Abstract
The species Inga laurina is native to the Brazilian Cerrado. There are no studies about the chemical composition and biological activities of extracts of this endangered species. The ethanolic extract and its successive fractions are rich in phenolic compounds and presented good antifungal activities. HPLC/MS-MS/MS and H1/C13 analysis led to the identification of seventeen compounds, most of which are gallic acid derivatives, myricetin and quercetin glycosides. The ethyl acetate fraction (EAF) contained high levels of total phenolics, expressed in milligrams of gallic acid equivalents per gram of extract (475.3 ± 1.9 mg GAE gextract−1) and flavonoids expressed in milligrams of quercetin equivalents per gram of extract (359.3 ± 10.6 mg QE gextract−1). This fraction was active against fungi of the Candida genus. The EAF showed MIC value 11.7 μg mL−1 against C. glabrata and a selectivity index of 1.6 against Vero cells. The flavonol glycoside myricetin-3-O-rhamnoside was isolated for the first time from the Inga laurina. These results make I. laurina a promising plant as a source of pharmaceutical and biological active antifungal compounds.
1. Introduction
Among the plants in danger of extinction from Brazilian savannah biome (Cerrado), the Inga spp. (Fabaceae) are found mainly in neotropical areas and many plants from this family have been reported to possess biological activities and several are used in folk medicine [1]. Leaves and roots of I. strigillosa are used by the indigenous tribes of the Amazon region for skin wounds, the flowers of I. cecropietorum are used for earache, and the flowers of I. rubiginosa are used against nasal congestion [2].
There are 131 species of this genus in Brazil, with 51 of them being endemic [3]. Several biological activities have been observed in Inga species, such as the antioxidant activity of I. edulis [4–6] and I. verna [2], the antifungal activity of I. marginata [7], the antimicrobial activity of I. fendleriana [8], the allelochemical effects in I. umbellifera [9], and the antitumoral activity in I. marginata [10].
The sp. I. laurina is found not only in the Cerrado biome but in different regions of Brazil: Amazon, Caatinga, and the Atlantic Coast. This plant is easily found in Brazil, but there are a few studies about its essential oil. The present work is based on a doctorate thesis and the aim of the present work was to identify the bioactive metabolites by Mass Spectrometry and to evaluate the antifungal activity against Candida genus and the cytotoxic activity on Vero cells ATCC CCL 81 using ethanolic extract and fractions of I. laurina leaves.
A few studies of plants from the Brazilian savannah have identified bioactive metabolites by Mass Spectrometry. The leaves of the plant Banisteriopsis laevifolia (A. Juss.) B. Gates presented mainly phenolic compounds, as flavonoid glycosides of quercetin derivatives [11].
2. Materials and Methods
2.1. Plant Material
Leaf samples of I. laurina (Sw.) Willd, Fabaceae, were collected near the BR-050 in Uberlândia, Minas Gerais State, Brazil (18°59′13.96′′S; 48°12′42.16′′W) in the month of February (2012). The specimens were identified by Prof. Dr. Glein Monteiro de Araújo, from the Biology Institute, Federal University of Uberlândia. An exsiccate was deposited at Herbarium Uberlandense (HUFU), under number 64050.
2.2. Extracts Preparation
Leaves were dried at 35°C and then shredded in a knife mill. The extract was prepared by maceration at room temperature with ethanol (95%). The mass of 900.0 grams of leaves were extracted over 48 hours (3x). Filtrates were joined, concentrated under vacuum (under 40°C), and freeze-dried (Lyophilizer LS 3000, TERRONI, Brazil). A yield of 54.0 grams was obtained. The ethanolic extract (EE) was stored under refrigeration until analysis.
2.3. Liquid-Liquid Extraction
The EE (42.0 g) was redissolved in 570.0 mL of methanol:water (9:1) and subjected to liquid-liquid extraction using solvents of increasing polarities (hexane, chloroform, ethyl acetate, and n-butanol). Three extractions were performed using 300.0 mL of solvent per extraction. Fractions were concentrated to dryness giving hexane (12.2 g), chloroform (4.1 g), ethyl acetate (3.2 g), and n-butanol (7.4 g) fractions. These fractions were freeze-dried and stored under refrigeration until analysis.
2.4. Spectrophotometric Analysis of Total Phenolics, Proanthocyanidins, and Flavonoids Contents
Total phenolics were determined by the Folin-Ciocalteu method described by Morais et al. [29] and the results were expressed in mg of gallic acid equivalents by gram of dry extract (mg GAE gextract−1). Proanthocyanidin content was determined by the sulfuric vanillin method according to Morais et al. [29] and expressed as milligrams of catechin equivalents per gram of dry extract (mg CE gextract−1). Flavonoid content was determined as described by Woisky and Salatino [30] and expressed in mg of quercetin equivalents per gram of dry extract (mg QE gextract−1). The readings were taken in a Thermo Scientific Genesys-10S spectrophotometer. All analyses were performed in triplicate.
2.5. Antifungal Activity
2.5.1. Microbial Strains and Minimum Inhibitory Concentration (MIC)
The following microorganisms obtained from the American Type Culture Collection (ATCC, Rockville MD, USA) were used: Candida albicans (ATCC 28366), Candida tropicalis (ATCC 13803), and Candida glabata (ATCC 15126). The MIC determination for antifungal assay was performed according to the CLSI (Clinical and Laboratory Standard Institute) using the broth dilution assay method [31]. This assay way followed the methods of Nunes et al. 2016 [11]. The Amphotericin B and strains ATCC 22019 (Candida parapsilosis) and ATCC 6258 (Candida krusei) were used as quality controls.
2.6. Cytotoxicity Assay (CC50) Using Vero Cells
The cell viability test was performed with Vero cells (ATCC CCL 81) (kidney fibroblasts, African green monkey). The cytotoxic activity was performed using the microplate dilution method [32]. Cell viability was calculated from the absorbance of each concentration tested according to the growth control. The cytotoxic concentration (CC50) (concentration that presents 50% cell viability) was calculated by means of a dose-response graph with nonlinear regression [33]. Controls of growth, solvent, samples, negative control (100% lysed cells), and control of the medium were performed. The assays were performed in triplicate. The selectivity index (SI) of each sample was defined as the ratio between the logarithm of CC50 and the MIC against each strain (SI = log[CC50]/[MIC]) [34, 35].
2.7. Ethyl Acetate Fraction (EAF) Isolation and Fractionation
Due to the fact that EAF of I. laurina showed promising results for inhibiting antifungal growth, it was selected for refractionation. Thus, a sample of EAF (0.60 g) was submitted to Column Chromatography with Sephadex LH-20 (34.0 g, 30.0 × 3.0 cm). The column was eluted with ethyl acetate:methanol in a stepwise gradient (300.0 mL 90:10; 300.0 mL 80:20; 300.0 mL 70:30; 200.0 mL 60:40; 200.0 mL 50:50; 200.0 mL 40:60; 200.0 mL 30:70; 100.0 mL 20:80; 100.0 mL 10:90). At the end, the column was washed with 300.0 mL of MeOH. The collected fraction volume was 15.0 mL and fractions with the same profile on TLC chromatograms were joined together. The fractionation of EAF yielded 8 subfractions (F1 to F8). Fractionation was repeated with another 0.60 g of EAF to obtain a higher mass of fraction. Both fractionations yielded about 500.0 mg of pure myricetin-3-O-rhamnoside in fraction 4 after TCL monitoring.
Myricetin-3-O-rhamnoside: M.p.: decomposes near 200°C. UV (MeOH): λmax 258 and 353 nm; 1H NMR (DMSO-d6, 400 MHz): δ 6.20 (1H, d, J = 2.0 Hz, H-6), 6.35 (1H, d, J = 2.0 Hz, H-8), 6.89 (2H, s, H-2′e H-6′), 5.20 (1H, d, J 1.2 Hz, H-1′′), 3.98 (1H, s, H-2′′), 3.55 (1H, dd, J 1.2 e 9.3 Hz, H-3′′), 3.16 (1H, m, H-4′′), 3.37 (1H, m, H-5′′), 0.84 (3H, d, J 6.2 Hz, H-6′′); 13C NMR (DMSO-d6, 100 MHz,): δ 156.4 (C-2), 136.5 (C-3), 177.8 (C-4), 161.3 (C-5), 98.7 (C-6), 164.2 (C-7), 93.5 (C-8), 157.5 (C-9), 104,1 (C-10), 119.6 (C-1′), 107.9 (C-2′), 145.8 (C-3′), 134.3 (C-4′), 145.8 (C-5′), 107.9 (C-6′), 101.9 (C-1′′), 70.0 (C-2′′), 70.4 (C-3′′), 71.3 (C-4′′), 70.6 (C-5′′), 17.5 (C-6′′). HPLC-ESI/MS2 m/z: 463.0889 ([M – H]–, C21H19O12– calc. 463.0882).
2.8. High Performance Liquid Chromatography–Electrospray Ionization–Tandem Mass Spectrometry (HPLC-ESI/MSn)
The assays were carried out in a Liquid Chromatography system (Agilent Infinity 1260) coupled to a High Resolution Mass Spectrometer with Quadrupole Time of Flight (QTOF) (Agilent® model 6520 B) with an Electrospray Ionization source (ESI). The chromatographic parameters were Agilent Zorbax column, 2.1 mm internal diameter, 5 cm long, 1.8 μm particles, mobile phase: water acidified with formic acid (0.1%, v v−1) (A) and methanol (B), with the following solvent gradient system: 2% B (0 min), 98% B (0-15 min); 100% B (15-17 min); 2% B (17-22 min). Nitrogen (N2) was used as a drying gas at a flow 8 L min−1 and as a nebulizing gas at a pressure of 58 psi. The nebulizer temperature was set at 220°C with a potential of 4.5 kV used on the capillary.
2.9. Nuclear Magnetic Resonance (NMR)
NMR spectra were carried out in the Bruker Model Ascend™ 400 Avance III HD (9.2 Tesla) spectrometer. The samples were solubilized in deuterated dimethyl sulfoxide (DMSO-d6) and tetramethylsilane (TMS) was used as an internal standard. Analyses were performed at 400 MHz for 1H NMR and at 100 MHz for 13C NMR. The following NMR analyses were performed: 1H, 13C, DEPT-135, COSY, and HSQC.
2.10. High Performance Liquid Chromatography (HPLC)
The EAF and subfractions from the leaves were analyzed by High Performance Liquid Chromatography coupled to a diode array detector (HPLC-DAD). A Shimadzu Chromatography system, model LC-6AD with C18 reverse phase column (Phenomenex Luna model, 4.6 mm internal diameter, 25 cm long, 5 μm particles with 100 Å diameter), was used. A volume of 20 μL of methanol solution was injected at 3,000 μg mL−1 of EAF and 1,000 μg mL−1 of subfraction (F1-F8). Deionized water (phase A) and methanol (phase B) were used as mobile phases using the following program: 50% B (25 min) at a flow rate of 0.8 ml min−1.
2.11. Statistical Analysis
The analyses were performed in triplicate and the results were evaluated using the Analysis of Variance (ANOVA) method. The results were considered statistically different when the significance level was lower than 5% (P<0.05). The Tukey test was used to determine the significant differences between the averages. Analyses were performed using the SigmaPlot 11.0 program.
3. Results and Discussion
3.1. Phenolics, Proanthocyanidins, and Flavonoids Contents
Spectrophotometric results for extracts and fractions of I. laurina leaves are shown in Table 1.
Table 1.
Total phenolics, proanthocyanidins, and flavonoids for extracts and fractions of I. laurina leaves.
| Samples | TP (mg GAE gextract−1) | P (mg CE gextract−1) | F (mg QE gextract−1) |
|---|---|---|---|
| EE | 127.7 ± 0.1 | 76.7 ± 1.3a | 133.1 ± 3.5 |
| HF | 35.0 ± 0.1 | 20.2 ± 1.9 | 33.6 ± 2.1a |
| CF | 82.2 ± 1.0 | 43.3 ± 4.5 | 205.9 ± 9.2 |
| EAF | 475.3 ± 1.9 | 68.1 ± 4.4a | 359.3 ± 10.6 |
| BF | 135.9 ± 1.5 | 59.7 ± 4.1 | 24.2 ± 1.2a |
EE = ethanol extract; HF = hexane fraction; CF = chloroform fraction; EAF = ethyl acetate fraction; BF = n-butanol fraction; TP = total phenolics; GAE = gallic acid equivalent; P = proanthocyanidins; CE = catechin equivalent; F = flavonoids; QE = quercetin equivalent. Results are presented as mean ± standard deviation for the triplicate assays. The analyses with the same letters did not show a significant difference between the averages by 5% Tukey test.
EE, EAF, and BF fractions presented the highest contents of total phenolics. The difference in these values can be explained by the polarity of the solvents used [6]. Polar solvents such as ethanol, ethyl acetate, and n-butanol are more able to extract phenolic compounds. EE and EAF have higher levels of total phenolics when compared to those of I. marginata, which has values of 31.63 mg and 8.37 of GAE gextract−1, respectively [36]. The methanolic extract (50%) of I. edulis presented total phenolics of 496.5 mg of GAE gextract−1, which is higher than that of EE from I. laurina [4]. Dias, Souza, and Rogez [6] reported values of 15.8 and 357.5 mg GAE gextract−1 of total phenolics for hexane and water fractions, respectively, for the acetone:water:acetic acid extract (70:28:2 v:v:v). The values of proanthocyanidins were lower when compared with total phenolics. No data were found for this technique in the literature concerning levels of proanthocyanidins for I. species.
The fractions CF and EAF presented the highest values of flavonoids among the other fractions. The good result obtained with the less polar fraction (chloroform) suggests the presence of aglycone flavonoids. Flavonols and flavones were confirmed by the test of complexation with aluminum ions (Al3+). This complexation reaction allows the quantification of flavonoids by reading the absorbance of the solution. In this reaction, the aluminum chloride shifts the wavelengths of bands I and II to a higher wavelength, a bathochromic shift [37]. EE has higher level of flavonoids when compared to that presented by I. marginata, whose value is 118 mg QE gextract−1 [38].
3.2. Antifungal and Cytotoxicity Activities
Values of MIC (minimum inhibitory concentration) for antifungal activity in μg mL−1 and cytotoxic activity in CC50 (cytotoxic concentration), in μg mL−1, for extract and fractions of leaves of I. laurina are shown in Table 2.
Table 2.
Results of antifungal activity (expressed as MIC in μg mL−1), cytotoxic activity (expressed as CC50 in μg mL−1), and selectivity index for extract and fractions of I. laurina leaves.
| MIC (μg mL−1) | |||||
|
| |||||
| Microorganisms | EE | HF | CF | EAF | BF |
|
| |||||
| C. albicans ATCC 28366 | 46.8 | 1,500 | >3,000 | 93.8 | 11.7 |
| C. glabrata ATCC 15126 | 11.7 | 187.5 | >3,000 | 11.7 | 23.4 |
| C. tropicalis ATCC 13803 | 93.8 | 3,000 | >3,000 | 93.8 | 46.8 |
|
| |||||
| CC 50 (μg mL−1) | |||||
|
| |||||
| Vero cells | 352 ± 5 | 384 ± 9 | >512 | >512 | >512 |
|
| |||||
| SI | |||||
|
| |||||
| Microorganisms | EE | HF | CF | EAF | BF |
|
| |||||
| C. albicans ATCC 28366 | 0.9 | - 0.6 | > - 0.8 | > 0.7 | > 1.6 |
| C. glabrata ATCC 15126 | 1.5 | 0.3 | > - 0.8 | > 1.6 | > 1.3 |
| C. tropicalis ATCC 13803 | 0.6 | - 0.9 | > - 0.8 | > 0.7 | > 1.0 |
EE = ethanol extract; HF = hexane fraction; CP = chloroform fraction; EAF = ethyl acetate fraction; BF = n-butanol fraction. SI: selectivity index. ATCC: American Type Culture Collection.
Fractions EE, EAF, and BF were the most active against the evaluated microorganism, showing MIC values lower than 100 μg mL−1, as shown in Table 2. In general, the BF fraction showed good activity against C. albicans (MIC 11.7 μg mL−1) and for C. glabrata (MIC 23.4 μg mL−1); the EE and EAF fractions were also very effective against C. glabrata (MIC 11.7 μg mL−1); and the CF fraction had no antifungal activity at the tested concentrations (MIC above 3,000 μg mL−1). The EE of the leaves from B. laevifolia showed good results for antifungal activity, showing MIC values of 31, 63, and 63 μg mL−1 for C. albicans, C. tropicalis, and C. glabrata, respectively [11].
Regarding the cytotoxic activity, the lower the CC50 value is, the higher the cytotoxicity against Vero cells will be, because a small concentration of the sample would inhibit the growth of cells by 50%. To correlate the antifungal activity with the cytotoxic concentration, the selectivity index (SI) was calculated. The SI indicates whether the sample is more selective for antifungal activity or more toxic for Vero cells. The more positive the SI value, the greater the selectivity to inhibit fungal growth; a negative value indicates that the sample is more toxic to Vero cells than selective for the inhibition of antimicrobial growth [34]. Therefore, the fractions EE, EAF, and BF were the most selective to the tested microorganisms, as they presented positive SI values for all of the fungi of the genus Candida; the best values were 1.5, 1.6, and 1.6, respectively. Nunes et al. [11] also found positive SI values for EE and n-butanol partition from B. laevifolia leaves, for the same microorganisms tested in the Table 2. The best results were found for E, which showed a positive SI value of 1.2, 0.9, and 0.9 for C. albicans, C. tropicalis, and C. glabrata, respectively.
Other Inga species were studied regarding antifungal activity. The EE of leaves of I. vera showed low inhibition against C. albicans, with an inhibition zone of 7 to 15 mm (i.d.), whereas a good inhibition would be larger than 20 mm [39]. The ethanolic extract of I. marginata leaves showed no antifungal activity against C. albicans and C. krusei but showed activity against C. tropicalis [40]. The promising antifungal activity of I. laurina can be related to the presence of phenolic compounds. The extracts and fractions showed a high quantity of total phenolics and flavonoids and these compounds have been frequently reported as potential antifungal agents [41–45].
3.3. High Performance Liquid Chromatography–Electrospray Ionization–Tandem Mass Spectrometry (HPLC-ESI/MS2) Analysis
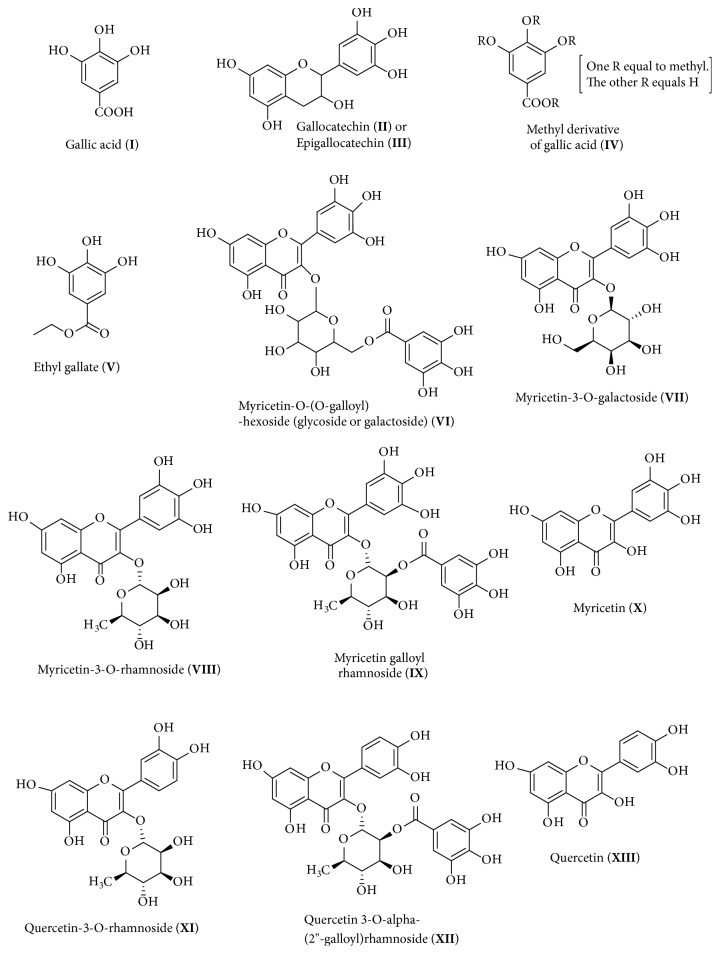
The EAF presented the highest level of phenolic compounds and flavonoids compared to the other fractions and showed good results for antifungal activity. Therefore, this polar fraction was submitted to High Performance Liquid Chromatography coupled to Electrospray Ionization (HPLC-ESI) analysis and sequential Mass Spectrometry (MS/MS) in the negative mode. The total ion chromatogram of EAF obtained by HPLC-ESI is shown in Figure 1. Thirteen compounds could be identified in this fraction using this technique (Table 3 and Figure 2).
Figure 1.
Total ion chromatogram of EAF obtained by HPLC-ESI.
Table 3.
Phenolic compounds identified in EAF and subfractions from I. laurina by HPLC-ESI/MS2.
| Fractions/Subfractions | tR(min) | [M – H] – | Exact mass | Error (ppm) |
Fragments m/z |
Molecular formula | Compound | References |
|---|---|---|---|---|---|---|---|---|
| EAF | 2.9 | 169.0154 | 169.0142 | -7.1 | 125 | C7H6O5 | Gallic acid (I) | [12, 13] |
| 5.4 | 305.0663 | 305.0667 | 1.3 | 261, 219, 167/165, 125 | C15H14O7 | Gallocatechin (II) or Epigallocatechin (III) | [14, 15] | |
| 6.9 | 183.0300 | 183.0299 | -0.5 | 168, 124 | C8H8O5 | Methyl derivative of gallic acid (IV) | [12] | |
| 9.1 | 197.0468 | 197.0455 | -6.6 | 169, 124/123 | C9H10O5 | Ethyl gallate (V) | [14, 16] | |
| 10.1 | 631.0956 | 631.0941 | -2.4 | 479, 316, 169 | C28H24O17 | Myricetin-O-(O-galloyl)-hexoside (glycoside or galactoside) (VI) | [17, 18] | |
| 10.7 | 479.0826 | 479.0831 | 1.0 | 316, 178 | C21H19O13 | Myricetin-3-O-galactoside (VII) | [17, 18] | |
| 11.1 | 463.0904 | 463.0888 | -3.5 | 316, 178 | C21H19O12 | Myricetin-3-O-rhamnoside (VIII) | [17] | |
| 12.2 | 615.1007 | 615.0992 | -2.4 | 463, 316/317, 178 | C28H24O16 | Myricetin galloyl rhamnoside (IX) | [18, 19] | |
| 12.4 | 317.0318 | 317.0303 | -4.7 | 178, 151 | C15H10O8 | Myricetin (X) | [13, 14, 20] | |
| 12.7 | 447.0955 | 447.0933 | -4.9 | 300/301 | C21H20O11 | Quercetin-3-O-rhamnoside (XI) | [17, 18] | |
| 13.5 | 599.1044 | 599.1042 | -0.3 | 447, 301, 169, 151 | C28H24O15 | Quercetin 3-O-alpha-(2′′-galloyl)rhamnoside (XII) | [21, 22] | |
| 13.8 | 301.0357 | 301.0354 | -1.0 | 178/179, 151 | C15H10O7 | Quercetin (XIII) | [14] | |
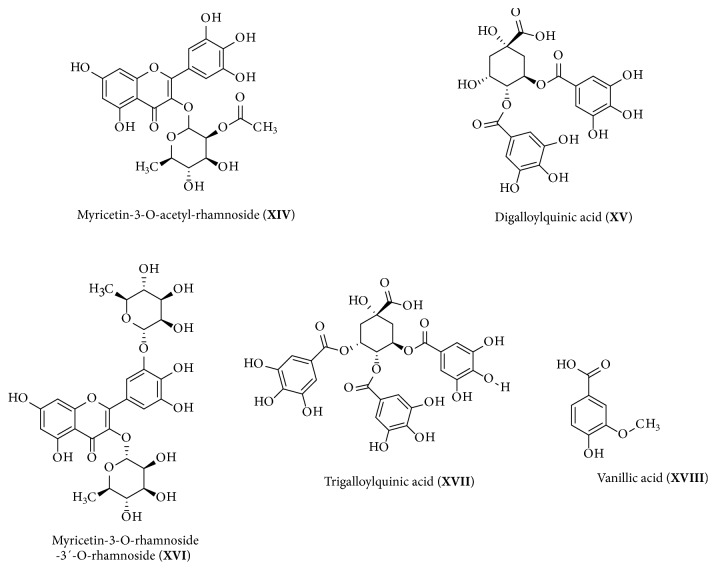
| F2 | 10.8 | 505.0999 | 505.0988 | - 2.2 | 463, 316, 271, 163 | C23H22O13 | Myricetin-3-O-acetyl-rhamnoside (XIV) | [23, 24] |
| F5 | 7.0 | 495.0786 | 495.0780 | - 1.2 | 343, 169 | C21H20O14 | Digalloylquinic acid (XV) | [25–27] |
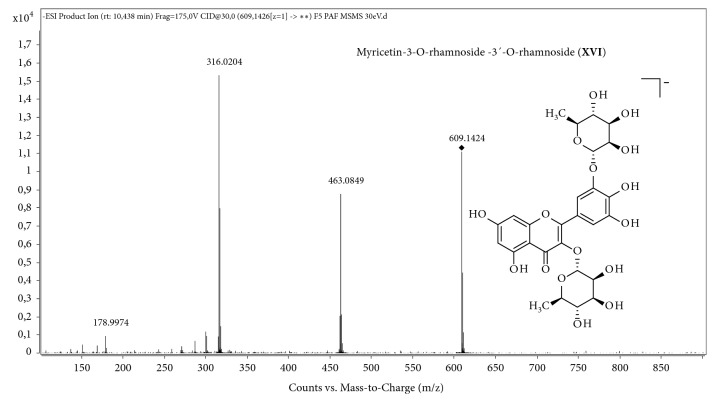
| F5 | 10.3 | 609.1482 | 609.1461 | - 3.4 | 463, 316, 178 | C27H30O16 | Myricetin-3-O- rhamnoside -3′-O-rhamnoside (XVI) | Structure proposed by the author |
| F6 | 7.7 | 647.0885 | 647.0890 | 0.8 | 495, 343, 325, 169 | C28H24O18 | Trigalloylquinic acid (XVII) | [26] |
| F7 | 8.3 | 167.0355 | 167.0350 | - 3.0 | 108 | C8H8O4 | Vanillic acid (XVIII) | [28] |
Figure 2.
Compounds identified in the EAF.
Column fractions (F1-F8) obtained from EAF were also analyzed by HPLC-ESI/MS2. Five different compounds were identified in addition to those identified in EAF (Table 3); their structures are shown in Figure 3. The compound myricetin-3-O-acetyl-rhamnoside (compound XIV) was identified in fraction F2 (m/z 505), digalloylquinic acid (compound XV, m/z 495) and myricetin-3-O-rhamnose-3′-O-rhamnoside (compound XVI, m/z 609) were identified in F5, trigalloylquinic acid (compound XVII, m/z 647) was identified in the F6, and vanillic acid (compound XVIII, m/z 167) was identified in F7 (Table 3). See Scheme S1 in the Supplementary Material for the comprehensive flowchart of purification, identification, and isolation of compounds from the EAF.
Figure 3.
Compounds identified in subfractions F2-F7.
The ion m/z 609 (compound XVI) forms the fragments m/z 463, 316, and 178 (Figure 4), which correspond to compound VIII, which was confirmed to be myricetin-3-O-rhamnoside (Figure 2). The loss of 146 Da (rhamnosyl group) of ion m/z 609 forms the ion m/z 463, which loses a further 146 Da to form the ion m/z 316. This finding is in accordance with the structure proposed for the ion m/z 609, which has 2 rhamnosyl groups bonded to the myricetin aglycone. The literature reports a structure for myricetin-3′-O-rhamnose-3-O-galactoside [46]; therefore, a similar structure carrying two rhamnosyl groups in positions 3 and 3′ of the aglycone was proposed: myricetin-3-O-rhamnose-3′-O-rhamnoside. No reports on the identification of compounds II, III, VI, VII, IX, XII, XIV, XV, XVI, or XVII in the Inga species have been found.
Figure 4.
MS/MS spectrum of ion m/z 609.
The Figures S1to S16 show mass spectrum of phenolic compounds acquired by HPLC-ESI/MS2. Figures S17to S22 show fragmentation patterns.
3.4. Structural Determination and Characterization of the Isolated Compound from Fraction F4 (Myricetin-3-O-Rhamnoside)
Fraction F4 was also analyzed by HPLC to check its purity. The HPLC chromatogram indicated the presence of only one intense peak in 8.1 min, while the UV/Vis spectrum shows two bands of absorption, characteristic for flavonoids (258 and 353 nm). See Figure S23 in the Supplementary Material to check the chromatogram and UV/Vis spectrum of F4.
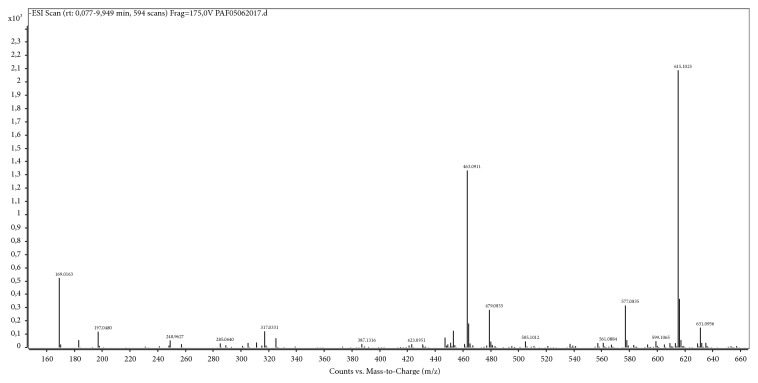
The high resolution mass spectrum and NMR spectra confirmed the structure of the isolated compound in fraction 4 as myricetin-3-O-rhamnoside, a glycosylated flavonoid with crystalline aspects, with the molecular formula C21H20O12 and MW 464.38 gmol−1 (compound VIII, Figure 2). There are no records for its melting point in the literature, but it decomposes close to 200°C and its color changes from yellow to orange and black.
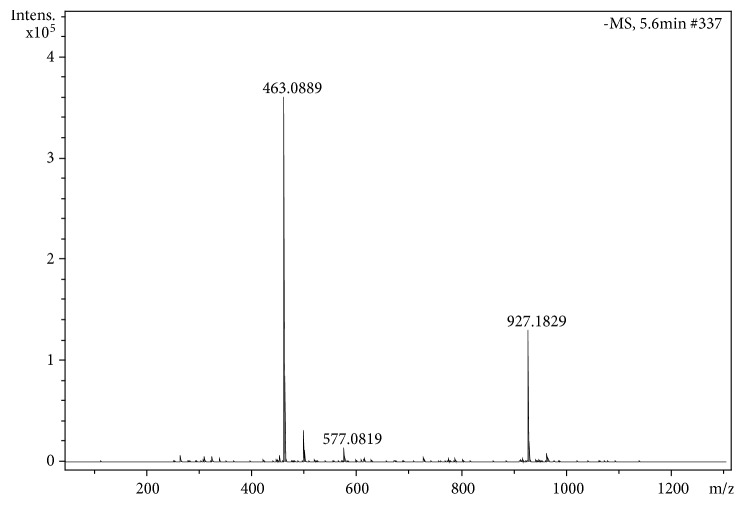
Figure 5 shows the high resolution mass spectrum of F4; it is possible to verify the molecular weight of the compound [M – H]− at ion m/z 463.0889 (acquired mass). The exact mass of compound VIII was m/z 463.0882 (C21H19O12)−, with an error of 1.5 ppm. The peak at m/z 927.1829 corresponds to the ion cluster formation of VIII.
Figure 5.
High resolution mass spectra of fraction F4.
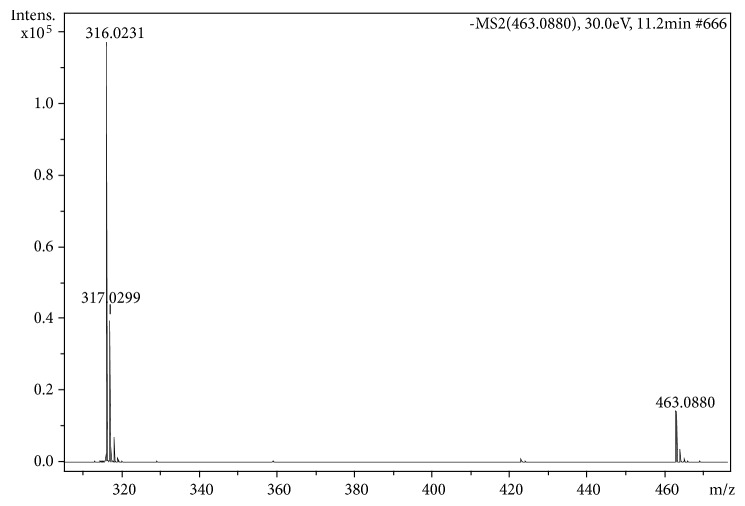
The main fragmentation of VIII is the loss of the rhamnoside radical, yielding the ion radical at m/z 316 [M− – 147]−; with lower intensity, the aglycone can be produced (M− –146]− after the loss of an unsaturated rhamnoside of mass 146 Da (Figure 6). The spectrum in Figure 5 is in good agreement with Saldanha et al. [17], where flavonoids in Myrcia bella Cambess were identified by using the same technique as in the present work (MS/ESI-MS/MS). The fragmentation pathway of myricetin-3-O-ramnoside corresponds to that proposed by [19] when they studied the methanol extract of Pistacia lentiscus leaves.
Figure 6.
MS/MS spectrum of the ion m/z 463.
1H NMR spectra showed 3 different signals attributed to aromatic hydrogens, two doublets were observed at δ 6.20 and δ 6.37, both with J = 2.0 Hz, typical of meta -couplings of carbons C-6 and C-8, respectively, in ring A. There is an intense singlet in δ 6.89 attributed to hydrogens H-2′ and H-6′ of ring B, which are equivalent. The signal at δ 12.00 corresponds to the hydroxyl hydrogen of carbonyl C-5, which has a hydrogen bond with a carbonyl carbon C-4, corresponding to a chelated hydroxyl.
In HSQC contour map analysis, it was possible to observe a signal at δ 5.2 (1H, d, J = 1.2 Hz), typical of a hydrogen bound to the anomeric carbon (δ 101.94, C-1′′) of rhamnose. This coupling constant value is typical of hydrogen in the equatorial position of the glycosidic ring which is coupled with the hydrogen H-2′′ (axial-equatorial or equatorial-equatorial). The DEPT-135 and 13C NMR spectra, respectively, allowed confirmation of the carbon signals in the aromatic region. The correlations of the hydrogens of rhamnose were attributed through the COSY spectrum. Thus, it can be inferred that the isolated compound is myricetin-3-O-rhamnoside [4, 47] (Figure 7). See Figures S24–S29 and Tables S1–S2 in the Supplementary Material to check the NMR spectra and NMR chemical shifts, respectively, of F4.
Figure 7.
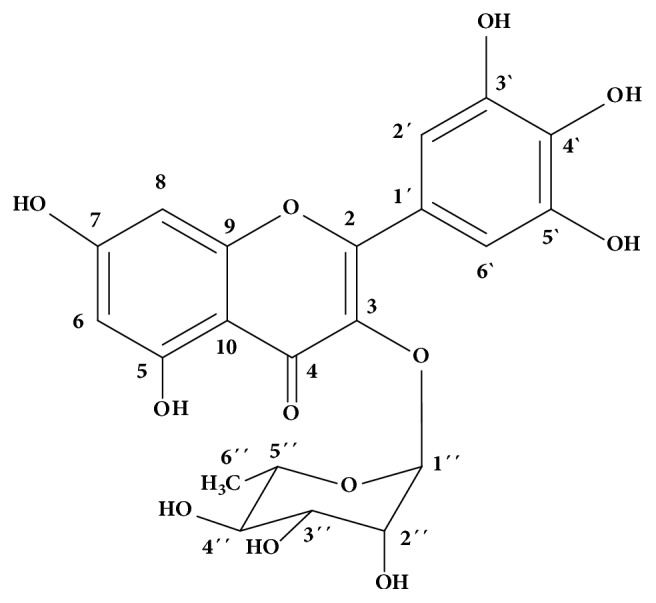
Structure of myricetin-3-O-rhamnoside compound isolated from the fraction F4.
Myricetin-3-O-rhamnoside inhibited the growth of all yeasts as shown in Table 2 at a concentration of 93.8 μg mL−1. This result confirms the antifungal activity for this compound. Salazar-Aranda and coworkers [48] also observed an MIC higher than 83 μg mL−1 for C. albicans and C. tropicalis, although the MIC values were higher for C. glabrata, 3.9 μg mL−1, and 7.8 μg mL−1 for clinical isolates of yeast.
The isolated compound presents the same MIC of EAF for the evaluated microorganism, except for C. glabrata, which has a lower value (11.7 μg mL−). From these results, it is possible to infer that myricetin-3-O-rhamnoside can contribute markedly to the antifungal activity observed for EAF against C. albicans and C. tropicalis, since they presented the same MIC values.
4. Conclusions
The EE from the leaves of Inga laurina and its fractions are rich in phenolic compounds and present good antifungal activities. HPLC/MS-MS/MS and H1/C13 analysis made it possible to identify seventeen compounds, most of which are gallic acid derivatives and myricetin and quercetin glycosides. The EAF contained a high level of total phenolics (475.3 ± 1.9 mg GAE gextract−1) and flavonoids (359.3 ± 10.6 mg QE gextract−1) and was active against fungus from the Candida genus. The best MIC results found for C. albicans, C. glabrata, and C. tropicalis were 11.7, 11.7, and 46.8 μg mL−1, respectively, and the best selectivity indexes for the three microorganisms, using Vero cells, were 1.6, 1.3, and 1.0, respectively. These results make I. laurina a promising plant for advanced antifungal studies.
Acknowledgments
This work was supported by the Foundation for Research Support of the Minas Gerais State (FAPEMIG-Brazil; APQ-01178-11). This study was financed in part by the Coordination for the Improvement of Higher Education Personnel (CAPES), Finance Code 001. The authors also thank the Nanobiotechnology Laboratory (IBTEC-UFU) for the UHPLC-MSn assays, the Chemistry Institute of the Federal University of Uberlândia (IQUFU) for the supporting infrastructure, and Professor Dr. Glein Monteiro de Araújo (Institute of Biology-UFU) for plant identification. This manuscript is based on the thesis of the authors.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request. All data in our manuscript is available for readers.
Conflicts of Interest
The authors declare no conflicts of interest.
Supplementary Materials
Supplementary Scheme S1: flowchart for purification, identification, and isolation of compounds from the leaves of I. laurina. Supplementary Figures S1–S12: mass spectrum of ion m/z of phenolic compounds identified in EAF from I. laurina by HPLC-ESI/MS2. Supplementary Figures S13–S16: mass spectrum of ion m/z of phenolic compounds identified in fractions F2, F5, F6, F7, respectively, from I. laurina by HPLC-ESI/MS2. Supplementary Figures S17–S22: Figures S17 and S18 show fragmentation patterns (m/z 609 and m/z 463) and fragmentation mechanisms of compounds identified in EAF. Supplementary Figure S23: chromatogram and UV/Vis spectrum of fraction 4 (F4). Supplementary Figure S24: 1H NMR spectra (400 MHz, DMSO-d6) of myricetin-3-O-rhamnoside. Supplementary Figure S25: 13C NMR spectra (100 MHz, DMSO-d6) of myricetin-3-O-rhamnoside. Supplementary Figure S26: HSQC contour map (DMSO-d6) of myricetin-3-O-rhamnoside in the aromatic region. Supplementary Figure S27: COSY contour map (DMSO-d6) of myricetin-3-O-rhamnoside. Supplementary Figure S28: amplification of the COSY (400 MHz, DMSO-d6) contour map in the region of glycosidic hydrogens of myricetin-3-O-rhamnoside. Supplementary Figure S29: DEPT-135 spectra (100 MHz, DMSO-d6) of myricetin-3-O-rhamnoside. Supplementary Table S1: 1H NMR (400 MHz, DMSO-d6,) data of myricetin-3-O-rhamnoside. Supplementary Table S2: 13C NMR (100 MHz, DMSO-d6) data of myricetin-3-O-rhamnoside.
References
- 1.Bermingham E., Dick C. The Inga - Newcomer or museum antiquity? Science. 2001;293(5538):2214–2216. doi: 10.1126/science.1065310. [DOI] [PubMed] [Google Scholar]
- 2.Vivot E., De Dios Muñoz J., Del Carmen Cruañes M., et al. Inhibitory activity of xanthine-oxidase and superoxide scavenger properties of Inga verna subsp. affinis. Its morphological and micrographic characteristics. Journal of Ethnopharmacology. 2001;76(1):65–71. doi: 10.1016/S0378-8741(01)00222-7. [DOI] [PubMed] [Google Scholar]
- 3.Garcia F. C. P., Fernandes J. M. Inga in Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. Disponivel em: http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB115295, 2015.
- 4.Souza J. N. S., Silva E. M., Da Silva M. N., Arruda M. S. P., Larondellea Y., Rogez H. Identification and antioxidant activity of several flavonoids of Inga edulis leaves. Journal of the Brazilian Chemical Society. 2007;18(6):1276–1280. doi: 10.1590/S0103-50532007000600025. [DOI] [Google Scholar]
- 5.Silva E. M., Souza J. N. S., Rogez H., Rees J. F., Larondelle Y. Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chemistry. 2006;101(3):1012–1018. doi: 10.1016/j.foodchem.2006.02.055. [DOI] [Google Scholar]
- 6.Dias A. L., Souza J. N., Rogez H. Enriquecimento de compostos fenólicos de folhas de Inga edulis por extração em fase sólida: quantificação de seus compostos majoritários e avaliação da capacidade antioxidante. Química Nova. 2010;33(1):38–42. doi: 10.1590/S0100-40422010000100008. [DOI] [Google Scholar]
- 7.Pinto J. M. A., Souza E. A., Oliveira D. F. Use of plant extracts in the control of common bean anthracnose. Crop Protection. 2010;29(8):838–842. doi: 10.1016/j.cropro.2010.03.006. [DOI] [Google Scholar]
- 8.Pistelli L., Bertoli A., Noccioli C., et al. Antimicrobial activity of Inga fendleriana extracts and isolated flavonoids. Natural Product Communications (NPC) 2009;4(12):1679–1683. [PubMed] [Google Scholar]
- 9.Lokvam J., Brenes-Arguedas T., Lee S. J., Coley P. D., Kursar T. A. Allelochemic function for a primary metabolite: The case of L-tyrosine hyper-production in Inga umbellifera (Fabaceae) American Journal of Botany. 2006;93(8):1109–1115. doi: 10.3732/ajb.93.8.1109. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez J. C., Serrano R. P., Ospina L. F., Torres L. A. A. Actividad Biológica de las saponinas de la corteza de Inga marginata Willd. Revista Colombiana de Ciencias Químico-Farmacéuticas. 1998;27:17–19. [Google Scholar]
- 11.Nunes B. C., Martins M. M., Chang R., et al. Antimicrobial activity, cytotoxicity and selectivity index of Banisteriopsis laevifolia (A. Juss.) B. Gates leaves. Industrial Crops and Products. 2016;92:277–289. doi: 10.1016/j.indcrop.2016.08.016. [DOI] [Google Scholar]
- 12.Abdel-Hameed E.-S. S., Bazaid S. A., Salman M. S. Characterization of the phytochemical constituents of taif rose and its antioxidant and anticancer activities. BioMed Research International. 2013;2013:13. doi: 10.1155/2013/345465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fracassetti D., Costa C., Moulay L., Tomás-Barberán F. A. Ellagic acid derivatives, ellagitannins, proanthocyanidins and other phenolics, vitamin C and antioxidant capacity of two powder products from camu-camu fruit (Myrciaria dubia) Food Chemistry. 2013;139(1-4):578–588. doi: 10.1016/j.foodchem.2013.01.121. [DOI] [PubMed] [Google Scholar]
- 14.Sun J., Liang F., Bin Y., Li P., Duan C. Screening non-colored phenolics in red wines using liquid chromatography/ultraviolet and mass spectrometry/mass spectrometry libraries. Molecules. 2007;12(3):679–693. doi: 10.3390/12030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mena P., Calani L., Dall'Asta C., et al. Rapid and comprehensive evaluation of (Poly)phenolic compounds in pomegranate (Punica granatum L.) Juice by UHPLC-MSn. Molecules. 2012;17(12):14821–14840. doi: 10.3390/molecules171214821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wyrepkowski C. C., Da Costa D. L. M. G., Sinhorin A. P., et al. Characterization and quantification of the compounds of the ethanolic extract from caesalpinia ferrea stem bark and evaluation of their mutagenic activity. Molecules. 2014;19(10):16039–16057. doi: 10.3390/molecules191016039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saldanha L. L., Vilegas W., Dokkedal A. L. Characterization of flavonoids and phenolic acids in Myrcia bella cambess. Using FIA-ESI-IT-MSn and HPLC-PAD-ESI-IT-MS combined with NMR. Molecules. 2013;18(7):8402–8416. doi: 10.3390/molecules18078402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taamalli A., Iswaldi I., Arráez-Román D., Segura-Carretero A., Fernández-Gutiérrez A., Zarrouk M. UPLC-QTOF/MS for a rapid characterisation of phenolic compounds from leaves of Myrtus communis L’. Phytochemical Analysis. 2014;25(1):89–96. doi: 10.1002/pca.2475. [DOI] [PubMed] [Google Scholar]
- 19.Rodríguez-Pérez C., Quirantes-Piné R., Amessis-Ouchemoukh N., Khodir M., Segura-Carretero A., Fernández-Gutierrez A. A metabolite-profiling approach allows the identification of new compounds from Pistacia lentiscus leaves. Journal of Pharmaceutical and Biomedical Analysis. 2013;77:167–174. doi: 10.1016/j.jpba.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 20.Abu-Reidah I. M., Ali-Shtayeh M. S., Jamous R. M., Arráez-Román D., Segura-Carretero A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chemistry. 2015;166:179–191. doi: 10.1016/j.foodchem.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Kim Y. J., Jung E. B., Seo S. J., Park K. H., Lee M. W., Lee C. S. Quercetin-3-O-(2"-galloyl)-a-L-rhamnopyranoside prevents TRAIL-induced apoptosis in human keratinocytes by suppressing the caspase-8- and Bid-pathways and the mitochondrial pathway. Chemico-Biological Interactions. 2013;204(3):144–152. doi: 10.1016/j.cbi.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Estrada O., Hasegawa M., Gonzalez-Mujíca F., et al. Evaluation of flavonoids from Bauhinia megalandra leaves as inhibitors of glucose-6-phosphatase system. Phytotherapy Research. 2005;19(10):859–863. doi: 10.1002/ptr.1703. [DOI] [PubMed] [Google Scholar]
- 23.Negri G., Tabach R. Saponins, tannins and flavonols found in hydroethanolic extract from Periandra dulcis roots. Revista Brasileira de Farmacognosia. 2013;23(6):851–860. doi: 10.1590/S0102-695X2013000600001. [DOI] [Google Scholar]
- 24.Faria A. F., Marques M. C., Mercadante A. Z. Identification of bioactive compounds from jambolão (Syzygium cumini) and antioxidant capacity evaluation in different pH conditions. Food Chemistry. 2011;126(4):1571–1578. doi: 10.1016/j.foodchem.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Sobeh M., ElHawary E., Peixoto H., et al. Identification of phenolic secondary metabolites from Schotia brachypetala Sond. (Fabaceae) and demonstration of their antioxidant activities in Caenorhabditis elegans. PeerJ. 2016;2016(11):1–25. doi: 10.7717/peerj.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pereira V. V., Da Fonseca F. A., Bento C. S. O., et al. Electrospray ionization mass spectrometry fingerprint of the Byrsonima species. Revista Virtual de Química. 2015;7(6):2539–2548. doi: 10.5935/1984-6835.20150152. [DOI] [Google Scholar]
- 27.Sannomiya M., Montoro P., Piacente S., Pizza C., Brito A. R. M. S., Vilegas W. Application of liquid chromatography/electrospray ionization tandem mass spectrometry to the analysis of polyphenolic compounds from an infusion of Byrsonima crassa Niedenzu. Rapid Communications in Mass Spectrometry. 2005;19(16):2244–2250. doi: 10.1002/rcm.2053. [DOI] [PubMed] [Google Scholar]
- 28.Sanz M., De Simón B. F., Cadahía E., et al. LC-DAD/ESI-MS/MS study of phenolic compounds in ash (Fraxinus excelsior L. and F. americana L.) heartwood. Effect of toasting intensity at cooperage. Journal of Mass Spectrometry. 2012;47(7):905–918. doi: 10.1002/jms.3040. [DOI] [PubMed] [Google Scholar]
- 29.de Morais S. A. L., de Aquino F. J. T., do Nascimento E. A., et al. Análise de compostos bioativos, grupos ácidos e da atividade antioxidante do café arábica (Coffea arabica) do cerrado e de seus grãos defeituosos (PVA) submetidos a diferentes torras. Food Science and Technology. 2008;28(suppl.):198–207. doi: 10.1590/S0101-20612008000500031. [DOI] [Google Scholar]
- 30.Woisky R. G., Salatino A. Analysis of propolis: some parameters and procedures for chemical quality control. Journal of Apicultural Research. 1998;37(2):99–105. doi: 10.1080/00218839.1998.11100961. [DOI] [Google Scholar]
- 31.CLSI. Reference method for broth dilution antifungal susceptibility testing of yeasts; Approved standard. 3rd. Wayne, Pa, USA: Clinical and Laboratory Standards Institute; 2011. (CLSI document M100-S17). [DOI] [Google Scholar]
- 32.Rolón M., Vega C., Escario J. A., Gómez-Barrio A. Development of resazurin microtiter assay for drug sensibility testing of Trypanosoma cruzi epimastigotes. Parasitology Research. 2006;99(2):103–107. doi: 10.1007/s00436-006-0126-y. [DOI] [PubMed] [Google Scholar]
- 33.Pillay P., Vleggaar R., Maharaj V. J., et al. Antiplasmodial hirsutinolides from Vernonia staehelinoides and their utilization towards a simplified pharmacophore. Phytochemistry. 2007;68(8):1200–1205. doi: 10.1016/j.phytochem.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Case R. J., Franzblau S. G., Wang Y., Cho S. H., Soejarto D. D., Pauli G. F. Ethnopharmacological evaluation of the informant consensus model on anti-tuberculosis claims among the Manus. Journal of Ethnopharmacology. 2006;106(1):82–89. doi: 10.1016/j.jep.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Cunha L. C. S., De Morais S. A. L., Martins C. H. G., et al. Chemical composition, cytotoxic and antimicrobial activity of essential oils from cassia bakeriana craib. against aerobic and anaerobic oral pathogens. Molecules. 2013;18(4):4588–4598. doi: 10.3390/molecules18044588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rieger S. C. Universidade Estadual de Londrina (Londrina), 2011.
- 37.Marcucci M. C., Woisky R. G., Salatino A. Uso de cloreto de alumínio na quantificação de flavonóides em amostras de própolis. Mensagem Doce. 1998;46:3–9. [Google Scholar]
- 38.Decian A. C., Dornelles R., Leal G. C., et al. in 1st Latin-American Congress of Clinical and Laboratorial Toxicology (TOXI-LATIN 2014), UFRGS, Porto Alegre/RS - Brazil, 2014.
- 39.Lozano C. M., Vasquez-Tineo M. A., Ramirez M., Jimenez F. In vitro antimicrobial activity screening of tropical medicinal plants used in Santo Domingo, Dominican Republic. Part I.'. Pharmacognosy Communications. 2013;3(2):64–69. doi: 10.5530/pc.2013.2.13. [DOI] [Google Scholar]
- 40.Daniel J. F., Iwasso D. R., Fiorini M. A., et al. Antimicrobial activity of Brazilian plants of the genera Leguminosae and Myrtaceae. Journal of Medicinal Plants Research. 2014;8(28):958–966. doi: 10.5897/JMPR2014.5385. [DOI] [Google Scholar]
- 41.Teodoro G. R., Ellepola K., Seneviratne C. J., Koga-Ito C. Y. Potential use of phenolic acids as anti-Candida agents: A review. Frontiers in Microbiology. 2015;6:1–11. doi: 10.3389/fmicb.2015.01420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martins N., Barros L., Henriques M., Silva S., Ferreira I. C. Activity of phenolic compounds from plant origin against Candida species. Industrial Crops and Products. 2015;74:648–670. doi: 10.1016/j.indcrop.2015.05.067. [DOI] [Google Scholar]
- 43.Daglia M. Polyphenols as antimicrobial agents. Current Opinion in Biotechnology. 2012;23(2):174–181. doi: 10.1016/j.copbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Sanches A. C. C., Lopes G. C., Nakamura C. V., Dias Filho B. P., De Mello J. C. P. Antioxidant and antifungal activities of extracts and condensed tannins from Stryphnodendron obovatum Benth. Revista Brasileira de Ciências Farmacêuticas. 2005;41(1):101–107. doi: 10.1590/S1516-93322005000100012. [DOI] [Google Scholar]
- 45.Ansari M. A., Anurag A., Fatima Z., Hameed S. In: Microbial pathogens and strategies for combating them: science, technology and education. Méndez-Vilas A., editor. Vol. 2. Spain: Formatex Research Center; 2013. [Google Scholar]
- 46.Arya R., Babu V., Ilyas M., Nasim K. T. Myricetin 3′-rhamnoside-3-galactoside from Buchanania lanzan (anacardiaceae) Phytochemistry. 1992;31(7):2569–2570. doi: 10.1016/0031-9422(92)83334-U. [DOI] [Google Scholar]
- 47.Hayder N., Bouhlel I., Skandrani I., et al. In vitro antioxidant and antigenotoxic potentials of myricetin-3-o-galactoside and myricetin-3-o-rhamnoside from Myrtus communis: Modulation of expression of genes involved in cell defence system using cDNA microarray. Toxicology in Vitro. 2008;22(3):567–581. doi: 10.1016/j.tiv.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 48.Salazar-Aranda R., Granados-Guzmán G., Pérez-Meseguer J., González G. M., De Torres N. W. Activity of polyphenolic compounds against candida glabrata. Molecules. 2015;20(10):17903–17912. doi: 10.3390/molecules201017903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Scheme S1: flowchart for purification, identification, and isolation of compounds from the leaves of I. laurina. Supplementary Figures S1–S12: mass spectrum of ion m/z of phenolic compounds identified in EAF from I. laurina by HPLC-ESI/MS2. Supplementary Figures S13–S16: mass spectrum of ion m/z of phenolic compounds identified in fractions F2, F5, F6, F7, respectively, from I. laurina by HPLC-ESI/MS2. Supplementary Figures S17–S22: Figures S17 and S18 show fragmentation patterns (m/z 609 and m/z 463) and fragmentation mechanisms of compounds identified in EAF. Supplementary Figure S23: chromatogram and UV/Vis spectrum of fraction 4 (F4). Supplementary Figure S24: 1H NMR spectra (400 MHz, DMSO-d6) of myricetin-3-O-rhamnoside. Supplementary Figure S25: 13C NMR spectra (100 MHz, DMSO-d6) of myricetin-3-O-rhamnoside. Supplementary Figure S26: HSQC contour map (DMSO-d6) of myricetin-3-O-rhamnoside in the aromatic region. Supplementary Figure S27: COSY contour map (DMSO-d6) of myricetin-3-O-rhamnoside. Supplementary Figure S28: amplification of the COSY (400 MHz, DMSO-d6) contour map in the region of glycosidic hydrogens of myricetin-3-O-rhamnoside. Supplementary Figure S29: DEPT-135 spectra (100 MHz, DMSO-d6) of myricetin-3-O-rhamnoside. Supplementary Table S1: 1H NMR (400 MHz, DMSO-d6,) data of myricetin-3-O-rhamnoside. Supplementary Table S2: 13C NMR (100 MHz, DMSO-d6) data of myricetin-3-O-rhamnoside.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request. All data in our manuscript is available for readers.