Abstract
Brain microinfarcts are common in aging and are associated with cognitive impairment. Anterior and posterior watershed border zones lie at the territories of the anterior, middle, and posterior cerebral arteries, and are more vulnerable to hypoperfusion than brain regions outside the watershed regions. However, little is known about microinfarcts in these regions and how they relate to cognition in aging. Participants from the Rush Memory and Aging Project (MAP), a community-based clinical-pathologic study of aging, underwent detailed annual cognitive evaluations. We examined 356 consecutive autopsy cases (mean age-at-death, 91 years (SD=6.16); 28% men) for microinfarcts from 3 watershed brain regions (2 anterior and 1 posterior) and 8 brain regions outside the watershed regions. Linear regression models were used to examine the association of cortical watershed microinfarcts with cognition, including global cognition and 5 cognitive domains. Microinfarcts in any region were present in 133 (37%) participants, of which 50 had microinfarcts in watershed regions. Persons with multiple microinfarcts in cortical watershed regions had lower global cognition (estimate=−0.56, SE=0.26, p=0.03) and lower cognitive function in the specific domains of working memory (estimate=−0.58, SE=0.27, p=0.03) and visuospatial abilities (estimate=−0.57, SE=0.27, p=0.03), even after controlling for microinfarcts in other brain regions, demographics, and age-related pathologies. Neither the presence nor multiplicity of microinfarcts in brain regions outside the cortical watershed regions were related to global cognition or any of the 5 cognitive domains. These findings suggest that multiple microinfarcts in watershed regions contribute to age-related cognitive impairment.
Keywords: Brain Watershed Regions, Microinfarcts, Vascular Pathology, Cognition
Introduction
Microinfarcts are focal areas of necrosis of ischemic origin that are not observed on gross examination, and are commonly found in the aging brain (Smith et al., 2012). While advances in neuroimaging modalities contribute to the detection of microinfarcts (van Veluw et al., 2015a, van Veluw et al., 2015b), the full spectrum, especially the smallest of microinfarcts, is most accurately identified by neuropathological examination. It is becoming increasingly recognized that microinfarcts, above and beyond, macroscopic infarcts, are important contributors to neurologic dysfunction, including cognitive impairment. Multiple large community-based studies show a higher frequency of microinfarcts in persons with dementia, with their presence being a relatively independently associated with dementia or cognitive impairment (Brundel et al., 2012, Kovari et al., 2004, Sonnen et al., 2007, Troncoso et al., 2008, White et al., 2002), and previous studies by our group and others, indicate persons with multiple microinfarcts have the most cognitive impairment(Arvanitakis et al., 2011, Sonnen et al., 2007). However, despite these latter studies which took account of common neuropathologies of aging and dementia, the clinical impact of microinfarcts, especially in the context of Alzheimer’s disease (AD) pathology, has also been challenged (Lee et al., 2000).
Precise mechanisms for microinfarct pathogenesis and their contribution to cognitive dysfunction are still largely unclear. Multiple underlying causes may contribute to microinfarct pathology including small vessel disease, hypoperfusion, and microemboli (van Veluw et al., 2017). In addition, some studies suggest the etiology of microinfarct pathology differs depending on the location of microinfarct burden (Arvanitakis et al., 2011, Kovari et al., 2004). Microinfarcts can be found in all brain regions, particularly in regions more vulnerable to hypoxic-ischemia. The cerebral hemisphere is supplied by the anterior, middle, and posterior cerebral arteries. The brain watershed regions are supplied by the distal arterial branches of 2 or more major arteries, and located the furthest from arterial supply, making them making more vulnerable to hypoxic-ischemic events than any other brain region (Miklossy, 2003, Suter et al., 2002). Based on their cortical location in the brain, watershed regions are often specified as either anterior watershed regions, located between the cortical territories of the anterior and middle cerebral arteries, or posterior watershed regions, located between the anterior-middle and posterior-middle cerebral arteries. In addition to cortical watershed regions, deep subcortical watershed regions have also been described, which overlie the territories of the deep arterial lenticulostriate and white matter perforators arising from the middle cerebral artery, and cerebellum watershed regions, bordered by the major cerebellar arteries (Lee et al., 2005, Mangla et al., 2011).
Due to their increased susceptibility to ischemia and hypoperfusion, watershed regions are interesting areas to investigate microvascular pathologies. In addition, older persons may be particularly vulnerable, because cerebral vessel disease pathology including atherosclerosis and arteriolosclerosis is common (Arvanitakis et al., 2017). There are limited data on the frequency of microvascular pathologies in watershed regions, and the role of watershed microinfarcts in cognitive impairment in older persons is yet to be explored. To address this gap in our knowledge, we collected data from 356 Rush Memory and Aging Project (MAP) participants to document the presence of microinfarcts (single vs. multiple) in cortical (anterior and posterior) watershed regions, and the association with cognitive impairment. Controlling for demographics and neuropathological factors, we investigated whether there was an independent association of cortical watershed microinfarcts, above and beyond the presence of microinfarcts in other brain regions, with global cognition and five different cognitive domains proximate to death.
Methods
Study Design
Brain specimens were obtained from a consecutive subset of deceased and autopsied participants of the Rush MAP, effective from January 1, 2013, as this was when watershed regions were added to the MAP study for neuropathological evaluation. Rush MAP is an ongoing epidemiologic clinical-pathological cohort study of aging, which began in 1997, and approved by the Institutional Review Board of the Rush University Medical Center (Chicago, IL). We used demographic, neuropathological, and clinical data. Participants are enrolled without known dementia, give informed consent to the study, and agree to brain donation at the time of death. Follow-up and autopsy rates exceed 80%.
Clinical Data
Structured and uniform baseline and clinical assessments were conducted annually, and included a medical history, neuropsychological testing, and a physical examination with a focus on the neurologic examination. A standard battery of neuropsychological tests was administered at baseline and each follow-up evaluation. All neuropsychological data were reviewed by clinicians blinded to previously data collected. In addition to the Mini-Mental State Examination, which was used for clinical descriptive purposes only, an additional 17 individual cognition tests were administered to create a composite summary indices for global cognition and 5 cognitive domains. Seven tests of episodic memory, 3 of semantic memory, 3 of working memory, 2 of perceptual speed, and 2 of visuospatial ability, were administrated as previously described (Bennett et al., 2006, Wilson et al., 2002). Raw individual of all test scores were converted to z scores and averaged to obtain a measure of global cognitive function score. Summary scores for each cognitive domain were derived by averaging the z scores of the neuropsychological tests specific to that particular cognitive domain.
Neuropathological Data
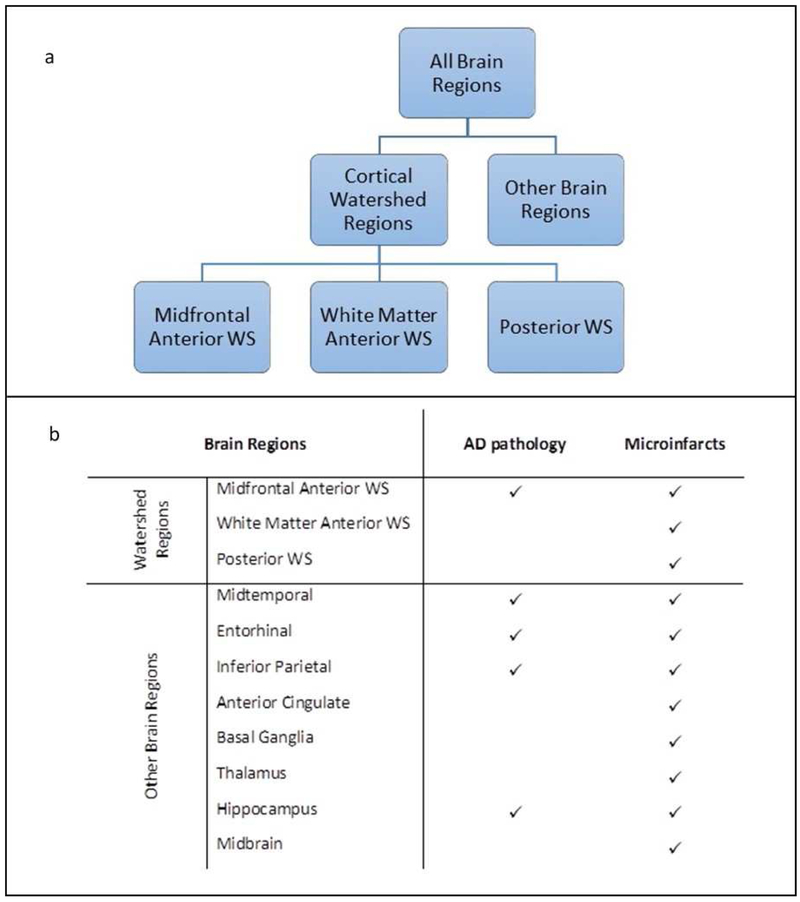
Brain autopsies were the performed at Rush University Medical Center (Bennett et al., 2012, Bennett et al., 2013) with an average post-mortem interval of 8.4 hours (SD=5.67). All neuropathologic data were collected at the Rush Alzheimer’s Disease Center laboratory. Following an external examination, one hemisphere was fixed for at least 48-72hrs in 4% paraformaldehyde in 0.1 M phosphate buffer. Paraformaldehyde-fixed cerebral and cerebellar hemispheres were cut into 1 cm coronal slabs and a standard set of 11 regions were blocked, including 3 cortical watershed regions (2 from the anterior and 1 from the posterior watershed areas), 4 cortical regions outside the watershed regions (middle temporal, entorhinal, inferior parietal, and anterior cingulate cortices), 2 subcortical regions (basal ganglia and thalamus), hippocampus, and midbrain. In addition, blocks were taken for any macroscopic infarcts observed during gross examination. All blocks were dehydrated, embedded in paraffin wax and sections (6μm) stained with hematoxylin-eosin were assessed for microscopic pathologies (AD pathology and microscopic infarcts) (Figure 1b).
Figure 1.
Summary of brain regions taken (a) for neuropathologic evaluation (b). Abbreviations: AD – Alzheimer’s disease; WS – watershed
Anatomic location of cortical watershed regions
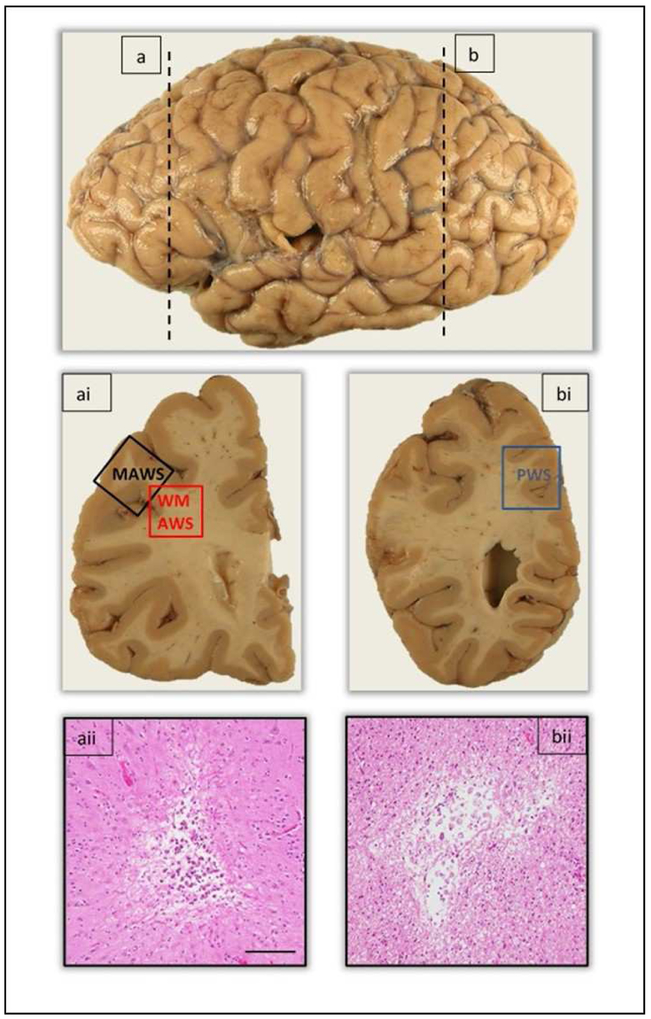
The focus of this study was to investigate microvascular pathologies in the cortical watershed regions, bordered by the large cerebral arteries. The anatomic location of the anterior watershed is located between the border zones of the anterior and middle cerebral arterial territories. For the purpose of this study, two anterior watershed regions were taken; the midfrontal gyrus (Brodmann area 9/46) and anterior white matter taken deep to the midfrontal cortex. We specify the two regions as midfrontal anterior watershed and white matter anterior watershed. The posterior watershed lies within the border zone of the middle-posterior and middle-anterior cerebral arterial territories. The posterior watershed region was taken medial to the posterior parietal cortex (Brodmann area 7), and included the parieto-occipital cortex with underlying white matter (Figure 2).
Figure 2.
Representation of the anatomic location of anterior and posterior watershed regions taken for evaluation of microscopic infarcts. The vertical dashed line (a) demarcates the level at which the anterior watershed regions, midfrontal and white matter anterior watershed regions were taken in the coronal brain slab (ai). Image aii shows the presence of a chronic microscopic infarct in the cortex of the midfrontal watershed region. The vertical dashed line (b) demarcates the level at which the posterior watershed region was taken in the coronal brain slab (bi). Image bii shows the presence of a chronic microscopic infarct in the white matter of the posterior watershed region. Abbreviations: MAWS - Midfrontal anterior watershed; WMAWS – White matter anterior watershed; PWS – Posterior watershed. Scale bar 100μm.
Post-mortem assessment of Alzheimer’s disease pathology
AD pathology was assessed in the midfrontal watershed, middle temporal, entorhinal, and inferior parietal cortices, and in the hippocampus. Paraffin-embedded blocks were sectioned into 6μm sections and stained with a modified Bielschowski stain. Manual counts of neuritic and diffuse plaques, and neurofibrillary tangles from all 5 regions were averaged across regions and divided by the SD to create a summary measure of the AD pathology score across the 5 regions in each case, as previously described (Schneider et al., 2004).
Post-mortem assessment of macroscopic and microscopic infarcts
Location, age, and size of macroscopic infarcts visible on gross examination were documented. Subsequently, the age of infarct was confirmed by microscopy and documented as acute, subacute, or chronic. Paraffin-embedded blocks for the standard set of 11 regions were sectioned into 6μm sections, stained with hematoxylin and eosin, and assessed for microinfarcts. Age (acute, subacute, and chronic) and location for all microinfarcts was documented as previously described (Arvanitakis et al., 2011). Because cognitive function is assessed on average about 1.1 (SD=1.41) years prior to death, only chronic microinfarcts were included in analyses. Acute and subacute infarcts, which by definition occur approximately 6 months or less before death were excluded.
Statistical Analyses
Six variables were created to indicate the presence of microinfarcts in the following locations:1) all brain regions including 3 cortical watershed and 8 regions outside watershed, 2) total cortical watershed (including three watershed regions), 3) all 8 brain regions outside the cortical watershed areas, 4) midfrontal watershed, 5) white matter anterior watershed, and 6) posterior watershed (Figure 1a). For each of these regions, we created a dichotomous variable indicating presence of any microinfarct vs. no microinfarcts present, as well as a 3-level variable indicating none, single, and multiple microinfarcts for variables 1, 2, and 3.
Initial bivariate associations of microinfarcts within and outside the cortical watershed regions with demographic, clinical, and neuropathology variables were examined with a Spearman correlations, chi-square, and t-tests. Linear regression models were used to investigate whether watershed microinfarcts were associated with level of global cognition and five cognitive domains, at the last available cognitive assessment proximate-to-death, after adjusting for microinfarcts in other brain regions, demographics (age, sex, education), and other pathologies (AD pathology and macroscopic infarcts). For total microinfarcts in any region, and then for microinfarcts split into watershed versus all other regions, we first ran models with the dichotomous (any vs none) variable, and then ran similar analyses with the 3-level variable including presence of none, single, and multiple microinfarcts. All analyses were programmed with SAS/STAT software version 9.4 (SAS Institute Inc, Cary, NC) using a Hewlett Packard server with a Linux operating system.
Results
Table 1 shows characteristics of study participants. Vascular lesions were frequent, with 125 (35%) participants having macroscopic infarcts, 133 (37%) participants having microinfarcts, including 51 (14%) participants having both macro- and microinfarcts. Of the 125 participants with macroscopic infarcts, 56 had cortical infarcts, 97 subcortical, and 23 had infarcts in brainstem and/or cerebellum. Twenty-seven (8%) participants had both macroinfarcts and cortical watershed microinfarcts, compared to 46 (13%) who had both macroscopic and microinfarcts in brain regions outside the watershed.
Table 1.
Demographic, clinical, and pathological characteristics of participants (N=356)
| Characteristics | Mean (SD) or N (%) |
|---|---|
| Clinical | |
| Age at death, years | 90.5 (6.16) |
| Age 90+ | 202 (56.7%) |
| Sex, men | 99 (27.8%) |
| Education, years | 14.6 (3.09) |
| Last MMSE score proximate to death | 20.5 (9.33) |
| Last clinical evaluation, years | 1.1 (1.41) |
| Cognition, proximate-to-death | |
| Global | −0.9 (1.15) |
| Episodic Memory | −0.9 (1.27) |
| Semantic Memory | −0.8 (1.34) |
| Working Memory | −0.8 (1.14) |
| Perceptual Speed | −1.05 (1.02) |
| Perceptual Orientation | −0.5 (1.10) |
| Neuropathologic | |
| PMI, hours | 8.4 (5.67) |
| AD pathology score | 0.8 (0.62) |
| Macroinfarcts presence | 125 (35.1%) |
| Cortical, n | 56 |
| Subcortical, n | 97 |
| Brainstem/cerebellum, n | 23 |
| Microinfarcts | 133 (37.4%) |
| Cortical WS | 50 (14.0%) |
| Location | |
| Midfrontal anterior WS, n | 18 |
| White matter anterior WS, n | 15 |
| Posterior WS, n | 24 |
| Quantity | |
| Single, n | 33 |
| Multiple, n | 17 |
| Other brain regions | 105 (29.5%) |
| Quantity | |
| Single, n | 71 |
| Multiple, n | 34 |
Abbreviations: PMI – post-mortem interval; WS – watershed; MMSE – Mini-Mental-State-Examination
Of the 133 participants with microinfarcts, 50 had microinfarcts located in cortical watershed regions and 97 in brain regions outside the watershed areas. These were not mutually exclusive. Over half (28/50 or 56%) had microinfarcts in only watershed regions, whereas 22 (44%) had microinfarcts in both watershed and other brain regions. Out of 50 participants with watershed microinfarcts, 18 had microinfarcts in the midfrontal anterior watershed, 15 in the white matter anterior watershed and 24 in the posterior watershed (Table 1).
Cortical watershed microinfarcts were positively associated with age-at death (p=0.04), while microinfarcts in brain regions outside the watershed regions were not (p=0.10). Microinfarcts in watershed regions were not associated with sex (p=0.32) or education (p=0.42). Association of microinfarcts in brain regions outside the watershed with sex did not reach standard threshold for clinical significance (p=0.06), and were not associated with education (p=0.83).
Cortical Watershed Microinfarcts and Cognition
We examined the relationship of microinfarcts with cognition using linear regression models adjusting for demographics (age, sex, and education), AD pathology score, and macroscopic infarcts. We classified microinfarcts by location (cortical watershed regions vs brain regions outside the watershed areas) to investigate if there is a differential relationship with cognition for microinfarcts in watershed regions. Presence of microinfarcts in any region was not associated with global cognition, although a trend for association with cortical watershed microinfarcts was noted (Table 2, Models 1 and 3).
Table 2.
Relation of watershed microinfarcts with global cognition
| Region | Model 1 | Model 2 | ||
|---|---|---|---|---|
| All Brain Regions | Any | −0.36 (0.12, 0.76) |
Single | 0.14 (0.13, 0.30) |
| Multiple |
−0.33 (0.16, 0.04*) |
|||
| Model 3 | Model 4 | |||
| Cortical Watershed | Any | −0.28 (0.16, 0.07) |
Single | −0.77 (0.19, 0.69) |
| Multiple |
−0.56 (0.26, 0.03*) |
|||
| Other Brain Regions | Any | 0.02 (0.12, 0.89) |
Single | 0.16 (0.14, 0.26) |
| Multiple | −0.22 (0.19, 0.25) |
|||
Values in cells are estimated coefficients (SE, p-value). Values based on linear regression models controlling for age, sex, education, AD pathology summary score, and macroscopic infarcts. Models 1 and 3 used dichotomous (none vs. any) variables, and models 2 and 4 used trichotomous (none, single, and multiple) variables for microinfarct measures. Significant (p<0.05) associations indicated with an asterisk*
We and others have previously shown that multiple microinfarcts have a stronger association with cognition then having a single microinfarct (Arvanitakis et al., 2011, Sonnen et al., 2007). Therefore, we ran similar analyses after categorizing the number of microinfarcts observed as none, single, and multiple. Compared to persons with no microinfarcts, persons with multiple microinfarcts in any brain region had lower cognition (Table 2, Model 2). When examining multiple microinfarcts by region, multiple microinfarcts in cortical watershed regions were associated with lower global cognition (estimate = −0.54; SE = 0.26, p-value = 0.04), while microinfarcts in other regions were not (estimate = 0.12; SE = 0.14, p-value = 0.40 for single microinfarct; estimate = −0.26; SE = 0.19, p-value = 0.16 for multiple microinfarcts). Finally, in a model including single and multiple microinfarcts from both locations, only multiple microinfarcts in cortical watershed regions were associated with lower cognition (Table 2, Model 4).
Cognitive function is complex and comprised of multiple related systems which may be related differentially with pathology. We therefore constructed linear regression models to examine associations of single and multiple microinfarcts in cortical watershed and in other brain regions with 5 different cognitive domains. In our analyses, multiple watershed microinfarcts were significantly associated with lower cognitive function for working memory and visuospatial ability (Table 3). Association with semantic memory did not reach the threshold for clinical significance, and no associations were observed with episodic memory and perceptual speed. Neither the presence of single nor multiple microinfarcts in other brain regions were significantly related to any of the cognitive domains.
Table 3.
Relation of watershed microinfarcts with cognitive domains
| Brain Regions |
Microinfarct Measure |
Episodic Memory |
Semantic Memory |
Working Memory |
Visuospatial Ability |
Perceptual Speed |
|---|---|---|---|---|---|---|
| Cortical Watershed | Single | −0.11 (0.21, 0.60) |
−0.22 (0.23, 0.44) |
−0.06 (0.20, 0.78) |
−0.15 (0.20, 0.46) |
−0.24 (0.18, 0.20) |
| Multiple | −0.35 (0.29, 0.22) |
−0.50 (0.31, 0.10) |
−0.58 (0.27, 0.03*) |
−0.57 (0.27, 0.03*) |
−0.08 (0.24, 0.75) |
|
| Other Brain Regions | Single | 0.15 (0.15, 0.32) |
0.06 (0.17, 0.72) |
0.24 (0.14, 0.10) |
0.15 (0.14, 0.29) |
0.08 (0.13, 0.52) |
| Multiple | −0.19 (0.21, 0.37) |
−0.17 (0.23, 0.46) |
−0.24 (0.20, 0.22) |
0.13 (0.20, 0.50) |
−0.15 (0.18, 0.41) |
Linear regression models with terms for age, sex, education, AD pathology summary score, and macroscopic infarcts. All models used trichotomous variables for microinfarct measures (none, single, and multiple). Values in cells are estimated coefficients (SE, p-value). Significant (p<0.05) associations indicated with an asterisk*
Discussion
In this autopsy study of 356 community-dwelling older persons, microinfarcts were present in nearly forty percent of subjects, of whom over a third had microinfarcts in cortical watershed regions. Subsequent analyses showed that the presence of multiple watershed microinfarcts were associated with lower cognitive function globally and in the domains of working memory and visuospatial ability, even after adjusting for microinfarcts in other brain regions. Further, we did not find that microinfarcts in other brain regions were associated with cognition. Associations of multiple watershed microinfarcts with cognition were observed after adjusting for demographics, Alzheimer’s pathology, and macroinfarcts, suggesting these watershed microinfarcts have an important independent association with cognition in aging.
A previous study investigated the involvement of cortical watershed microinfarcts with dementia (Suter et al., 2002). Investigators showed a significant association between watershed microinfarcts and AD dementia; however, the association was not independent of demographics or other age-related neuropathologies. We and others have found significant associations between cortical microinfarcts with cognition in large community-based cohorts (Arvanitakis et al., 2011, Ince et al., 2016, Launer et al., 2011, Troncoso et al., 2008); however information regarding the involvement of watershed microinfarcts with cognitive function is lacking, and to our knowledge the relationship between watershed microinfarcts with cognition or cognitive domains has not been explored. Though the numbers are still relatively small, this current study provides support for the hypothesis that watershed microinfarcts are important contributors to cognitive impairment in aging and that their effect may even be stronger than microinfarcts in other brain regions. This suggests watershed microinfarcts may be partly driving previously observed associations between microinfarcts and cognition. Indeed, it may also be that watershed microinfarcts serve as the proximate and the most apparent marker of the adverse impact of global cerebral ischemia on neuronal and synapse function.
We are extending our previous findings by adding watershed regions to other commonly investigated regions for microinfarcts in aging. Previous examination of microinfarcts in Rush MAP did not specifically evaluate white matter anterior or posterior watershed regions. By including these regions in our protocol, overall frequency of microinfarcts increased to 37%, a more complete representation of microinfarct burden than our previous studies (24% in Rush MAP (Schneider et al., 2007) and 30% in the Religious Orders Study (ROS) (Arvanitakis et al., 2011)). Our estimate is also slightly higher than other population-based cohorts including Baltimore Longitudinal Study of Aging (BLSA) (22%) (Troncoso et al., 2008) and similar to the MRC Cognitive Function and Aging Study (MRC-CFAS) (36%) (Ince et al., 2016). However, the Honolulu Asian Aging Study (HAAS) documented a much higher frequency of microinfarcts (64%) (Launer et al., 2011), which may be attributed to the study being based on Japanese- American men, who have been shown to have higher rates of vascular disease. While sampling more regions may drive up the number of participants recognized to have microinfarcts, variation in estimated proportions in cases with microinfarcts is unlikely to be entirely determined by sampling strategy, as one study documented a frequency of 19% when 38 regions were sampled (White et al., 2002) and another study documented 30% when 9 regions were sampled (Arvanitakis et al., 2011). In the current study microinfarcts are common in watershed regions, particularly favoring the posterior watershed region, an expected observation, given the posterior watershed region overlies the edges of all three cerebral arterial territories, predisposing this area to increased ischemic events. We suggest cortical watershed brain regions may prove to be important regions to sample when evaluating microvascular pathologies, given their prevalence and relation to cognition.
It is important to note that while both neuropathological examination and neuroimaging modalities can detect the presence of microinfarcts, the standards differ. Neuropathological examination can detect the smallest of lesions, from 100μm to a few mm, whereas advanced neuroimaging techniques can generally detect lesions greater than > 1mm in size (van Veluw et al., 2017). Use of both modalities have facilitated studies investigating the association of microinfarcts and neurologic function (Hilal et al., 2016, Wang et al., 2016). However, it remains unclear whether microinfarct subtypes detected by different modalities have the same underlying pathogenesis. We have previously showed that multiple microinfarcts across cortical and subcortical regions were associated with lower cognitive function, and lower function in several cognitive domains including perceptual speed and episodic and semantic memory (Arvanitakis et al., 2011). In this current study, presence of multiple microinfarcts in watershed regions lowered global cognitive function and was associated with lower levels of working memory and visuospatial ability. While working memory is often considered a frontal predominant function, we will need further numbers to determine whether the cognitive profile of executive impairment differs in cortical watershed vs. brain regions outside the watershed zones, and also within individual watershed regions. It is interesting to note that watershed microinfarcts were associated with visuospatial skills but not language in the present study. The lower performance in visuospatial abilities may be attributed to the disruption of frontal – parietal networks (Oh et al., 2012), an effect perhaps secondary to the inclusion of posterior watershed regions, as the posterior parietal cortex is presumed to be associated with visuospatial perception (Constantinidis et al., 2013). In extension to our previous studies, we were able to examine the effects of watershed microinfarcts vs. microinfarcts in other brain regions with cognition and in specific cognitive domains. Only microinfarcts in the watershed regions were significantly associated with cognitive decrements, suggesting there may be heterogeneity within microinfarct pathology spectrum. Indeed, the relatively small numbers in this study could also still remain a factor. Future studies investigating mechanistic differences underlying watershed microinfarcts, if any, are warranted. Indeed, neuroimaging efforts may serve well to further understand watershed microinfarct-associated changes.
The contribution of vascular disease to cognitive decline and dementia is complex and diverse. In addition, the high prevalence of vascular disease provides a compelling target for intervention, from a public health standpoint (Satizabal et al., 2016). There is an increasing need in the field of aging to accurately quantify total burden of microinfarcts in the brain to further understand the pathogenic mechanisms involved and their contribution to vascular disease and cognition. Indeed, there is an emerging literature associating microinfarcts with a number of cardiac diseases and vascular risk factors, including a clinical history of stroke (Ince et al., 2016), atrial fibrillation (Wang et al., 2016), ischemic heart disease, congestive heart failure (Hilal et al., 2017), hypertension (Ince et al., 2016), and blood pressure (Graff-Radford et al., 2017, Wang et al., 2009). Together, these may be important factors when considering risk factors for watershed microinfarct pathogenesis. In an episode of perturbed hemodynamic factors, watershed regions are most vulnerable to low cerebral perfusion (hypoperfusion) and most likely the first brain regions to be affected (Miners et al., 2015). Experimental animal model studies of ischemic injury provide compelling evidence linking chronic cerebral hypoperfusion, which may be mediated by accelerated Aβ deposition, with cognitive impairment (Duncombe et al., 2017, Okamoto et al., 2012). Alternatively, increased blood pressure or high arterial pulsations in older persons also have important ramifications for microvascular disease (Kim et al., 2017). Prospective studies investigating the relationship between watershed microvascular pathology with cerebral blood flow would be informative concerning mechanisms of vascular disease, in particular cerebral hypoperfusion.
It is plausible that an unrecognized large burden of microinfarcts (Westover et al., 2013) induces peri-lesional neural deficits which can lead to structural alterations (Summers et al., 2017), resulting in possible cognitive impairment. Further, there may also be diffuse changes in the brain associated with microinfarcts, such as, microbleeds (Lauer et al., 2016) and white matter demyelination/hyperintensities (Wang et al., 2016), disrupting cognitive networks. Vessel abnormalities, small- and large vessel disease, are both important contributors to vascular cognitive impairment and microinfarct burden. Where arteriosclerosis and atherosclerosis increases the odds for subcortical microinfarcts, significant cerebral amyloid angiopathy has been shown to increase the odds for cortical microinfarcts (Arvanitakis et al., 2017), suggesting etiology of microinfarct pathology may be different depending on their location in the brain; further supporting the idea of heterogeneity within the microinfarct pathology spectrum. Indeed, there may also be non-structural vascular abnormalities which cause vasoconstriction, consequently leading to hypoperfusion (Love and Miners, 2016). Taken together, many factors may cause microinfarcts, including manifestations of both small and large vessel disease. Future work to further examine vessel pathologies, vascular risk factors, and cardiac disease in relation to watershed border zones will further aid and refine the understanding of vascular contribution to cognitive impairment.
There are several limitations with this study. Microinfarct assessment was performed on 11 brain regions from one hemisphere, a relatively limited number of regions (Smith et al., 2012, Westover et al., 2013). However, including additional cortical watershed regions to our methodology provides a more accurate estimation of microinfarct burden. It is important to note that there is variability in the location of watershed border zones across individuals and therefore it may be difficult to identify the correct anatomic location of cortical watershed regions. Variability may include a shift due to the development of leptomeningeal collaterals, smaller arteries branching of the distal ends of the anterior, middle, and posterior cerebral arteries (Momjian-Mayor and Baron, 2005, Tariq and Khatri, 2008). Lastly, due to the small number of microinfarcts in the individual cortical watershed regions, we were unable to detect potential effects or to examine the relationship between individual watershed regions with global cognition and/or cognitive domains. Ongoing neuropathological assessments of these brain watershed regions will provide further power to address these questions.
This study also has notable strengths. Detailed systematic neuropathological assessment was performed blinded to clinical data. Participants underwent an annual detailed battery of neuropsychological tests, measures of global cognition and 5 different cognitive domains. MAP is a community-based cohort comprised of both men and women with and without dementia at autopsy and has a high clinical follow up and autopsy rates, and therefore is not subjected to selection bias. Finally, we are using new data from watershed regions, which have not been previously documented in this cohort.
Brain microinfarcts are common in older persons.
Microinfarcts are common in cortical watershed brain regions.
Cortical watershed microinfarcts are related to cognitive impairment.
Acknowledgements
We are thankful to all the participants enrolled in the Rush Memory and Aging Project. We also thank the Rush Alzheimer’s Disease Center staff, in particular Karen Skish for laboratory management, John Gibbons for data management, and Traci Colvin for study coordination of the cohort.
Funding sources: This study was funded by the following sources; P30AG010161, R01AG017917, R01AG047976, UH2NS100599, R01AG034374, R01NS084965, R01 AG040039, and K01AG050823.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: JAS: Consultant to Eli Lilly, AVID radiopharmaceuticals, Grifols, and Genetech.
References
- Arvanitakis Z, Capuano AW, Leurgans SE, Buchman AS, Bennett DA, Schneider JA, 2017. The Relationship of Cerebral Vessel Pathology to Brain Microinfarcts. Brain Pathol 27, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA, 2011. Microinfarct pathology, dementia, and cognitive systems. Stroke. 42, 722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS, 2006. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 66, 1837–1844. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS, 2012. Overview and findings from the rush Memory and Aging Project. Curr.Alzheimer Res 9, 646–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Wilson RS, Arvanitakis Z, Boyle PA, de Toledo-Morrell L, Schneider JA, 2013. Selected findings from the Religious Orders Study and Rush Memory and Aging Project. J.Alzheimers Dis 33 Suppl 1, S397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundel M, de Bresser J, van Dillen JJ, Kappelle LJ, Biessels GJ, 2012. Cerebral microinfarcts: a systematic review of neuropathological studies. J.Cereb.Blood Flow Metab 32, 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis C, Bucci DJ, Rugg MD, 2013. Cognitive functions of the posterior parietal cortex. Front.Integr.Neurosci 7, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncombe J, Kitamura A, Hase Y, Ihara M, Kalaria RN, Horsburgh K, 2017. Chronic cerebral hypoperfusion: a key mechanism leading to vascular cognitive impairment and dementia. Closing the translational gap between rodent models and human vascular cognitive impairment and dementia. Clin.Sci.(Lond). 131, 2451–2468. [DOI] [PubMed] [Google Scholar]
- Graff-Radford J, Raman MR, Rabinstein AA, Przybelski SA, Lesnick TG, Boeve BF, Murray ME, Dickson DW, Reichard RR, Parisi JE, Knopman DS, Petersen RC, Jack CR Jr, Kantarci K, 2017. Association Between Microinfarcts and Blood Pressure Trajectories. JAMA Neurol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilal S, Chai YL, van Veluw S, Shaik MA, Ikram MK, Venketasubramanian N, Richards AM, Biessels GJ, Chen C, 2017. Association Between Subclinical Cardiac Biomarkers and Clinically Manifest Cardiac Diseases With Cortical Cerebral Microinfarcts. JAMA Neurol 74, 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilal S, Sikking E, Shaik MA, Chan QL, van Veluw SJ, Vrooman H, Cheng CY, Sabanayagam C, Cheung CY, Wong TY, Venketasubramanian N, Biessels GJ, Chen C, Ikram MK, 2016. Cortical cerebral microinfarcts on 3T MRI: A novel marker of cerebrovascular disease. Neurology. 87, 1583–1590. [DOI] [PubMed] [Google Scholar]
- Ince PG, Minett T, Forster G, Brayne C, Wharton SB, Medical Research Council Cognitive Function and Ageing Neuropathology Study, 2016. Microinfarcts in an older population-representative brain donor cohort (MRC CFAS): Prevalence, relation to dementia and mobility, and implications for the evaluation of cerebral Small Vessel Disease. Neuropathol.Appl.Neurobiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MO, Li Y, Wei F, Wang J, O'Rourke MF, Adji A, Avolio AP, 2017. Normal cerebral vascular pulsations in humans: changes with age and implications for microvascular disease. J.Hypertens 35, 2245–2256. [DOI] [PubMed] [Google Scholar]
- Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Michel JP, Bouras C, Giannakopoulos P, 2004. Cortical microinfarcts and demyelination significantly affect cognition in brain aging. Stroke. 35, 410–414. [DOI] [PubMed] [Google Scholar]
- Lauer A, van Veluw SJ, William CM, Charidimou A, Roongpiboonsopit D, Vashkevich A, Ayres A, Martinez-Ramirez S, Gurol EM, Biessels GJ, Frosch M, Greenberg SM, Viswanathan A, 2016. Microbleeds on MRI are associated with microinfarcts on autopsy in cerebral amyloid angiopathy. Neurology. 87, 1488–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launer LJ, Hughes TM, White LR, 2011. Microinfarcts, brain atrophy, and cognitive function: the Honolulu Asia Aging Study Autopsy Study. Ann.Neurol 70, 774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Olichney JM, Hansen LA, Hofstetter CR, Thal LJ, 2000. Small concomitant vascular lesions do not influence rates of cognitive decline in patients with Alzheimer disease. Arch.Neurol 57, 1474–1479. [DOI] [PubMed] [Google Scholar]
- Lee PH, Oh SH, Bang OY, Joo IS, Huh K, 2005. Pathogenesis of deep white matter medullary infarcts: a diffusion weighted magnetic resonance imaging study. J.Neurol.Neurosurg.Psychiatry 76, 1659–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love S, Miners JS, 2016. Cerebrovascular disease in ageing and Alzheimer's disease. Acta Neuropathol 131, 645–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangla R, Kolar B, Almast J, Ekholm SE, 2011. Border zone infarcts: pathophysiologic and imaging characteristics. Radiographics. 31, 1201–1214. [DOI] [PubMed] [Google Scholar]
- Miklossy J, 2003. Cerebral hypoperfusion induces cortical watershed microinfarcts which may further aggravate cognitive decline in Alzheimer's disease. Neurol.Res 25, 605–610. [DOI] [PubMed] [Google Scholar]
- Miners JS, Palmer JC, Love S, 2015. Pathophysiology of hypoperfusion of the precuneus in early Alzheimer's disease. Brain Pathol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momjian-Mayor I, Baron JC, 2005. The pathophysiology of watershed infarction in internal carotid artery disease: review of cerebral perfusion studies. Stroke. 36, 567–577. [DOI] [PubMed] [Google Scholar]
- Oh H, Gentili RJ, Reggia JA, Contreras-Vidal JL, 2012. Modeling of visuospatial perspectives processing and modulation of the fronto-parietal network activity during action imitation. Conf.Proc.IEEE Eng.Med.Biol.Soc. 2012, 2551–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y, Yamamoto T, Kalaria RN, Senzaki H, Maki T, Hase Y, Kitamura A, Washida K, Yamada M, Ito H, Tomimoto H, Takahashi R, Ihara M, 2012. Cerebral hypoperfusion accelerates cerebral amyloid angiopathy and promotes cortical microinfarcts. Acta Neuropathol 123, 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satizabal C, Beiser AS, Seshadri S, 2016. Incidence of Dementia over Three Decades in the Framingham Heart Study. N.Engl.J.Med 375, 93–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA, 2007. Subcortical infarcts, Alzheimer's disease pathology, and memory function in older persons. Ann.Neurol 62, 59–66. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA, 2004. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 62, 1148–1155. [DOI] [PubMed] [Google Scholar]
- Smith EE, Schneider JA, Wardlaw JM, Greenberg SM, 2012. Cerebral microinfarcts: the invisible lesions. Lancet Neurol 11, 272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, Craft S, Leverenz JB, Montine TJ, 2007. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann.Neurol 62, 406–413. [DOI] [PubMed] [Google Scholar]
- Summers PM, Hartmann DA, Hui ES, Nie X, Deardorff RL, McKinnon ET, Helpern JA, Jensen JH, Shih AY, 2017. Functional deficits induced by cortical microinfarcts. J.Cereb.Blood Flow Metab 37, 3599–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter OC, Sunthorn T, Kraftsik R, Straubel J, Darekar P, Khalili K, Miklossy J, 2002. Cerebral hypoperfusion generates cortical watershed microinfarcts in Alzheimer disease. Stroke. 33, 1986–1992. [DOI] [PubMed] [Google Scholar]
- Tariq N, Khatri R, 2008. Leptomeningeal collaterals in acute ischemic stroke. J.Vasc.Interv.Neurol 1, 91–95. [PMC free article] [PubMed] [Google Scholar]
- Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, O'Brien RJ, 2008. Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Ann.Neurol 64, 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veluw SJ, Hilal S, Kuijf HJ, Ikram MK, Xin X, Yeow TB, Venketasubramanian N, Biessels GJ, Chen C, 2015a. Cortical microinfarcts on 3T MRI: Clinical correlates in memory-clinic patients. Alzheimers Dement. [DOI] [PubMed] [Google Scholar]
- van Veluw SJ, Jolink WM, Hendrikse J, Geerlings MI, Luijten PR, Biessels GJ, Klijn CJ, 2015b. Cortical microinfarcts on 7 T MRI in patients with spontaneous intracerebral hemorrhage. J.Cereb.Blood Flow Metab 35, 1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veluw SJ, Shih AY, Smith EE, Chen C, Schneider JA, Wardlaw JM, Greenberg SM, Biessels GJ, 2017. Detection, risk factors, and functional consequences of cerebral microinfarcts. Lancet Neurol 16, 730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LY, Larson EB, Sonnen JA, Shofer JB, McCormick W, Bowen JD, Montine TJ, Li G, 2009. Blood pressure and brain injury in older adults: findings from a community-based autopsy study. J.Am.Geriatr.Soc 57, 1975–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, van Veluw SJ, Wong A, Liu W, Shi L, Yang J, Xiong Y, Lau A, Biessels GJ, Mok VC, 2016. Risk Factors and Cognitive Relevance of Cortical Cerebral Microinfarcts in Patients With Ischemic Stroke or Transient Ischemic Attack. Stroke. 47, 2450–2455. [DOI] [PubMed] [Google Scholar]
- Westover MB, Bianchi MT, Yang C, Schneider JA, Greenberg SM, 2013. Estimating cerebral microinfarct burden from autopsy samples. Neurology. 80, 1365–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White L, Petrovitch H, Hardman J, Nelson J, Davis DG, Ross GW, Masaki K, Launer L, Markesbery WR, 2002. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann.N.Y.Acad.Sci 977, 9–23. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Mendes de Leon CF, Aggarwal NT, Schneider JS, Bach J, Pilat J, Beckett LA, Arnold SE, Evans DA, Bennett DA, 2002. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 59, 364–370. [DOI] [PubMed] [Google Scholar]