Abstract
Background
No prior study has measured or compared self‐reported and objectively measured physical activity trajectories in prostate cancer survivors before and after treatment.
Methods
Clinically localized prostate cancer patients treated with radical prostatectomy were recruited between 2011 and 2014. Of the 350 participants enrolled at the main site, 310 provided self‐reported physical activity at baseline before radical prostatectomy, and 5 weeks, 6 months, and 12 months after radical prostatectomy. A subset of participants (n = 81) provided objectively measured physical activity at all study time points by wearing an accelerometer for 7 days each. Changes in activity over time were compared using Friedman’s test. Agreement between self‐reported and objective measures was evaluated using Spearman’s rank correlation coefficient.
Results
Self‐reported moderate‐to‐vigorous physical activity was high at baseline (median, 32.1 min/day), followed by a decline at 5 weeks (median, 15.0 min/day) and a recovery at 6 and 12 months (median, 32.1‐47.1 min/day). In contrast, objectively measured moderate‐to‐vigorous physical activity was low at all 4 time points (median, 0.0‐5.2 min/day), with no overall change across study assessments (global P = .29). Self‐reported moderate‐to‐vigorous physical activity tended to be more closely related to objectively measured light‐intensity physical activity (ρ = 0.29‐0.42) than to objectively measured moderate‐to‐vigorous physical activity (ρ = 0.07‐0.27, P = .009‐.32).
Conclusions
In our population of prostate cancer survivors with critically low moderate‐to‐vigorous physical activity levels, self‐reported measures greatly overestimated moderate‐to‐vigorous physical activity and may have been more reflective of light‐intensity physical activity. Because cancer survivor guidelines are derived from self‐reported data, our findings may imply that intensities of physical activity below moderate, such as light intensity, still have health benefits.
Keywords: prostate cancer, prostatectomy, accelerometer, free‐living physical activity, sedentary behavior
Short abstract
In a population of 81 prostate cancer survivors with critically low moderate‐to‐vigorous physical activity levels, self‐reported measure greatly overestimates moderate‐to‐vigorous physical activity and may be more reflective of light‐intensity physical activity. Because prostate cancer guidelines are derived from self‐reported moderate‐to‐vigorous physical activity, the findings imply that lower intensities of physical activity, such as light‐intensity physical activity, may still have health benefits.
Introduction
In cancer survivors (defined as anyone diagnosed with cancer),1 regular and sustained participation in physical activity is associated with reduced cancer recurrence and improved survival, as well as a range of physical and psychological outcomes and health‐related quality of life.1, 2, 3, 4, 5 The majority of knowledge about the benefits of physical activity among cancer survivors has been derived from self‐reported,1, 2, 3, 4 as opposed to objectively measured,6, 7, 8 physical activity data. Although self‐reported measures are useful for ranking participants by their relative physical activity levels and for monitoring changes in activity over time, they are less useful for estimating absolute levels of free‐living physical activity. Self‐reported measures have lesser accuracy for measuring light‐intensity physical activity (eg, routine domestic tasks) than objective measures, require highly complex cognitive processing, and are susceptible to social desirability and recall biases.9 Moreover, many of these factors may be more pronounced in cancer survivors. For example, cancer survivors in general may have greater difficulty recalling physical activity because of cancer‐ or therapy‐related cognitive impairments, such as lack of concentration and short‐term memory loss.10, 11 However, no study to date has compared self‐reported with objectively measured physical activity in cancer survivors to inform the degree of discrepancy between these 2 measures.
A key challenge for the development of cancer survivor physical activity guidelines is to identify the appropriate timing of physical activity engagement, given that little is known about cancer survivors’ physical activity levels before and after therapy. Although a few studies have documented declined physical activity levels following a cancer diagnosis using self‐reported measures,12, 13, 14, 15 these may not reflect actual levels for the aforementioned reasons. Moreover, discrepancies between self‐reported and objective measures may be exacerbated further by the influence of therapy on survivors’ activity levels and cognitive function. A limited number of studies have collected repeated measures of objective physical activity in cancer survivors to inform actual trajectories, but most of these studies were small in size (eg, <30 participants16, 17, 18, 19, 20) or lacked pretreatment data.21, 22 Therefore, additional studies of objectively measured physical activity trajectories in cancer survivors are needed to document their actual physical activity levels throughout their survivorship.
To begin to address these gaps, we investigated and compared levels and patterns of self‐reported and objectively measured physical activity in a large cohort of men undergoing radical prostatectomy for clinically localized prostate cancer, the most commonly diagnosed cancer in men in the United States.
Patients and Methods
Study Population and Design
The Prostatectomy, Incontinence, and Erectile Function study was a multicenter longitudinal clinical cohort study based at 2 sites in the United States: Washington University School of Medicine and Brigham & Women’s Hospital. Men scheduled to undergo radical prostatectomy for clinically localized prostate cancer were recruited between September 2011 and January 2014. All men undergoing prostatectomy were eligible except for those who had previously undergone treatment for prostate cancer, radiation treatment to the pelvic region (including bladder, rectum, or prostate), major pelvic surgery, or placement of a penile implant or artificial urinary sphincter. We also excluded men with known urethral stricture or colostomy, men who were unable to urinate and required chronic urinary catheterization, and men who did not speak English. In total, we enrolled 350 men at Washington University and 76 at Brigham & Women’s Hospital.
Before their surgery, participants were asked to complete a questionnaire that included questions on recent physical activity, weight history, insurance status, urinary and sexual function and bother, medications that can impact urinary and sexual function, and a range of socio‐demographic characteristics. Participants completed a similar questionnaire 5 weeks, 6 months, and 12 months after surgery. Comorbidity data and clinical prostate cancer information (including stage, Gleason score, and pretreatment prostate‐specific antigen levels) were abstracted from participants’ medical records. Baseline height and weight were measured at the preoperative clinic visit by nursing staff. Participants enrolled at Washington University were also given the option of wearing an activity monitor. Those who agreed were asked to wear an Actigraph GT3X+ accelerometer for 7 days at each of the study time points: baseline before radical prostatectomy and 5 weeks, 6 months, and 12 months after radical prostatectomy. Participants enrolled at Brigham & Women’s Hospital were not given the option of wearing an accelerometer and were thus not included in the present analyses. The Prostatectomy, Incontinence, and Erectile Function Study was approved by the institutional review boards at Washington University School of Medicine and Brigham & Women’s Hospital. All participants provided informed consent.
Self‐Reported Physical Activity
Recent physical activity was assessed using the Community Healthy Activities Model Program for Seniors (CHAMPS) physical activity questionnaire for older adults. This instrument measures physical activities that participants may have engaged in “in a typical week in the past four weeks”. The CHAMPS instrument has been found to have good reproducibility in older men (Pearson’s r = 0.58‐0.67)23 and has been validated in racial/ethnic minorities.24, 25 It was administered at all 4 study time points. We used this questionnaire to summarize the total daily self‐reported amount of moderate‐to‐vigorous physical activity in minutes for each participant at each study assessment.
Objectively Measured Physical Activity
A subsample of participants agreed to wear a waist‐worn accelerometer (Actigraph GT3X+, 1 second epochs) for 7 consecutive days to measure free‐living physical activity objectively. The Actigraph GT3X+ is a small, lightweight, extensively validated device that provides detailed information about the intensity, frequency, and duration of physical activity.22, 26, 27, 28 The epoch length was set at 1 second, and the Actigraph recorded count data for physical activity in the form of counts per minute (cpm). Nonwearing time was defined as 60 minutes of consecutive zero counts with allowance for 1 or 2 minutes with <100 cpm. A recording of at least 10 hours of data (about two thirds of waking hours per day) was defined as a valid day, and 3 or more valid days measured at any time during the 7‐day wearing period were required for the analysis.22
Actigraph data were processed using ActiLife software based on the Freedson equation29 to derive 1) total wearing time, 2) “raw” minutes spent in moderate‐to‐vigorous physical activity bouts (>1951 cpm) of at least 10 minutes, and 3) sedentary behavior (<100 cpm). Raw minutes spent in light‐intensity physical activity were calculated by subtracting raw minutes spent in moderate‐to‐vigorous physical activity bouts of 1 minute and sedentary behavior from the total wearing time, namely 100 to 1951 cpm. Adjusted minutes were computed by dividing raw minutes by total wearing time and multiplying the resulting fraction by the average wearing time for all participants. We summarized the adjusted total daily amount of moderate‐to‐vigorous physical activity, light‐intensity physical activity, and sedentary behavior in minutes for each participant at all 4 study time points.
Statistical Analysis
Descriptive statistics (means and percentages) were used to compare participants who did and did not provide valid objectively measured physical activity data at the Washington University site. Participants were compared with respect to socio‐demographic characteristics, prostate cancer‐related factors, and surgery‐related factors. Medians and interquartile ranges were used to summarize participants’ self‐reported moderate‐to‐vigorous physical activity and objectively measured time spent in moderate‐to‐vigorous physical activity, light‐intensity physical activity, and sedentary behavior. Changes in activity over time were compared using Friedman’s test. Post hoc analyses using Wilcoxon’s signed‐rank tests were performed to identify times when changes occurred. Finally, agreement between self‐reported and objectively measured physical activity was evaluated using Spearman’s rank correlation coefficient. Data analyses were conducted using STATA, SAS, and R.
Results
In total, 350 participants completed the baseline survey before radical prostatectomy at the Washington University site, and 310 (88.6%) provided complete survey data at all 4 study time points. Of these men, 203 agreed to wear an accelerometer, and 193 provided valid objectively measured physical activity data at baseline, 143 at 5 weeks, 119 at 6 months, and 108 at 12 months, for a total of 81 men with complete objectively measured physical activity data at all 4 time points (Fig. 1). These 81 participants were similar to those who did not provide valid objectively measured physical activity data, with the exception of race, body mass index, marital status, presurgical prostate‐specific antigen concentration, and possibly education. Of the 26 (8.4% of 310) African American participants who completed the study, only 1 (1.2% of 81) provided objectively measured physical activity data (Table 1). In addition, men who did not provide valid objectively measured physical activity data were more likely to be obese, live alone, and have a higher presurgical prostate‐specific antigen concentration than participants who did provide valid objectively measured physical activity data. They were also nonsignificantly less likely to have completed a graduate education. Overall, the majority of the 81 analyzed men were overweight (49.4%) or obese (35.8%), had completed a college education or more (50.3%), earned ≥$75,000 (52.6%), were married or living as married (82.6%), and had clinical stage T1 disease (76.8%), with a median presurgical prostate‐specific antigen level of 5.2 ng/mL (interquartile range, 4.1‐6.9).
Figure 1.
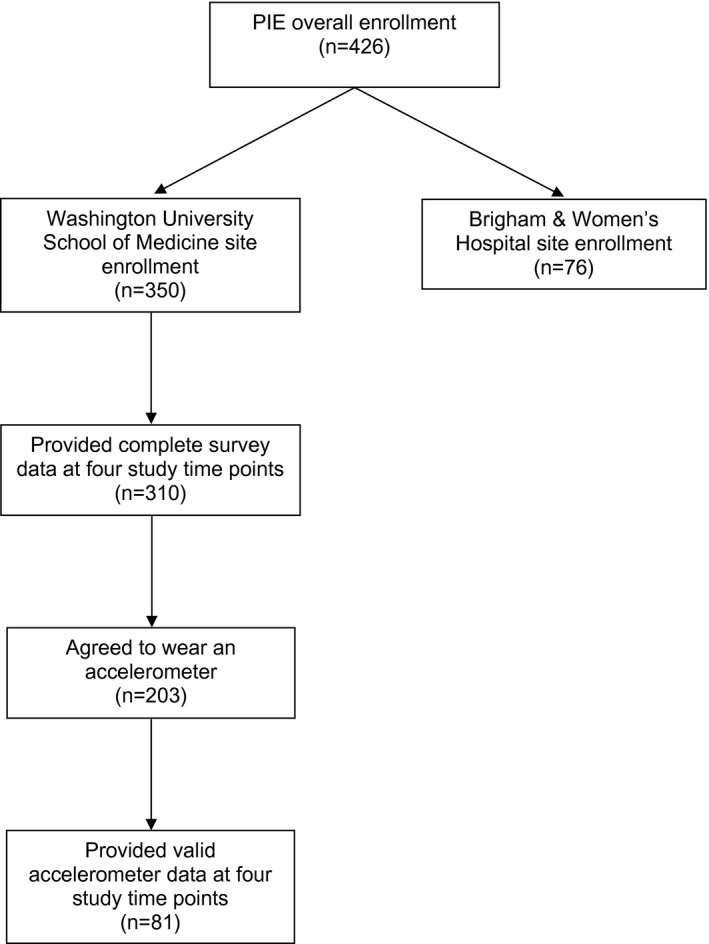
Flow diagram illustrating prostate cancer survivors in the Prostatectomy, Incontinence, and Erectile Function Study (PIE) population and analyzed sample.
Table 1.
Baseline Characteristics of Participants in the Prostatectomy, Incontinence, and Erectile Function Study
| Sociodemographic | Provided Valid Objectively Measured Physical Activity Data | |||
|---|---|---|---|---|
| Total (N = 310) | No (n = 229) | Yes (n = 81) | P | |
| Age, y, mean (SD) | 61.1 (6.9) | 60.8 (7.0) | 61.9 (6.4) | .27 |
| Race, n (%) | .02 | |||
| White | 283 (91.3) | 203 (88.7) | 80 (98.8) | |
| African American | 26 (8.4) | 25 (10.9) | 1 (1.2) | |
| Asian | 1 (0.3) | 1 (0.4) | 0 (0) | |
| BMI, n (%) | .005 | |||
| Normal weight (18.5‐24.9 kg/m2) | 46 (14.8) | 27 (11.8) | 19 (23.5) | |
| Overweight (25.0‐29.9 kg/m2) | 153 (49.4) | 110 (48.0) | 43 (53.1) | |
| Obese (≥30 kg/m2) | 111 (35.8) | 92 (40.2) | 19 (23.4) | |
| Education, n (%) | .12 | |||
| High school degree or less | 56 (18.1) | 46 (20.1) | 10 (12.3) | |
| Some college | 98 (31.6) | 76 (33.2) | 22 (27.2) | |
| College degree | 75 (24.2) | 54 (23.6) | 21 (25.9) | |
| Postgraduate | 81 (26.1) | 53 (23.1) | 28 (34.6) | |
| Household income, n (%) | .38 | |||
| $50,000‐<$75,000 | 147 (47.4) | 112 (48.9) | 35 (43.2) | |
| ≥$75,000 | 163 (52.6) | 117 (51.1) | 46 (56.8) | |
| Marital status, n (%) | .02 | |||
| Married or living with partner | 256 (82.6) | 182 (79.5) | 74 (91.4) | |
| Living alone | 54 (17.4) | 47 (20.5) | 7 (8.6) | |
| Cigarette smoking status, n (%) | .30 | |||
| Never smoked | 179 (57.8) | 132 (57.7) | 47 (59.3) | |
| Former smoker | 108 (34.8) | 77 (33.6) | 31 (34.2) | |
| Current smoker | 23 (7.4) | 20 (8.7) | 3 (5.5) | |
| T1 stage before surgery, n (%) | 238 (76.8) | 173 (75.6) | 65 (82.3) | .39 |
| PSA level before surgery, median (IQR) | 5.2 (4.1‐6.9) | 5.4 (4.3‐7.2) | 4.6 (3.9‐5.8) | .04 |
| Surgical procedure, n (%) | .20 | |||
| Laparoscopic | 150 (48.4) | 117 (51.1) | 33 (40.7) | |
| Open | 18 (5.8) | 14 (6.1) | 4 (5.0) | |
| Robotic | 142 (45.8) | 98 (42.8) | 44 (54.3) | |
Abbreviations: BMI, body mass index; IQR, interquartile range; PSA, prostate‐specific antigen; SD, standard deviation.
Self‐Reported Physical Activity
At baseline, participants’ self‐reported levels of daily moderate‐to‐vigorous physical activity were high (median = 32.1 minutes), with 72.8% meeting general physical activity guidelines (ie, 150 min/wk of moderate‐to‐vigorous physical activity) (Table 2). These levels declined 5 weeks after radical prostatectomy (15.0 minutes; post hoc P < .001 compared with baseline; 38.3% meeting physical activity guidelines), followed by a recovery at 6 months (32.1 minutes; post hoc P < .001 compared with 5 weeks after radical prostatectomy; 70.4% meeting physical activity guidelines). Self‐reported physical activity levels remained stable at 12 months (47.1 minutes; post hoc P = ..30 compared with 6 months post‐RP; 71.6% meeting physical activity guidelines).
Table 2.
Median and Interquartile Range of Activity Patterns Among Prostate Cancer Survivors in the Prostatectomy, Incontinence, and Erectile Function Study (n = 81)
| Baseline | 5 weeks | 6 months | 12 months | P [Link] | |
|---|---|---|---|---|---|
| Questionnaire (CHAMPS[Link]) | |||||
| Moderate‐to‐vigorous intensity physical activity | |||||
| Daily minutes | 32.1 (15.0‐77.1) | 15.0 (0.0‐32.1) | 32.1 (15.0‐64.3) | 47.1 (15.0‐79.3) | <.001 |
| Meeting physical activity guideline | 72.8% | 38.3% | 70.4% | 71.6% | |
| Post hoc P [Link] | <.001 | <.001 | .30 | ||
| Accelerometer (Actigraph GTX 3) | |||||
| Moderate‐to‐vigorous intensity physical activity | |||||
| Daily minutes | 3.9 (0.0‐11.5) | 5.2 (0.0‐11.4) | 0.0 (0.0‐7.5) | 3.1 (0.0‐11.1) | .29 |
| Meeting physical activity guideline | 11.1% | 11.1% | 8.6% | 6.2% | |
| Post hoc P [Link] | .96 | .03 | .45 | ||
| Light‐intensity physical activity | |||||
| Daily minutes | 195.6 (159.7‐240.6) | 180.4 (141.1‐214.3) | 201.2 (161.5‐244.9) | 190.1 (165.9‐243.6) | .008 |
| Post hoc P [Link] | <.001 | .01 | .61 | ||
| Sedentary behavior | |||||
| Daily minutes | 488.8 (448.8‐529.3) | 511.5 (467.3‐554.8) | 489.3 (445.7‐525.3) | 501.5 (439.2‐529.1) | .02 |
| Post hoc P [Link] | .001 | .02 | .58 |
Friedman’s test.
Community Healthy Activities Model Program for Seniors.
Wilcoxon’s signed‐rank test to compare the change with the previous data point.
Objectively Measured Physical Activity
In contrast to self‐reported moderate‐to‐vigorous physical activity, objective measures were low at all 4 time points (median, 0.0‐5.2 min/day; 6.2%‐11.1% meeting physical activity guidelines) and showed no overall change across study assessments (global P = .29) (Table 2). However, stepwise comparisons indicated a statistically significant but small decline in objectively measured daily moderate‐to‐vigorous physical activity from 5 weeks (5.2 minutes) to 6 months after radical prostatectomy (0.0 minutes; P = .03).
With regard to objectively measured light‐intensity physical activity, a similar pattern of findings was observed as for self‐reported moderate‐to‐vigorous physical activity. Participants engaged in a median of 195.6 minutes of daily light‐intensity physical activity before radical prostatectomy, after which they experienced a significant decline 5 weeks after radical prostatectomy (180.4 minutes; post hoc P < .001 compared with baseline). Levels of daily light‐intensity physical activity recovered 6 months after radical prostatectomy (201.2 minutes; post hoc P = .01 compared with 5 weeks after radical prostatectomy), and remained stable at 12 months (190.1 minutes; post hoc P = .61 compared with 6 months after radical prostatectomy).
Finally, the opposite pattern of findings was observed for sedentary behavior as for light‐intensity physical activity. Participants accumulated higher levels of sedentary behavior 5 weeks after radical prostatectomy (median, 511.5 minutes) compared with baseline (488.8 minutes; post hoc P = .001), after which their levels returned to baseline (6 months after radical prostatectomy: 489.3 minutes; post hoc P = .02 compared with 5 weeks after radical prostatectomy; post hoc P = .84 compared with baseline; and 12 months after radical prostatectomy: 501.5 minutes; post hoc P = .87 compared with baseline).
Agreement Between Self‐Reported and Objectively Measured Physical Activity
Agreement between self‐reported and objectively measured moderate‐to‐vigorous physical activity was poor at all 4 time points (Ρ = 0.07‐0.27) (Table 3), as expected based on their differing patterns of findings over study follow‐up. In contrast, self‐reported moderate‐to‐vigorous physical activity and objectively measured light‐intensity physical activity were in considerably better agreement (Ρ = 0.29‐0.42, P = .009‐0.32 compared with self‐reported and objectively measured moderate‐to‐vigorous physical activity agreement).
Table 3.
Agreement (ρ) of Self‐Reported Moderate‐to‐Vigorous Physical Activity With Accelerometer‐Measured Moderate‐to‐Vigorous Physical Activity and Light‐Intensity Physical Activity, Respectively, in Prostate Cancer Survivors in the Prostatectomy Incontinence and Erectile Function Study (n = 81)
| Self‐reported moderate‐to‐vigorous physical activity | Accelerometer Measured (min/day) | |
|---|---|---|
| Moderate‐to‐Vigorous Physical Activity | Light‐Intensity Physical Activity | |
| Baseline | 0.22 | 0.29 |
| 5 wk after radical prostatectomy | 0.27 | 0.39 |
| 6 mo after radical prostatectomy | 0.07 | 0.42 |
| 12 mo after radical prostatectomy | 0.16 | 0.35 |
Discussion
The findings from our prospective cohort study of prostate cancer survivors show a disagreement between self‐reported and objectively measured levels of moderate‐to‐vigorous physical activity both before and after surgery. Whereas self‐reported moderate‐to‐vigorous physical activity levels were high and generally above physical activity guidelines at all study time points except for 5 weeks after radical prostatectomy, objectively measured levels were low and generally below physical activity guidelines at all 4 study time points. Interestingly, similar patterns of change were observed for self‐reported moderate‐to‐vigorous physical activity as for objectively measured light‐intensity physical activity and a stronger correlation was observed between self‐reported moderate‐to‐vigorous physical activity and objectively measured light‐intensity physical activity than between self‐reported and objectively measured moderate‐to‐vigorous physical activity, suggesting that self‐reported moderate‐to‐vigorous physical activity may have been more likely to capture physical activity at light intensity than moderate or vigorous intensities in this study population with critically low moderate‐to‐vigorous physical activity.
To the best of our knowledge, our study is the first to report trajectories of physical activity (either self‐reported or objectively measured) in prostate cancer survivors, precluding a comparison to previous studies. However, our findings for individual physical activity measures and values are consistent with those from previous studies. For example, our observed decline shortly after surgery is consistent with findings from a study of Australian breast and prostate cancer survivors in which participants reported a decline in self‐reported weekly moderate‐to‐vigorous physical activity of 72 minutes in the 6 weeks after diagnosis.12 In addition, our findings of generally low objectively measured moderate‐to‐vigorous physical activity at all time points after radical prostatectomy are consistent with those from a previous nationally representative cross‐sectional sample of American prostate cancer survivors in various stages of their recovery that observed low levels of physical activity (6.0 minutes of moderate‐to‐vigorous physical activity, 4.0 hours of light‐intensity physical activity, and 9.9 hours of sedentary behavior) using the same cpm cutoffs for accelerometer data used in our study.30 These values are within 10 minutes of our values; therefore, it is likely that they are clinically similar.31 Therefore, given these similarities in findings for individual physical activity measures, it is likely that our observed discrepancy between self‐reported and objectively measured moderate‐to‐vigorous physical activity (ie, overestimation of moderate‐to‐vigorous physical activity by self‐report), and perhaps our better agreement between self‐reported moderate‐to‐vigorous physical activity and objectively measured light‐intensity physical activity, would also generalize to these study populations.
Self‐reported measures may overestimate objectively measured moderate‐to‐vigorous physical activity for several reasons. The first is the nature of the questionnaire design. For example, the CHAMPS questionnaire includes a few sets of different items that may in fact capture the same activity (for example, walking fast, walking leisurely, walking for errands, and walking uphill), which may lead to double counting and overestimation of physical activity. This concern is particularly problematic for common activities, such as walking (prevalence in our sample: walking fast, 32%‐42%; walking leisurely, 51%‐69%; walking for errands, 44%‐48%; and walking uphill, 30%‐33%). It has also been found to be more common in men than in women.32 Another reason self‐reported moderate‐to‐vigorous physical activity may overestimate actual values, particularly in cancer survivors, is response bias.33 Unlike the general population, cancer survivors experience major changes in aerobic capacity, body composition, and mental burden from their cancer and its treatment, which may influence their perceptions of physical activity intensity dramatically. For example, we speculate that men who suffer from radical prostatectomy‐related adverse effects may perceive the same activity that was “light” before radical prostatectomy (eg, “work on your car, truck, lawn mower, or other machinery”) as “heavy” after radical prostatectomy. Finally, it is also possible that men may report a longer duration of activity because of their strong desire to be active, an important component of perceived normalcy for men after prostate cancer treatment.34
Considering our findings in light of cancer survivor physical activity guidelines (ie, 150 minutes of weekly moderate‐to‐vigorous physical activity35), our results for self‐reported data tend to meet these guidelines, but those for objectively measured moderate‐to‐vigorous physical activity fall far short. However, because previous physical activity recommendations for prostate cancer survivors were derived from self‐reported data,36 which appears to overestimate actual moderate‐to‐vigorous physical activity and may instead capture light‐intensity activity, it is possible that clinically relevant health benefits could still be accrued at intensities and durations of physical activity below currently recommended levels.37 This conclusion, which is in line with a growing body of research on the benefits of light‐intensity physical activity,38, 39 would be helpful for prostate cancer survivors for several reasons. First, large amounts of moderate‐to‐vigorous physical activity may be difficult for prostate cancer survivors to achieve, particularly those who engage in low levels of moderate‐to‐vigorous physical activity before surgery (ie, the large majority of our cohort). This unrealistic increase in moderate‐to‐vigorous physical activity may partially explain the low adherence rates seen in many previous physical activity interventions.40 Second, increases in physical activity at light intensity rather than moderate or vigorous intensities may be more achievable because of their lesser physiological stress and ease of performance even in the presence of adverse effects. This hypothesis is supported by our observation of a recovery in light‐intensity physical activity for prostate cancer survivors between 5 weeks and 6 months after radical prostatectomy. Thus, given these potential benefits, both in terms of “achievability” and health, additional research is warranted examining the influence of light‐intensity physical activity on prostate cancer survival, particularly using measurement tools capable of capturing physical activities of various intensities accurately. Additionally, an in‐depth understanding of the biological consequences of these activities—and perhaps more importantly the biological consequences of the energy expenditure associated with these activities—according to disease factors and personal characteristics is required. Such knowledge is critical to design effective physical activity interventions that are achievable in wider cancer survivor populations unlikely to engage in moderate‐to‐vigorous physical activity.
Our study population consisted of prostate cancer survivors with relatively high educational attainment and socioeconomic status who were treated for early‐stage prostate cancer by minimally invasive radical prostatectomy procedures. Therefore, our observed degree of discrepancy between self‐reported and objectively measured physical activity and our observed absolute physical activity levels may not generalize to men with lesser educational attainment or socioeconomic status or men treated for later‐stage prostate cancer or by open or different therapeutic procedures with a greater impact on physical and mental function. In fact, it is likely that a higher degree of discrepancy and lesser absolute physical activity levels would have been observed in these men. Nonetheless, we believe our overall conclusion of a possible benefit of light‐intensity physical activity might also apply to these men, because they are even less likely to engage in high levels of moderate‐to‐vigorous physical activity. In addition, although our study population differs from the general population of prostate cancer survivors, it is similar to study populations in which the influence of physical activity on prostate cancer survival has been studied,3, 4, 41, 42 possibly making our findings more relevant to the interpretation of these previous studies. Finally, given the potential implications of our findings (ie, that light‐intensity physical activity may still be beneficial for cancer survival), researchers should explore the possible benefit of this intensity of physical activity for survivors of other cancers in older men, as well as those that affect women and younger individuals.
There are a number of strengths to this study. These include its prospective study design, collection of objectively measured physical activity data, moderate to high follow‐up rate for accelerometer measures (5 weeks = 74.1%, 6 months = 61.7%, 12 months = 56.0%), and large sample size. In fact, our study is the largest study to date to collect repeated objective measures of physical activity before and after treatment.
Our study also has some limitations. Lower limb movements (such as cycling) or strength training activities may be underestimated by hip‐worn accelerometers, resulting in underestimated levels of objective physical activity. Although these types of activities are not expected to be prevalent in men 5 weeks after surgery, they may have been more common once participants recovered from their surgery at 6 and 12 months follow‐up. Discrepancies between self‐reported and objective measures of physical activity may have also been introduced by the possibly differing time frames of assessment of the CHAMPS questionnaire (activities in “a typical week during the past 4 weeks,” which participants may have interpreted as the most recent week) and accelerometers (activity over a 7‐day period near in time to questionnaire completion). This concern may be especially problematic for the 5‐week assessment when participants’ activity levels likely varied markedly from week to week as they recovered from their surgery. Nevertheless, our observed higher agreement at this time point suggests this may have been less of a concern, possibly because of participants’ overall low physical activity levels 5 weeks after surgery. Finally, although the magnitude of our findings may not generalize to populations with different demographic or clinical characteristics as our study population, we believe the inferences of our findings have broader generalizability, as described earlier.
In conclusion, in this prospective study of prostate cancer survivors, objectively measured moderate‐to‐vigorous physical activity levels were critically low from baseline before to 12 months after radical prostatectomy. In contrast, self‐reported levels were considerably higher and above the physical activity guidelines at all 4 time points for a large proportion of men, potentially reflecting light‐intensity rather than moderate‐to‐vigorous physical activity levels. Because physical activity guidelines were derived from self‐reported moderate‐to‐vigorous physical activity, it is possible that these guidelines may be too high for many men and that lower intensities of physical activity may still be beneficial. Therefore, additional research into the potential benefits of physical activity at light intensity is warranted. Such research could lead to interventions that are achievable in a wider survivor population challenged to engage in physical activity at moderate or vigorous intensities.
Funding Support
The Prostatectomy, Incontinence, and Erectile Function Study was supported by the Transdisciplinary Research on Energetics and Cancer (TREC) Center at Washington University in St. Louis, Missouri. The TREC Center was funded by the National Cancer Institute of the National Institutes of Health (grant U54 CA155496), Washington University, the Alvin J. Siteman Cancer Center (grant P30 CA091842), and the Foundation for Barnes‐Jewish Hospital. This work was supported in part by the National Institute of Food and Agriculture, US Department of Agriculture.
Conflict of Interest Disclosures
The authors made no disclosures.
Author Contributions
Lee Smith: Design of the present analysis, conceptualization, writing (original draft), and writing (review and editing). Jung Ae Lee: Methodology, data analysis, and writing (review and editing). Junbae Mun: Methodology, data analysis, and writing (review and editing). Ratna Pakpahan: Data curation and formal analysis. Kellie R. Imm: Data curation and writing (review and editing). Sonya Izadi: Data curation and writing (review and editing). Adam S. Kibel: Funding acquisition, parent study design, study oversight, and writing (review and editing). Graham A. Colditz: Funding acquisition, parent study design, study oversight, and writing (review and editing). Robert L. Grubb III: Funding acquisition, parent study design, study oversight, and writing (review and editing). Kathleen Y. Wolin: Funding acquisition, parent study design, study oversight, and writing (review and editing). Siobhan Sutcliffe: Design of the present analysis, conceptualization, and writing (review and editing). Lin Yang: Design of the present analysis, conceptualization, methodology, data curation, writing (review and editing), supervision, and primary responsibility for the study and the integrity of the content contained herein.
The final 2 authors contributed equally to this article.
We acknowledge all of the participants of this study.
Contributor Information
Lee Smith, Email: lee.smith@anglia.ac.uk.
Lin Yang, Email: lin.yang@muv.ac.at.
References
- 1. Thorsen L, Courneya KS, Stevinson C, Fossa SD. A systematic review of physical activity in prostate cancer survivors: outcomes, prevalence, and determinants. Support Care Cancer. 2008;16:987‐997. [DOI] [PubMed] [Google Scholar]
- 2. Davies NJ, Batehup L, Thomas R. The role of diet and physical activity in breast, colorectal, and prostate cancer survivorship: a review of the literature. Br J Cancer. 2011;105(suppl 1):S52‐S73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang Y, Jacobs EJ, Gapstur SM, et al. Recreational physical activity in relation to prostate cancer‐specific mortality among men with nonmetastatic prostate cancer. Eur Urol. 2017;72:931‐939. [DOI] [PubMed] [Google Scholar]
- 4. Friedenreich CM, Wang Q, Neilson HK, Kopciuk KA, McGregor SE, Courneya KS. Physical activity and survival after prostate cancer. Eur Urol. 2016;70:576‐585. [DOI] [PubMed] [Google Scholar]
- 5. Farris MS, Kopciuk KA, Courneya KS, McGregor SE, Wang Q, Friedenreich CM. Associations of postdiagnosis physical activity and change from prediagnosis physical activity with quality of life in prostate cancer survivors. Cancer Epidemiol Biomarkers Prev. 2017;26:179‐187. [DOI] [PubMed] [Google Scholar]
- 6. Gaskin CJ, Craike M, Mohebbi M, et al. Associations of objectively measured moderate‐to‐vigorous physical activity and sedentary behavior with quality of life and psychological well‐being in prostate cancer survivors. Cancer Causes Control. 2016;27:1093‐1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thraen‐Borowski KM, Gennuso KP, Cadmus‐Bertram L. Accelerometer‐derived physical activity and sedentary time by cancer type in the United States. PLoS ONE. 2017;12:e0182554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peddle‐McIntyre CJ, Cavalheri V, Boyle T, et al. A review of accelerometer‐based activity monitoring in cancer survivorship research. Med Sci Sports Exerc. 2018;50:1790‐1801. [DOI] [PubMed] [Google Scholar]
- 9. Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med. 2003;37:197‐206; discussion 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reid‐Arndt SA, Matsuda S, Cox CR. Tai Chi effects on neuropsychological, emotional, and physical functioning following cancer treatment: a pilot study. Complement Ther Clin Pract. 2012;18:26‐30. [DOI] [PubMed] [Google Scholar]
- 11. Moore HC. An overview of chemotherapy‐related cognitive dysfunction, or “chemobrain”. Oncology (Williston Park). 2014;28:797‐804. [PubMed] [Google Scholar]
- 12. Humpel N, Iverson DC. Depression and quality of life in cancer survivors: is there a relationship with physical activity? Int J Behav Nutr Phys Act. 2007;4:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Irwin ML, McTiernan A, Bernstein L, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36:1484‐1491. [PMC free article] [PubMed] [Google Scholar]
- 14. Williams K, Steptoe A, Wardle J. Is a cancer diagnosis a trigger for health behaviour change? Findings from a prospective, population‐based study. Br J Cancer. 2013;108:2407‐2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hawkes AL, Lynch BM, Youlden DR, Owen N, Aitken JF. Health behaviors of Australian colorectal cancer survivors, compared with noncancer population controls. Support Care Cancer. 2008;16:1097‐1104. [DOI] [PubMed] [Google Scholar]
- 16. Broderick JM, Hussey J, Kennedy MJ, O’Donnell DM. Testing the “teachable moment” premise: does physical activity increase in the early survivorship phase? Support Care Cancer. 2014;22:989‐997. [DOI] [PubMed] [Google Scholar]
- 17. De Jesus S, Fitzgeorge L, Unsworth K, et al. Feasibility of an exercise intervention for fatigued breast cancer patients at a community‐based cardiac rehabilitation program. Cancer Manag Res. 2017;9:29‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fouladiun M, Korner U, Gunnebo L, Sixt‐Ammilon P, Bosaeus I, Lundholm K. Daily physical‐rest activities in relation to nutritional state, metabolism, and quality of life in cancer patients with progressive cachexia. Clin Cancer Res. 2007;13:6379‐6385. [DOI] [PubMed] [Google Scholar]
- 19. Gell NM, Grover KW, Humble M, Sexton M, Dittus K. Efficacy, feasibility, and acceptability of a novel technology‐based intervention to support physical activity in cancer survivors. Support Care Cancer. 2017;25:1291‐1300. [DOI] [PubMed] [Google Scholar]
- 20. Tonosaki A, Ishikawa M. Physical activity intensity and health status perception of breast cancer patients undergoing adjuvant chemotherapy. Eur J Oncol Nurs. 2014;18:132‐139. [DOI] [PubMed] [Google Scholar]
- 21. Sabiston CM, Brunet J, Vallance JK, Meterissian S. Prospective examination of objectively assessed physical activity and sedentary time after breast cancer treatment: sitting on the crest of the teachable moment. Cancer Epidemiol Biomarkers Prev. 2014;23:1324‐1330. [DOI] [PubMed] [Google Scholar]
- 22. Skender S, Schrotz‐King P, Bohm J, et al. Repeat physical activity measurement by accelerometry among colorectal cancer patients—feasibility and minimal number of days of monitoring. BMC Res Notes. 2015;8:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stewart AL, Verboncoeur CJ, McLellan BY, et al. Physical activity outcomes of CHAMPS II: a physical activity promotion program for older adults. J Gerontol A Biol Sci Med Sci. 2001;56:M465‐M470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Resnicow K, McCarty F, Blissett D, Wang T, Heitzler C, Lee RE. Validity of a modified CHAMPS physical activity questionnaire among African‐Americans. Med Sci Sports Exerc. 2003;35:1537‐1545. [DOI] [PubMed] [Google Scholar]
- 25. Harada ND, Chiu V, King AC, Stewart AL. An evaluation of three self‐report physical activity instruments for older adults. Med Sci Sports Exerc. 2001;33:962‐970. [DOI] [PubMed] [Google Scholar]
- 26. van Roekel EH, Winkler EA, Bours MJ, et al. Associations of sedentary time and patterns of sedentary time accumulation with health‐related quality of life in colorectal cancer survivors. Prev Med Rep. 2016;4:262‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vallance JK, Boyle T, Courneya KS, Lynch BM. Accelerometer‐assessed physical activity and sedentary time among colon cancer survivors: associations with psychological health outcomes. J Cancer Surviv. 2015;9:404‐411. [DOI] [PubMed] [Google Scholar]
- 28. Vallance JK, Boyle T, Courneya KS, Lynch BM. Associations of objectively assessed physical activity and sedentary time with health‐related quality of life among colon cancer survivors. Cancer. 2014;120:2919‐2926. [DOI] [PubMed] [Google Scholar]
- 29. Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications. Inc. accelerometer. Med Sci Sports Exerc. 1998;30:777‐781. [DOI] [PubMed] [Google Scholar]
- 30. Lynch BM, Dunstan DW, Winkler E, Healy GN, Eakin E, Owen N. Objectively assessed physical activity, sedentary time and waist circumference among prostate cancer survivors: findings from the National Health and Nutrition Examination Survey (2003–2006). Eur J Cancer Care (Engl). 2011;20:514‐519. [DOI] [PubMed] [Google Scholar]
- 31. Rock CL, Doyle C, Demark‐Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:243‐274. [DOI] [PubMed] [Google Scholar]
- 32. Hekler EB, Buman MP, Haskell WL, et al. Reliability and validity of CHAMPS self‐reported sedentary‐to‐vigorous intensity physical activity in older adults. J Phys Act Health. 2012;9:225‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ainsworth BE, Caspersen CJ, Matthews CE, Masse LC, Baranowski T, Zhu W. Recommendations to improve the accuracy of estimates of physical activity derived from self report. J Phys Act Health. 2012;9(suppl 1):S76‐S84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Imm KR, Williams F, Housten AJ, et al. African American prostate cancer survivorship: Exploring the role of social support in quality of life after radical prostatectomy. J Psychosoc Oncol. 2017:1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Irwin ML. Physical activity interventions for cancer survivors. Br J Sports Med. 2009;43:32‐38. [DOI] [PubMed] [Google Scholar]
- 36. Downer MK. Why epidemiological studies of physical activity in prostate cancer often underestimate its benefits. Eur Urol. 2017;72:940‐941. [DOI] [PubMed] [Google Scholar]
- 37. Warburton DER, Bredin SSD. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017;32:541‐556. [DOI] [PubMed] [Google Scholar]
- 38. Buman MP, Hekler EB, Haskell WL, et al. Objective light‐intensity physical activity associations with rated health in older adults. Am J Epidemiol. 2010;172:1155‐1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fuzeki E, Engeroff T, Banzer W. Health benefits of light‐intensity physical activity: a systematic review of accelerometer data of the National Health and Nutrition Examination Survey (NHANES). Sports Med. 2017;47:1769‐1793. [DOI] [PubMed] [Google Scholar]
- 40. Stull VB, Snyder DC, Demark‐Wahnefried W. Lifestyle interventions in cancer survivors: designing programs that meet the needs of this vulnerable and growing population. J Nutr. 2007;137:243s‐248s. [DOI] [PubMed] [Google Scholar]
- 41. Richman EL, Kenfield SA, Stampfer MJ, Paciorek A, Carroll PR, Chan JM. Physical activity after diagnosis and risk of prostate cancer progression: data from the cancer of the prostate strategic urologic research endeavor. Cancer Res. 2011;71:3889‐3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kenfield SA, Stampfer MJ, Giovannucci E, Chan JM. Physical activity and survival after prostate cancer diagnosis in the health professionals follow‐up study. J Clin Oncol. 2011;29:726‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]


