Abstract
Introduction: The aim of this research was to study the impact of various doxorubicin (Dox)-containing nanofluids, e.g. singlewalled carbon nanotube (SWCNT)+Dox, graphene oxide (GO)+Dox and DextranPNIPAM (copolymer)+Dox mixtures on HeLa cells (human transformed cervix epithelial cells, as a model for cancer cells) depending on their concentration.
Methods: Structural analysis of GO+Dox complex was accomplished using Hartree-Fock level of theory in 6-31G** basis set in Gaussian. Dynamic light scattering (DLS), zeta-potential, scanning electron microscopy and confocal laser scanning microscopy were used. The cell viability was analyzed by the MTT assay.
Results: The viability of HeLa cells was studied with the MTT assay after the incubation with various Dox-containing dispersions depending on their concentration. The size of the particles was determined by DLS. The morphology of the nanoparticles (NPs) was studied by scanning electron microscopy and their uptake into cells was visualized by confocal laser scanning microscopy. It was found that the Dextran-PNIPAM+Dox nanofluid in contrast to Dox alone showed higher toxicity towards HeLa cells up to 80% after 24 hours of incubation, whereas the SWCNT+Dox and GO+Dox nanofluids at the same concentrations protected cells from Dox.
Conclusion: The importance of Dextran-PNIPAM copolymer as a universal platform for drug delivery was established, and the huge potential of Dextran-PNIPAM+Dox NPs as novel anticancer agents was noted. Based on the in vitro study of the SWCNT+Dox and GO+Dox nanofluids, it was concluded that SWCNT and GO NPs can be effective cytoprotectors against the highly toxic drugs.
Keywords: Cytotoxicity, Dextran-PNIPAM copolymer, Doxorubicin, Graphene oxide, HeLa cells, Single-walled carbon nanotube
Introduction
Nanoparticles (NPs) have the advantage of targeting cancer by simply being effectively accumulated and entrapped in tumors due to the phenomenon of enhanced permeability and retention caused by leaky tumor angiogenetic vessels and poor lymphatic drainage.1,2 This effect constitutes one of the major advantages of NPs against multiple drug resistance (MDR) mechanisms.3 Many of the drugs available to circumvent MDR were in the past unavailable to target this mechanism due to low solubility and/or stability. NPs made these drugs a possible strategy to target MDR. They have also provided an effective platform to deliver high loads of drugs in a specific and controlled way (using stimuli-sensitive and pH-responsive), with surface modified to improve circulation time, to prevent the uptake by the reticuloendothelial system and to ensure specific targeting (using bioactive agents like antibodies, receptor ligands, siRNA). Another advantage of using NPs for MDR regression is the drastic reduction of the IC50 value for drugs thus reducing the clinical doses. In fact, the lipid nanocapsules, polymeric micelles, metal and carbon NPs have been reported to circumvent drug resistance.4,5 In this regard, the conjugation of anticancer drugs, for example, doxorubicin (Dox) as a ‘gold standard’ of chemotherapy, with the representatives of carbon NPs could be promising for targeted drug delivery, overcoming of tumor cells drug resistance and reduction of side toxic effects.6-8
In the present work, we studied the impact of various Dox-containing nanofluids, e.g. single-walled carbon nanotube (SWCNT)+Dox, graphene oxide (GO)+Dox and Dextran-PNIPAM (copolymer)+Dox mixtures on HeLa cells (human transformed cervix epithelial cells, as a model for cancer cells) depending on their concentration.
Materials and Methods
Material preparation
Dox (Doxorubicin hydrochloride; Sigma-Aldrich, USA) was dissolved in saline with a maximum concentration of 0.15 mg mL-1.
An aqueous solution of carboxylated SWCNT (Sigma-Aldrich, USA) in a maximum concentration of 0.15 mg mL-1 was prepared as described in previously published papers.9,10
GO (Sigma-Aldrich, USA) was dispersed in ultrapure water to prepare a stock solution with a maximum concentration of 0.15 mg mL-1. Then this aqueous solution was sonicated for 2 hours (40 kHz).11
Preparation of the SWCNT+Dox and GO+Dox mixtures were executed according to the protocol: SWCNT or GO and Dox were mixed in a 1:1 volume ratio (0.075:0.075 mg), and the resulting aqueous solution was treated in the ultrasonic disperser for 15 minutes, and after that stirred magnetically for 24 hours at room temperature.
The Dextran-PNIPAM copolymer used as a nanocarrier for Dox has been described in a recently published paper.12 This copolymer is star-like with Dextran core and PNIPAM grafts (Mw=1.03×106 g mol-1; Mw/Mn=1.52; Dh=38 nm). Dextran-PNIPAM exhibits a lower critical solution temperature (LCST). The polymer is soluble in water below LCST and undergoes a phase transition above LCST becoming partially hydrophobic. The conformational transition for this copolymer was registered in the temperature range of 32.6-33.4°C, that higher than typical LCST point for linear PNIPAM of similar molecular weight and polydispersity and is closer to physiological temperature (37°C).13 A possible tuning of the hydrophobicity of star-like polymer, the regulation of the region of phase transition and size of hydrophobic domains by variation of copolymer internal structure was reported.12
A stock solution of Dextran-PNIPAM (0.15 mg mL-1) was prepared in distilled water. This concentration is lower of a crossover concentration.13 For obtaining an aqueous solution of Dextran-PNIPAM+Dox, the Dextran-PNIPAM and Dox were mixed in a 1:1 volume ratio (0.075:0.075 mg).
Structural calculations
The structural parameters of GO molecule were taken from14 and the initial structure was built with an aid of HyperChem8 software. After that, the structure of Dox molecule, taken from Protein Data Bank (PDB ID 1D12), was added in HyperChem. The planes of GO sheet and Dox chromophore were located parallel to each other at a distance of 3.4 Å which is typical of aromatic-aromatic stacking. The longitudinal axis of the Dox molecule was located above the central row of carbon cycles of the GO sheet. Preliminary optimization of this structure by means of molecular mechanics was performed in HyperChem using AMBER force field. After that full minimization by the energy of the spatial structure of GO+Dox complex was accomplished using Hartree-Fock level of theory in 6-31G** basis set in Gaussian.
Dynamic light scattering and zeta potential determination
Measurements of size distribution and zeta potential for various aqueous mixtures containing different particles were performed by dynamic light scattering (DLS) on a Zetasizer Nano-ZS90 (Malvern, Worcestershire, UK) at T=298 K. The instrument was equipped with a He-Ne laser (5 mW) operating at the wavelength of 633 nm. The autocorrelation function of the scattered light intensity was analyzed by the Malvern Zetasizer software with the Smoluchowski approximation.
Scanning electron microscopy
NPs presented in the studied samples were visualized by scanning electron microscopy (SEM) with an ESEM Quanta 400 instrument (FEI, The Netherlands) on gold/palladium-sputtered samples.
Cell culture
HeLa cells were cultured in Dulbecco’s modified Eagle medium (DMEM), supplemented with 10% fetal calf serum (FCS), 100 U mL-1penicillin, and 100 mg mL-1 streptomycin at 37°C in humidified atmosphere with 5% CO2. 12 hours prior to the uptake experiments, the cells were trypsinized and seeded in 24-well plates with 5∙104 cells per well in 0.5 mL DMEM with FCS for HeLa cells.
The cell line was obtained from the Collection of Microorganisms and Cell Cultures of the University of Duisburg-Essen.
Confocal laser scanning microscopy
The uptake studies of NPs presented in the samples were carried out as follows: 30 μL of the nanofluid was added to the cells. The cells were incubated with the NPs for 3 and 48 hours, respectively. Immediately after the indicated time, the cells were washed three times with phosphate-buffered saline (PBS) in order to remove all dispersed NPs, fixed with 4% paraformaldehyde at room temperature for 10 minutes, then stained with fluorescent dye DAPI for the cell nucleus and washed three times with PBS. Finally, the cells were studied by confocal laser scanning microscopy (CLSM; analysis of z-stacks), which gave us information whether the NPs were able to enter the cells. CLSM was performed on a TCS SP8 AOBS system controlled by the LASX software (Leica Microsystems). The laser lines were used for the excitation by DPSS 561 nm (for Dox) and Diode 405 nm (DAPI). Images were acquired with a 20x objective.
MTT assay
The cell viability was analyzed by the MTT assay 24 and 48 hours after the incubation of cells with the NPs, so the final concentration of NPs per well after dilution in the medium was: 1.5, 3 and 6 µg mL-1. MTT (3-(4.5-dimethylthiazol-2-yl)-2.5-diphenyl-tetrazolium bromide; Sigma, Taufkirchen, Germany) was dissolved in PBS (5 mg/mL) and then diluted to 1 mg mL-1 in the cell culture medium. The cell culture medium of the incubated cells was replaced by 300 μL of the MTT solution. The cells were then incubated for 1 hour at 37°C under 5% CO2 in a humidified atmosphere. About 300 μL of dimethyl sulfoxide (DMSO) was added to the cells. After 30 min, a 100 μL aliquot was taken for spectrophotometric analysis with a Multiscan FC instrument (Thermo Fisher Scientific, Vantaa, Finland) at λ=570 nm. The absorption of incubated cells was normalized to that of control (untreated) cells, thereby indicating the relative level of cell viability.
Statistics
Statistical analysis was performed by conventional methods of variation statistics. The significance of the differences between the control and experimental measurements was estimated within the framework of the Student’s t-test using Origin 8.0 software (OriginLab Corporation, USA). The difference between the compared values was considered to be significant at P < 0.05.
Results and Discussion
Material characterization
The freshly prepared aqueous mixtures were characterized by DLS and SEM, in order to determine the aggregation state of NPs. Such information is considered important as the particles size is known to correlate with their bioactivity.15-19
Table 1 shows the experimental hydrodynamic sizes of various particles in aqueous solutions at 25°C. The Dextran-PNIPAM+Dox and SWCNT+Dox systems contain NPs with a hydrodynamic size of 69 to 81 nm. The GO+Dox mixture formed particles with hydrodynamic size 158 nm. The polydispersity index (PDI) falls in between 0.3-0.5 for all of the studied samples and indicates the degree of particle’s aggregation.
Table 1. The hydrodynamic diameter of particles (Z-average size), polydispersity index (PDI) and zeta potential for various aqueous systems at T=25°C .
| Sample | Size (nm) | PDI | Zeta potential (mV) |
| Dextran-PNIPAM+Dox | 69 | 0.36 | -9 |
| SWCNT+Dox | 81 | 0.49 | -13 |
| GO+Dox | 158 | 0.39 | -10 |
The zeta potential study has shown that at 25°C for the Dextran-PNIPAM+Dox, GO+Dox and SWCNT+Dox aqueous systems it takes values in between -9 and -13 mV (see Table 1). A negative charge of the individual particles plays a significant role in the stabilization of the studied aqueous systems. It contributes to the electrostatic repulsion between the negatively charged clusters, thus disfavoring the aggregation and making the solution colloidally stable. For all studied systems, no signs of sediment and changes in UV-Vis absorption spectra were noted within the period of the experiment, suggesting the stability of the investigated mixtures.
Fig. 1 shows the SEM data for the studied samples, which provides additional confirmation of their polydispersity.
Fig. 1.
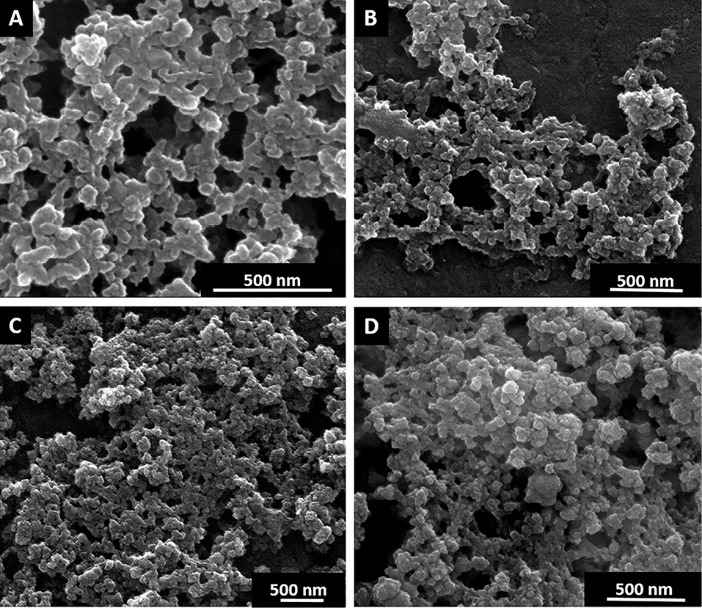
Scanning electron microscopy images of Dox (A), SWCNT+Dox (B), GO+Dox (C) and Dextran-PNIPAM+Dox (D).
Structural analysis of the GO+Dox nanofluid was performed by means of a molecular modeling approach. It is known that both, GO and SWCNT, bind small aromatic molecules by formation of π-stacks with them in solution.6,20,21 The structure of the SWCNT+Dox complex is already known6 and was not investigated in this work. Hence, further analysis was focused on GO+Dox system.
It was initially assumed that the GO+Dox nanofluid contain GO+Dox complexes, which were studied by theoretical calculation of their spatial structures minimized by energy. Fig. 2B contains the resultant structure of 1:1 complex. As a consequence of the structure optimization, the initially flat sheet of GO has become bent accepting the shape of groove with the bending line passing over the central row of the carbon cycles (see dashed line in Fig. 2A). Such bending of GO sheets is well known (e.g.).22 The optimized structure features the average distance between the carbon atoms of the Dox chromophore equal to 4.3 Å. The longitudinal axis of the Dox chromophore has shifted and located above the neighboring row of the carbon cycles (shown in Fig. 2A by the solid line). In general, as a consequence of energy minimization the stacking of GO and Dox molecules within the 1:1 complex has been preserved, although the resultant distance has appeared to be relatively high (~4.5 Å).23 In addition, the obtained geometry of the studied complex allows the formation of 4 intermolecular hydrogen bonds between the GO and Dox molecules (see Fig. 2B), which, obviously, make a significant contribution to its stabilization. The possibility of intermolecular H-bonding within the π-stacked complexes of aromatic molecules including Dox is well known,24 and therefore, may be expected in GO+Dox nanofluid as well.
Fig. 2.
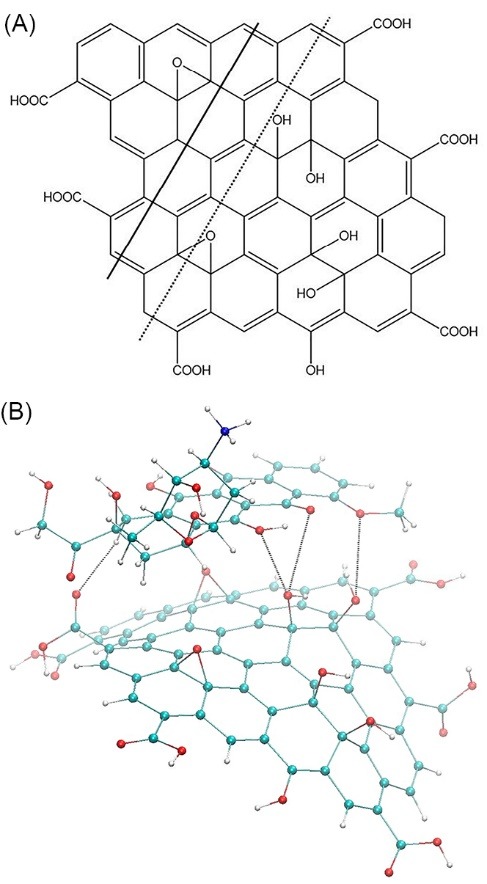
The structure of GO molecule (A) and the minimized structure of GО+Dox complex (B)
In vitro experiment
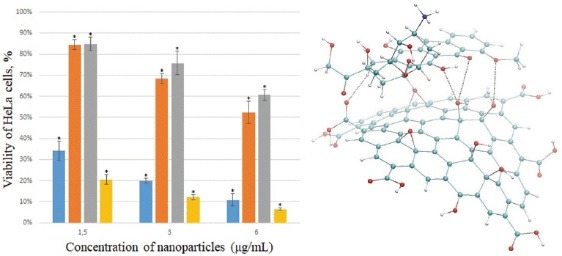
As can be seen from the Fig. 3A, Dox already kills about 65% of cells for 24 h at the lowest used concentration of 1.5 mg mL-1, and Dextran-PNIPAM+Dox almost 80% at the statistically significant level of difference. The effectiveness of these therapeutic drugs only increases with increasing concentration (Fig. 3A, B). As one can see, the Dextran-PNIPAM+Dox nanofluid shows a much higher toxicity towards HeLa cells in comparing with Dox alone.
Fig. 3.
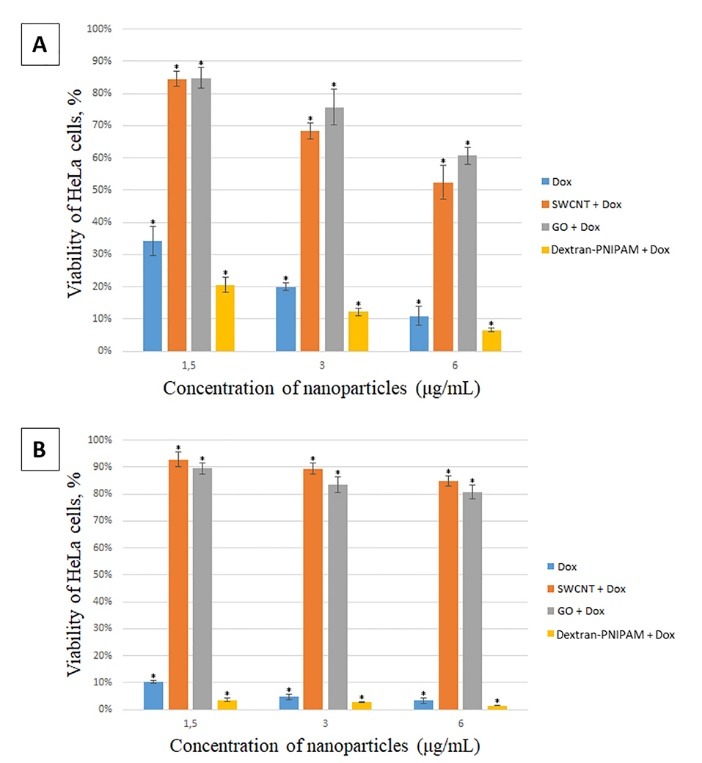
MTT assay on HeLa cells after incubation with studied NPs at different concentrations for 24 hours (A) and 48 hours (B). *significant differences compared to the control (untreated cells) at P < 0.05.
At the same time, the SWCNT+Dox and GO+Dox nanofluids at the concentrations used are of low toxicity to cells, especially at the lowest concentration 1.5 mg mL-1 (Fig. 3A, B), and also with increasing time of incubation time with the cells (Fig. 3B). Thus, it can be stated that the SWCNT and GO NPs protect cells from Dox. This observation can be explained using the following rationale. The results of the structural analysis performed above, as well as the literature data (e.g.6,20,21), suggest the complex formation of the SWCNT and GO NPs with Dox. We assume that this effect reduces the concentration of free Dox molecules able to induce cell death, thus leading to cell protection from the antibiotic action when Dox is administered together with NPs, as compared with the Dox action as a single agent. The mechanism described is well known in literature with respect to small molecules, called as ‘interceptors’ or ‘scavengers’, which can bind with Dox via π-stacking (see24,25 for details), and has recently been revealed for Dox binding with another carbon nanoparticle, i.e. C60 fullerene.7,8 Most likely, the molecular complexations act here as the universal physicochemical factor, which is independent of the type of biosystem under study.
Finally, the Dox, Dextran-PNIPAM+Dox, SWCNT+Dox and GO+Dox NPs demonstrated even more pronounced treatment and protective effects (about 80-90% cells in both cases), respectively, when incubated with the cells for 48 hours (Fig. 3B).
It should be noted that SWCNT, GO and Dextran-PNIPAM NPs are non-toxic in vitro and in vivo in the concentration range under study (up to 6 mg mL-1).26-28
It is known that Dox-induced toxicity and resistance are major obstacles in chemotherapeutic approaches. The mechanisms of toxicity and resistance are respectively related to induction of reactive oxygen species and up-regulation of ATP-binding cassette transporter.29 Thus, one can assume that coadministration of SWCNT and GO NPs with a high Dox dose is capable of ameliorating its cytotoxicity through their antioxidant potential.15,26,28,30,31
It is important to emphasize that the Dextran-PNIPAM copolymer is thermosensitive with a transition temperature from the hydrophilic to the hydrophobic state of the macromolecule in the region of physiological temperatures.12 Therefore, one can assume that the anticancer activity of Dextran-PNIPAM+Dox nanofluid sharply increases due to a change in the conformation of the Dextran-PNIPAM copolymer, which promotes the release of Dox molecules. Thus, the novel Dextran-PNIPAM copolymer can be used as a potential carrier of anticancer drugs (for example, Dox) as opposed to SWCNT and GO NPs. As shown above, both GO and SWCNT act as interceptors of Dox molecules due to the formation of GO+Dox and SWCNT+Dox complexes, thus reducing the concentration of monomeric Dox molecules able to induce cell death.
The in vitro uptake of NPs by HeLa cells was studied by CLSM. For this purpose, we used Dox as a fluorescing marker (~500 nm; Dox gives a red fluorescence).31,32 Fig. 4 shows the cellular uptake and the distribution of SWCNT+Dox (A) and GO+Dox (B) after 48 h of incubation on HeLa cells. NPs were thoroughly washed in all these cases to remove adhering or dispersed NPs. Fig. 5 demonstrates the z-stack through the cells, which were carried out to prove the accumulation of GO+Dox inside the cells and not on their surface. It is important to note that in the case of Dextran-PNIPAM+Dox NPs, the results for CLSM almost are no different from those we obtained earlier for pure Dox at 3 hours of incubation with HeLa cells.33 This again confirms the fact that the water-soluble Dextran-PNIPAM copolymer acts as the Dox molecules transporter into cells, actively releasing them to the intracellular space at physiological temperature.12 Thus, one may conclude that the Dextran-PNIPAM+Dox NPs can be used for further pre-clinical trials.
Fig. 4.
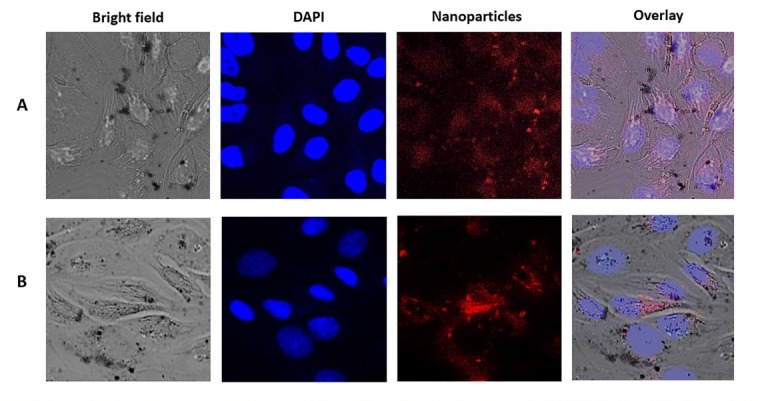
Laser scanning confocal microscopy images of HeLa cells after incubation with SWCNT+Dox (A, time of incubation 48 h; concentration 6 μg mL-1) and GO+Dox (B; 48 h; 6 μg mL-1) NPs.
Fig. 5.
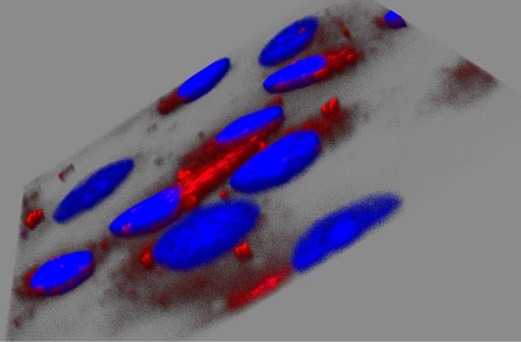
Analysis of z-stacks carried out by laser confocal scanning microscopy on HeLa cells after incubation with GO+Dox (B; 48 h; 6 μg mL-1) NPs. This information could prove, that NPs were able to go inside a cell.
Conclusion
This study clearly demonstrates that water-soluble Dextran-PNIPAM+Dox NPs sharply decreased the viability of HeLa cells at the low concentrations (1.5-6 µg mL-1) for 24-48 hours. This result indicates on the importance of Dextran-PNIPAM copolymer as a universal platform for drug delivery, and, in particular, the huge potential of Dextran-PNIPAM+Dox NPs as novel anticancer agents.
At the same time, the SWCNT+Dox and GO+Dox nanofluids are of low toxicity to HeLa cells at the same conditions, and hence, the SWCNTs and GO NPs can be effective cytoprotectors against the high toxic drugs. Moreover, the SWCNTs, as well as GO NPs, offer a great potential for upcoming nanomedicines nanoscaled devices, including biosensor and bioimaging techniques.
Finally, the uptake of the NPs into cancer cells was shown by CLSM.
Acknowledgments
TM is grateful to DAAD for financial support within the Leonhard-Euler program. NK is grateful to the Ukrainian State Fund for Fundamental Research for financial support (grant NF76/64-2017). We thank the Imaging Centre Campus Essen (ICCE) for access to the confocal laser scanning microscope. We thank Dr Kateryna Loza for recording the SEM images.
Funding sources
This work was, in part, supported by grant 5889.2018.3 to leading research groups (ME).
Ethical approval
None to be declared.
Competing interests
Authors declare no conflict of interest.
Authors contribution
The work presented here was carried out in collaboration between all the authors. SP, YH, NK and UR synthesized and characterized nanomaterials. VS and TM performed in vitro and microscopy studies. VK and ME performed the computer simulation. M. Epple and YP coordinated the experimental work, analyzed the data, performed the statistical analysis, and wrote the manuscript. All authors discussed the results and commented on the manuscript. All authors read and approved the final manuscript.
Research Highlights
What is the current knowledge?
√ Importance of Dextran-PNIPAM copolymer as a universal platform for drug delivery is established
What is new here?
√ Water-soluble Dextran-PNIPAM+Dox NPs sharply decrease the viability of HeLa cells at low concentration;
√ GO particles can be effective cytoprotectors against the high toxic drugs.
References
- 1.Kumari P, Ghosh B, Biswas S. Nanocarriers for cancer-targeted drug delivery. J Drug Target. 2016;24(3):179–91. doi: 10.3109/1061186X.2015.1051049. [DOI] [PubMed] [Google Scholar]
- 2.Bhattarai P, Hameed S Hameed S, Dai Z. Recent advances in anti-angiogenic nanomedicines for cancer therapy. Nanoscale. 2018;10(12):5393–423. doi: 10.1039/c7nr09612g. [DOI] [PubMed] [Google Scholar]
- 3.Kunjachan S, Rychlik B, Storm G, Kiessling F, Lammers T. Multidrug resistance: physiological principles and nanomedical solutions. Adv Drug Deliv Rev. 2013;65(13-14):1852–65. doi: 10.1016/j.addr.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayers D, Nasti A. Utilisation of nanoparticle technology in cancer chemoresistance. J Drug Deliv. 2012;2012:265691. doi: 10.1155/2012/265691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao J, Feng SS, Guo YJ. Nanomedicine against multidrug resistance in cancer treatment. Nanomedicine. 2012;7(4):465–8. doi: 10.2217/nnm.12.11. [DOI] [PubMed] [Google Scholar]
- 6.Buchelnikov AS, Voronin DP, Kostjukov VV, Deryabina TA, Khrapatiy SV, Prylutskyy. et al. Complexation of aromatic drugs with single-walled carbon nanotubes. J Nanopart Res. 2014;16:2472. doi: 10.1007/s11051-014-2472-5. [DOI] [Google Scholar]
- 7.Prylutskyy YuI, Evstigneev MP, Cherepanov VV, Kyzyma OA, Bulavin LA, Davidenko NA. et al. Structural organization of С60 fullerene, doxorubicin and their complex in physiological solution as promising antitumor agents . J Nanopart Res. 2015;17:45. doi: 10.1007/s11051-015-2867-y. [DOI] [Google Scholar]
- 8.Prylutska S, Skivka L, Didenko G, Prylutskyy Yu, Evstigneev M, Potebnya G. et al. Complex of C60 fullerene with doxorubicin as a promising agent in antitumor therapy . Nanoscale Res Lett. 2015;10(1):499. doi: 10.1186/s11671-015-1206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritter U, Scharff P, Dmytrenko OP, Kulish NP, Prylutskyy YuI, Belyi Belyi. et al. Radiation damage and Raman vibrational modes of single-walled carbon nanotubes. Chem Phys Lett. 2007;447:252–6. doi: 10.1016/j.cplett.2007.09.010. [DOI] [Google Scholar]
- 10.Prylutska S, Bilyy R, Shkandina T, Rotko D, Bychko A, Cherepanov V. et al. Сomparative study of membranotropic action of single- and multi-walled carbon nanotubes. J Biosci Bioeng. 2013;115(6):674–9. doi: 10.1016/j.jbiosc.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Kang Y, Liu J, Wu J, Yin Q, Liang H, Chen A. Graphene oxide and reduced graphene oxide induced neural pheochromocytoma-derived PC12 cell lines apoptosis and cell cycle alterations via the ERK signaling pathways. Int J Nanomed. 2017;12:5501–10. doi: 10.2147/IJN.S141032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chumachenko V, Kutsevol N, Harahuts Yu, Rawiso M, Marinin A, Bulavin L. Star-like dextran-graft-pnipam copolymers Effect of internal molecular structure on the phase transition. J Mol Liq. 2017;235:77–82. doi: 10.1016/j.molliq.2017.02.098. [DOI] [Google Scholar]
- 13.Halperin A, Kroger M, Winnik FM. Poly(N-isopropylacrylamide) phase diagrams: fifty years of research. Angew Chem Int Ed. 2015;54:1534215367. doi: 10.1002/anie.201506663. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Zhang X, Liu Z, MaY MaY, Huang Y, Chen Y. High-efficiency loading and controlled release of doxorubicin hydrochloride on graphene oxide. J Phys Chem C. 2008;112:17554–8. doi: 10.1021/jp806751k. [DOI] [Google Scholar]
- 15.Lyon DY, Adams LK, Falkner JC, Alvarez PJ. Antibacterial activity of fullerene water suspensions: Effects of preparation method and particle size. J Environ Sci Tech. 2006;40:4360–6. doi: 10.1021/es0603655. [DOI] [PubMed] [Google Scholar]
- 16.Prylutska SV, Grynyuk II, Grebinyk SM, Matyshevska OP, Prylutskyy YuI, Ritter U. et al. Comparative study of biological action of fullerenes C60 and carbon nanotubes in thymus cells . Mat-wiss u Werkstofftech. 2009;40:238–41. doi: 10.1002/mawe.200900433. [DOI] [Google Scholar]
- 17.Zhang B, Bian W, Pal A, He Y. Macrophage apoptosis induced by aqueous C60 aggregates changing the mitochondrial membrane potential . Environ Toxicol Pharmacol. 2015;39:237–46. doi: 10.1016/j.etap.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Bai RG, Ninan N, Muthoosamy K, Manickam S. Graphene: a versatile platform for nanotheranostics and tissue engineering. Progress Mater Sci. 2018;91:24–69. doi: 10.1016/j.pmatsci.2017.08.004. [DOI] [Google Scholar]
- 19.Dryn DO. Dryn DOMelnyk MI, Kury LAl, Prylutskyy YuI, Ritter U, Zholos AVС60 fullerenes disrupt cellular signalling leading to TRPC4 and TRPC6 channels opening by the activation of muscarinic receptors and G-proteins in small intestinal smooth muscles . Cell Signal. 2018;43:40–6. doi: 10.1016/j.cellsig.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 20.McCallion C, Burthem J, Rees-Unwin K, Golovanov A, Pluen A. Graphene in therapeutics delivery: Problems, solutions and future opportunities. Eur J Pharm Biopharm. 2016;104:235–50. doi: 10.1016/j.ejpb.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Robinson JT, Tabakman SM, Yang K, Dai H. Carbon materials for drug delivery and cancer therapy. Mater Today. 2011;14:316–23. doi: 10.1016/S1369-7021(11)70161-4. [DOI] [Google Scholar]
- 22.Alarcуn LM, Malaspina DC, Schulz EP, Frechero MA, Appignanesi GA. Structure and orientation of water molecules at model hydrophobic surfaces with curvature: From graphene sheets to carbon nanotubes and fullerenes. Chem Phys. 2011;388:47–56. doi: 10.1016/j.chemphys.2011.07.019. [DOI] [Google Scholar]
- 23.McGaughey GB, Gagne M, Rappe AK. π-Stacking Interactions Alive and Well in Proteins. J Biol Chem. 1998;273(25):15458–63. doi: 10.1074/jbc.273.25.15458. [DOI] [PubMed] [Google Scholar]
- 24.Evstigneev MP. Hetero-association of aromatic molecules in aqueous solution. Int Rev Phys Chem. 2014;33:229–273. doi: 10.1080/0144235X.2014.926151. [DOI] [Google Scholar]
- 25.Woziwodzka A, Golunski G, Wyrzykowski D, Kazmierkiewicz R, Piosik J. Caffeine and other methyxanthines as interceptors of food-borne aromatic mutagens: inhibition of Trp-P-1 and Trp-P-2 mutagenic activity. Chem Res Toxicol. 2013;26(11):1660–73. doi: 10.1021/tx4002513. [DOI] [PubMed] [Google Scholar]
- 26.Shapoval LM, Prylutska SV, Kotsyuruba AV, Dmitrenko OV, Prylutskyy YuI, Sagach VF. et al. Single-walled carbon nanotubes modulate cardiovascular control in rats. Mat-wiss u Werkstofftech. 2016;47:208–15. doi: 10.1002/mawe.201600484. [DOI] [Google Scholar]
- 27.Volkov Y, McIntyre J, Prina-Mello A. Graphene toxicity as a double-edged sword of risks and exploitable opportunities: A critical analysis of the most recent trends and developments. 2D Mater. 2017;4:022001. doi: 10.1088/2053-1583/aa5476. [DOI] [Google Scholar]
- 28.Telegeev G, Kutsevol N, Chumachenko V, Naumenko A, Telegeeva P, Filipchenko S. et al. Dextran-Polyacrylamide as matrices for creation of anticancer nanocomposite. Int J Polymer Sci. 2017:4929857. doi: 10.1155/2017/4929857. [DOI] [Google Scholar]
- 29.Mohajeri M, Sahebka A. Protective effects of curcumin against doxorubicin-induced toxicity and resistance: A review. Crit Rev Oncol Hematol. 2018;122:30–51. doi: 10.1016/j.critrevonc.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Amani H, Habibey R, Hajmiresmail SJ, Latifi S, Pazoki-Toroudi H, Akhavan O. Antioxidant nanomaterials in advanced diagnoses and treatments of ischemia reperfusion injuries. J Mater Chem B. 2017;5:9452–76. doi: 10.1039/C7TB01689A. [DOI] [PubMed] [Google Scholar]
- 31.Sin DP, Herrera CE, Singh B, Singh S, Singh RK, Kumar R. Graphene oxide: An efficient material and recent approach for biotechnological and biomedical applications. Mater Sci Eng C. 2018;86:173–97. doi: 10.1016/j.msec.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Prylutskyy YuI, Evstigneev MP, Pashkova IS, Wyrzykowski D, Woziwodzka A, Gołuński G. et al. Characterization of C60 fullerene complexation with antibiotic doxorubicin . Phys Chem Chem Phys. 2014;16:23164–72. doi: 10.1039/c4cp03367a. [DOI] [PubMed] [Google Scholar]
- 33.Prylutskyy Y, Bychko A, Sokolova V, Prylutska S, Evstigneev M, Rybalchenko V. et al. Interaction of C60 fullerene complexed to doxorubicin with model bilipid membranes and its uptake by HeLa cells . Mater Sci Eng C. 2016;59:398–403. doi: 10.1016/j.msec.2015.10.049. [DOI] [PubMed] [Google Scholar]


