. A randomized, placebo- and active-controlled trial of baze-doxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause 2010;17:281–9. [DOI] [PubMed] [Google Scholar]
- 15.Mirkin S, Komm BS, Pan K, et al. Effects of baze-doxifene/conjugated estrogens on endometrial safety and bone in postmenopausal women. Climacteric 2013;16:338–46. [DOI] [PubMed] [Google Scholar]
- 16.Pickar JH, Yeh I-T, Bachmann G, et al. Endomerial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapy. Fertil Steril 2009;92:1018–24. [DOI] [PubMed] [Google Scholar]
- 17.Pinkerton JV, Utian WH, Constantine GD, et al. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause 2009;16:1116–24. [DOI] [PubMed] [Google Scholar]
- 18.Pinkerton JV, Harvey JA, Lindsay R, et al. Effects of bazedoxifene/conjugated estrogens on the endometrium and bone: a randomized trial. J Clin Endocrinol Metab 2014;99:E189–98. [DOI] [PubMed] [Google Scholar]
- 19.Mirkin SPJ, Kagan R, Thompson JR, et al. Gynecologic safety of conjugated estrogens plus baze-doxifene: pooled analysis of five phase 3 trials. J Womens Health (Larchmt) 2016;25:431–42. [DOI] [PubMed] [Google Scholar]
- 20.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst 1998;90:1371–88. [DOI] [PubMed] [Google Scholar]
- 21.Vogel VG, Costantino JP, Wickerham D, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP study of tamoxifen and raloxifene (star) p-2 trial. JAMA 2006;295: 2727–41. [DOI] [PubMed] [Google Scholar]
- 22.Song Y, Santen RJ, Wang J-P, et al. Effects of the conjugated equine estrogen/bazedoxifene tissue selective estrogen complex (TSEC) on mammary gland and breast cancer in mice. Endocrinology 2012;153:5706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gullino PM, Pettigrew HM, Grantham FH. N-nitrosomethylurea as mammary gland carcinogen in rats. J Natl Cancer Inst 1975;54:401–14. [PubMed] [Google Scholar]
- 24.Thompson HJ, McGinley J, Rothhammer K, et al. Ovarian hormone dependence of pre-malignant and malignant mammary gland lesions induced in pre-pubertal rats by 1-methyl-1-nitrosourea. Carcinogenesis 1998;19:383–6. [DOI] [PubMed] [Google Scholar]
- 25.Shull JD, Spady TJ, Snyder MC, et al. Ovaryintact, but not ovariectomized female ACI rats treated with 17beta-estradiol rapidly develop mammary carcinoma. Carcinogenesis 1997;18:1595–601. [DOI] [PubMed] [Google Scholar]
- 26.Li SA, Weroha SJ, Tawfik O, et al. Prevention of solely estrogen-induced mammary tumors in female ACI rats by tamoxifen: evidence for estrogen receptor mediation. J Endocrinol 2002;175:297–305. [DOI] [PubMed] [Google Scholar]
- 27.Peano BJ, Crabtree JS, Komm BS, et al. Effects of various selective estrogen receptor modulators with or without conjugated estrogens on mouse mammary gland. Endocrinology 2009;150:1897–903. [DOI] [PubMed] [Google Scholar]
- 28.Yue W, Wang J-P, Hamilton CJ, et al. In situ aromatization enhances breast tumor estradiol levels and cellular proliferation. Cancer Res 1998; 58:927–32. [PubMed] [Google Scholar]
- 29.Haisenleder DJ, Schoenfelder AH, Marcinko ES, et al. Estimation of estradiol in mouse serum samples: evaluation of commercial estradiol immunoassays. Endocrinology 2011;152:4443–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q, Rangiah K, Mesaros C, et al. Ultrasensitive quantification of serum estrogens in postmenopausal women and older men by liquid chromatography-tandem mass spectrometry. Steroids 2015;96:140–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim T, Park H, Yue W, et al. Tetra-methoxystilbene modulates ductal growth of the developing murine mammary gland. Breast Cancer Res Treat 2011;126:779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson HJ, Adlakha H. Dose-responsive induction of mammary gland carcinomas by the intraperitoneal injection of 1-methyl-1-nitrosourea. Cancer Res 1991;51:3411–5. [PubMed] [Google Scholar]
- 33.Russo J Significance of rat mammary tumors for human risk assessment. Toxicol Pathol 2015;43:145–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allred DC, Medina D. The Relevance of mouse models to understanding the development and progression of human breast cancer. J Mammary Gland Biol Neoplasia 2008;13:279. [DOI] [PubMed] [Google Scholar]
- 35.Medina D Premalignant and malignant mammary lesions induced by MMTV and chemical carcinogens. J Mammary Gland Biol Neoplasia 2008;13:271–7. [DOI] [PubMed] [Google Scholar]
- 36.Levy MJ, Boyne MT, Rogstad S, et al. Market-place analysis of conjugated estrogens: determining the consistently present steroidal content with LC-MS. AAPS J 2015;17:1438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song RX-D, Mor G, Naftolin F, et al. Effect of long-term estrogen deprivation on apoptotic responses of breast cancer cells to 17β-estradiol. J Natl Cancer Inst 2001;93:1714–23. [DOI] [PubMed] [Google Scholar]
- 38.Lewis JS, Meeke K, Osipo C, et al. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. J Natl Cancer Inst 2005;97:1746–59. [DOI] [PubMed] [Google Scholar]
- 39.Jordan VC. The new biology of estrogen-induced apoptosis applied to treat and prevent breast cancer. Endocr Relat Cancer 2015;22:R1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the women’s health initiative randomized trials. JAMA 2017;318:927–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmberg C Decision making in the context of breast cancer chemoprevention: patient perceptions and the meaning of risk. Am Soc Clin Oncol Educ Book 2015;35:e59–64. [DOI] [PubMed] [Google Scholar]
- 42.Russo J, Tahin Q, Lareef MH, et al. Neoplastic transformation of human breast epithelial cells by estrogens and chemical carcinogens. Environ Mol Mutagen 2002;39:254–63. [DOI] [PubMed] [Google Scholar]
- 43.Tan-Chiu E, Wang J, Costantino JP, et al. Effects of tamoxifen on benign breast disease in women at high risk for breast cancer. J Natl Cancer Inst 2003;95:302–7. [DOI] [PubMed] [Google Scholar]
- 44.Smith DC, Prentice R, Thompson DJ, Herrmann WL. Association of exogenous estrogen and endometrial carcinoma. New Engl J Med 1975; 293:1164–7. [DOI] [PubMed] [Google Scholar]
- 45.Ziel HK, Finkle WD. Increased risk of endometrial carcinoma among users of conjugated estrogens. New Engl J Med 1975;293:1167–70. [DOI] [PubMed] [Google Scholar]
- 46.Wood CE, Clarkson TB, Chen H, et al. Comparative effects of oral conjugated equine estrogens and micronized 17beta-estradiol on breast proliferation: a retrospective analysis. Menopause 2008;15:978. [DOI] [PubMed] [Google Scholar]
- 47.Lugar CW, Magee D, Adrian MD, et al. B-Ring unsaturated estrogens: biological evaluation of 17α-Dihydroequilein and novel B-Nor-6-thiaequilenins as tissue selective estrogens. Bioorg Med Chem Lett 2003;13:4281–4. [DOI] [PubMed] [Google Scholar]
- 48.Bhavnani BR, Stanczyk FZ. Pharmacology of conjugated equine estrogens: efficacy, safety and mechanism of action. J Steroid Biochem Mol Biol 2014;142:16–29. [DOI] [PubMed] [Google Scholar]
- 49.Santen RJ, Yue W, Heitjan DF. Modeling of the growth kinetics of occult breast tumors: role in interpretation of studies of prevention and menopausal hormone therapy. Cancer Epidemiol Biomarkers Prev 2012;21:1038–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhathal PS, Brown RW, Lesueur GC, et al. Frequency of benign and malignant breast lesions in 207 consecutive autopsies in Australian women. Br J Cancer 1985;51:271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.