Abstract
Background:
Following primary breast cancer treatment, endocrine therapy (ET) is prescribed for hormone-receptor positive cancers. Despite ET treatment recommendations of 5–10 years, there is little prospective study of its impact on cognitive function over an extended period of time. ET has known pharmacologic effects on the brain. Cognitive side effects are a concern for many women, with mixed findings in various studies. This prospective longitudinal study examined the neuropsychological effects of ET over time, up to 6 years after treatment.
Methods:
189 early-stage breast cancer survivors (BCS) enrolled in the study prior to initiating ET if prescribed, and were followed at 6-months (n=175), 12-months (n=173), and 3–6 years (n=102) with self-report and neuropsychological assessments. Using linear mixed models, we examined whether neuropsychological performance or impairment rates differed over time based on whether or not ET was received.
Results:
We did not find any effect of ET on neuropsychological performance or impairment at any time point, compared to women who did not take ET. However, those who participated in the 3–6 year visit exhibited better executive function at baseline.
Conclusions:
In this observational cohort study, we did not identify a detrimental effect of ET on cognitive function in BCS receiving treatment compared to those who did not take ET.
Keywords: breast cancer, survivors, cognition, endocrine therapy, neuropsychological
Condensed abstract:
We found no cognitive differences over time between breast cancer survivors taking endocrine therapy or not. Further studies accounting for methodological complexities are needed to corroborate the safety of these medications on the brain and cognitive health.
Introduction
Endocrine therapies (ET) are widely used in breast cancer adjuvant treatment to reduce recurrence and improve survival, with recommended treatment up to 5–10 years.1 These treatments systematically interfere with action of estrogen in the body and brain, including possible neurocognitive dysfunction.2 Our team was among the first to report worse neuropsychological functioning in breast cancer survivors (BCS) 2–5 years after diagnosis related to adjuvant tamoxifen treatment.3 Several cross-sectional and longitudinal studies have since found mixed results.4–10 However, sample sizes across studies tend to be small and longitudinal studies often have limited follow-up durations. There remains substantial uncertainty about the cognitive effects of ET given its protracted use.2
The Mind Body Study (MBS) was a prospective longitudinal study specifically designed to evaluate the effects of ET on neuropsychological function in BCS. BCS were evaluated within three months of completing primary cancer treatment but before initiating ET if it was indicated, followed by 6- and 12-month follow-up assessments. Follow-up of the MBS cohort was extended beyond the first year, including an additional in-person assessment visit that took place ~3–6 years after their baseline evaluation. We have previously reported finding worse cognitive complaints at 6 months among those taking ET, associated with diminished improvement in psychomotor speed.11 In analysis of self-reported symptoms over the first year of the study, we also found worse cognitive complaints among participants taking ET compared to those not taking this treatment at 6 and 12 months12, but have not yet reported neuropsychological outcomes. The current study is a report of the longitudinal neuropsychological data across all four-time points, the longest prospective study of the cognitive effects of ET to date. Given our prior findings, we hypothesized that BCS on ET would exhibit diminished neuropsychological functioning over time compared to those not taking ET.
Methods
The MBS methods are described in detail elsewhere, and will be briefly summarized.16,18,21 Between 2007 and 2011, newly-diagnosed, early stage breast cancer patients were recruited through clinical oncology practices and rapid case ascertainment using the Los Angeles County Surveillance, Epidemiology, and End Results Program registry with collaborating physicians and hospitals. The baseline visit occurred within 3 months of completing primary cancer treatment with surgery, radiation, and/or chemotherapy but before initiation of ET if prescribed. Identical follow-up visits occurred at 6 and 12 months after baseline. At each study visit, we administered a questionnaire battery, collected blood for genetic and inflammatory markers, and administered a comprehensive neuropsychological battery. At the end of the 12-month visit, we re-consented all active participants for longer-term follow-up that included annual questionnaires and a final in-person neuropsychological assessment. This final visit occurred about 3–6 years after baseline (the fourth and final time point in the present analysis). See Figure 1 for a schematic of the study in-person visits. Inclusion criteria were 21–65 years of age, diagnosis of stage 0, I, II, or IIIA breast cancer, primary breast cancer treatments completed within the past 3 months, available for 12-month follow-up, and English proficiency. We excluded participants who had a history of prior cancer diagnosis or chemotherapy, whole brain irradiation or surgery, history of a neurological disease (e.g., seizures or epilepsy, dementia, multiple sclerosis), , head trauma with prolonged loss of consciousness, a current or past psychotic-spectrum disorder or current uncontrolled major affective disorder, an active autoimmune disorder, insulin-dependent diabetes, uncontrolled allergic condition or asthma, chronic steroid use, and hormone therapy (estrogen, progestin compounds) other than vaginal estrogen. This study was approved by the UCLA Institutional Review Board and all participants signed informed consent.
Figure 1:
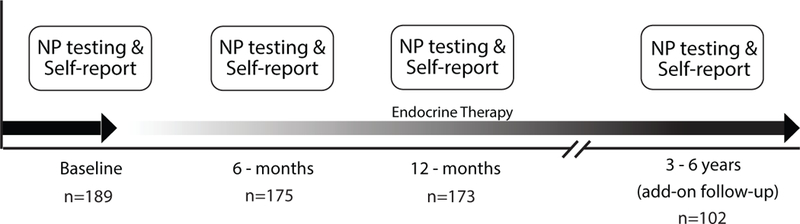
Study Diagram
Measures
We obtained clinical information through medical record review. Participants completed self-report questionnaires and a standard neuropsychological (NP) battery, presented in Table 1 by domain. Participants’ NP test scores were transformed into z-scores using appropriate published normative data, and then averaged into cognitive domains scores. We examined the frequency of test scores that were < −1.5 or < −2 z-score based on standard clinical practice, and characterized impairment based on International Cognition and Cancer Task Force guidelines.13 The battery changed slightly at the final 3–6 year visit (described in Table 1), but domains were comparable and treated as equivalent in analyses in order to capitalize on this unique and valuable long-term assessment.
Table 1:
Neuropsychological Tests by Domain
| Domain | Test/Measure |
|---|---|
| Learning | CVLT-II List A Total Trials 1–5 |
| WMS-III LM I | |
| BVMT-R Total Trials 1–3 | |
| Memory | CVLT-II List A Long Delay Free Recall |
| WMS-III LM II | |
| BVMT-R Delayed Recall | |
| ROCFT 3-minute Delayed Recall | |
| Attention | WAIS-III Digit Span, Coding, Letter-Number Sequencing |
| TMT A completion time | |
| PASAT Trial 2 total errors | |
| Visuospatial | ROCFT copy |
| WAIS-III Block Design | |
| Executive Functioning | TMT B |
| Stroop Color Word Interference13 | |
| Verbal Fluency (FAS) | |
| Processing Speed | Grooved Pegboard14 |
| Stroop Word Reading & Color Naming | |
BVMT-R=Brief Visuospatial Memory Test-Revised15; CVLT-II=California Verbal Learning Test-II16;TMT=Trail Making Test17; ROCFT=Rey-Osterreith Complex Figure Test18,19; Paced Auditory Serial Attention Test20; WAIS-III=Wechsler Adult Intelligence Scale-3rd Edition21; WMS-III=Wechsler Memory Scale-3rd Edition22
Note: WAIS and WMS tests at BL, 6-, and 12-month visits are the 3rd edition; 4th edition tests were used at the 3–6 year visit, which are highly correlated to the 3rd edition tests. The Stroop test was not administered at the 3–6 year visit. Also of note, 20 participants were missing WAIS-III Digit Span data at BL.
Statistical Analyses
We used T-tests and chi square tests to examine group differences on demographic and clinical variables. We divided participants into two groups based on whether or not they started ET shortly after primary cancer treatment (i.e., ET Yes/ET No). The ET factor in models is time-invariant, akin to an intent-to-treat approach; the rationale being our focus is the effects of any ET exposure initiated after primary treatment. Most women planning to initiate ET treatment did so within the first few months after the baseline assessment and the majority continued on some ET without interruption. However, not all women consistently took their medications or took the same type of ET, so we present a breakdown of ET use patterns. To test group differences among cognitive domains over time, we conducted linear mixed effect models for repeated measures, which accommodates missing data within participants. We examined various covariance structures and diagonal was consistently superior. All models included random intercepts, fixed effects included time, age, IQ, chemotherapy exposure, BDI-II, and race, as well as the terms of interest - the main effects of ET and ET x time. We modelled time as a categorical factor (i.e., baseline, 6-months, 12-months, and 3–6 years). We examined if −2 Restricted Log Likelihood criteria improved with the addition of the two ET terms. We also tested ET group differences in neuropsychological impairment variables using ANCOVA and binary logistic regression at each time point; these analyses controlled for age, IQ, race, BDI-II, and chemotherapy exposure. We used IBM SPSS v. 24 software, and statistical significance was set at p<.05.
Results
We enrolled 191 BCS in the MBS study. We excluded two participants’ data based on incomplete testing and English being a second language. Five participants missed their 6-month evaluation, and five participants dropped out after their 6-month evaluation. Two participants were missing neuropsychological data at 12 months. The resulting analyzable sample size was 189 at baseline (63 ET No, 126 ET Yes, i.e., prescribed subsequently), 175 at 6-months (49 ET No, 126 ET Yes), 173 at 12-months (52 ET No, 121 ET Yes), and 102 at 3–6 years (28 ET No, 74 ET Yes). Table 2 presented the baseline demographic and clinical characteristics of the total sample, and by ET group. Participants in the two groups were comparable across most demographic and clinical variables, with expected differences of higher stage of disease and higher frequency of radiation treatment in women prescribed ET.
Table 2:
Sample Characteristics
| Whole Sample (n=189) | ET No (n=63) | ET Yes (n=126) | P value | ||
|---|---|---|---|---|---|
| Age, mean(SD), range | 51.29(8.35), 30–66* | 50.56(9.25), 32–66* | 51.66(7.88), 30–65 | 0.39 | |
| Education, n(%) | < College | 34(18%) | 15(24%) | 19(15%) | 0.33 |
| College Degree | 56(30%) | 17(27%) | 39(31%) | ||
| > College | 99(52%) | 31(49%) | 68(36%) | ||
| Marital Status, n(%) married, n=188 | 124(66%) | 41(66%) | 83(66%) | 0.97 | |
| Race n (%) White | 150(79%) | 46(73%) | 102(81%) | 0.13 | |
| Annual Income, n(%; n=186) | >$100,000 | 73(39%) | 29(47%) | 44(35%) | 0.22 |
| <$100,000 | 112(60%) | 32(50%) | 80(64%) | ||
| Employment Status, n(%) employed FT or PT | 121(64%) | 41(65%) | 80(63%) | 0.34 | |
| Surgery | Lumpectomy | 125(66%) | 37(59%) | 88(70%) | 0.13 |
| Mastectomy | 64(34%) | 26(41%) | 38(30%) | ||
| Anthracycline treatment, n | 24 | 9 | 15 | 0.26 | |
| Stage at Diagnosis, n (% of group) | 0 | 25(13%) | 15(24%) | 1(8%) | 0.02 |
| 1 | 87(46%) | 24(38%) | 63(50%) | ||
| 2 | 59(31%) | 18(28%) | 41(33%) | ||
| 3 | 18(10%) | 6(10%) | 12(9%) | ||
| Radiation treatment, n(%) | 141(75%) | 41(65%) | 100(79%) | 0.03 | |
| Chemotherapy treatment, n(%) | 98(52%) | 28(44%) | 70(55%) | 0.15 | |
| Post-menopausal, n(%) | 101(53%) | 32(51%) | 69(55%) | 0.61 | |
| Ever took HRT (n=185) | 54(28%) | 14(23%) | 40 | 0.19 | |
| BDI-II, mean (SD) (n=188) | 8.77(6.87) | 8.82(7.06) | 8.75 (6.67) | 0.95 | |
| State Anxiety Inventory, mean (SD) (n=188) | 35.45(8.74) | 35.63(8.79) | 35.36(8.75) | 0.84 | |
| IQ WTAR, mean (SD) (n=188) | 114.31(9.07) | 113.55(9.92) | 114.68(8.63) | 0.42 | |
BDI-II = Beck Depression Inventory, 2nd edition; WTAR = Wechsler Test of Adult Reading
Inclusion criteria for age is 21–65; one participant turned 66 between screening and baseline
Of the original study sample, 102 women (54%) consented to undergo neuropsychological evaluation at the longer-term follow-up; participants were 4.3 (±0.66) years from baseline on average (range 3–6 years), and there was no difference in follow-up period between ET groups (p=.24). The rest of the initial sample either declined or were not reachable. To examine participation bias, we compared baseline differences between those who consented to return for neuropsychological testing and those who did not. We found that those who consented to the add-on follow-up were comparable to those who did not on age (p=.27), IQ (p=.74), education (p=.93), chemotherapy treatment (p=.56), and BDI-II scores (p=.34). However we did find that those who consented to the add-on follow-up exhibited higher Executive Function scores at baseline than those who did not (p=.02), but comparable performance in other domains. We did not see any differences in participation in the add-on follow-up based on ET use or type of ET medication.
Endocrine Therapy Use
Of those who returned for a follow-up visit, 126 had started ET and 49 had not, which defined the grouping variable used in models. The majority of those starting ET (97%) began treatment after their baseline evaluation and before their 6-month evaluation, and 4 started after their 6-month but before their 12-month evaluation. Two participants began ET after their 12 month evaluation but before 3–6 years (one started an AI and one tamoxifen), whom we grouped as No ET since our analyses address ET exposure right after primary cancer treatment. Since this was a naturalistic study several participants did go on and off treatment or switched treatments over the course of the study. At 6-months, 61 were on tamoxifen, 57 on an AI, and 4 on ovarian suppression. The majority of those who started an AI were post-menopausal (93%); the majority who started Tamoxifen or ovarian suppression were pre-menopausal (77% and 100%, respectively). At 12 months, 54 continued tamoxifen, 52 continued an AI, and all four continued ovarian suppression – others newly started (n=4), switched medications (n=4), or stopped altogether (n=3). Of those on ET who returned for the 3–6 year visit, most remained on one (n=63, 85%), but some switched agents (n=17). A few went on and off ET over time points (n=11, 15%). At 3–6 years, 15/25 taking tamoxifen and 30/46 taking an AI had been consistently taking the same medication type at prior visits; the one person on ovarian suppression had switched from Tamoxifen.
Neuropsychological Performance by ET Group
We examined neuropsychological performance over time based on ET group using mixed effects models that included the main effects of age, IQ, BDI-II, race, and chemotherapy exposure as well as random intercepts. The F-tests for the fixed effects of Time and ET, and the ET x Time term are presented in Table 3. Figure 2 displays graphs of each domain by ET group across all time points (estimates from linear mixed effects models). As portrayed in the table and graphs, we did not observe a significant main effect of ET or a significant ET x Time interaction in any domain. Significant main effects of time generally portray improvement from baseline in the first year in most domains.
Table 3:
Tests of Fixed Effects in Mixed Effects Models of Cognitive Domains
| Learning | Memory | Attention | Visuospatial | Executive Function | Processing Speed | |
|---|---|---|---|---|---|---|
| Time | F(3,226.22)=42.06, p<.01 | F(3,250.16)=56.55, p<.01 | F(3,246.64)=44.26, p<.01 | F(3,264.52)=22.66, p<.01 | F(3,180.76)=41.96, p<.01 | F(3,186.75)=40.72, p<.01 |
| ET | F(1,203.61)=2.46, p=.12 | F(1, 196.79)=1.19, p=.28 | F(1,193.02)=.003, p=.96 | F(1,196.43)=1.19, p=.28 | F(1,232.68)=.001, p=.97 | F(1,242.486)=2.05, p=.15 |
| ET x Time | F(3,200.11)=1.77, p=.15 | F(3,218.36)=1.75, p=.16 | F(3,219.61)=.17, p=.92 | F(3, 237.62)=1.55, p=.20 | F(3,163.32)=.94, p=.42 | F(3,166.36)=.29, p=.83 |
Fixed effects included in the models were: age, IQ, chemotherapy exposure, race, and BDI-II, and all models included a random intercept.
Time = Baseline, 6-month, 12-month, and 3–6 year visits (categorical, with Baseline as referent); ET = Endocrine therapy (no/yes)
Figure 2:
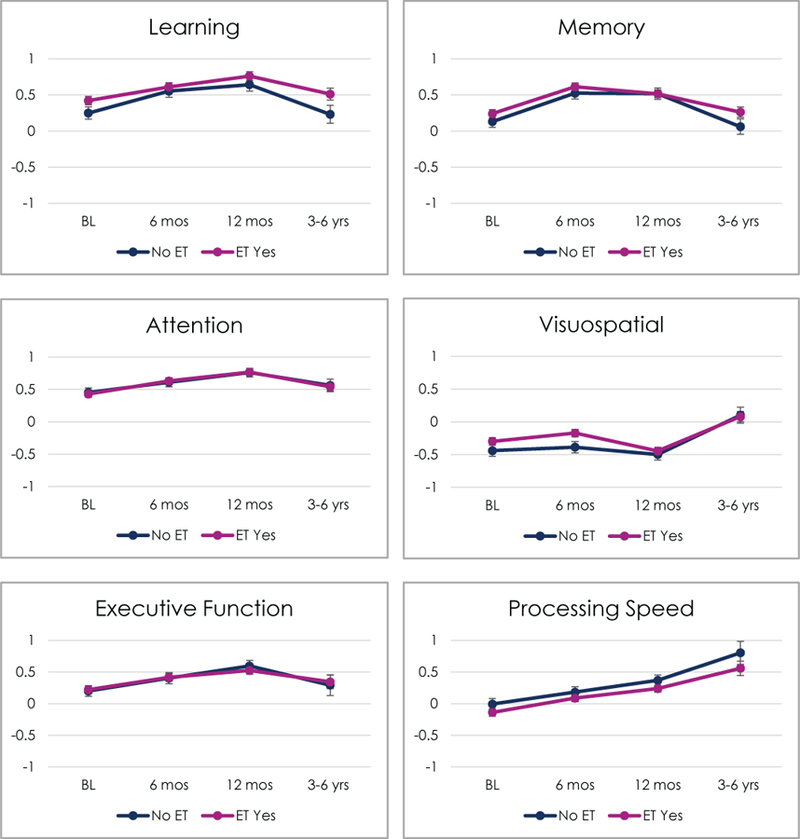
Cognitive Domains by ET Use Over Time
Impairment
We also examined rates of impairment on neuropsychological tests using standard cut-offs and impairment based on ICCTF criteria at each time point by ET use, controlling for age, IQ, BDI-II, race, and chemotherapy exposure. As displayed in Table 4, we did not detect any significant differences in the number of impaired tests or number of impaired participants by ICCTF criteria based on ET group at any time point.
Table 4:
Impairment Rates by ET Group
| No ET | ET Yes | Statistica | |||||
|---|---|---|---|---|---|---|---|
| BL | # of tests < −1.5, mean(SD) | 1.69(1.73) | 1.28(1.72) | F(1,181)=2.23,p=.14 | |||
| # of tests < −2, mean(SD) | 0.85(1.23) | 0.73(1.38) | F(1,181)=.15,p=.70 | ||||
| Impaired by ICCTF, n(%) | 35(55%) | 53(42%) | OR=.59(95% CI .31–1.11); Wald χ2=2.69,p=.10 |
||||
| n | 64 | 126 | |||||
| 6 mos | # of tests < −1.5, mean (SD) | 0.86(0.96) | 0.90(1.45) | F(1,167)=.17,p=.68 | |||
| # of tests < −2, mean (SD) | 0.45(0.68) | 0.46(1.08) | F(1,167)=.05,p=.83 | ||||
| Impaired by ICCTF, n(%) | 17(37%) | 33(26%) | OR=.71(95% CI .33–1.49); Wald χ2=.84,p=.36 |
||||
| n | 49 | 126 | |||||
| 12 mos | # of tests < −1.5, mean (SD) | 1.04(0.92) | 1.24(1.68) | F(1,167)=1.00,p=.32 | |||
| # of tests < −2, mean (SD) | 0.51(0.67) | 0.62(1.10) | F(1,167)=.63,p=.43 | ||||
| Impaired by ICCTF, n(%) | 24(45%) | 48(39%) | OR=.92(95% CI .46–1.83); Wald χ2=.06,p=.80 |
||||
| n | 53 | 122 | |||||
| TF | # of tests < −1.5, mean (SD) | 1.18(1.72) | .74(1.32) | F(1,95)=1.66,p=.20 | |||
| # of tests < −2, mean (SD) | 0.57(0.96) | 0.43(0.89) | F(1,95)=.47,p=.49 | ||||
| Impaired by ICCTF, n(%) | 10(36%) | 19(26%) | OR=.72(95% CI .26–1.99); Wald χ2=.39,p=.53 |
||||
| n | 28 | 74 |
Covariates in ANCOVA and binary logistic regression models included age, IQ, BDI-II, race, and chemotherapy exposure
ICCTF = International Cancer and Cognition Task Force impairment guidelines
Note: ICCTF impairment missing in the No ET group for 3 participants at BL, 2 at 6 and 12 mos.
Discussion
This prospective longitudinal study examined whether ET affected neuropsychological performance a well-characterized sample of BCS. We were able to extend our original study from a one-year follow-up period to include a neuropsychological assessment 3–6 years after baseline, making it the longest longitudinal cognitive study of ET to date. Based on our previous findings, we hypothesized that those taking ET would exhibit diminished neuropsychological performance compared to those not taking ET in models accounting for age, IQ, race, depression, and chemotherapy exposure.3,11 Contrary to our prediction, we observed no differences in any neuropsychological domain based on ET use in the first year after completing primary cancer treatments or at a subsequent follow-up 3–6 years later. Across most domains, both groups exhibited improvement at similar rates in the first year, followed by a return to baseline levels at the later 3–6 year follow-up. We also examined neuropsychological impairment in a few ways, again failing to find group differences in the rate of neuropsychological impairment over time. For the sake of simplifying analyses and interpretation, we used a time invariant variable for ET based on whether the participant started a medication shortly after completing primary treatment, i.e., not necessarily recent use although most were. We did also conduct sensitivity analyses using a time varying grouping variable (data not shown), i.e., group membership reflected recent ET use, and observed similar results across domains. Although side effects of ET have been a concern for patients and providers4,14, these negative neuropsychological test results contribute to reassurances about the safety of these medications.
While reassuring, our findings are somewhat surprising and require measured interpretation. The role of endogenous estrogen in the brain is complex and thought to be neuroprotective.15 ET agents cross the blood brain barrier and are known to interfere with the action of estrogen in the brain based on pre-clinical studies (for review see2). Several prior studies have suggested that ET leads to adverse cognitive outcomes, including work from our lab.3,6,8,10,11,16–18 Some studies suggest Tamoxifen may have cognitive effects,46,19 whereas other studies find evidence for AIs16 or both10. Cognitive data from well-controlled randomized clinical trials portray a similarly mixed picture, with some suggestion that Tamoxifen particularly leads to adverse cognitive effects6,18,20 but not AI.21 However, our study is one of the largest and longest to date and joins other cross-sectional and longitudinal studies that have similarly failed to find any cognitive effects of ET in the year after treatment.22,23 Likewise, results from a recent meta-analysis did not yield compelling evidence that ET has significant cognitive effects, finding only a cross-sectional effect on verbal memory and failing to find a longitudinal effect in any domain.24
Most studies of the cognitive effects of ET are complicated by methodological issues. First, most focus on post-menopausal women or a mixed sample of both pre- and post-menopausal women as in the current study. Our early report of worse cognition in pre-menopausal women taking Tamoxifen was then joined by only a few other studies.3,5,25 This methodological issue may be obscuring effects: menopausal status itself is associated with cognitive changes26 in addition to influencing the type of ET agent recommended, leading to different alterations of estrogen-related functioning in the brain2. Further, chemotherapy can induce early menopause22 and may have other unknown additive effects. Since many prior studies exclude chemotherapy treatment2, it remains unknown whether its neurotoxic effects interact with ET. Further, the role of prior hormone-replacement therapy is poorly understood. The current study was powered and designed for broader questions than these, when there was scarce evidence in this area. Thus, we need future nuanced investigations to better rule-out or isolate medication effects, and determine if we are missing vulnerable subgroups.
Second, as we have previously argued, the weaknesses of traditional neuropsychological tests may undercut efforts to detect subtle cognitive effects in cancer.27 Neuropsychological tests are subject to practice effects (i.e., “benefit” from prior test exposure28), complicating longitudinal observations. The pattern of improvement observed in this study may represent some early recovery from the effects of primary cancer treatments, but it seems likely that the initial improvement was rather due to practice effects, with later diminishment practice effects observed after a years-long break from testing. Future studies can better account for practice effects using methodologies not available in this study (e.g., a control group). Recently, others have similarly pointed out that “noise” in repeated neuropsychological assessments preclude adequate sensitivity to subtle effects in cancer samples.29 Other methods may be more useful, considering patients’ self-report of cognitive changes11, and promising but understudied neuroimaging approaches.30,31 Our prior reports from this sample have revealed worse self-reported cognitive symptoms at 6 months among those on ET32,33, and we will next systematically examine self-report measures across all time points.
Regarding study limitations, the sample is predominantly white and highly educated. Studies of the cognitive effects of ET particularly in diverse samples are needed, as effects may not be uniform. We do not have a pre-cancer treatment assessment, which is important for isolating effects and recovery from other treatments. Our study was naturalistic and treatment regimens followed clinical recommendations; therefore, women who experience more severe side effects may have opted to discontinue ET or switch to a different medication, potentially diminishing observable effects. Importantly, initial study retention was good, but we detected participation bias among those who consented to return for the add-on follow-up several years later, with better baseline executive function among those who returned. Other longitudinal studies of the cognitive effects of ET have similarly found that those who dropped out were more cognitively vulnerable6, or more likely on ET34. In the cognitive aging field it is well-known that attrition is often disproportionately observed in those with cognitive compromise.35 Notably, the recent meta-analysis only found a cognitive effect of ET in cross-sectional studies, not longitudinal.24 Longitudinal studies of ET such as this one may be missing important data from vulnerable women who choose to avoid lengthy and challenging cognitive assessments. Additionally, the final time point captures a wide timeframe from baseline (i.e., 3–6 years), reflecting the range of the initial study enrollment period, and future studies are needed to more carefully establish cognitive risk over time.
In sum, our findings suggest that there are not significant neuropsychological differences in performance or impairment rates between BCS who do or do not start taking ET after primary cancer treatment, even years after starting these medications. This provides some reassurance to the many thousands of women taking these medications who may be concerned about cognitive effects. However, in the context of a mixed literature and important methodological considerations, our results highlight the need for more nuanced study of these treatments and the possibility of vulnerable subgroups. Given the broad and complex effects of estrogen function in the brain, intensive study of ET’s risk to brain health remains a priority.
Acknowledgments
Funding: This work was supported by the National Cancer Institute at the National Institute of Health grant number R01 CA109650, P30 CA16042, L30 CA220873, the American Cancer Society (KVD), and the Breast Cancer Research Foundation (PAG).
Footnotes
Conflict of Interest: Dr. Ganz is a Scientific Advisory Board member for the Breast Cancer Research Foundation.
References
- 1.Runowicz CD et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. CA. Cancer J. Clin 66, 43–73 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Zwart W, Terra H, Linn SC & Schagen SB Cognitive effects of endocrine therapy for breast cancer: keep calm and carry on? Nat Rev Clin Oncol 12, 597–606 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Castellon SA et al. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol 26, 955–969 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Boele FW, Schilder CMT, de Roode M-L, Deijen JB & Schagen SB Cognitive functioning during long-term tamoxifen treatment in postmenopausal women with breast cancer. Menopause 22, 17–25 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Chen X et al. Decision-making impairments in breast cancer patients treated with tamoxifen. Horm. Behav 66, 449–456 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Schilder CM et al. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J. Clin. Oncol 28, 1294–1300 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Bender CM et al. Cognitive impairment associated with adjuvant therapy in breast cancer. Psycho‐Oncology 15, 422–430 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Bender CM et al. Patterns of change in cognitive function with anastrozole therapy. Cancer 121, 2627–2636 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debess J, Riis JØ, Engebjerg MC & Ewertz M Cognitive function after adjuvant treatment for early breast cancer: a population-based longitudinal study. Breast Cancer Res. Treat 121, 91–100 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Collins B, Mackenzie J, Stewart A, Bielajew C & Verma S Cognitive effects of hormonal therapy in early stage breast cancer patients: a prospective study. Psycho‐Oncology 18, 811–821 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Ganz PA et al. Cognitive function after the initiation of adjuvant endocrine therapy in early-stage breast cancer: an observational cohort study. J. Clin. Oncol 32, 3559–67 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganz PA, Petersen L, Bower JE & Crespi CM Impact of Adjuvant Endocrine Therapy on Quality of Life and Symptoms: Observational Data Over 12 Months From the Mind-Body Study. J. Clin. Oncol 34, 816–24 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wefel JS, Vardy J, Ahles T & Schagen SB International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol 12, 703–708 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Bender CM et al. Influence of patient and treatment factors on adherence to adjuvant endocrine therapy in breast cancer. Oncol. Nurs. Forum 41, 274–85 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hara Y, Waters EM, McEwen BS & Morrison JH Estrogen Effects on Cognitive and Synaptic Health Over the Lifecourse. Physiol. Rev 95, 785–807 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bender CM et al. Memory impairments with adjuvant anastrozole versus tamoxifen in women with early-stage breast cancer. Menopause (New York, NY) 14, 995 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkins V, Shilling V, Fallowfield L, Howell A & Hutton S Does hormone therapy for the treatment of breast cancer have a detrimental effect on memory and cognition? A pilot study. Psychooncology 13, 61–66 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Phillips KA et al. Cognitive function in postmenopausal breast cancer patients one year after completing adjuvant endocrine therapy with letrozole and/or tamoxifen in the BIG 1–98 trial. Breast Cancer Res Treat 126, 221–226 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eberling JL, Wu C, Tong-Turnbeaugh R & Jagust WJ Estrogen- and tamoxifen-associated effects on brain structure and function. Neuroimage 21, 364–371 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Phillips K-A et al. Cognitive function in postmenopausal women receiving adjuvant letrozole or tamoxifen for breast cancer in the BIG 1–98 randomized trial. Breast 19, 388–95 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins VA et al. Effects of anastrozole on cognitive performance in postmenopausal women: a randomised, double-blind chemoprevention trial (IBIS II). Lancet Oncol 9, 953–961 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Hermelink K et al. Short‐term effects of treatment‐induced hormonal changes on cognitive function in breast cancer patients. Cancer 113, 2431–2439 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Breckenridge LM, Bruns GL, Todd BL & Feuerstein M Cognitive limitations associated with tamoxifen and aromatase inhibitors in employed breast cancer survivors. Psychooncology (2010). 10.1002/pon.1860 [DOI] [PubMed] [Google Scholar]
- 24.Underwood EA et al. Cognitive sequelae of endocrine therapy in women treated for breast cancer: a meta-analysis. Breast Cancer Res. Treat 1–12 (2017). 10.1007/s10549-017-4627-4 [DOI] [PubMed] [Google Scholar]
- 25.Palmer JL, Trotter T, Joy AA & Carlson LE Cognitive effects of Tamoxifen in pre-menopausal women with breast cancer compared to healthy controls. J. Cancer Surviv 2, 275–282 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Greendale GA et al. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology 72, 1850–7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dyk K Van & Ganz PA Doctor, Now That My Chemotherapy Treatment Is Over, When Will My ‘Chemofog’ Lift? J. Clin. Oncol 35, 482–484 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Duff K, Callister C, Dennett K & Tometich D Practice Effects: A Unique Cognitive Variable. Clin. Neuropsychol 26, 1117–1127 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Andreotti C et al. Reliable change in neuropsychological assessment of breast cancer survivors. Psychooncology 25, 43–50 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurria A et al. The Effect of Aromatase Inhibition on the Cognitive Function of Older Patients With Breast Cancer. Clin. Breast Cancer 14, 132–140 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bayer J et al. The effect of estrogen synthesis inhibition on hippocampal memory. Psychoneuroendocrinology 56, 213–225 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Ganz PA et al. Cognitive complaints after breast cancer treatments: examining the relationship with neuropsychological test performance. J. Natl. Cancer Inst 105, djt073 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ganz PA, Petersen L, Bower JE & Crespi CM Im pact of Adjuvant Endocrine Therapy on Quality of Life and Symptoms: Observational Data Over 12 Months From the Mind-Body Study. J. Clin. Oncol 34, 816–824 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hedayati E, Alinaghizadeh H, Schedin A, Nyman H & Albertsson M Effects of adjuvant treatment on cognitive function in women with early breast cancer. Eur. J. Oncol. Nurs 16, 315–322 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Yao C, Stawski RS, Hultsch DF & MacDonald SWS Selective attrition and intraindividual variability in response time moderate cognitive change. J. Clin. Exp. Neuropsychol 38, 227–37 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]


