Abstract
Phosphatidylcholine (PC) is a primary phospholipid and major source of secondary lipid messengers and also serves as a biosynthetic precursor for other membrane phospholipids. Phosphocholine cytidylyltransferase (CCT) is the rate-limiting enzyme responsible for catalyzing the formation of PC. Changes in CCT activity have been associated with lipid dysregulation across various neurological disorders. Additionally, intermediates in PC synthesis, such as CDP-choline, have been suggested to attenuate drug craving during cocaine addiction. Recent work from our group demonstrated that cocaine exposure and conditioning alter the level of PC in the brain, specifically in the cerebellum and hippocampus. The present study examines the role of CCT expression in the brain and determines the effect of cocaine exposure on CCT expression. Immunohistochemical analysis (IHC) was performed to assess region-specific expression of CCT, including both of its isoforms; alpha (CCTα) and beta (CCTβ). IHC did not detect any staining of CCTα throughout the rat brain. In contrast, CCTβ expression was detected in the Purkinje cells of the cerebellum with decreases in expression following cocaine exposure. Collectively, these data demonstrate the region- and cell-specific localization of CCTα and CCTβ in the rat brain, as well as the altered expression of CCTβ in the cerebellum following cocaine exposure.
Keywords: phosphatidylcholine cytidylyltransferase, cocaine, hippocampus, cerebellum
Introduction
Phosphatidylcholine (PC) is a primary membrane phospholipid in eukaryotes, and is a major source of secondary lipid messengers (i.e. diacylglycerol [DAG], phosphatidic acid), and also serves as the biosynthetic precursor for other membrane phospholipids (Cornell and Ridgway, 2015; Vance, 2002). The major pathway responsible for PC biosynthesis is the CDP-choline or Kennedy pathway, where the main regulator is cytidine triphosphate (CTP):phosphocholine cytidylyltransferase [CCT] (Kent, 1997). The CCT enzyme plays an essential role in the catalysis of the transfer of choline from phosphocholine to CDP-choline, which can proceed to donate the phosphocholine moiety to DAG and result in the formation of PC (Kent, 1997). Thus, the regulation of PC homeostasis in mammalian cells is heavily reliant on CCT.
CCT proteins are characterized by their N- and C-termini depending on the presence (CCTα) or absence (CCTβ) of a nuclear localization signal (NLS), extent of phosphorylation, or charge (Cornell and Ridgway, 2015; Jackowski and Fagone, 2005). Regarding cellular localization, CCT is an amphitropic enzyme distributed between the cytosol and membrane. Generally, the NLS directs most of CCTα to the nucleus but it can also be found in the cytoplasm associated with other organelle membranes, although this can differ across cells and tissues (Jackowski and Fagone, 2005; Karim et al., 2003). CCTβ, however, is reported to exclusively localize outside of the nucleus in HeLa cells (Lykidis et al., 1999).
Regulation of CCT activity is well-characterized by the translocation from the inactive, soluble form to the active, membrane-bound form, which then becomes subject to lipid-dependent feed-forward and feedback mechanisms (Pelech and Vance, 1984). All isoforms maintain the same biochemical function; however, expression varies across tissue type (Karim et al., 2003). CCTα is ubiquitously expressed throughout various tissues, whereas CCTβ expression is generally low, with the exception of the brain and gonadal tissue (Jackowski et al., 2004; Jackowski and Fagone, 2005). The heightened expression of CCTβ in the brain suggests an important functional role in this tissue.
Alterations in CCT activity have been associated with lipid dysregulation across some neurological disorders (Adibhatla and Hatcher, 2005; Jackowski and Fagone, 2005). The accumulation of cellular glucosylceramide observed in Gaucher disease was shown to directly activate PC synthesis (Bodennec et al., 2002). CCT has also been implicated in neuronal development with an essential role in the PC biosynthesis of axons (Strakova et al., 2011). The essential intermediate in PC synthesis, cytidine-5’-diphosphocholine (CDP-choline or citicholine), has been extensively studied for its potential to remedy membrane damage and provide therapeutic effects across a variety of CNS disorders and injury (Adibhatla and Hatcher, 2005). For example, CDP-choline was suggested to have beneficial effects in cocaine addiction where treatment attenuated drug craving in cocaine-dependent subjects, suggesting a role for PC synthesis within the context of drug dependence (Renshaw et al., 1999). Furthermore, Ross et al., reported reduced CCT activity in postmortem brain tissue obtained from cocaine users (Ross et al., 2002), identifying a potential rationale for the efficacy of CDP-choline treatment.
Prior work from our laboratory demonstrated the persisting effects of cocaine conditioning on brain phospholipid alterations where the majority of changes occurred in PC species, region-specifically in the hippocampus and the cerebellum (Cummings et al., 2015a). The hippocampus is characterized by its role in learning and memory and is directly involved in the reinstatement of drug-seeking behavior. It also plays a major role in the retention of memories of addiction, which may involve membrane remodeling and lipid alterations (Buzsaki and Moser, 2013; Cummings et al., 2015a; Vorel et al., 2001). Although the cerebellum has not received much attention in the addiction literature, quite a few studies have suggested a role for the cerebellum in drug reward and reinstatement of drug-seeking (Koob and Volkow, 2010; Moreno-Rius and Miquel, 2017; Moulton et al., 2014; Wagner et al., 2017). These studies report consistent cerebellar activation in response to drug-cues via neuroimaging as well as bidirectional connections with regions mediating drug reward such as the striatum and ventral tegmental area function (Moreno-Rius and Miquel, 2017; Moulton et al., 2014). The cerebellum was also found to have a role in nonmotor functions, and more recently, Wagner, et. al demonstrated that granule cells can convey information about the expectation of reward (Wagner et al., 2017).
To identify a potential mechanism mediating these changes, this study further investigates the effects of cocaine exposure on region-specific CCT expression in the hippocampus and cerebellum. Additionally, while the distribution of CCT isoforms in human tissues has been reported, little information is available pertaining to the abundance as well as localization of CCT isoforms in the rodent brain (Lykidis et al., 1999; Xu et al., 2002). To address this gap, the present work assessed the localization and expression of CCT isoforms in the rat brain using immunohistochemical analyses.
1. Results
2.1. Expression of CCT Isoforms
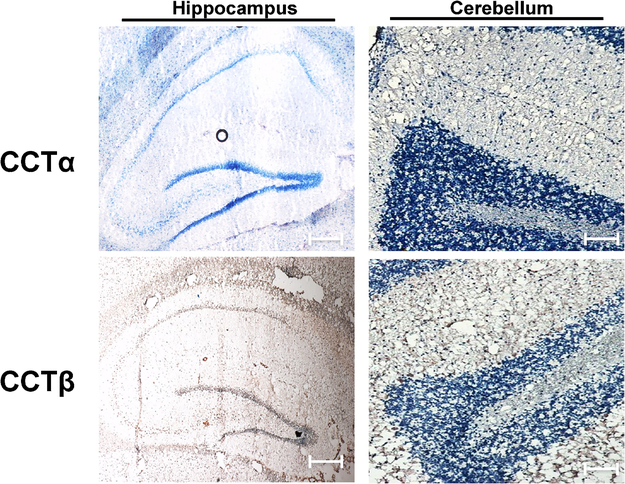
Immunohistochemistry (IHC) was performed on 10-μm sagittal rat brain sections using antibodies for both CCTα and CCTβ. CCTα expression was not detected in either structure (Figure 1), nor was CCTα expression detected throughout the brain. In contrast, CCTβ expression was detected in both the hippocampus and cerebellum (Figure 1 and Supplemental Figure 1).
Figure 1. Distribution of CCT isoforms throughout the rat brain.
Immunohistochemical analysis of 10-μm sagittal sections (magnification: X20) demonstrated detection of CCTβ but not CCTα, in both the hippocampus and cerebellum where positive staining is indicated by DAB (brown). Scale bar, 50 μm. Images are representative of sections analyzed from at least 3 (n = 3) animals.
2.2. Cerebellar Localization
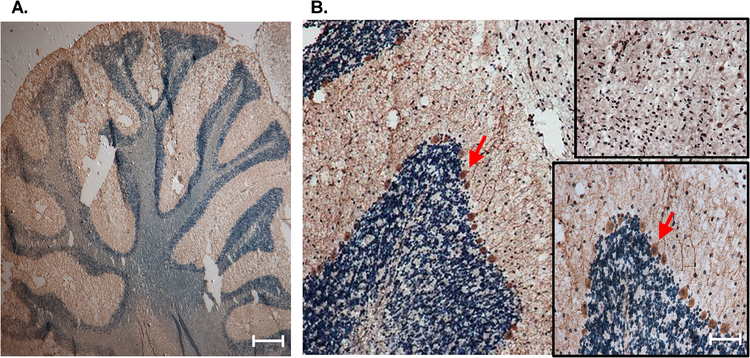
Although levels of CCTβ expression are known to be more abundant in the brain, there is little information regarding the cellular localization of CCTβ throughout neural structures. As such, we further analyzed CCTβ expression in the cerebellum to identify the specific cell type in which this protein was expressed (Figure 2A). Interestingly, and yet unreported, CCTβ expression appeared to localize predominately to the Purkinje cells (Figure 2B), a class of inhibitory projection neurons. Further, CCTβ expression appears to be cytosolic within these Purkinje cells (Figure 2B, inset 2). Previously, CCTβ has been reported to be localized outside of the nucleus (Lykidis et al., 1999); however, our IHC analysis indicates region-specific cellular localization throughout the rat brain. Increased magnification (50X, Figure 2B, inset 1), indicated nuclear CCTβ staining throughout peripheral midbrain
Figure 2. Localization of CCTβ in cerebellar Purkinje cells of rat brain.
Immunohistochemical analysis (magnification: X20) of 10-μm sagittal sections demonstrated a relatively high expression of CCTβ in Purkinje cells of the cerebellum, which are indicated by red arrows, with enlarged inset view (magnification: X50). Top inset (magnification: X50) indicates nuclear CCT staining throughout peripheral midbrain. Scale bar, 50 μm. Images are representative of sections analyzed from at least 3 (n = 3) separate animals.
2.3. Protein Expression of CCTβ following Cocaine Exposure
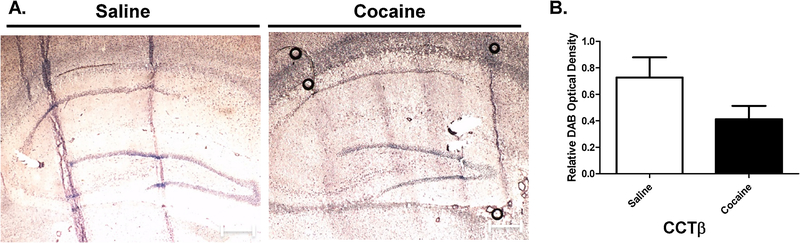
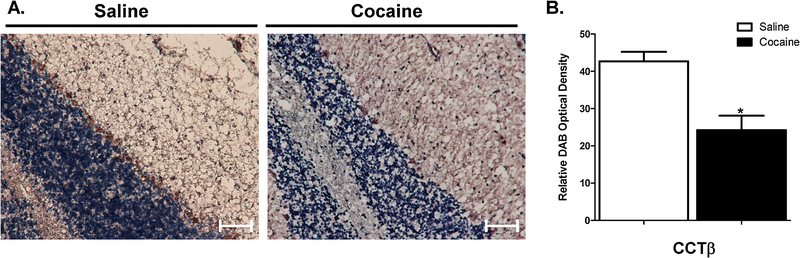
Previous studies, including our own (Cummings et al., 2015a), have implicated a role for altered phosphatidylcholine (PC) metabolism within the context of addiction. Thus, this study examined the effects of cocaine exposure on region-specific CCT protein expression in the hippocampus and cerebellum (Figures 3 & 4). IHC quantification and image analysis was performed using the ImageJ-based software package FIJI to obtain the relative optical density of DAB staining values. Relative optical density (O.D.) was calculated based on the maximum density of DAB staining values, which was derived based on the maximum intensity of an 8-bit image. Exposure of rats to cocaine did not significantly alter the expression of CCTβ in the hippocampus (Figure 3). In contrast, cocaine exposure did appear to decrease the expression of CCTβ in the Purkinje cell layer (Figure 4). These data suggest that cocaine exposure alters the expression of CCTβ.
Figure 3. Immunohistochemical analysis of cocaine exposure on hippocampal CCTβ expression.
(A) IHC was performed on 10-μm sagittal sections to assess CCTβ protein expression in the hippocampus of saline- and cocaine-treated rats (magnification: X20). (B) Quantification and image analysis was performed using the ImageJ-based software package FIJI to obtain the relative optical density of DAB staining values. Relative optical density (O.D.) was calculated based on the maximum density of DAB staining values, which was derived based on the maximum intensity of an 8-bit image. Scale bar, 50 μm. Data indicate results from at least 3 separate animals (n=3) per group and are expressed as mean values ± SEM.
Figure 4. Immunohistochemical analysis of cocaine exposure on cerebellar CCTβ expression.
(A) IHC was performed on 10-μm sagittal sections to assess CCTβ protein expression in the Purkinje layer of the cerebellum in saline- and cocaine-treated rats (magnification: X20). (B) Quantification and image analysis was performed using the ImageJ-based software package FIJI to obtain the relative optical density of DAB staining values. Relative optical density (O.D.) was calculated based on the maximum density of DAB staining values, which was derived based on the maximum intensity of an 8-bit image. Scale bar, 50 μm. Data indicate results from 3 separate animals (n=3) per group and are expressed as mean values ± SEM. *Indicates a significant difference (p < 0.05) as compared to saline control rats.
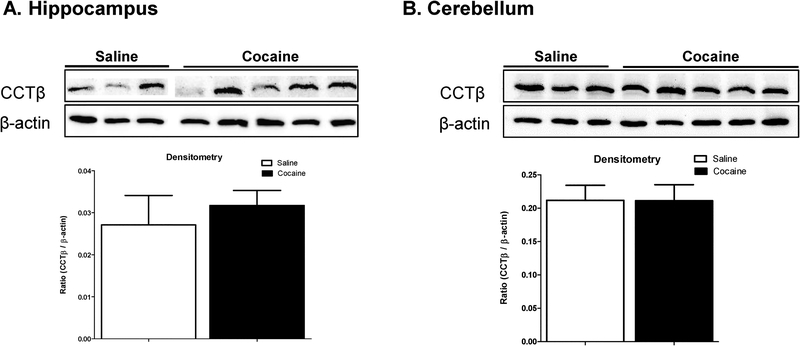
Immunoblot analysis of whole tissue lysate was performed to validate the IHC data. In agreement with the IHC data, cocaine exposure did not alter CCTβ exposure in the hippocampus in any animal tested (Figure 5A). Surprisingly, cocaine exposure also did not alter CCTβ expression in whole tissue lysate isolated from the cerebellum, which is in direct contrast to the IHC results (Figure 5B). The lack of changes in CCTβ protein expression as detected using immunoblot analysis, may be due to the fact that whole tissue lysate was used, as opposed to tissue directly from the Purkinje cell layer.
Figure 5. Effect of cocaine exposure on hippocampal and cerebellar CCTβ expression.
CCTβ protein expression was assessed in the (A) hippocampus and (B) cerebellum of saline- and cocaine-treated rats using immunoblot analysis. Quantitative densitometry is presented as the CCTβ/β-actin ratio. Data indicate results from at least 3 separate animals (n=3) per group and are expressed as mean values ± SEM.
3. Discussion
CCT, a critical enzyme in PC synthesis, is responsible for the regulation of PC homeostasis within mammalian cells. CCT has been well studied across various pathologies including neurological disorders such as ischemia and addiction. However, there is little information regarding the basic distribution and cellular localization of CCT isoforms in the rodent brain. Within the context of cocaine dependence, Ross et al. reported a reduction of CCT activity in cocaine users (Ross et al., 2002). Additionally, our lab previously demonstrated the effects of cocaine conditioning on region-specific alterations of PC species in the rat brain (Cummings et al., 2015a). With these studies in mind, this study examined the expression of the PC synthesis protein, CCT, including both isoforms alpha (CCTα) and beta (CCTβ), as well as region-specific alterations following cocaine exposure.
To our knowledge, this is the first report to use immunohistochemistry to determine the expression of CCTα and CCTβ in the brain as well as to assess its regional distribution. Although significant CCTα expression was not detected in either the hippocampus of the cerebellum, this does not preclude the possibility that CCTα is expressed in other areas of the rat brain or at different levels compared to CCTβ. This possibility is supported by earlier studies suggesting that rodent brains may contain a mixture of both α and β isoforms, possibly varying in different ratios in different brain regions (Bodennec et al., 2002; Xiong et al., 2000). The finding that CCTβ is the dominantly expressed isoform within the hippocampus and cerebellum supports the assertion that CCTβ has an essential biological role in the brain (Jackowski et al., 2004; Jackowski and Fagone, 2005).
The cytosolic expression of CCTβ in Purkinje cells is also a novel finding. The fact that expression was localized between the molecular and granule layers of the cerebellar cortex was interesting given that its expression was predominantly nuclear in the hippocampus and structures throughout the midbrain. The expression of CCTβ in the cytosol in the cerebellum agrees with previously published studies demonstrating the exclusive cytosolic expression of CCTβ in other brain areas (Lykidis et al., 1999). The nuclear localization of CCTβ is intriguing since CCTβ lacks a NLS and was previously reported to be located outside the nucleus in HeLa cells (Lykidis et al., 1999). These differences cannot be attributed to the CCTβ antibody as the same antibody was used in both studies. These differences may arise due to differences in the cell-types being studied along with the use of tissue for IHC analysis as opposed to cells. It should also be mentioned that HeLa cells are cancerous in origin and the differential expression and localization of CCT isoforms between normal and cancerous tissues is not well studied.
Regardless of its nuclear or cytosolic localization, the expression of CCTβ in Purkinje cells raises questions relating to its functional role. As mentioned above, CCT is responsible for regulating PC synthesis. Studies using mass spectrometric imaging with matrix-assisted laser desorption/ionization (MALDI) have reported abundant PC levels in the cerebellum of rodent brains (Mikawa et al., 2009; Sugiura et al., 2009). For example, Sugiura, et al. showed that polyunsaturated fatty acid (PUFA)-containing PCs are distributed in cerebellar Purkinje cells (Sugiura et al., 2009), while Mikawa, et al. reported very high levels of PC 32:0 and PC 34:1 in the molecular layer of the cerebellum (Mikawa et al., 2009). It is possible that higher levels of CCT expression within Purkinje cells is needed to maintain the cellular distribution of these lipids.
The discrepancy between IHC and immunoblot analysis regarding the persisting effects of cocaine exposure on CCT expression is puzzling. Although IHC staining indicated a decrease in CCTβ expression in the Purkinje cell layer of the cerebellum, this was not supported by immunoblot analysis. It should be noted that immunoblot analysis was performed using whole tissue lysate from the entire cerebellum. It’s possible that the decrease in CCTβ expression observed within Purkinje cells is cellular specific, and that the decrease in CCTβ in these cells may not be abundant enough to alter the overall protein expression in the cerebellum. This would include CCTβ expression throughout the molecular and granule layers, which did not appear to change when assessed by IHC. Further studies are needed to address this possibly, such as laser caption microdissection PCR. Dissection of the Purkinje cell layer followed by immunoblot analysis is difficult due to the small amount of tissue from each animal.
The primary significance of this study is that it is one of the first to demonstrate the region- and cell-specific localization of CCTα and CCTβ in the rat brain. A secondary finding is the suggestion that CCTβ expression in the Purkinje cells is decreased by cocaine exposure. The decrease in CCTβ expression may alter the overall lipidome of the cerebellum, or at least the Purkinje cell layer, after cocaine exposure. Theoretically, a decrease in CCTβ induced by cocaine would decrease PC levels and decrease variability in overall lipids. This is in fact what we reported in our previous studies (Cummings et al., 2015b). As such, the current data suggest that changes in CCTβ may be one mechanisms mediating lipidomic changes in the cerebellum. It should be noted though that changes in CCTβ did not correlate to changes in conditioned place preference (CPP, data not shown). Thus, the effect of CCTβ on behavioral changes induced by cocaine is not known. Future studies, increasing the sample size are needed to address this possibility.
The cocaine exposure protocol used in this study is well established to increase locomotor activity and induce CPP. Thus, it is possible that the change in both CTTβ, as reported in this study, and in the lipidomic profile reported in our previous work, is a side effect of the behavioral changes induced by cocaine, as opposed to a driver. It is doubtful that changes in CCTβ are mediating any cellular damage as the dose of cocaine used in this study is not believed to induce any toxicity.
In conclusion, these data demonstrate the novel finding that CCTβ, but not CCTα is expressed in the rat hippocampus and the cerebellum. These data also demonstrate the novel finding that CCTβ is expressed in the Purkinje cell layer and suggest that cocaine exposure decrease CCTβ expression specifically in these cells. The functional consequence of decreased CCTβ expression on cocaine-induced behavioral changes are not known, but it’s possible that this event may drive cocaine-induced alterations in the lipid profile, as reported in our recent studies (Cummings et al., 2015b). These data also support the emerging consensus that cocaine has substantial effects on the cerebellum.
4.1. Animals
Adult (12–13 weeks) male Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) were housed in pairs in clear plastic cages and maintained on a 12-hour light/dark cycle (0700/1900 hours) with food and water available ad libitum. Animals were allowed to acclimate to their home cages for at least 1 week and were habituated to handling (3 days) prior to testing. All studies were approved by the University of Georgia Institutional Animal Care and Use Committee and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals. Cocaine hydrochloride was obtained from the National Institutes of Health National Institute on Drug Abuse (RTI International, Research Triangle Park, NC), and was dissolved in 0.9% saline and filter sterilized prior to use. Animals were injected intraperitoneally with either cocaine (15 mg/kg on conditioning days, 10 mg/kg on reinstatement days) or 0.9% saline.
4.2. Conditioned place preference
Behavioral testing took place in two-compartment chambers with clear plastic walls and a smooth solid floor divided by a black partition containing a guillotine door (Med Associates Inc., St. Albans, VT), described previously (Scholpa et al., 2016). Briefly, each chamber was located in a sound-attenuating box equipped with two house lights, ventilation fan, and photo beam banks for horizontal activity detection. Beam breaks were counted using Activity Monitor software (Med Associates, Inc.). For conditioning sessions, the door was closed in order to confine the animal’s activity. To obtain equal preference between compartments, one compartment contained rod-like steel bar flooring and transparent walls while the other compartment contained a wire mesh grid floor and transparent walls.
The experimental design for the behavioral regimen was modified based on that described in previous studies (Scholpa et al., 2016; Seymour and Wagner, 2008) by incorporating intervals of extinction, reinstatement, and prolonged abstinence to establish a model resembling the pattern of repeat relapse observed in humans. Following a pretest session, conditioning sessions took place for 4 consecutive days. Rats were divided into two groups: saline-treated and cocaine-treated. Animals were injected with either saline or cocaine (15 mg/kg, I.P.), placed in one of the two insert compartments for 15 minutes, and then returned to their home cage. Four hours later, animals were injected with either saline or cocaine and were confined to the opposite compartment for the second daily conditioning session. After the 4 days of conditioning, the rats underwent a drug-free place preference post-test to determine the shift in time spent in the drug-paired compartment as compared with the pretest session assessed prior to conditioning. Over the next 7 days, these animals then experienced six consecutive days of extinction (saline conditioning) followed by a cocaine-primed reinstatement assessment. Rats were then housed in their home cage environment for 30 days of abstinence, followed by a final reinstatement session. The results of these behavioral assessments are under review as part of a separate study beyond the scope of this work.
4.3. Tissue extraction
Region-specific brain tissue was extracted 7 days after the final exposure to cocaine, as described previously (Scholpa et al., 2016). Briefly, animals were anesthetized with halothane prior to decapitation in compliance with protocols approved by the University of Georgia Animal Care and Use guidelines. The brain was removed and regions of interest were extracted and flash frozen in liquid nitrogen. For immunohistochemistry studies (n ≥ 3 rats per group), the left hemisphere was placed midline down on a coverslip, flash frozen in liquid nitrogen, and kept at −80°C until cryosectioning.
4.4. Immunohistochemistry
Immunohistochemical staining was performed on sagittal, 10-μm frozen sections fixed in 2% paraformaldehyde at room temperature for 20 min. The manufacturer’s protocol was followed using the Vectastain Universal Elite ABC HRP Kit (Vector Laboratories, Burlingame, CA) as described previously (Scholpa et al., 2016). Sections were incubated overnight with primary antibody against CCTβ and/or CCTα (1:50 dilution (Lykidis et al., 1999); obtained as a generous gift from Dr. S. Jackowski) in 0.1% bovine serum albumin (BSA) in PBS. Secondary staining was performed with 3,3’-diaminobenzidine (DAB) as a substrate (Vector Laboratories), and slides were subsequently counterstained using Gill’s hematoxylin. Sections were mounted with Fluoromount (SigmaAldrich, St. Louis, MO) and visualized using a Nikon AZ100 microscope (Tokyo, Japan). Quantification and image analysis was subsequently performed using the ImageJ-based software package FIJI (Schindelin et al., 2012). Using the ‘colour deconvolution’ with ‘H DAB’ settings, DAB and Gill’s hematoxylin staining were deconvoluted, inverted, and converted into 8-bit gray scale images. Regions of interest (ROI) were defined to ensure uniform quantitative comparisons across sections and structures. For example, the entire hippocampus and Purkinje cell layer were set as the ROIs for hippocampal and cerebellar comparisons, respectively. Relative optical density (O.D.) was calculated based on DAB staining values where mean intensity was calculated using the ‘Measure’ function. A reciprocal intensity was derived based on the maximum intensity of an 8-bit image (250) and log transformed to yield an O.D. directly proportional to the amount of chromogen present.
4.5. Immunoblot analysis
Protein isolation was performed region specifically using 10–15 mg flash-frozen tissue as described previously (Scholpa et al., 2016). Protein concentrations were determined using the BCA assay and samples (40 mg) were separated on SDS-PAGE gels and transferred to nitrocellulose membranes. The membranes were blocked in 5% (w/v) milk powder in Tris-buffered saline/Tween 20 for 2 hours and exposed to primary antibodies against CCTβ or CCTα (1:2000 dilution; obtained as a generous gift from Dr. S. Jackowski) overnight. Membranes were then incubated with secondary antibody (1:2500 dilution; Promega, Madison, WI) for 2 hours and bands were visualized using SuperSigna Chemiluminescent Substrate (Thermo Scientific, Waltham, MA). Intensities were quantified using an Alpha Innotech FluorChem HD2 system (ProteinSimple, Santa Clara, CA) and normalized to β-actin (1:2000 dilution). Samples were compared against positive (liver tissue lysate) and negative (no primary antibody) controls. The CCTβ antibody recognized major regions in the cerebellum and in the hippocampus (Supplemental Figure 2).
4.6. Statistical Analysis
All statistical analyses were compiled using GraphPad Prism for windows version 5.04 (GraphPad Software, Inc., La Jolla, CA). For all analysis, the experimental unit was one tissue section from an individual animal and sections obtained from a total of 3–4 animals/group point were assessed. For all analyses, significance was set at p ≤ 0.05 where data are expressed as mean ± SEM based on t-test for pairwise analysis.
Supplementary Material
Highlights:
CCTβ, but not CCTα, expression was demonstrated in the rat hippocampus and cerebellum.
CCTβ cellular localization was region-specific, with cytosolic expression detected in Purkinje cells, and nuclear expression detected in the hippocampus and midbrain.
CCTβ expression was not significantly decreased by cocaine exposure in the hippocampus, corpus callosum or midbrain, but was significantly decreased in the Purkinje cell layer of the cerebellum.
Acknowledgements
We would like to thank Dr. Suzanne Jackowski (St. Jude Children’s Research Hospital) for kindly providing us with CCT-specific antibodies.
Funding
This project was supported by in part by the National Institutes of Health (NIH); National Institute of Biomedical Imaging and Bioengineering (BIBIB (EB0160100)) and an US Department of Defense Prostate Cancer Research Program Idea Development Award (DOD PC150431, GRANT11996600) to BSC.
Abbreviations:
- BSA
bovine serum albumin
- CTP
cytidine triphosphate
- CCT
CTP:phosphocholine cytidylyltransferase
- CCTα
CCT alpha
- CCTβ
CCT beta
- DAB
3,3’-diaminobenzidine
- DAG
diacylglycerol
- IHC
immunohistochemistry
- MALDI
matrix-assisted laser desorption/ionization
- NLS
nuclear localization sequence
- OD
optical density
- PC
phosphatidylcholine
- PUFA
polyunsaturated fatty acid
- ROI
region of interest
Footnotes
Declarations of interest
The authors of this manuscript declare they have no declarations of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adibhatla RM, Hatcher J, 2005. Cytidine 5′-diphosphocholine (CDP-choline) in stroke and other CNS disorders. Neurochemical research. 30, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodennec J, Pelled D, Riebeling C, Trajkovic S, Futerman AH, 2002. Phosphatidylcholine synthesis is elevated in neuronal models of Gaucher disease due to direct activation of CTP: phosphocholine cytidylyltransferase by glucosylceramide. The FASEB Journal. 16, 1814–1816. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Moser EI, 2013. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 16, 130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell RB, Ridgway ND, 2015. CTP: phosphocholine cytidylyltransferase: Function, regulation, and structure of an amphitropic enzyme required for membrane biogenesis. Progress in lipid research. 59, 147–171. [DOI] [PubMed] [Google Scholar]
- Cummings BS, Pati S, Sahin S, Scholpa NE, Monian P, Trinquero PM, Clark JK, Wagner JJ, 2015a. Differential effects of cocaine exposure on the abundance of phospholipid species in rat brain and blood. Drug and alcohol dependence. 152, 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BS, Pati S, Sahin S, Scholpa NE, Monian P, Trinquero PM, Clark JK, Wagner JJ, 2015b. Differential effects of cocaine exposure on the abundance of phospholipid species in rat brain and blood. Drug Alcohol Depend. 152, 147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski S, Rehg JE, Zhang Y-M, Wang J, Miller K, Jackson P, Karim MA, 2004. Disruption of CCTβ2 expression leads to gonadal dysfunction. Molecular and cellular biology. 24, 4720–4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski S, Fagone P, 2005. CTP: phosphocholine cytidylyltransferase: paving the way from gene to membrane. Journal of Biological Chemistry. 280, 853–856. [DOI] [PubMed] [Google Scholar]
- Karim M, Jackson P, Jackowski S, 2003. Gene structure, expression and identification of a new CTP: phosphocholine cytidylyltransferase β isoform. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 1633, 1–12. [DOI] [PubMed] [Google Scholar]
- Kent C, 1997. CTP: phosphocholine cytidylyltransferase. Biochimica et Biophysica Acta (BBA)-Lipids and Lipid Metabolism. 1348, 79–90. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2010. Neurocircuitry of addiction. Neuropsychopharmacology. 35, 217–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykidis A, Baburina I, Jackowski S, 1999. Distribution of CTP: phosphocholine cytidylyltransferase (CCT) isoforms identification of a new CCTβ splice variant. Journal of Biological Chemistry. 274, 26992–27001. [DOI] [PubMed] [Google Scholar]
- Mikawa S, Suzuki M, Fujimoto C, Sato K, 2009. Imaging of phosphatidylcholines in the adult rat brain using MALDI-TOF MS. Neuroscience letters. 451, 45–49. [DOI] [PubMed] [Google Scholar]
- Moreno-Rius J, Miquel M, 2017. The cerebellum in drug craving. Drug and Alcohol Dependence. 173, 151–158. [DOI] [PubMed] [Google Scholar]
- Moulton EA, Elman I, Becerra LR, Goldstein RZ, Borsook D, 2014. The cerebellum and addiction: insights gained from neuroimaging research. Addiction biology. 19, 317–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelech SL, Vance DE, 1984. Regulation of phosphatidylcholine biosynthesis. Biochimica et Biophysica Acta (BBA)-Reviews on Biomembranes. 779, 217–251. [DOI] [PubMed] [Google Scholar]
- Renshaw PF, Daniels S, Lundahl LH, Rogers V, Lukas SE, 1999. Short-term treatment with citicoline (CDP-choline) attenuates some measures of craving in cocaine-dependent subjects: a preliminary report. Psychopharmacology. 142, 132–138. [DOI] [PubMed] [Google Scholar]
- Ross BM, Moszczynska A, Peretti FJ, Adams V, Schmunk GA, Kalasinsky KS, Ang L, Mamalias N, Turenne SD, Kish SJ, 2002. Decreased activity of brain phospholipid metabolic enzymes in human users of cocaine and methamphetamine. Drug and Alcohol Dependence. 67, 73–79. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A, 2012. Fiji: an open-source platform for biological-image analysis. Nat Meth. 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholpa NE, Briggs SB, Wagner JJ, Cummings BS, 2016. Cyclin-Dependent Kinase Inhibitor 1a (p21) Modulates Response to Cocaine and Motivated Behaviors. Journal of Pharmacology and Experimental Therapeutics. 357, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour CM, Wagner JJ, 2008. Simultaneous expression of cocaine-induced behavioral sensitization and conditioned place preference in individual rats. Brain research. 1213, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakova J, Demizieux L, Campenot RB, Vance DE, Vance JE, 2011. Involvement of CTP: phosphocholine cytidylyltransferase-β2 in axonal phosphatidylcholine synthesis and branching of neurons. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 1811, 617–625. [DOI] [PubMed] [Google Scholar]
- Sugiura Y, Konishi Y, Zaima N, Kajihara S, Nakanishi H, Taguchi R, Setou M, 2009. Visualization of the cell-selective distribution of PUFA-containing phosphatidylcholines in mouse brain by imaging mass spectrometry. Journal of lipid research. 50, 1776–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE, 2002. Phospholipid biosynthesis in eukaryotes. New comprehensive biochemistry. 36, 205–232. [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL, 2001. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 292, 1175–8. [DOI] [PubMed] [Google Scholar]
- Wagner MJ, Kim TH, Savall J, Schnitzer MJ, Luo L, 2017. Cerebellar granule cells encode the expectation of reward. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Liu X. l., Wang Y, Du Y.-c., 2000. Cloning of cytidine triphosphate: phosphocholine cytidylyltransferase mRNA upregulated by a neuropeptide arginine-vasopressin (4–8) in rat hippocampus. Neuroscience letters. 283, 129–132. [DOI] [PubMed] [Google Scholar]
- Xu KY, Xiong Y, Xie ZQ, Bian W, Jing NH, Du YC, 2002. Localization of CCTbeta in rat brain and overexpression in insect cells. Acta Pharmacol Sin. 23, 349–54. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.