Abstract
Cancer is the second most frequent cause of death worldwide. It is considered to be one of the most dangerous diseases, and there is still no effective treatment for many types of cancer. Since cancerous cells have a high proliferation rate, it is pivotal for their proper functioning to have the well-functioning protein machinery. Correct protein processing and folding are crucial to maintain tumor homeostasis. Endoplasmic reticulum (ER) stress is one of the leading factors that cause disturbances in these processes. It is induced by impaired function of the ER and accumulation of unfolded proteins. Induction of ER stress affects many molecular pathways that cause the unfolded protein response (UPR). This is the way in which cells can adapt to the new conditions, but when ER stress cannot be resolved, the UPR induces cell death. The molecular mechanisms of this double-edged sword process are involved in the transition of the UPR either in a cell protection mechanism or in apoptosis. However, this process remains poorly understood but seems to be crucial in the treatment of many diseases that are related to ER stress. Hence, understanding the ER stress response, especially in the aspect of pathological consequences of UPR, has the potential to allow us to develop novel therapies and new diagnostic and prognostic markers for cancer.
1. Introduction
Cancer refers to any of a large number of diseases characterized by the development of abnormal cells that divide uncontrollably and have the ability to infiltrate and destroy normal body tissue. In the context of rapidly proliferating cells, there is a large demand for protein synthesis [1]. The endoplasmic reticulum (ER) is a cellular organelle responsible for the synthesis and proper folding of transmembrane proteins [2]. Many insults, including hypoxia, nutrient starvation, acidosis, redox imbalance, loss of calcium homeostasis, or exposure to drugs or other compounds, are capable of disturbing ER homeostasis, resulting in diminished capacity for proper protein folding.
These factors can result in unfolded and improperly folded proteins, termed ER stress. Upon ER stress conditions, the activated master regulators of the unfolded protein response (UPR) communicate to the nucleus to regulate the transcription of genes involved in protein folding and processing to increase the ER protein folding capacity, ERAD, and autophagy components. This further leads to reduction in ER workload and cell survival and death factors to determine the fate of the cell depending on the ER stress condition [3]. Cancerous cells rely on these UPR pathways to adapt to perturbations in ER folding capacity due to the hostile tumor microenvironment as well as the increase in unfolded and misfolded proteins [4]. When the UPR fails to restore ER homeostasis and attenuate ER stress, the UPR activation induces apoptosis [5]. Therefore, UPR can be therapeutically exploited to reduce the survivability of malignant cells or tip the balance towards apoptosis.
In this review, we have discussed the studies on ER stress-induced UPR signaling in cancer as well as other various diseases and applications of ER stress-modulating molecules in therapy. The use of PERK kinase inhibitors appears to be a chance for a modern personalized therapy for people for whom other therapies have failed. This article is a short analysis of publications published so far in this field.
2. ER Stress, UPR, and Their Role in the Disease Development
The stress of the endoplasmic reticulum (ER) can be induced by various factors. In response to it, the UPR pathway is activated. It is responsible for preservation of cell homeostasis. This ER balance can be perturbed by physiological and pathological insults such as high protein demand, infections, environmental toxins, inflammatory cytokines, and mutant protein expression resulting in the accumulation of misfolded and unfolded proteins in the ER lumen, a condition termed as ER stress.
The stress of the endoplasmic reticulum is associated with the activation of three factors: PKR-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring enzyme 1 (IRE1α). Studies on the role of this pathway and the effects of its inhibition show different results depending on the activated factor and the type of cancer.
The regulation of the protein synthesis process in response to stress conditions is based on the phosphorylation of the eIF2α factor by PERK kinase [6]. Interestingly, higher levels of the phosphorylated eIF2α protein have been discovered in the course of neoplastic diseases, e.g., breast cancer [7]. Activation of the UPR pathway results in the restoration of cellular homeostasis by increasing the translation of ATF4 mRNA which is responsible for the expression of proadaptive genes needed to transmit a signal that allows the cell to survive during stressful conditions [8]. The prolonged stress of the endoplasmic reticulum results in an increased transcription of the CCAAT-enhancer-binding protein homologous (CHOP) protein [9]. It is a factor that can both direct the cell to the pathway of programmed death (by weakening the expression of antiproapoptotic Bcl-2 proteins and activation of BIM proteins that bring cells to the apoptosis pathway and enable cell survival by inducing the expression of the GADD34 and ERO1α genes [6, 10]. On the other hand, it is responsible for the weakening of the UPR associated with PERK kinase and the proapoptotic response induced by the CHOP protein [11, 12].
Other pathway that partially has a crosstalk with the PERK branch of UPR is IRE1α. IRE1α is a kinase that undergoes autotransphosphorylation upon ER stress conditions, leading to endoRNase activation. Active IRE1 introduces nicks in X-box-binding protein-1 (XBP1) mRNA, and ligation of the remaining 5' and 3' fragments resulting in the activation of XBP1s (spliced form) Lu et al. [13]. It modulates the expression of several UPR target genes involved in ER folding, glycosylation, and ER-associated degradation (ERAD) [14]. Moreover, the IRE1/endo-RNAse activity can affect mRNAs and microRNAs and cause regulated IRE1-dependent decay (RIDD). RIDD has emerged as a novel UPR regulatory component that controls cell fate under ER stress [15].
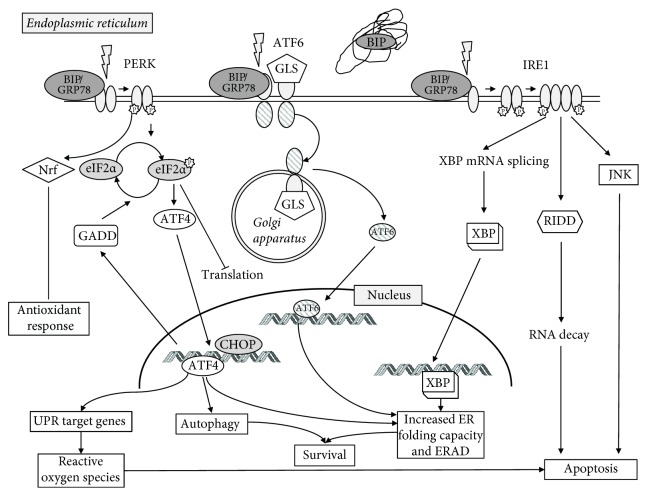
The last branch of ER stress-induced cellular response via UPR is the activation of ATF6. It was primarily identified as a cytoprotective factor during ER stress [16]. ATF6 is activated by proteolysis and acts as a transcriptional factor for regulating the downstream expression of genes responsible for stresses [17]. Studies have shown that activated ATF6 signaling is correlated with lower OS of patients with various types of tumors, cancer recurrence, metastatic lesions, tumor growth, and resistance to radio- and chemotherapy [18]. The UPR signaling cascade is shown in Figure 1.
Figure 1.
The UPR signaling cascade. UPR pathways are activated through competitive binding of the chaperone immunoglobulin heavy-chain-binding protein (BiP) also known as glucose-regulated protein 78 (GRP78) to the receptors. Accumulation of misfolded or unfolded proteins in the endoplasmic reticulum (ER) leads to the dissociation of BiP from 3 transducers: PERK (double-stranded RNA-activated protein kinase-like ER kinase), ATF6 (activating transcription factor 6), and IRE1 (inositol-requiring enzyme). Upon activation, PERK phosphorylates and deactivates the eukaryotic initiation factor (eIF2α), which results in an increased level of ATF4. This triggers the activation of C/EBP homologous protein (CHOP). Subsequently, DNA damage-inducible protein transcript (GADD) expression is also elevated, what negatively regulates eIF2α phosphorylation and restores translation. While initially contributing to cellular survival in conditions of ER stress, PERK is considered proapoptotic due to strong induction of CHOP in chronic or terminal ER stress. PERK also regulates several transcription factors including NRF-2 that upregulate the antioxidant response and ATF4 which can lead to both protective and apoptotic signaling. Upon activation, ATF6 is released from BiP that is trafficked to the Golgi apparatus (it consists of two Golgi localization signals, GLS) and cleaved by the proteases into two subunits. Then it translocates to the nucleus where it is the promoter region of UPR target genes termed the endoplasmic reticulum stress element (ERSE), activating genes responsible for the components of the UPR response and leads to the induction of molecular chaperones (e.g., GRP78, Grp94, and calreticulin, as well as CHOP and XBP1). The various ER chaperones are part of a protective adaptive response that regulates protein folding and other components of the UPR. ATF6 is primarily considered prosurvival due to its role in promoting the transcription of chaperones and XBP1. IRE-1 activation is responsible for in the unconventional splicing of XBP-1 mRNA. Spliced XBP-1 encodes a transcription factor that activates the expression of UPR genes, such as chaperones and ER-associated degradation proteins (ERAD). These include the activation of the cell death machinery, degradation of ER-localized mRNAs that encode secreted and membrane proteins through the RIDD (regulated Ire1-dependent decay) pathway, and induction of autophagosomes. This signaling cascade increases the folding capacity of the ER and causes degradation of misfolded proteins. IRE1 is mainly considered as a prosurvival pathway, but it also can contribute to apoptosis through the activation of JNK-dependent pathway.
3. Major Inducers of ER Stress
UPR is a factor of known prosurvival factor of tumor cells that can act via adaptive mechanism during cancer progression. In the context of cancer, different extrinsic (hypoxia, nutrient deprivation, and acidosis) and intrinsic (oncogene activation) factors cause endoplasmic reticulum stress and trigger the UPR.
One of the major factors inducing the UPR pathway is hypoxia. The tumor microenvironment is characterized by low oxygen concentration that is related to rapid tumor growth. Cancer cells in this environment show a high proliferative potential and, together with the increase in oxygen concentration, an increasingly aggressive phenotype. Previous studies suggest that hypoxia weakens protein biosynthesis due to the stress of the endoplasmic reticulum, which leads to the activation of the response pathway to UPR. Activation of the UPR in hypoxic tumors leads to increased autophagy [19]. Autophagy liberates amino acids from long-lived proteins and damaged organelles. In multiple cell lines, PERK mediates the upregulation of LC3 and autophagy-related gene 5 via ATF4 and CHOP, respectively, promoting phagophore formation.
Oxidative stress is also one of the main factors causing ER stress. Reactive oxygen species (ROS), i.e., molecules having an unpaired electron, such as hydroxyl (OH) and superoxide (O2) radicals, are formed endogenously during the processes occurring in the respiratory chain in the mitochondria; hence, their increased amount can be observed in cells with high-energy demand. O2 may form nitrate (ONOO-) together with nitric oxide (NO), which is an extremely overreactive molecule and may interfere with proteins and DNA causing their oxidation or nitration [20]. They arise in large quantities under hypoxia conditions, which stimulate mitochondrial activity. Free radicals can also be delivered to the body exogenously by eating fried and grilled products. Their production is also induced by smoking cigarettes. Free radicals in the human body perform many roles such as signaling, regulation of gene expression, or modulating the level of calcium in the cell [20]. Their excess, however, can be harmful. Oxidative stress interferes with the process of protein folding, leading to the formation of deposits of unfolded proteins, which induces ER stress [21]. Studies carried out on mice may confirm this directly [22]. In transgenic animals that overexpressed the superoxide dismutase (SOD) gene, ATF4 and CHOP levels were observed to be lower than in wild type. It follows that the apoptotic death of hippocampal cells after ischemia associated with ER stress in these mice occurs to a lesser extent if the process of eliminating free radicals is more efficient.
Induction of oxidative stress is closely related to inflammatory processes. Chronic inflammation can lead to the release of inflammatory factors such as prostaglandins, production of ROS, and secretion of tumor-promoting cytokines. These molecules promote the survival, growth, and metastasis of tumor cells through NFKB/NFkB (nuclear factor kappa B; mediators downstream of the UPR), STAT3 (signal transducer and activator of transcription 3), and AP-1 (AP-1 transcription factor) signaling pathways as well as cytokines such as IL1B/IL1b, IL6, IL11, and IL23A [23]. Experiments performed on pancreatic islet cells and in mice with type 2 diabetes mellitus showed that cytokines such as IL-1B, IL-23, and IL-24 can induce ER stress [21]. By administering serum with antibodies against this particular interleukin, an improved glycemic control and a reduction in ER stress were achieved. The experiments carried out in 2010 by scientists from Belgium, Germany, Greece, and USA have also shown that interferons can cause disturbances leading to excessive ER stress [24].
Other factor that can induce ER stress is ionizing radiation (IR). It is proven that IR can evoke the activation of the PERK-eIF2α pathway and subsequently cell death [25, 26].
During cancer genesis, an acute demand of protein synthesis is also needed to support different cellular functions, such as tumor proliferation, migration, and differentiation, often driven by oncogenic activation [27]. Eukaryotic cells react to the nutrient starvation by activation of the integrated stress response (ISR). It is driven by kinases (including GCN2 and PERK kinase) that induce eIF2α phosphorylation and translation of ATF4 [28]. ATF4 regulates adaptation to amino acid deprivation (AAD) by regulation of amino acid transporter expression (SLC3A2, SLC7A5, and GLYT1) and enzymes of amino acids metabolism. Additionally, activation of ATF4 is also vital for suppressing oxidative stress through the induction of glutathione biosynthesis [29]. ATF4 is a protein necessary for cancer cells growth proliferation. Data has shown that ATF4-deficient cell cultures have to be supplemented with antioxidants and necessary amino acids to survive [30, 31]. GCN2 activation/overexpression and increased phospho-eIF2α were observed in human and mouse tumors compared with normal tissues and abrogation of ATF4 or GCN2 expression significantly inhibited tumor growth in vivo [31]. Additionally, Wang et al. [32] showed that amino acid deprivation promotes tumor angiogenesis through the GCN2/ATF4 pathway [32].
UPR can also be induced by glucose deprivation and subsequent acidosis. Tumor cells adapt to low glucose levels by switching to a high rate of aerobic glycolysis, which is correlated with the expression of glucose transporter GLUT1 [33]. The resulting lactic acid production reduces the pH and thus causes acidosis. It is an important feature of the tumor microenvironment that can increase tumor survival rate and its progression by the regulation of CHOP and BCL-2 (B-cell leukemia/lymphoma-2) protein family members [34]. The glucose-regulated protein family, which includes the master UPR regulator GRP78, was discovered due to the upregulation of its members in response to glucose deprivation [35]. Also, elevated XBP1 splicing level was observed upon exposure to a nonmetabolizable glucose analog that simulates glucose deprivation [36].
The most potent intrinsic factors that induce UPR are activated oncogenes. We will discuss three of them: RAS, BRAF, and c-MYC.
Data show that oncogenic HRAS induces and activates the IRE1α RNase in primary epidermal keratinocytes through the MEK-ERK pathway and that IRE1α and Xbp1 splicing are elevated in mouse cutaneous squamous tumors [37]. Moreover, HRAS(G12V)-driven senescence was mediated by the activation of all arms the ER-associated unfolded protein response. It was also found that oncogenic forms of HRAS (HRAS(G12V)), but not its downstream target BRAF (BRAF(V600E)), engaged a rapid cell-cycle arrest and were associated with massive vacuolization and expansion of the ER [38]. ATF4-deficient MEFs transformed with SV40 large T antigen and HRAS(G12V) oncogenes displayed a slow growth, failed to form colonies on soft agar, and formed significantly smaller tumors in vivo due to suppressing expression of the INK4a/ARF [39]. Transformation of PERK-deficient cells by SV40 large T antigen and K-RAS (G12V) did not affect growth and anchorage-independent growth, suggesting that ATF4 could have some PERK-independent functions during transformation [40]. Increased levels of p-eIF2α, XPB1s, and GRP78 were observed in Nf1/p53 mutant mouse model of malignant peripheral nerve sheath tumors (MPNSTs), suggesting that the UPR is activated in HRAS-driven tumors in vivo [41].
The BRAF(V600E) mutation is present in up to 70% of malignant melanoma and other cancers and results in an increased activation of the kinase, leading to enhanced MEK/ERK signaling in the absence of extracellular signals [42]. It was proven that the presence of this mutation increased protein synthesis and activated XBP1 and GRP78 in human melanocytes. Activation of the UPR was dependent on protein synthesis, as suppression of protein synthesis attenuates the activation of XBP1s and GRP78 as well as induced autophagy via IRE1 and PERK [43–45].
c-Myc drives important biological processes such as cell growth, proliferation, and its metabolism (especially protein synthesis) and regulates apoptosis [46]. Recent studies showed that cell autonomous stress, such as activation of the protooncogene MYC/c-Myc, can also trigger the UPR. It was demonstrated that c-Myc and N-Myc activated the PERK/eIF2α/ATF4 arm of the UPR, leading to an increased cell survival via the induction of cytoprotective autophagy. Inhibition of PERK significantly reduced Myc-induced autophagy, colony formation, and tumor formation. Moreover, pharmacologic or genetic inhibition of autophagy resulted in increased Myc-dependent apoptosis [47]. Dey et al. [48] also observed EIF2AK3/PERK-dependent induction of cytoprotective autophagy in MYC-overexpressing cells. The deregulated expression of Myc drives tumor progression in most human cancers, and UPR and autophagy have been implicated in the survival of Myc-dependent cancer cells. Data obtained in the animal model (Drosophila melanogaster) show that UPR, autophagy, and p62/Nrf2 signaling are required for Myc-dependent cell growth [49].
A number of studies confirm the role of the excess of unfolded proteins in the induction of the PERK kinase-dependent pathway. ER stress is induced to restore cell homeostasis by inhibiting translation.
4. Cancer Cell Targeting via Apoptosis Pathway or Promoting Cell Survival
The stress of the endoplasmic reticulum is associated with the activation of three factors: PERK, ATF6, and IRE1a. Studies on the role of this pathway and the effects of its inhibition show different results depending on the activated factor and the type of cancer.
The role of ER stress as an important factor in cancer development has been proposed in 2004, and since then there are more and more evidence confirming this thesis [50]. For instance, increased expression levels of the major components of the UPR such as PERK and ATF6, IRE1α, both unspliced and spliced XBP1, were observed in tissue sections from a variety of human tumors including brain, breast, gastric, kidney, liver, lung, and pancreatic cancers [51–58]. Moreover, the chaperone GRP78 that is linked to higher tumor grades dissemination/metastasis of human tumors and reduced overall survival (OS).
Rubio-Patino et al. [59] showed that in mice with colorectal malignancies, activation of the IRE1-associated UPR pathway led to reduced tumor growth and increased survival [59]. This study, through a low-protein diet, induced ER stress in tumor cells. During the experiment, it also turned out that under such conditions the immune response is much more efficient; these mice had an increased number of NK cells and CD3 + CD8 + lymphocytes infiltrating the tumor. Inhibition of this pathway by the inhibitor resulted in a reduction in the beneficial effect of the low-protein diet, which suggests that the UPR-related pathway associated with IRE1a directed the cells to the pathway of apoptosis and increased sensitivity to the immune system.
It should be noted that studies regarding the role of IRE1a activated in the group of patients with breast cancer showed that splicing XBP1 associated with the above-mentioned factor leads to the adaptation of cells to the conditions of hypoxia [60]. Such tumors are characterized by a worse prognosis. This underlines the very important role of accurate determination of the impact of UPR pathway activation on tumor progression.
In patients with chronic B-chronic lymphocytic leukemia (B-CLL), it was shown that the induction of the UPR pathway associated with ER stress (activation of PERK kinase) leads to apoptotic death of tumor cells. This effect was confirmed by the influence of commercially available ER stress inducers (thapsigargin and tunicamycin) on the progression of tumor growth. Researchers have shown that these compounds induce apoptosis of cells in patients with B-CLL [61]. On the other hand, ER stress also triggers survival signals in B-CLL cells by increasing BiP/GRP78 expression.
The branch of the UPR pathway associated with PERK kinase is responsible for the induction of blood vessel formation in tumor cells under hypoxic conditions. Angiogenesis is mediated by ATF4, which induces the expression of vascular endothelial growth factor (VEGF) [62]. Data have shown that ATF4 binds to the regulatory site of VEGF [63]. Moreover, in vitro studies revealed that partially blocking UPR signaling by silencing PERK or ATF4 significantly reduced the production of angiogenesis mediators induced by glucose deprivation [63].
In the melanoma patients, the role of the UPR pathway in promoting cell survival has been confirmed [64]. It induces the expression of proadaptive proteins and at the same time lowers proapoptotic proteins. It also increases the process of autophagy, which allows cancer cells to recover the necessary components, such as amino acids, and remove damaged organelles from cells that are older and more damaged.
It has also been confirmed that the UPR pathway associated with PERK promotes the progression of colorectal tumors. It has been shown that PERK plays an important role in tumor cell adaptation to hypoxic stress by regulating the translation of molecules that promotes cellular adhesion, integrin binding, and capillary morphogenesis necessary for the development of functional microvessels [65]. The association of ATF4 factor promoting angiogenesis and proadaptive gene expression is suspected, and GADD34, which prevents apoptosis induction during prolonged ER stress, by lowering overtranslation of proteins [66].
Pancreatic cancer cells are under permanent high hypoxic state caused by large volume of the tumor, and only a small fraction of cancer cells are at the normal oxygenation levels of the surrounding normal pancreas [67]. Choe et al. (2011) showed that in pancreatic cancer cells, activation of the PERK and IRE1 arms of the UPR are delayed in the presence of ER stressors, compared to normal pancreatic cells. This was attributed to an abundance of protein-folding machinery, such as chaperones. Additionally, once activated, the prosurvival XBP1 was noted to be activated for a longer period of time in cancer cells when compared to normal cells [68]. Moreover, the unfolded protein response seems to play a predominant homeostatic role in response to mitochondrial stress in pancreatic stellate cells. Su et al. evaluated AMPK/mTOR signaling, autophagy, and the UPR to cell fate responses during metabolic stress induced by mitochondrial dysfunction [69]. Rottlerin treatment induced rapid and sustained PERK/CHOP UPR signaling, causing loss of cell viability and cell death. As well as adapting to chronic ER stress, it has been recently postulated that anterior-gradient 2 (AGR2) may contribute to the initiation and development of PDAC [70].
In addition, the experiment conducted by Liu et al. [71] showed that activation of the UPR pathway leads to the change in ATF6α, PERK, and IRE1α expression and is associated with progression of prostate cancer, worse prognosis, and more aggressive growth [71].
The summary and additional information of the UPR involvement in the pathogenesis and progression of various types of cancer is presented in Table 1.
Table 1.
UPR involvement in cancers.
| UPR linked to cancer | Cancer type | Branch of the UPR | References |
|---|---|---|---|
| Cancer initiation | CRC | PERK/eIF2α axis activation is associated with the loss of stemness IRE1α pathway induces intestinal stem cell expansion |
[72, 73] |
| Colitis-associated cancer model | XBP1 loss in epithelial cells results in intestinal stem cell hyperproliferation | ||
| Tumor quiescence and aggressiveness | Prostate cancer | Change in ATF6α, PERK, and IRE1α expression | [60, 61, 71, 74, 75] |
| B-CLL | BiP/GRP78 overexpression triggers survival signals and prevents apoptosis | ||
| Triple-negative breast cancers | Constitutively active IRE1α/XBP1s axis confers higher aggressiveness due to XBP1-mediated hypoxia-inducible factor-1α activation | ||
| Glioblastoma (GBM) | IRE1α endoribonuclease activity regulates the extracellular matrix protein SPARC (secreted protein acidic and rich in cysteine) involved in GBM tumor invasion | ||
| Tumor epithelial-to-mesenchymal transition | Breast tumors thyroid cell glioblastoma (GBM) | Increased expression of XBP1s in metastatic tumors correlates with the EMT inducer SNAIL (snail-related protein) LOXL2 (lysyl oxidase-like 2)/GRP78 activates the IRE1-XBP1 signaling induce EMT-linked transcription factors expression: SNAI1 (snail family transcriptional repressor), SNAI2, ZEB2 (zinc-finger E-box-binding homeobox 2), and TCF3 (transcription factor 3) Serpin B3, a serine/cysteine protease inhibitor overexpression, is associated with chronic UPR induction leading to nuclear factor-κB activation and interleukin-6 production PERK constitutive activation correlates with the overexpression of the TWIST (twist-related protein) transcription factor |
[76–78] |
| Tumor angiogenesis | Human head and neck squamous cell carcinoma | Amino acid deprivation promotes tumor angiogenesis through the GCN2/ATF4 pathway | [32, 63, 65, 79–82] |
| Human head and neck squamous cell carcinoma, breast cancer, and glioma cell lines | Glucose deprivation-induced UPR activation promotes upregulation of proangiogenic mediators (VEGF, FGF2, and IL6) and downregulation of several angiogenic inhibitors (THBS1, CXCL14, and CXCL10) through the PERK/ATF4 | ||
| Colorectal cancer | Hypoxic stress-induced PERK overexpression stimulates the creation of microvessels | ||
| Glioblastoma (GBM) | IRE1α signaling induce vascular endothelial growth factor-A (VEGF-A), interleukin-1β, and interleukin-6 IRE1α-mediated mRNA cleavage of the circadian gene PERIOD1,92 an important mediator of regulation of the CXCL3 chemokine supports tumor angiogenesis PERK-ATF4 branch upregulates VEGF in hypoxia |
||
| Prostatic and glioma cancer cells | Chaperone ORP150 (oxygen-regulated protein 150) controls tumor angiogenesis by promoting the secretion of VEGF | ||
| Tumor metabolic processes | Triple-negative breast cancer cells | Hypoxia-inducible factor-1α activation, XBP1 upregulates glucose transporter 1 expression promotes glucose uptake of IRE1α, XBP1s downstream activates enzymes of the hexosamine biosynthetic pathway expression | [83] |
| Tumor autophagy | Triple-negative breast cancer cells | PERK/eIF2α/ATF4 pathway activation protect tumor cells through autophagy induction via LC3B (autophagy protein microtubule-associated protein 1 light chain 3b) and ATG5 (autophagy protein 5) TNF receptor associated factor 2 (TRAF2)/IRE1α activates c-Jun N-terminal protein kinase induces autophagy |
[19, 83] |
5. ER Stress and Cancer Treatment—Novel UPR Modulating Factors
ER stress plays a large role in both progression and moderation of response to cancer chemo- and radiotherapy. Activation of the UPR pathway takes place under the influence of many factors, which are subjected to a cancer cell: unfolded proteins (protein economy is intensified during cancer, which is a very dynamic process), hypoxia (associated with excessively fast nascent tumor mass), pH changes, or chemotherapy [84].
GPR78 as the chaperone protein is an interesting target for the anticancer therapy, especially in cancer steam cells, and was partially effective in head and neck cancer treatment [85]. An immune adjuvant therapy seems to be effective since monoclonal antibody against GRP78 was shown to suppress signaling through the PI3K/Akt/mTOR pathway, which is responsible for radiation resistance in nonsmall cell lung cancer and glioblastoma multiforme (GBM). It was shown that ionizing radiation increased GRP78 expression through the induction of ER stress, and treatment with the monoclonal antibody along with ionizing radiation in mouse xenograph models showed a significant tumor growth delay [86]. Other study reveals that using a phage, displaying a ligand specific to GRP78 with the antiviral drug ganciclovir, prostate cancer bone metastasis tumors were reduced by an average of 50% [87].
A group of patients with AML has been studied for molecular changes that allow survival and resistance to treatment. The results clearly indicate the role of the proadaptive pathway associated with ER stress mediated by PERK kinase. In the case of PERK, selective ATP-competitive PERK kinase inhibitors such as GSK2606414 or GSK2656157 were antiproliferative in multiple cancer models in vivo including multiple myeloma [88, 89]. In the AML cells obtained from the mouse model in which GSK2656157, a PERK inhibitor, was used, the response to treatment was better. An 80% greater decrease in tumor colony growth was obtained against the group in which the UPR pathway occurred correctly [90]. In case of human multiple myeloma, other ER stress modulator STF-083010, a small-molecule inhibitor of Ire1, is a promising target for anticancer therapy [91].
It has been demonstrated that tyrosine kinase inhibitors (TKIs) on Hodgkin's lymphoma are correlated to increase in ER stress and ER stress-induced apoptosis. After treatment of L-428, L-1236, and KM-H2 cells with the TKI sorafenib, the elevated level of p-PERK and phosphorylation of eIF2α were observed. In addition, proapoptotic signaling molecules GADD34 and CHOP were noted to be upregulated after incubation with sorafenib [92].
It has been also proven that PERK regulates glioblastoma sensitivity to ER stress through promoting radiation resistance [25]. By inhibiting PERK, it was determined that ionizing radiation- (IR-) induced PERK activity led to eIF2α phosphorylation. IR enhanced the prodeath component of PERK signaling in cells treated with Sal003, an inhibitor of phospho-eIF2α phosphatase. Mechanistically, ATF4 mediated the prosurvival activity during the radiation response. The data support the notion that induction of ER stress signaling by radiation contributes to adaptive survival mechanisms during radiotherapy.
Adaptation to an environment conducive to ER stress is essential for survival and propagation of pancreatic cancer cells. In vitro studies of diindolylmethane derivatives have shown similar ER stress induction activity as thapsigargin followed by subsequent apoptosis via death receptor 5 (DR5) through induction by CHOP [93]. Other compound, a proteasome inhibitor called bortezomib, was increasing the levels of GRP78, CHOP, and c-Jun NH2 terminal kinase (JNK) in L3.6pl pancreatic cancer cells, yet interestingly at the same time was blocking PERK autophosphorylation, and thus inhibiting phosphorylation of eIF2α [94].
ER stress can be a factor supporting the progression of colorectal cancer. It has been proven that in cell lines of colorectal cancer it plays an important role in the loss of the intestinal stem cell (ISC) phenotype. Activation of the PERK eIF2α branch in response to ER stress leads to the transformation of CRC cells to a more aggressive type [84].
It has been shown that activation of the UPR pathway and adaptation to stress conditions lead to the emergence of a chemotherapy-resistant phenotype HT-29/MDR [95]. This process takes place by activating the PERK/Nrf2/MRP1 axis. MRP1 is a protein belonging to membrane transporters. Its activity is inversely proportional to the concentration of doxorubicin in the cell. The induction of MRP1 protein expression by PERK kinase under ER stress conditions was associated with a lower concentration of the chemotherapeutic agent in the cell and hence resistance to treatment [95].
Activation of the UPR pathway in response to ER stress involves targeting the cell both to the apoptosis pathway and to enable its survival. The pathways leading to cell survival allow clones to resist both treatment [84] and those more susceptible depending on the type of chemotherapy and tumor phenotype used [96].
The research conducted by Wielenga et al. [96] in colorectal cancer cells showed that the induction of tumor cell differentiation before stress ER leads to the formation of clones that are more susceptible to chemotherapy [96]. Cell lines taken from patients with colorectal cancer were exposed to an ER stress inducer (subtilase cytotoxin AB, SubAB). The results were as follows: in vitro activation of the UPR pathway led to the differentiation of tumor cells whose colonies had increased sensitivity to chemotherapy in the form of oxaliplatin. In vivo, supportive treatment in the form of SubAB was shown to improve the tumor response to oxaliplatin, but the experiment did not prove in this model that this was directly due to the changes in the phenotype of the derived cells.
The induction of ER stress with various substances, moderating the course, blocking the branches of the UPR pathway is currently used in in vitro and in vivo models to assess their impact on growth and progression of CRC. Treatment trials are divided into two streams of ER stress use. One of them induces it with compounds that activate the proapototic pathway. The other uses the assumption that CRC stem cells, thanks to the PERK/eIF2α pathway, differentiate into more aggressive phenotypes and the fact that the primary role of the UPR pathway is to restore homeostasis in the cell and allow it to survive under stress conditions through its other branches.
Yang et al. [97] using levistolide A induced the formation of free radicals that caused ER stress [97]. The wild type and p53-/- CRC colonies treated with this compound were reduced, since the cells were subject to apoptosis. Administration of N-acetylcysteine, which blocked the action of levistolide A, had an effect in the reduction of tumor mass.
The effect of tolfenamic acid, which belongs to the NSAIDs group, was also investigated on the development of CRC [98]. Tolfenamic acid promotes ER stress, resulting in the activation of the unfolded UPR signaling pathway, of which PERK-mediated phosphorylation of eukaryotic translation initiation factor 2α (eIF2α) induces the repression of cyclin D1 translation. In mice with FAP syndrome, the apoptosis in CRC cells was induced through the branch associated with ATF4. It also correlated positively with the decrease in the concentration of cyclin D1 and the activity of Rb oncogene. The result of this study may suggest a likely mechanism of beneficial effects of NSAIDs on the risk of CRC.
Other study confirms the positive effect on CRC tumor regression, due to the activation of the branches associated with CHOP, Bax, and caspase 3 in andrographolide therapies [99]. This compound increases the production of free radicals and induces ER stress, which leads cells to the path of apoptosis. In addition, decreased concentrations of cyclins have also been demonstrated, which in turn inhibits the progression of the cell cycle.
Studies are not limited to the UPR modulators mentioned above. Since it is a very promising target for novel anticancer therapy, more and more new molecules are being tested. A significant amount of them are naturally occurring chemicals that are present also in plants. Due to the abundance of the compounds affecting UPR in Table 2, we have summarized the literature review on tested modulators in various cancer cell lines.
Table 2.
UPR-modulating factors inducing ER stress activity in cancer cells.
| Agents | Mechanism | Cancer type/cell lines | References |
|---|---|---|---|
| GSK2606414 and GSK2656157 | p-PERK↓, p-elF2α↓ | Multiple myeloma | [88, 89] |
| STF-083010 | Ire1 inhibitor | Multiple myeloma | [91] |
| Sorafenib tyrosine kinase inhibitor (TKI) | CHOP↑ GADD34↑; p-PERK↑; p-elF2α↑ | L-428, L-1236, and KM-H2 cells | [92] |
| Sal003, inhibitor of phospho-eif2α phosphatase | ATF4; p-elF2α↑ | Glioblastoma cells | [25] |
| Diindolylmethane derivatives | CHOP↑; DR5↑ | Pancreatic cancer cells | [93] |
| Bortezomib proteasome inhibitor | GRP78↑, CHOP↑, JNK↑, p-eIF2α↓ | L3.6pl pancreatic cancer cells | [94] |
| Levistolide A | ROS↑; CHOP↑ | Colorectal cancer cells | [97] |
| Andrographolide | ROS↑; CHOP↑ | Colorectal cancer cells | [99] |
| Tolfenamic acid | eIF2α↑; ATF4↑ | Colorectal cancer cells | [98] |
| Cantharidin | GRP78/BiP ↑, IRE1α ↑, IRE1β ↑, ATF6α ↑, XBP1 ↑ | H460 | [100] |
| Carnosic acid | ROS↑; CHOP↑; ATF4↑ | Renal carcinoma Caki cells | [101] |
| Casticin | CHOP ↑, p-eIF2α ↑, eIF2α ↑, GRP78/BiP ↑ | BGC-823 | [102] |
| Cryptotanshinone | p-eIF2α ↑, GRP94 ↑, GRP78 ↑, CHOP ↑, ROS↑ | MCF7 | [103] |
| Curcumin | CHOP ↑, GRP78/BiP ↑, ROS ↑ | NCI-H460, HT-29, AGS | [104, 105] |
| Flavokawain B | CHOP ↑, ATF4 ↑ | HCT116 | [106] |
| Fucoidan | CHOP ↑, ATF4 ↑, p-eIF2α ↑, GRP78/BiP ↓, p-IRE1 ↓, XBP1 ↓ | MDA-MB-231 HCT116 | [107] |
| Furanodiene | CHOP ↑, BIP ↑ | A549, 95-D | [108] |
| 2-3,4 Dihydroxyphenylethanol | IRE1 ↑, XBP1 ↑, GRP78/BiP ↑, PERK ↑, eIF2α ↑, CHOP ↑ | HT-29 | [109] |
| 7-Dimethoxyflavone | CHOP ↑, GPR78/BiP ↑, ATF4 ↑ | Hep3B | [110] |
| SMIP004 (N-(4-butyl-2-methyl-phenyl) acetamide) | ROS↑ IRE1↑; p-38↑; p-elF2α↑ | Prostate cancer cells | [111] |
| Licochalcone A | ATF6 ↑, eIF2α ↑, IRE1α ↑, CHOP ↑, GRP94 ↑, XBP1 ↑, GRP78/BiP ↑ | HepG2 | [112] |
| Neferine | GRP78/BiP ↑ | Hep3B | [113] |
| Paeonol | GRP78 ↑, CHOP ↑ | HepG2 | [114] |
| Pardaxin | ROS↑; p-PERK↑; p-elF2α↑ | HeLa cells | [115] |
| Parthenolide | ATF4 ↑, p-eIF2a ↑, eIF2α ↑ | A549, Calu-1, H1299, H1792 | [116] |
| Piperine | IRE1α ↑, CHOP ↑, GPR78/BiP ↑ | HT-29 | [117] |
| Polyphenon E | ATF4 ↑, PERK ↑, p-eIF2α ↑, eIF2α ↑, GRP78/BiP ↑, CHOP ↑, XBP1 ↑, ROS ↑ | PC3, PNT1a | [118] |
| Polyphyllin D | CHOP ↑, GRP78/BiP ↑, PDI ↑ | NCI-H460 | [119] |
| Resveratrol | GRP78/BiP ↑, CHOP ↑, XBP1 ↑, eIF2α ↑ | HT29 | [120] |
| Dehydrocostuslactone | p-PERK ↑, GRP78/BiP ↑, IRE1 ↑, CHOP ↑, XBP-1 ↑, ROS ↑ | NCI-H460 A549 | [121] |
| γ-Tocotrienol | CHOP ↑, GRP78/BiP ↑, XBP1 ↑ | MDA-MB-231; MCF-7 | [122] |
| Ω-Hydroxyundec-9-enoic Acid (ω-HUA) | ROS↑; CHOP↑ | Lung cancer cells (H1299, A549, HCC827) | [123] |
| Ampelopsin | ROS↑ GRP78↑; p-PERK↑; p-elF2α↑ | Breast cancer cells (MCF-7; MDA-MB-231) | [124] |
| Ardisianone | GRP78/BiP ↑ | PC3 | [125] |
| Genistein | CHOP ↑, GRP78/BiP ↑ | Hep3B | [126] |
| Guttiferone H | ATF4 ↑, XBP1 ↑, CHOP ↑ | HCT116 | [127] |
| Guggulsterone | ROS↑; p-eIF2α↑; CHOP↑ DR5↑ | Liver cancer cells (Hep3B; HepG2) | [128] |
| Marchantin M | GRP78/BiP, CHOP ↑, XBP1 ↑, p-eIF2α ↑, eIF2α ↑, ATF4 ↑, ATF6 ↑, ERAD ↓ | PC3, DU145, LNCaP | [129] |
| Sarsasapogenin | ROS↑; CHOP↑ | HeLa cells | [130] |
| Saxifragifolin | IRE1α ↑, XBP1 ↑, CHOP ↑, GRP78/BiP ↑, ROS↑ | MDA-MB-231, MCF7 | [131] |
| Prodigiosin | ROS↑; CHOP↑; p-eIF2α↑; PERK↑; GRP78↑; ATF6α↑, IRE1 ↑, eIF2a ↑ | Pancreatic (8898); breast cancer cells (MCF-7 and MDA-MB-231) | [132, 133] |
| Quercetin | GRP78/BiP ↑, ATF4 ↑, IRE1α ↑ ATF6 ↑ | PC3 | [134] |
| Honokiol (HNK) | ROS↑ p-eIF2α↑; GRP78↑ CHOP↑ | Chondrosarcoma (JJ012 and SW1353); gastric (AGS, SCM-1 and MKN-45) cancer cells | [135–139] |
| Brefeldin A (BFA) | ROS↑, IRE1α ↑, PERK ↑, XBP1↑; GRP78↑ CHOP↑ | Ovarian (OVCAR-3); lung (A549); colorectal (colo 205); breast (MDA-MB-231) cancer cells | [140–142] |
| A-tocopheryl succinate | SGC-7901 | GRP78/BiP ↑, CHOP ↑ | [143] |
| Verrucarin A | GRP78/BiP ↑, p-PERK ↑, p-eIF2α ↑, CHOP ↑ | Hep3B, HepG2 | [144] |
| Vitamin E succinate | GRP78/BiP ↑, GRP94 ↓, PERK ↑, ATF4 ↑, ATF6 ↑, XBP1 ↑, CHOP ↑ | SGC-7901 | [145] |
| Ultrafine | p-eIF2α ↑, GRP78/BiP ↑ | SNU-484 | [146] |
| Zerumbone | ATF4 ↑, CHOP ↑, GRP78/BiP ↑, p-PERK ↑, PERK ↑ eIF2α ↑, p-eIF2α ↑ | HCT116-p53null, SW480, PC3 | [68, 147] |
6. Conclusion
Stress of the endoplasmic reticulum is a process commonly occurring under the influence of various factors (free radicals, unfolded or misfolded proteins). The UPR pathway is the physiological response of the cell to the stress conditions affecting the cell. ER stress response has been highlighted as a key factor (next to the mutations) occurring at various stages of the disease progression and the individual response to the treatment. Cancers are a very heterogeneous group in which the UPR pathway can lead to adaptation to stress conditions (e.g., hypoxia in rapidly growing tumors), apoptosis (strengthening the immune response in colorectal cancer cells or induction of apoptosis in B-CLL cells). At the same time, depending on the circumstances and cell's condition, it can lead to resistance to treatment and production of clones less sensitive to chemotherapy. UPR activation is a vital step for oncogenic transformation, as UPR signaling molecules interact with well-established oncogene and tumor suppressor gene networks to modulate their function during cancer development.
UPR modulators are a promising hope for a personalized therapy for patients in whom chemotherapy or radiotherapy have failed. It can become an innovative way to fight several different types of cancer. The response to a given compound depends on the phenotype of tumor cells, the severity of the disease, and the chemotherapy used so far.
It is emphasized that further experiments and analyses should be carried out using a variety of compounds that have the ability to inhibit and induce the UPR pathway in different types of cancers. It could also be useful in the treatment of noncancerous diseases.
Acknowledgments
This work was supported by the National Science Centre (grant number 2016/23/B/NZ5/02630) and the grant of the Medical University of Lodz for Young Researchers (grant number 502-03/5-108-05/502-54-194).
Conflicts of Interest
The authors declare no conflict of interests.
References
- 1.Dolfi S. C., Chan L. L.-Y., Qiu J., et al. The metabolic demands of cancer cells are coupled to their size and protein synthesis rates. Cancer & metabolism. 2013;1(1):20–20. doi: 10.1186/2049-3002-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braakman I., Hebert D. N. Protein folding in the endoplasmic reticulum. Cold Spring Harbor Perspectives in Biology. 2013;5(5, article a013201) doi: 10.1101/cshperspect.a013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodsky J. L., Skach W. R. Protein folding and quality control in the endoplasmic reticulum: recent lessons from yeast and mammalian cell systems. Current Opinion in Cell Biology. 2011;23(4):464–475. doi: 10.1016/j.ceb.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Drie J. H. Protein folding, protein homeostasis, and cancer. Chinese Journal of Cancer. 2011;30(2):124–137. doi: 10.5732/cjc.010.10162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oslowski C. M., Urano F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods in enzymology. 2011;490:71–92. doi: 10.1016/B978-0-12-385114-7.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui W., Li J., Ron D., Sha B. The structure of the PERK kinase domain suggests the mechanism for its activation. Acta Crystallographica Section D Biological Crystallography. 2011;67(5):423–428. doi: 10.1107/S0907444911006445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo L., Chi Y., Xue J., Ma L., Shao Z., Wu J. Phosphorylated eIF2α predicts disease-free survival in triple-negative breast cancer patients. Scientific Reports. 2017;7(1, article 44674) doi: 10.1038/srep44674. https://www.nature.com/articles/srep44674#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bobrovnikova-Marjon E., Grigoriadou C., Pytel D., et al. PERK promotes cancer cell proliferation and tumor growth by limiting oxidative DNA damage. Oncogene. 2010;29(27):3881–3895. doi: 10.1038/onc.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han J., Back S. H., Hur J., et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nature Cell Biology. 2013;15(5):481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oyadomari S., Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death & Differentiation. 2004;11(4):381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 11.Marciniak S. J., Yun C. Y., Oyadomari S., et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes & Development. 2004;18(24):3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandewynckel Y. P., Laukens D., Geerts A., et al. The paradox of the unfolded protein response in cancer. Anticancer Research. 2013;33(11):4683–4694. [PubMed] [Google Scholar]
- 13.Lu Y., Liang F.-X., Wang X. A synthetic biology approach identifies the mammalian UPR RNA ligase Rtc B. Molecular Cell. 2014;55(5):758–770. doi: 10.1016/j.molcel.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walter P., Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 15.Maurel M., Chevet E., Tavernier J., Gerlo S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends in Biochemical Sciences. 2014;39(5):245–254. doi: 10.1016/j.tibs.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto K., Sato T., Matsui T., et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6α and XBP1. Developmental Cell. 2007;13(3):365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Reviews Molecular Cell Biology. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 18.Dadey D. Y., Kapoor V., Khudanyan A., et al. The ATF6 pathway of the ER stress response contributes to enhanced viability in glioblastoma. Oncotarget. 2016;7(2):2080–2092. doi: 10.18632/oncotarget.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rouschop K. M. A., van den Beucken T., Dubois L., et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. The Journal of Clinical Investigation. 2010;120(1):127–141. doi: 10.1172/JCI40027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karpinska A., Gromadzka G. Oxidative stress and natural antioxidant mechanisms: the role in neurodegeneration. From molecular mechanisms to therapeutic strategies. Postepy Hig Med Dosw. 2013;67:43–53. doi: 10.5604/17322693.1029530. [DOI] [PubMed] [Google Scholar]
- 21.Hasnain S. Z., Prins J. B., McGuckin M. A. Oxidative and endoplasmic reticulum stress in beta-cell dysfunction in diabetes. Journal of Molecular Endocrinology. 2016;56(2):R33–R54. doi: 10.1530/jme-15-0232. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi T., Saito A., Okuno S., Ferrand-Drake M., Dodd R. L., Chan P. H. Damage to the endoplasmic reticulum and activation of apoptotic machinery by oxidative stress in ischemic neurons. Journal of Cerebral Blood Flow & Metabolism. 2005;25(1):41–53. doi: 10.1038/sj.jcbfm.9600005. [DOI] [PubMed] [Google Scholar]
- 23.Garg A. D., Kaczmarek A., Krysko O., Vandenabeele P., Krysko D. V., Agostinis P. ER stress-induced inflammation: does it aid or impede disease progression? Trends in Molecular Medicine. 2012;18(10):589–598. doi: 10.1016/j.molmed.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Allagnat F., Christulia F., Ortis F., et al. Sustained production of spliced X-box binding protein 1 (XBP1) induces pancreatic beta cell dysfunction and apoptosis. Diabetologia. 2010;53(6):1120–1130. doi: 10.1007/s00125-010-1699-7. [DOI] [PubMed] [Google Scholar]
- 25.Dadey D. Y. A., Kapoor V., Khudanyan A., Thotala D., Hallahan D. E. PERK regulates glioblastoma sensitivity to ER stress although promoting radiation resistance. Molecular Cancer Research. 2018;16(10):1447–1453. doi: 10.1158/1541-7786.mcr-18-0224. [DOI] [PubMed] [Google Scholar]
- 26.Kim K. W., Moretti L., Mitchell L. R., Jung D. K., Lu B. Endoplasmic reticulum stress mediates radiation-induced autophagy by perk-eIF2alpha in caspase-3/7-deficient cells. Oncogene. 2010;29(22):3241–3251. doi: 10.1038/onc.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dejeans N., Barroso K., Fernandez-Zapico M. E., Samali A., Chevet E. Novel roles of the unfolded protein response in the control of tumor development and aggressiveness. Seminars in Cancer Biology. 2015;33:67–73. doi: 10.1016/j.semcancer.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Wek R. C., Jiang H. Y., Anthony T. G. Coping with stress: eIF2 kinases and translational control. Biochemical Society Transactions. 2006;34(1):7–11. doi: 10.1042/bst20060007. [DOI] [PubMed] [Google Scholar]
- 29.Yu X., Long Y. C. Crosstalk between cystine and glutathione is critical for the regulation of amino acid signaling pathways and ferroptosis. Scientific Reports. 2016;6(1, article 30033) doi: 10.1038/srep30033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harding H. P., Zhang Y., Zeng H., et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Molecular Cell. 2003;11(3):619–633. doi: 10.1016/S1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 31.Ye J., Kumanova M., Hart L. S., et al. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. The EMBO Journal. 2010;29(12):2082–2096. doi: 10.1038/emboj.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y., Ning Y., Alam G. N., et al. Amino acid deprivation promotes tumor angiogenesis through the GCN2/ATF4 pathway. Neoplasia. 2013;15(8):989–997. doi: 10.1593/neo.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amann T., Hellerbrand C. GLUT1 as a therapeutic target in hepatocellular carcinoma. Expert Opinion on Therapeutic Targets. 2009;13(12):1411–1427. doi: 10.1517/14728220903307509. [DOI] [PubMed] [Google Scholar]
- 34.Ryder C. B., McColl K., Distelhorst C. W. Acidosis blocks CCAAT/enhancer-binding protein homologous protein (CHOP)- and c-Jun-mediated induction of p53-upregulated mediator of apoptosis (PUMA) during amino acid starvation. Biochemical and Biophysical Research Communications. 2013;430(4):1283–1288. doi: 10.1016/j.bbrc.2012.11.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiu R. P., Pouyssegur J., Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proceedings of the National Academy of Sciences of the United States of America. 1977;74(9):3840–3844. doi: 10.1073/pnas.74.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spiotto M. T., Banh A., Papandreou I., et al. Imaging the unfolded protein response in primary tumors reveals microenvironments with metabolic variations that predict tumor growth. Cancer Research. 2010;70(1):78–88. doi: 10.1158/0008-5472.can-09-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blazanin N., Son J., Craig-Lucas A. B., et al. ER stress and distinct outputs of the IRE1α RNase control proliferation and senescence in response to oncogenic Ras. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(37):9900–9905. doi: 10.1073/pnas.1701757114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Denoyelle C., Abou-Rjaily G., Bezrookove V., et al. Anti-oncogenic role of the endoplasmic reticulum differentially activated by mutations in the MAPK pathway. Nature Cell Biology. 2006;8(10):1053–1063. doi: 10.1038/ncb1471. [DOI] [PubMed] [Google Scholar]
- 39.Horiguchi M., Koyanagi S., Okamoto A., Suzuki S. O., Matsunaga N., Ohdo S. Stress-regulated transcription factor ATF4 promotes neoplastic transformation by suppressing expression of the INK4a/ARF cell senescence factors. Cancer Research. 2012;72(2):395–401. doi: 10.1158/0008-5472.can-11-1891. [DOI] [PubMed] [Google Scholar]
- 40.Bi M., Naczki C., Koritzinsky M., et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. The EMBO Journal. 2005;24(19):3470–3481. doi: 10.1038/sj.emboj.7600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Raedt T., Walton Z., Yecies J. L., et al. Exploiting cancer cell vulnerabilities to develop a combination therapy for ras-driven tumors. Cancer Cell. 2011;20(3):400–413. doi: 10.1016/j.ccr.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies H., Bignell G. R., Cox C., et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 43.Corazzari M., Rapino F., Ciccosanti F., et al. Oncogenic BRAF induces chronic ER stress condition resulting in increased basal autophagy and apoptotic resistance of cutaneous melanoma. Cell Death and Differentiation. 2015;22(6):946–958. doi: 10.1038/cdd.2014.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Croft A., Tay K. H., Boyd S. C., et al. Oncogenic activation of MEK/ERK primes melanoma cells for adaptation to endoplasmic reticulum stress. The Journal of Investigative Dermatology. 2014;134(2):488–497. doi: 10.1038/jid.2013.325. [DOI] [PubMed] [Google Scholar]
- 45.Ma X. H., Piao S. F., Dey S., et al. Targeting ER stress-induced autophagy overcomes BRAF inhibitor resistance in melanoma. The Journal of Clinical Investigation. 2014;124(3):1406–1417. doi: 10.1172/jci70454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen H., Liu H., Qing G. Targeting oncogenic Myc as a strategy for cancer treatment. Signal Transduction and Targeted Therapy. 2018;3(1):p. 5. doi: 10.1038/s41392-018-0008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hart L. S., Cunningham J. T., Datta T., et al. ER stress–mediated autophagy promotes Myc-dependent transformation and tumor growth. The Journal of clinical investigation. 2012;122(12):4621–4634. doi: 10.1172/JCI62973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dey S., Tameire F., Koumenis C. PERK-ing up autophagy during MYC-induced tumorigenesis. Autophagy. 2013;9(4):612–614. doi: 10.4161/auto.23486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagy P., Varga A., Pircs K., Hegedus K., Juhasz G. Myc-driven overgrowth requires unfolded protein response-mediated induction of autophagy and antioxidant responses in Drosophila melanogaster. PLoS Genetics. 2013;9(8, article e1003664) doi: 10.1371/journal.pgen.1003664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma Y., Hendershot L. M. The role of the unfolded protein response in tumour development: friend or foe? Nature Reviews Cancer. 2004;4(12):966–977. doi: 10.1038/nrc1505. [DOI] [PubMed] [Google Scholar]
- 51.Al-Rawashdeh F. Y., Scriven P., Cameron I. C., Vergani P. V., Wyld L. Unfolded protein response activation contributes to chemoresistance in hepatocellular carcinoma. European Journal of Gastroenterology & Hepatology. 2010;22(9):1099–1105. doi: 10.1097/MEG.0b013e3283378405. [DOI] [PubMed] [Google Scholar]
- 52.Epple L. M., Dodd R. D., Merz A. L., et al. Induction of the unfolded protein response drives enhanced metabolism and chemoresistance in glioma cells. PLoS One. 2013;8(8, article e73267) doi: 10.1371/journal.pone.0073267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fu W., Wu X., Li J., et al. Upregulation of GRP78 in renal cell carcinoma and its significance. Urology. 2010;75(3):603–607. doi: 10.1016/j.urology.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 54.Fujimoto T., Yoshimatsu K., Watanabe K., et al. Overexpression of human X-box binding protein 1 (XBP-1) in colorectal adenomas and adenocarcinomas. Anticancer Research. 2007;27(1a):127–131. [PubMed] [Google Scholar]
- 55.Genovese G., Carugo A., Tepper J., et al. Synthetic vulnerabilities of mesenchymal subpopulations in pancreatic cancer. Nature. 2017;542(7641):362–366. doi: 10.1038/nature21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kong B., Wu W., Valkovska N., et al. A common genetic variation of melanoma inhibitory activity-2 labels a subtype of pancreatic adenocarcinoma with high endoplasmic reticulum stress levels. Scientific Reports. 2015;5(1, article 8109) doi: 10.1038/srep08109. https://www.nature.com/articles/srep08109#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee E., Nichols P., Spicer D., Groshen S., Yu M. C., Lee A. S. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Research. 2006;66(16):7849–7853. doi: 10.1158/0008-5472.can-06-1660. [DOI] [PubMed] [Google Scholar]
- 58.Tsai H. Y., Yang Y. F., Wu A. T., et al. Endoplasmic reticulum ribosome-binding protein 1 (RRBP1) overexpression is frequently found in lung cancer patients and alleviates intracellular stress-induced apoptosis through the enhancement of GRP78. Oncogene. 2013;32(41):4921–4931. doi: 10.1038/onc.2012.514. [DOI] [PubMed] [Google Scholar]
- 59.Rubio-Patino C., Bossowski J. P., De Donatis G. M., et al. Low-protein diet induces IRE1α-dependent anticancer immunosurveillance. Cell Metabolism. 2018;27(4):828–842.e7. doi: 10.1016/j.cmet.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 60.Davies M. P., Barraclough D. L., Stewart C., et al. Expression and splicing of the unfolded protein response gene XBP-1 are significantly associated with clinical outcome of endocrine-treated breast cancer. International Journal of Cancer. 2008;123(1):85–88. doi: 10.1002/ijc.23479. [DOI] [PubMed] [Google Scholar]
- 61.Rosati E., Sabatini R., Rampino G., et al. Novel targets for endoplasmic reticulum stress-induced apoptosis in B-CLL. Blood. 2010;116(15):2713–2723. doi: 10.1182/blood-2010-03-275628. [DOI] [PubMed] [Google Scholar]
- 62.Zhu K., Jiao H., Li S., et al. ATF4 promotes bone angiogenesis by increasing VEGF expression and release in the bone environment. Journal of bone and mineral research. 2013;28(9):1870–1884. doi: 10.1002/jbmr.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y., Alam G. N., Ning Y., et al. The unfolded protein response induces the angiogenic switch in human tumor cells through the PERK/ATF4 pathway. Cancer Research. 2012;72(20):5396–5406. doi: 10.1158/0008-5472.CAN-12-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hersey P., Zhang X. D. Adaptation to ER stress as a driver of malignancy and resistance to therapy in human melanoma. Pigment Cell & Melanoma Research. 2008;21(3):358–367. doi: 10.1111/j.1755-148X.2008.00467.x. [DOI] [PubMed] [Google Scholar]
- 65.Blais J. D., Addison C. L., Edge R., et al. Perk-dependent translational regulation promotes tumor cell adaptation and angiogenesis in response to hypoxic stress. Molecular and Cellular Biology. 2006;26(24):9517–9532. doi: 10.1128/MCB.01145-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iwasaki N., Sugiyama Y., Miyazaki S., Nakagawa H., Nishimura K., Matsuo S. An ATF4-signal-modulating machine other than GADD34 acts in ATF4-to-CHOP signaling to block CHOP expression in ER-stress-related autophagy. Journal of Cellular Biochemistry. 2015;116(7):1300–1309. doi: 10.1002/jcb.25085. [DOI] [PubMed] [Google Scholar]
- 67.Koong A. C., Mehta V. K., Le Q. T., et al. Pancreatic tumors show high levels of hypoxia. International Journal of Radiation Oncology Biology Physics. 2000;48(4):919–922. doi: 10.1016/S0360-3016(00)00803-8. [DOI] [PubMed] [Google Scholar]
- 68.Edagawa M., Kawauchi J., Hirata M., et al. Role of activating transcription factor 3 (ATF3) in endoplasmic reticulum (ER) stress-induced sensitization of p53-deficient human colon cancer cells to tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis through up-regulation of death receptor 5 (DR5) by zerumbone and celecoxib. Journal of Biological Chemistry. 2014;289(31):21544–21561. doi: 10.1074/jbc.M114.558890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Su H. Y., Waldron R. T., Gong R., Ramanujan V. K., Pandol S. J., Lugea A. The unfolded protein response plays a predominant homeostatic role in response to mitochondrial stress in pancreatic stellate cells. PLoS One. 2016;11(2, article e0148999) doi: 10.1371/journal.pone.0148999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dumartin L., Alrawashdeh W., Trabulo S. M., et al. ER stress protein AGR2 precedes and is involved in the regulation of pancreatic cancer initiation. Oncogene. 2017;36(22):3094–3103. doi: 10.1038/onc.2016.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu J., Xiao M., Li J., et al. Activation of UPR signaling pathway is associated with the malignant progression and poor prognosis in prostate cancer. Prostate. 2017;77(3):274–281. doi: 10.1002/pros.23264. [DOI] [PubMed] [Google Scholar]
- 72.Niederreiter L., Fritz T. M., Adolph T. E., et al. ER stress transcription factor Xbp1 suppresses intestinal tumorigenesis and directs intestinal stem cells. The Journal of Experimental Medicine. 2013;210(10):2041–2056. doi: 10.1084/jem.20122341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vermeulen L., Snippert H. J. Stem cell dynamics in homeostasis and cancer of the intestine. Nature Reviews Cancer. 2014;14(7):468–480. doi: 10.1038/nrc3744. [DOI] [PubMed] [Google Scholar]
- 74.Chen X., Iliopoulos D., Zhang Q., et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1α pathway. Nature. 2014;508(7494):103–107. doi: 10.1038/nature13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dejeans N., Pluquet O., Lhomond S., et al. Autocrine control of glioma cells adhesion and migration through IRE1α-mediated cleavage of SPARC mRNA. Journal of Cell Science. 2012;125(18):4278–4287. doi: 10.1242/jcs.099291. [DOI] [PubMed] [Google Scholar]
- 76.Feng Y. X., Sokol E. S., Del Vecchio C. A., et al. Epithelial-to-mesenchymal transition activates PERK–eIF2α and sensitizes cells to endoplasmic reticulum stress. Cancer Discovery. 2014;4(6):702–715. doi: 10.1158/2159-8290.cd-13-0945. [DOI] [PubMed] [Google Scholar]
- 77.Sheshadri N., Catanzaro J. M., Bott A. J., et al. SCCA1/SERPINB3 promotes oncogenesis and epithelial–mesenchymal transition via the unfolded protein response and IL6 signaling. Cancer Research. 2014;74(21):6318–6329. doi: 10.1158/0008-5472.can-14-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ulianich L., Garbi C., Treglia A. S., et al. Retraction: ER stress is associated with dedifferentiation and an epithelial-to-mesenchymal transition-like phenotype in PC Cl3 thyroid cells. Journal of Cell Science. 2016;129(18):p. 3518. doi: 10.1242/jcs.196584. [DOI] [PubMed] [Google Scholar]
- 79.Drogat B., Auguste P., Nguyen D. T., et al. IRE1 signaling is essential for ischemia-induced vascular endothelial growth factor-A expression and contributes to angiogenesis and tumor growth in vivo. Cancer Research. 2007;67(14):6700–6707. doi: 10.1158/0008-5472.can-06-3235. [DOI] [PubMed] [Google Scholar]
- 80.Miyagi T., Hori O., Koshida K., et al. Antitumor effect of reduction of 150-kDa oxygen-regulated protein expression on human prostate cancer cells. International Journal of Urology. 2002;9(10):577–585. doi: 10.1046/j.1442-2042.2002.00519.x. [DOI] [PubMed] [Google Scholar]
- 81.Ozawa K., Tsukamoto Y., Hori O., et al. Regulation of tumor angiogenesis by oxygen-regulated protein 150, an inducible endoplasmic reticulum chaperone. Cancer Research. 2001;61(10):4206–4213. [PubMed] [Google Scholar]
- 82.Pluquet O., Dejeans N., Bouchecareilh M., et al. Posttranscriptional regulation of PER1 underlies the oncogenic function of IREα. Cancer Research. 2013;73(15):4732–4743. doi: 10.1158/0008-5472.can-12-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ferrer C. M., Lynch T. P., Sodi V. L., et al. O-Glc NAcylation regulates cancer metabolism and survival stress signaling via regulation of the HIF-1 pathway. Molecular Cell. 2014;54(5):820–831. doi: 10.1016/j.molcel.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Avril T., Vauléon E., Chevet E. Endoplasmic reticulum stress signaling and chemotherapy resistance in solid cancers. Oncogene. 2017;6(8, article e373) doi: 10.1038/oncsis.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu M. J., Jan C. I., Tsay Y. G., et al. Elimination of head and neck cancer initiating cells through targeting glucose regulated protein 78 signaling. Molecular Cancer. 2010;9(1):p. 283. doi: 10.1186/1476-4598-9-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dadey D. Y. A., Kapoor V., Hoye K., et al. Antibody targeting GRP78 enhances the efficacy of radiation therapy in human glioblastoma and non-small cell lung cancer cell lines and tumor models. Clinical Cancer Research. 2017;23(10):2556–2564. doi: 10.1158/1078-0432.ccr-16-1935. [DOI] [PubMed] [Google Scholar]
- 87.Ferrara F., Staquicini D. I., Driessen W. H. P., et al. Targeted molecular-genetic imaging and ligand-directed therapy in aggressive variant prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(45):12786–12791. doi: 10.1073/pnas.1615400113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Atkins C., Liu Q., Minthorn E., et al. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Research. 2013;73(6):1993–2002. doi: 10.1158/0008-5472.can-12-3109. [DOI] [PubMed] [Google Scholar]
- 89.Axten J. M., Medina J. R., Feng Y., et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) Journal of Medicinal Chemistry. 2012;55(16):7193–7207. doi: 10.1021/jm300713s. [DOI] [PubMed] [Google Scholar]
- 90.Zhou C., Di Marcantonio D., Martinez E., et al. C-Jun regulates ER stress signaling to promote chemotherapy resistance in acute myeloid leukemia. Blood. 2015;126(23, article 2464) [Google Scholar]
- 91.Papandreou I., Denko N. C., Olson M., et al. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117(4):1311–1314. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Holz M. S., Janning A., Renne C., Gattenlohner S., Spieker T., Brauninger A. Induction of endoplasmic reticulum stress by sorafenib and activation of NF-κB by lestaurtinib as a novel resistance mechanism in Hodgkin lymphoma cell lines. Molecular Cancer Therapeutics. 2013;12(2):173–183. doi: 10.1158/1535-7163.mct-12-0532. [DOI] [PubMed] [Google Scholar]
- 93.Abdelrahim M., Newman K., Vanderlaag K., Samudio I., Safe S. 3, 3′-Diindolylmethane (DIM) and its derivatives induce apoptosis in pancreatic cancer cells through endoplasmic reticulum stress-dependent upregulation of DR5. Carcinogenesis. 2006;27(4):717–728. doi: 10.1093/carcin/bgi270. [DOI] [PubMed] [Google Scholar]
- 94.Nawrocki S. T., Carew J. S., Dunner K., Jr., et al. Bortezomib inhibits PKR-like endoplasmic reticulum (ER) kinase and induces apoptosis via ER stress in human pancreatic cancer cells. Cancer Research. 2005;65(24):11510–11519. doi: 10.1158/0008-5472.can-05-2394. [DOI] [PubMed] [Google Scholar]
- 95.Salaroglio I. C., Panada E., Moiso E., et al. PERK induces resistance to cell death elicited by endoplasmic reticulum stress and chemotherapy. Molecular Cancer. 2017;16(1):p. 91. doi: 10.1186/s12943-017-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wielenga M. C. B., Colak S., Heijmans J., et al. ER-stress-induced differentiation sensitizes colon cancer stem cells to chemotherapy. Cell Reports. 2015;13(3):489–494. doi: 10.1016/j.celrep.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 97.Yang Y., Zhang Y., Wang L., Lee S. Levistolide A induces apoptosis via ROS-mediated ER stress pathway in colon cancer cells. Cellular Physiology and Biochemistry. 2017;42(3):929–938. doi: 10.1159/000478647. [DOI] [PubMed] [Google Scholar]
- 98.Zhang X., Lee S.-H., Min K.-W., et al. The involvement of endoplasmic reticulum stress in the suppression of colorectal tumorigenesis by tolfenamic acid. Cancer Prevention Research. 2013;6(12):1337–1347. doi: 10.1158/1940-6207.capr-13-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Banerjee A., Banerjee V., Czinn S., Blanchard T. Increased reactive oxygen species levels cause ER stress and cytotoxicity in andrographolide treated colon cancer cells. Oncotarget. 2017;8(16):26142–26153. doi: 10.18632/oncotarget.15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hsia T. C., Yu C. C., Hsu S. C., et al. Cantharidin induces apoptosis of H460 human lung cancer cells through mitochondria-dependent pathways. International Journal of Oncology. 2014;45(1):245–254. doi: 10.3892/ijo.2014.2428. [DOI] [PubMed] [Google Scholar]
- 101.Min K.-J., Jung K.-J., Kwon T. K. Carnosic acid induces apoptosis through reactive oxygen species-mediated endoplasmic reticulum stress induction in human renal carcinoma Caki cells. Journal of cancer prevention. 2014;19(3):170–178. doi: 10.15430/JCP.2014.19.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou Y., Tian L., Long L., Quan M., Liu F., Cao J. Casticin potentiates TRAIL-induced apoptosis of gastric cancer cells through endoplasmic reticulum stress. PLoS One. 2013;8(3, article e58855) doi: 10.1371/journal.pone.0058855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Park I. J., Kim M. J., Park O. J., et al. Cryptotanshinone induces ER stress-mediated apoptosis in HepG2 and MCF7 cells. Apoptosis. 2012;17(3):248–257. doi: 10.1007/s10495-011-0680-3. [DOI] [PubMed] [Google Scholar]
- 104.Cao A., Li Q., Yin P., et al. Curcumin induces apoptosis in human gastric carcinoma AGS cells and colon carcinoma HT-29 cells through mitochondrial dysfunction and endoplasmic reticulum stress. Apoptosis. 2013;18(11):1391–1402. doi: 10.1007/s10495-013-0871-1. [DOI] [PubMed] [Google Scholar]
- 105.Wu S. H., Hang L. W., Yang J. S., et al. Curcumin induces apoptosis in human non-small cell lung cancer NCI-H460 cells through ER stress and caspase cascade- and mitochondria-dependent pathways. Anticancer Research. 2010;30(6):2125–2133. [PubMed] [Google Scholar]
- 106.Kuo Y. F., Su Y. Z., Tseng Y. H., Wang S. Y., Wang H. M., Chueh P. J. Flavokawain B, a novel chalcone from Alpinia pricei Hayata with potent apoptotic activity: involvement of ROS and GADD153 upstream of mitochondria-dependent apoptosis in HCT116 cells. Free Radical Biology and Medicine. 2010;49(2):214–226. doi: 10.1016/j.freeradbiomed.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 107.Chen S., Yang Z., Yu Z., Zhang D. Fucoidan induces cancer cell apoptosis by modulating the endoplasmic reticulum stress cascades. PLoS One. 2014;9, article e108157(9) doi: 10.1371/journal.pone.0108157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xu W. S., Dang Y. Y., Guo J. J., et al. Furanodiene induces endoplasmic reticulum stress and presents antiproliferative activities in lung cancer cells. Evidence-Based Complementary and Alternative Medicine. 2012;2012:8. doi: 10.1155/2012/426521.426521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guichard C., Pedruzzi E., Fay M., et al. Dihydroxyphenylethanol induces apoptosis by activating serine/threonine protein phosphatase PP2A and promotes the endoplasmic reticulum stress response in human colon carcinoma cells. Carcinogenesis. 2006;27(9):1812–1827. doi: 10.1093/carcin/bgl009. [DOI] [PubMed] [Google Scholar]
- 110.Yang J. F., Cao J. G., Tian L., Liu F. 5, 7-Dimethoxyflavone sensitizes TRAIL-induced apoptosis through DR5 upregulation in hepatocellular carcinoma cells. Cancer Chemotherapy and Pharmacology. 2012;69(1):195–206. doi: 10.1007/s00280-011-1686-9. [DOI] [PubMed] [Google Scholar]
- 111.Rico-Bautista E., Zhu W., Kitada S., et al. Small molecule-induced mitochondrial disruption directs prostate cancer inhibition via UPR signaling. Oncotarget. 2013;4(8):1212–1229. doi: 10.18632/oncotarget.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Choi A. Y., Choi J. H., Hwang K. Y., et al. Licochalcone A induces apoptosis through endoplasmic reticulum stress via a phospholipase Cγ1-, Ca2+-, and reactive oxygen species-dependent pathway in HepG2 human hepatocellular carcinoma cells. Apoptosis. 2014;19(4):682–697. doi: 10.1007/s10495-013-0955-y. [DOI] [PubMed] [Google Scholar]
- 113.Yoon J. S., Kim H. M., Yadunandam A. K., et al. Neferine isolated from Nelumbo nucifera enhances anti-cancer activities in Hep3B cells: molecular mechanisms of cell cycle arrest, ER stress induced apoptosis and anti-angiogenic response. Phytomedicine. 2013;20(11):1013–1022. doi: 10.1016/j.phymed.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 114.Fan L., Song B., Sun G., Ma T., Zhong F., Wei W. Endoplasmic reticulum stress-induced resistance to doxorubicin is reversed by paeonol treatment in human hepatocellular carcinoma cells. PLoS One. 2013;8(5, article e62627) doi: 10.1371/journal.pone.0062627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huang T. C., Chen J. Y. Proteomic analysis reveals that pardaxin triggers apoptotic signaling pathways in human cervical carcinoma HeLa cells: cross talk among the UPR, c-Jun and ROS. Carcinogenesis. 2013;34(8):1833–1842. doi: 10.1093/carcin/bgt130. [DOI] [PubMed] [Google Scholar]
- 116.Zhao X., Liu X., Ling S. Parthenolide induces apoptosis via TNFRSF10B and PMAIP1 pathways in human lung cancer cells. Journal of experimental & clinical cancer research. 2014;33(1):p. 3. doi: 10.1186/1756-9966-33-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yaffe P. B., Power Coombs M. R., Doucette C. D., Walsh M., Hoskin D. W. Piperine, an alkaloid from black pepper, inhibits growth of human colon cancer cells via G1 arrest and apoptosis triggered by endoplasmic reticulum stress. Molecular Carcinogenesis. 2015;54(10):1070–1085. doi: 10.1002/mc.22176. [DOI] [PubMed] [Google Scholar]
- 118.Rizzi F., Naponelli V., Silva A., et al. Polyphenon E (R), a standardized green tea extract, induces endoplasmic reticulum stress, leading to death of immortalized PNT1a cells by anoikis and tumorigenic PC3 by necroptosis. Carcinogenesis. 2014;35(4):828–839. doi: 10.1093/carcin/bgt481. [DOI] [PubMed] [Google Scholar]
- 119.Siu F. M., Ma D. L., Cheung Y. W., et al. Proteomic and transcriptomic study on the action of a cytotoxic saponin (polyphyllin D): induction of endoplasmic reticulum stress and mitochondria-mediated apoptotic pathways. Proteomics. 2008;8(15):3105–3117. doi: 10.1002/pmic.200700829. [DOI] [PubMed] [Google Scholar]
- 120.Park J. W., Woo K. J., Lee J. T., et al. Resveratrol induces pro-apoptotic endoplasmic reticulum stress in human colon cancer cells. Oncology Reports. 2007;18(5):1269–1273. doi: 10.3892/or.18.5.1269. [DOI] [PubMed] [Google Scholar]
- 121.Hung J. Y., Hsu Y. L., Ni W. C., et al. Oxidative and endoplasmic reticulum stress signaling are involved in dehydrocostuslactone-mediated apoptosis in human non-small cell lung cancer cells. Lung Cancer. 2010;68(3):355–365. doi: 10.1016/j.lungcan.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 122.Park S. K., Sanders B. G., Kline K. Tocotrienols induce apoptosis in breast cancer cell lines via an endoplasmic reticulum stress-dependent increase in extrinsic death receptor signaling. Breast Cancer Research and Treatment. 2010;124(2):361–375. doi: 10.1007/s10549-010-0786-2. [DOI] [PubMed] [Google Scholar]
- 123.Yang K. M., Kim B. M., Park J. B. ω-Hydroxyundec-9-enoic acid induces apoptosis through ROS-mediated endoplasmic reticulum stress in non-small cell lung cancer cells. Biochemical and Biophysical Research Communications. 2014;448(3):267–273. doi: 10.1016/j.bbrc.2014.04.111. [DOI] [PubMed] [Google Scholar]
- 124.Zhou Y., Shu F., Liang X., et al. Ampelopsin induces cell growth inhibition and apoptosis in breast cancer cells through ROS generation and endoplasmic reticulum stress pathway. PLoS One. 2014;9(2, article e89021) doi: 10.1371/journal.pone.0089021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yu C. C., Wu P. J., Hsu J. L., et al. Ardisianone, a natural benzoquinone, efficiently induces apoptosis in human hormone-refractory prostate cancers through mitochondrial damage stress and survivin downregulation. Prostate. 2013;73(2):133–145. doi: 10.1002/pros.22548. [DOI] [PubMed] [Google Scholar]
- 126.Yeh T. C., Chiang P. C., Li T. K., et al. Genistein induces apoptosis in human hepatocellular carcinomas via interaction of endoplasmic reticulum stress and mitochondrial insult. Biochemical Pharmacology. 2007;73(6):782–792. doi: 10.1016/j.bcp.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 127.Protiva P., Hopkins M. E., Baggett S., et al. Growth inhibition of colon cancer cells by polyisoprenylated benzophenones is associated with induction of the endoplasmic reticulum response. International Journal of Cancer. 2008;123(3):687–694. doi: 10.1002/ijc.23515. [DOI] [PubMed] [Google Scholar]
- 128.Moon D. O., Park S. Y., Choi Y. H., Ahn J. S., Kim G. Y. Guggulsterone sensitizes hepatoma cells to TRAIL-induced apoptosis through the induction of CHOP-dependent DR5: involvement of ROS-dependent ER-stress. Biochemical Pharmacology. 2011;82(11):1641–1650. doi: 10.1016/j.bcp.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 129.Jiang H., Sun J., Xu Q., et al. Marchantin M: a novel inhibitor of proteasome induces autophagic cell death in prostate cancer cells. Cell Death & Disease. 2013;4(8, article e761) doi: 10.1038/cddis.2013.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Farooqi A. A., Li K.-T., Fayyaz S., et al. Anticancer drugs for the modulation of endoplasmic reticulum stress and oxidative stress. Tumour biology. 2015;36(8):5743–5752. doi: 10.1007/s13277-015-3797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shi J. M., Bai L. L., Zhang D. M., et al. Saxifragifolin D induces the interplay between apoptosis and autophagy in breast cancer cells through ROS-dependent endoplasmic reticulum stress. Biochemical Pharmacology. 2013;85(7):913–926. doi: 10.1016/j.bcp.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 132.Liu Y., Zhou H., Ma X., et al. Prodigiosin inhibits proliferation, migration, and invasion of nasopharyngeal cancer cells. Cellular Physiology and Biochemistry. 2018;48(4):1556–1562. doi: 10.1159/000492278. [DOI] [PubMed] [Google Scholar]
- 133.Pan M. Y., Shen Y. C., Lu C. H., et al. Prodigiosin activates endoplasmic reticulum stress cell death pathway in human breast carcinoma cell lines. Toxicology and Applied Pharmacology. 2012;265(3):325–334. doi: 10.1016/j.taap.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 134.Liu K. C., Yen C. Y., Wu R. S., et al. The roles of endoplasmic reticulum stress and mitochondrial apoptotic signaling pathway in quercetin-mediated cell death of human prostate cancer PC-3 cells. Environmental Toxicology. 2014;29(4):428–439. doi: 10.1002/tox.21769. [DOI] [PubMed] [Google Scholar]
- 135.Chen Y. J., Wu C. L., Liu J. F., et al. Honokiol induces cell apoptosis in human chondrosarcoma cells through mitochondrial dysfunction and endoplasmic reticulum stress. Cancer Letters. 2010;291(1):20–30. doi: 10.1016/j.canlet.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 136.Hahm E. R., Sakao K., Singh S. V. Honokiol activates reactive oxygen species-mediated cytoprotective autophagy in human prostate cancer cells. Prostate. 2014;74(12):1209–1221. doi: 10.1002/pros.22837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pan H. C., Lai D. W., Lan K. H., et al. Honokiol thwarts gastric tumor growth and peritoneal dissemination by inhibiting Tpl2 in an orthotopic model. Carcinogenesis. 2013;34(11):2568–2579. doi: 10.1093/carcin/bgt243. [DOI] [PubMed] [Google Scholar]
- 138.Sheu M. L., Liu S. H., Lan K. H. Honokiol induces calpain-mediated glucose-regulated protein-94 cleavage and apoptosis in human gastric cancer cells and reduces tumor growth. PLoS One. 2007;2(10, article e1096) doi: 10.1371/journal.pone.0001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Weng T. I., Wu H. Y., Chen B. L., Liu S. H. Honokiol attenuates the severity of acute pancreatitis and associated lung injury via acceleration of acinar cell apoptosis. Shock. 2012;37(5):478–484. doi: 10.1097/SHK.0b013e31824653be. [DOI] [PubMed] [Google Scholar]
- 140.Moon J. L., Kim S. Y., Shin S. W., Park J. W. Regulation of brefeldin A-induced ER stress and apoptosis by mitochondrial NADP+-dependent isocitrate dehydrogenase. Biochemical and Biophysical Research Communications. 2012;417(2):760–764. doi: 10.1016/j.bbrc.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 141.Tseng C. N., Hong Y. R., Chang H. W., et al. Brefeldin A reduces anchorage-independent survival, cancer stem cell potential and migration of MDA-MB-231 human breast cancer cells. Molecules. 2014;19(11):17464–17477. doi: 10.3390/molecules191117464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tseng C. N., Huang C. F., Cho C. L., et al. Brefeldin A effectively inhibits cancer stem cell-like properties and MMP-9 activity in human colorectal cancer Colo 205 cells. Molecules. 2013;18(9):10242–10253. doi: 10.3390/molecules180910242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Huang X., Li L., Zhang L., et al. Crosstalk between endoplasmic reticulum stress and oxidative stress in apoptosis induced by α-tocopheryl succinate in human gastric carcinoma cells. British Journal of Nutrition. 2013;109(4):727–735. doi: 10.1017/s0007114512001882. [DOI] [PubMed] [Google Scholar]
- 144.Moon D. O., Asami Y., Long H., et al. Verrucarin A sensitizes TRAIL-induced apoptosis via the upregulation of DR5 in an eIF2α/CHOP-dependent manner. Toxicology In Vitro. 2013;27(1):257–263. doi: 10.1016/j.tiv.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 145.Huang X., Zhang Z., Jia L., Zhao Y., Zhang X., Wu K. Endoplasmic reticulum stress contributes to vitamin E succinate-induced apoptosis in human gastric cancer SGC-7901 cells. Cancer Letters. 2010;296(1):123–131. doi: 10.1016/j.canlet.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 146.Ahn J., Lee J. S., Yang K. M. Ultrafine particles of Ulmus davidiana var. japonica induce apoptosis of gastric cancer cells via activation of caspase and endoplasmic reticulum stress. Archives of Pharmacal Research. 2014;37(6):783–792. doi: 10.1007/s12272-013-0312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chan M. L., Liang J. W., Hsu L. C., Chang W. L., Lee S. S., Guh J. H. Zerumbone, a ginger sesquiterpene, induces apoptosis and autophagy in human hormone-refractory prostate cancers through tubulin binding and crosstalk between endoplasmic reticulum stress and mitochondrial insult. Naunyn-Schmiedeberg's Archives of Pharmacology. 2015;388(11):1223–1236. doi: 10.1007/s00210-015-1152-z. [DOI] [PubMed] [Google Scholar]