Abstract
Statins, 3-hydroxy-methylglutaryl coenzyme A reductase inhibitors, have been used for decades for the prevention of coronary artery disease and stroke. They act primarily by lowering serum cholesterol through the inhibition of cholesterol synthesis in the liver, which results in the upregulation of low-density lipoprotein receptors in the liver. This results in the removal of low-density lipoproteincholesterol. Studies have suggested that statins may demonstrate additional effects that are independent of their effects on low-density lipoprotein-cholesterol. These have been termed “pleiotropic” effects. Pleiotropic effects may be due to the inhibition of isoprenoid intermediates by statins. Isoprenoid inhibition has effects on the small guanosine triphosphate binding proteins Rac and Rho which in turn effects nicotinamide adenine dinucleotide phosphate oxidases. Therefore, there are changes in endothelial nitric oxide synthase expression, atherosclerotic plaque stability, pro-inflammatory cytokines and reactive oxygen species production, platelet reactivity, and cardiac fibrosis and hypetrophy development. Recently, statins have been compared to the ezetimibe and the recently published outcomes data on the proprotein convertase subtilisin kexin type 9 inhibitors has allowed for a reexamination of statin pleiotropy. As a result of these diverse effects, it has been suggested that statins also have anti-arrhythmic effects. This review focuses on the mechanisms of statin pleiotropy and discusses evidence from the statin clinical trials as well as examining the possible anti-arrhythmic effects atrial fibrillation and ventricular tachyarrhythmias.
Keywords: Statins, pleiotropy, coronary artery disease, stroke, atrial fibrillation, low density lipoprotein, cholesterol
1. INTRODUCTION
Cardiovascular diseases are the leading cause of death and low-density lipoprotein cholesterol (LDL-C) is responsible for atherogenesis [1, 2]. 3-hydroxy-3-methyl-glutarylcoenzyme A (HMG-CoA) reductase inhibitors, statins, decrease LDL-C levels and have been used for over 30 years for both the primary and secondary prevention of coronary artery disease (CAD) [3–7]. It has been hypothesized that statins also have effects independent of LDL-C lowering, termed pleiotropic effects [8]. Recent outcomes studies have focused on non-statin medications, such as ezetimide, the inhibitor of Niemann—Pick C1 like protein and the proprotein convertase subtilisin kexin 9 inhibitors (PCSK9i) evolocumab, bococizumab, and alirocumab [9–12]. These agents allow a re-examination of statin pleiotropy. While the contribution of the pleiotropic effects of statins to clinical outcomes remains uncertain due to the overwhelming benefits of LDL-C reduction in preventing CAD, this review focuses on both non-LDL-C lowering effects for CAD and for cardiovascular diseases where the causal link of elevated LDL-C is less certain, such as atrial fibrillation (AF), ventricular tachyarrhythmias, and stroke.
2. PHARMACOKINETIC PROPERTIES OF STATINS
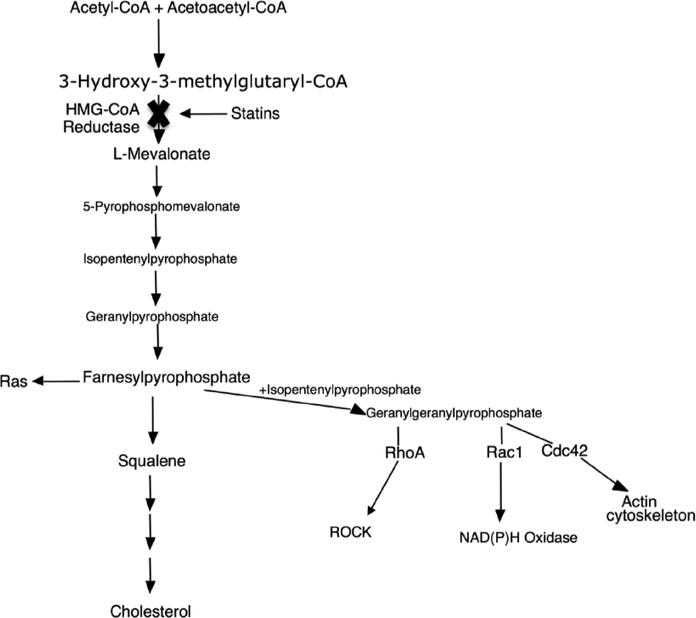
Statins are reversible competitive inhibitors of HMGCoA reductase, which is the rate limiting enzyme for cholesterol biosynthesis in the liver and therefore inhibit the production of mevalonate [13]. Inhibition of cholesterol synthesis leads to decreased cholesterol production and upregulation of the LDL receptor [3]. By inhibiting mevalonate synthesis statins prevent isoprenoid intermediate sythensis, including farnesylpyrophosphate (FPP) and geranylgeranylpyrophosphate (GGPP) (Fig. 1)[14]. The inhibition of FPP and GGPP production is central to statin pleiotropy.
Fig. (1).
Statin Mechanism of Action. Rock – Rho Kinase; NADPH – nicotinamide adenine dinucleotide phosphate.
GGPP and FPP are involved in the post-translational modification of G proteins such as Rho and Ras [15]. Ras and Rho effect cell functions, such as differentiation, proliferation, the cytoskeleton, and apoptosis [16]. Rho translocation depends on geranylgeranylation, whereas Ras translocation depends on on farnesylation [17, 18].
Statins can be divided into two groups. There are lipophilic statins (lovastatin, simvastatin, fluvastatin, atorvastatin, and pitavastatin) which cross cell membranes by passive diffusion and are relatively non-selective for hepatic tissues. The other group of statins are hydrophilic (rosuvastatin and pravastatin) and are unable to cross cell membranes and therefore require activated carrier-mediated transport. Given that the statin transporters are not present in all tissues, hydrophilic statins are more selective for hepatic tissues [19–21]. It is not clear whether statin pleiotropy is due to hepatic or non-hepatic isoprenoid inhibition effects, but it has been suggested the both lipophilic and hydrophilic statins demonstrate pleiotropy.
By affecting Rho gtpases, statins also inhibit Rho kinase’s (ROCKs) activity [22]. ROCKs effect myosin light chain phosphorylation and the actin cytoskeletan. ROCK inhibition limits cardiac fibrosis and pathological remodeling [23]. Statins also inhibit Rac, a monomeric G protein which is included in the Rho GTPase subfamily [24]. Rac1 leads to left ventricular hypertrophy (LVH) by activating nicotin-amide adenine dinucleotide phosphate (NADPH) oxidase which produces reactive oxygen species (ROS) [25]. ROS may be responsible for forming oxidized LDL, which leads to foam cell formation [26]. Additionally, statins activate peroxisome proliferator activated receptor γ (PPAR-γ), which acts to reduce ROS as well as cardiac fibrosis and may be independent of LDL-C lowering [27, 28]. The diverse statin targets have effects on different cell types (Fig. 2).
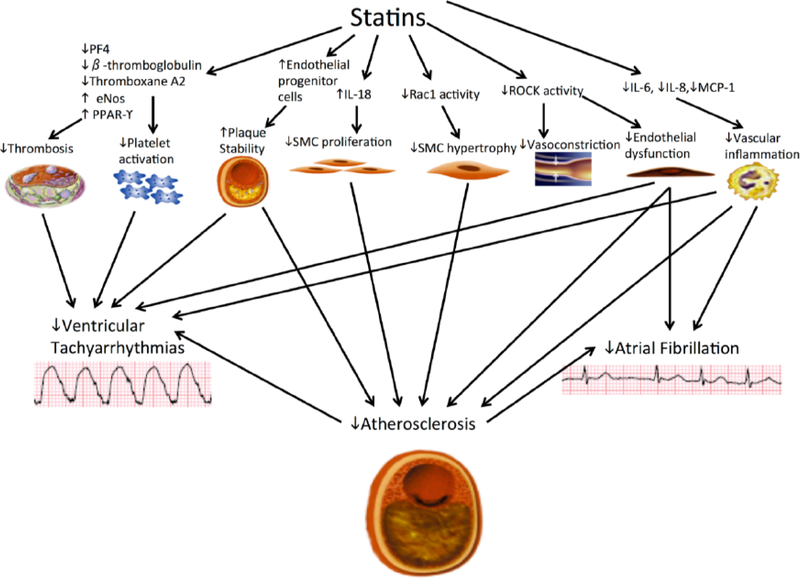
Fig. (2).
Effects of Statins by Different Tissues and Cell Types: There is interplay between the pleiotropic effects of statins and the reduction in atherosclerosis also plays a role in the possible reduction of atrial fibrillation or ventricular tachyarrhythmias that may be seen with statin use. SMC – smooth muscle cell, IL- interleukin, ROCK – rho kinase, PF – platelet factor, eNOS – endothelial nitric oxide synthase.
3. STATIN CELLULAR EFFECTS
3.1. Endothelial Effects of Statins
Hypercholesterolemia causes endothelial dysfunction. Impaired bioavailability of endothelial-derived nitric oxide (NO) characterizes endothelial dysfunction. Endothelial NO is involved in vascular smooth muscle cell (SMC) proliferation, leukocyte/endothelium interactions and platelet aggregation [29]. Statins upregulate endothelial NO synthase (eNOS) and therefore increase NO bioavailability [18, 30].
Statins up-regulate eNOS, increasing NO bioavailability, through multiple mechanisms. One mechanism is through ROCK inhibition. ROCK downregulates eNOS and ROCK inhibitors increase eNOS expression [31, 32]. A second mechanism that Statins increase eNOS activity is through activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway. Akt phosphorylates eNOS, and activation of the pathway therefore increases eNOS activity [33]. Caveolae are invaginations of the plasma cell mebranes. Caveolin-1 binds to eNOS in caveolae and decrease eNOS activity. Statins decrease caveolin-1 expression, and therefore increase eNOS activity [34]. Statins also induce kruppel-like factor 2 in endothelial cells (ECs), which appears to be required for eNOS expression [35]. Lastly, endothelial progenitor cells express eNOS and therefore increase NO levels. Statins increase endothelial progenitor cells; however, the increase is seen only at low statin concentrations. High statin concentrations have angiostatic effects [36, 37].
3.2. Vascular Smooth Muscle and Statins
Vascular smooth muscle cells (SMCs) are required for vascular lesion pathogenesis. Statins affect vascular SMCs, which may be one way which statins affect diseases not related to hyperlipidemia [38]. For example, cardiac transplant arteriosclerosis is not dependent on hyperlipidemia but rather is an immune mediated response against donor vascular SMCs and ECs, which nonetheless is reduced by statins [39].
Statins reduce atherosclerosis by reducing intimal thickening, cellular proliferation, and platelet activation in mice without LDL receptor [40]. Statins also increase the effects of interleukin (IL) – 18, which inhibits nuclear factor- κB activation, SMC migration, and matrix metalloproteinase-9 expression [41]. Lastly, statins inhibit the migration of pulmonary artery SMCs [42].
3.3. Statins and the Myocardium
Ras, Rac, and Rho are important for cardiac hypertrophy and are affected by statins [43]. Rac1 is necessary for myocardial hypertrophy, increased NADPH oxidase activity, and increased mineralocorticoid receptor activity and contributes to doxorubicin related cardiotoxicity [44–46]. Statins decrease Rac1 levels, independent of lipid-lowering and atorvastatin inhibited NADPH in atrial myocardium, through a Rac1 related mechanism [47, 48].
RhoA and ROCK increase cell apoptosis and myocardial fibrosis, which may be associated with heart failure and LVH. Increased expression of RhoA results in apoptosis with increased caspase-9 activation, which is blocked by the inhibition of ROCK [49]. Mice lacking ROCK had less ischemia related fibrosis then normal nice as well as less LVH and apoptosis when exposed to stress [50, 51]. Human leukocyte ROCK activity is higher in patients with LVH then those without LVH [52, 53]. NO bioavailability is increased by statins which results in increased myocardial blood flow dur ing hypoxia [30]. Statins also reduce mitochondrial dysfunction and cardiomyocyte death [54].
Despite the above studies there is no clear evidence that statins improve outcomes in non-ischemic cardiomyopathy. Small studies have demonstrated improved ejection fraction, symptoms, lower inflammatory markers, and decreased mortality [55, 56]. Both large randomized control trials that have been conducted in heart failure, GISSI-HF and CORONA, did not show any mortality benefit [57, 58]. However, it is possible that there may be a benefit for statins if they are started earlier in the course of the disease.
3.4. Statins and Platelets
Platelet reactivity is increased in hypercholesterolemia and platelets are essential for acute coronary syndrome. Platelet reactivity and thrombin generation are decreased by statins in LDL-C dependent and independent mechanisms [59]. Statins increase eNOS while down-regulating β-thromboglobulin and platelet factor 4 in platelets [60]. Platelet factor 4 and β-thromboglobulin are both released by activated platelets and are chemoattractants for inflammatory cells. Arachidonic acid increases platelet aggregation, which is inhibited by statins compared to colestimide [61, 62]. In another study statins inhibited platelet recruitment, decreased thromboxane A2 and Rac1 while increasing NO levels [63]. In the JUPITER trial, rosuvastatin decreased thrombotic events, which appeared to be independent of LDL-C lower ing as hyperlipidemia is not a strong risk factor for thrombosis [64].
3.5. The Immune Effects of Statins
Increased LDL-C initiates the chronic inflammatory process of atherosclerosis, which is mediated by macrophages, SMCs, and T and B lymphocytes [65]. Statins decrease Rac1-mediated ROS production which reduces inflammation that is sensitive to oxidation [66]. Statins also reduce the pro-inflammatory cytokines monocyte chemotactic protein-1, IL-6, and IL-8 [67, 68]. Statins decrease matrix metalloproteinases, in both macrophages and SMCs [69]. The effect of statins on the matrix metalloproteinases may explain one mechanism through which they promote plaque stability.
Statins also effect T-cell differentiation. Statins reduce the differentiation of pro-inflammatory IL-17 helper T cells and increase forkhead box P3+ Cd4+ regulatory T cells [70]. Forkhead box P3+ Cd4+ regulatory T cells are decreased in acute coronary syndrome and their decrease is associated with worsened atherosclerotic lesions. Statins also inhibit Smad6 and Smad7, which are transforming growth factor- inhibitors. This results in increased transforming growth factor-β expression, and induces forkhead box P3+ Cd4+ regulatory T cells [71]. Cd4+ T lymphocytes in acute coronary syndrome induce EC apoptosis, which statins block and be a mechanism for statin related plaque stability [72].
Statins decrease the interactions between leukocytes and ECs. One mechanism is by inhibiting vascular cell adhesion molecule-1 which is mediated by increased NO production [73]. Inhibiting RhoA decreases the adhesion of ECs to monocytes and decreases the clustering of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 [74]. Statins regulate the expression of EC and platelet adhesion molecule-1, which was reversed by mevalonate and GGP [75].
4. CLINICAL EVIDENCE OF STATIN PLEIOTROPY
4.1. Coronary Artery Disease
The concept of anti-inflammatory statin pleiotropy has been examined by looking at the effects of statins on c-reactive protein (CRP). The JUPITER trial was placebo controlled, randomized, and involved primary prevention treatment with rosuvastatin that included 17, 802 patients with a LDL <130 mg/dL and a CRP ≥ 2.0 mg/L [76]. Rosuvastatin decreased the primary end point by 44%, CRP by 37%, and LDL-C by 50%. In contrast, the HOPE-3 study was also a primary prevention trial with rosuvastatin, but there was no CRP or LDL-C levels for inclusion. Rosuvastatin reduced the primary outcomes by 24% and 25% and LDL-C by 26.5%, and while rosuvastatin lowered CRP in the trial, the benefit of rosuvastatin was seen in patients with both high and normal CRP levels, which indicates the primary rosuvastatin benefit may be due to LDL-C reduction [77]. The A-Z trial included patients with acute coronary syndrome who were randomized to either 40 mg of simvastatin, titrated to 80 mg after one month versus placebo for four months followed by 20 mg of simvastatin. The higher dose simvastatin lowered LDL-C by a greater amount, but there was no change in CRP at one month and the A-Z trial failed to achieve its primary end-point [78]. However, after month four, CRP was reduced in the high intensity group, and there was a stronger trend to benefit then earlier in the trial [78]. Both groups had relatively low CRP at one month, which may be responsible for the negative result. The MIRACL trial was a placebo controlled randomized trial with atorvastatin 80 mg in patients with acute coronary syndrome and lowered its primary endpoint in both normal and elevated LDL-C and reduced CRP by 83% [79, 80].
The contribution of statin pleiotropy to clinical outcomes remains unclear, however, because of the strong association between elevated LDL-C and CAD. As already discussed, much of statin pleiotropy is due to isoprenoid inhibition, which occurs at the same time as cholesterol synthesis inhibition and makes it difficult to determine the clinical benefit of statin pleiotropy. Additionally, in the clinical trials of newer therapies, such as ezetimibe and the PCSK9i, the new medication is tested in addition to statin therapy and compared against statins therapy, which prevents quantification of any pleiotropic effects since both the intervention and control groups are receiving statin therapy.
Ezetimibe has been compared with statins, in multiple small studies with various surrogate endpoints, to help determine how much of the clinical benefit of statins is due to pleiotropy. One problem with this approach is that ezetimibe lowers LDL-C by 15–20%, which is similar to low intensity statins, which makes it difficult to see vascular effects. Several studies have randomized patients to high intensity statins or lower intensity statins with ezetimibe and have found more improvement in vascular function and endothelial function with high intensity statins, despite similar LDL-C decrease in both groups [81–85]. But other studies have shown the opposite result [86, 87]. While these studies are interesting, they do not conclusively answer whether statins have clinical pleiotropic effects in addition to LDL-C reduction.
The PCSK9i lower LDL-C levels by approximately 60%, which is comparable to high dose statins and recent outcomes trials have been completed that allow for comparison with statin therapy [88, 89]. PCSK9i lower LDL-C by increasing the LDL receptor-mediated uptake of ApoB-containing lipoproteins, which is similar in mechanism to statins. But, since they have no effect on the mevalonate pathway they should not demonstrate pleiotropic effects that result from the inhibition of isoprenoid intermediate formation. The PCSK9i do not lower inflammatory markers, such as CRP [90]. High dose statin therapy has previously demonstrated plaque reduction, which was felt to be due to the anti-inflammatory effects of statins. The GLAGOV trial tested the PCSK9i evolocumab against placebo in addition to statin therapy and there was a reduction of LDL-C to 36.6 mg/dL compared to 93.0 mg/dL in the placebo group and there was also coronary plaque regression, indicating the plaque regression may not be due to statin pleiotropy and may be due to LDL-C reduction [10].
The large PCSK9i outcomes trials, SPIRE 1, 2 and FOURIER have been recently published [11, 12]. SPIRE 1, 2 were two randomized controlled trials that compared bococizumab in addition to background statin therapy with placebo [12]. SPIRE 1 examined high risk primary prevention or secondary prevention CAD patients with an LDL-C > 70 mg/dL and found no difference at 7 months in the primary composite endpoint of myocardial infarction (MI), stroke, revascularization, and death despite a 51.5% reduction in LDL-C. SPIRE 2 examined a similar population, but with an LDL-C > 100 mg/dL and did show a reduction in the primary composite endpoint. It should be noted that bococizumab, unlike evolocumab and alirocumab, is a humanized monoclonal antibody. The SPIRE trials were terminated early due to evidence of decreased LDL-C lowering at 1 year resulting from anti-drug antibody development in some patients, which has not been seen in the fully human monoclonal antibodies and the drug is no longer undergoing development. FOURIER compared evolocumab to placebo in those with atherosclerotic disease and LDL-C > 70 mg/dL on background statin therapy and showed a 59% reduction in LDL-C as well as a reduced risk of the primary endpoint at 48 weeks by 15% [11]. However, there was no reduction in either cardiovascular death or death from any cause during the trial [11]. The reduction seen in the primary endpoint was lower than expected based on the previous statin trials, given the significant LDL-C lowering seen with evolocumab, and raises the question if there are clinical effects of statin pleiotropy, or if there is less benefit seen at extremely low LDLC levels. Given that all of the PCSK9i outcomes trials have been done on background statin therapy, it is currently not possible to say if statin pleiotropy has clinical effects, but a study could be designed to compare PCSK9i with statin naïve patients to further investigate the hypothesis.
Trials are currently ongoing, or recently published, that either currently provide, or will provide more evidence for statin pleiotropy. The CANTOS trial is a 10, 061-patient secondary prevention randomized clinical trial that included patients who had a previous MI and elevated CRP and compared the IL-1β inhibitor, Canakinumab, to placebo. The trial was recently published and found that Canakinumab reduced CRP levels compared to placebo, but had no effect on lipid levels at 48 months [91]. Both of the higher dose regimens of Canakinumab reduced the primary composite endpoint of nonfatal MI, non-fatal stroke, and death compared to placebo. There was a higher rate of infection in the treatment group and no change in mortality. This trial provides the strongest current clinical evidence that anti-inflammatory medications reduce cardiovascular events, providing additional support to the hypothesis that inflammation plays a lipid independent role in promoting atherosclerosis. The OSYSEEY Outcomes, testing alirocumab and CIRT examining methotrexate, are both currently ongoing and will provide further information regarding statin pleiotropy, LDL-C lowering, and the clinical effects of anti-inflammatory medications on CAD.
4.2. Stroke
The link between elevated LDL-C and ischemic stroke is not as strong as it is for CAD [92]. However, statins reduce the risk of stroke by 25% in the Treating to New Targets study and the Heart Protection Study and 48% in JUPITER [93–95]. The SPARCL trial showed that atorvastatin works for the secondary prevention of stroke [96]. Recently there has also been trial evidence regarding the non-statin medications and stroke reduction. The IMPROVE-IT trial demon strated that ezetimibe added to statin therapy reduced stroke by 21% and FOURIER showed that evolocumab reduced stroke by 21% [9, 11]. Therefore is not possible to say, based on clinical trial data that stroke reduction is due to statin pleiotropy.
Statin pleiotropy in stroke may be related to the effect of statins on eNOS, given that mice that lack eNOS have increased infarct size, and ROCK inhibitors also increase eNOS and cerebral blood flow which indicates that the effect of statins in stroke may be mediated through Rho/ROCK [30, 32].
4.3. Atrial Fibrillation
AF is a multifactorial disorder that is often the result of a variety of predisposing cardiac and non-cardiac factors such as atherosclerosis, obesity, diabetes, and other pro-inflammatory diseases. AF is associated with increased atrial fibrosis and abnormal autonomic nervous system activity, and despite its association with cardiac risk factors, is not associated with elevated LDL-C [97]. Animal models show that statins may reduce AF by increasing the atrial refractory period, reducing pro-inflammatory markers such as CRP, and desensitization to beta adrenergic stimuli, and the effect was abolished by both GGPP and mevalonate [98, 99]. AF has also been associated with increased atrial oxidative stress and NADPH oxidase activity [100]. Rac1 GTPase regulates NADPH oxidase activity and is associated with AF and statin treatment reduces Rac1 and NADPH oxidase activity as well as the incidence of AF in a mouse model [101].
Based on the basic science data, statin use for the prevention of AF has been examined in statin clinical trials. A JUPITER post-hoc analysis showed a 27% reduced risk of developing AF, and increased AF incidence was associated with increased CRP – which suggests that the anti-inflammatory effects of statins may reduce AF [102]. There was also a 13% reduced incidence of AF in an ancillary analysis of GISSI-HF [103]. There was a non-statistically significant trend to reduced AF in WOSCOPS [104]. Reduced AF incidence, however has not been seen in other statin trials [105–107]. Multiple meta-analysis have also been done, also with conflicting results regarding AF prevention [108, 109]. While the benefit of statins to prevent AF is not universally seen, it is important to note that in these studies AF was not the primary outcome and was only detected on clinic electrocardiograms or by the discretion of the treating physician and was not evaluated in a systematic way. This tends to reduce the detection of AF, as shown in recent data [110]. With more diligent monitoring, with either event monitors or implantable loop recorders it may be easier to detect an effect of statins.
The effect of statins on AF recurrence in patients with known AF has also been studied. A small randomized placebo controlled trial enrolled patients with an elevated CRP and a previous diagnosis of paroxysmal AF and found a 65% reduction of AF recurrence and a significant reduction in CRP after 6 months of treatment with atorvastatin [111]. J-RHYTHM was a randomized Japanese trial between rate control and rhythm control strategies for the treatment of paroxysmal AF [112]. A post-hoc analysis of the trial examining patients on statins did not show any reduction in AF recurrence in those on statins, but it should be noted that the statin users in the trial were significantly older, more likely to be female, more likely to have CAD, more likely to have a history of ischemic stroke, and more likely to have hypertension then statin non-users, characteristics which may make people more susceptible to AF recurrence [113]. Two small randomized controlled trials have examined the effect of statins after electrical cardioversion for AF and both found a reduced recurrence of AF at 3 months in the statin treated groups [114, 115]. However, reduction in AF recurrence was not seen in other trials of recurrence after cardioversion [116–119]. One randomized trial has been done after AF ablation and found no difference in recurrence rate at 3 months, however given the low recurrence rates in each group (95% free from AF in the atorvastatin group and 93.5% in the placebo group) and the short follow up time, the clinical results of the trial are difficult to interpret [120]. Given the small sizes of the trials and conflicting results, two meta-analysis of controlled trials have been performed and both showed that statins both reduce the recurrence of AF in general and specifically in patients undergoing electrical cardioversion [108, 121]. The heterogeneity of the patient populations, follow up time, and small trial populations make the data on AF recurrence difficult to interpret and a large randomized controlled trial would help provide clarity.
Post-operative AF (POAF) is common after cardiac surgery and is thought to be related both to myocardial injury during surgery and a pro-inflammatory post-operative state [122]. Two large randomized controlled trials have examined statin efficacy for preventing POAF. ARMYDA-3 randomized 200 statin-naive people to atorvastatin or placebo, starting 7 days before surgery and found a 35% reduction in POAF [123]. The STICS trial randomized 1922 patients to rosuvastatin or placebo and found no difference in the incidence of POAF [124]. While STICS is the larger trial, there are important differences between the two trials that may explain the results. While ARMYDA-3 included only statin naïve patients, 34% of the patients in STICS were taking statins prior to trial enrollment, which may have reduced the benefit of statin therapy. Furthermore, while patients in ARMYDA-3 all received a statin for 7 days pre-operatively, in STICS most patients received less then 7 days of therapy, with roughly 44% of the population receiving either statin or placebo for less then 3 days pre-operatively, which may not have allowed for enough time to see the effect of statin therapy on POAF prevention. Additionally, a meta-analysis of the 13 trials of statins in cardiac surgery not including STICS showed an odds ratio (OR) of 0.39 with 95% confidence intervals (CI) of 0.29–0.51 for POAF incidence [124]. Regardless of the differences between trials, there is currently not enough evidence to support that statin therapy reduces POAF after cardiac surgery. The majority of patients undergoing cardiac surgery meet other indications for statin use, and therefore many patients are taking statins prior to cardiac surgery regardless of the results of the STICS trial.
While the incidence of POAF is lower after non-cardiac surgery, statins have been studied in this setting as well. Observational data has suggested that statins may reduce POAF incidence after non-cardiac surgery [125]. Two small randomized controlled trials have been done. One examined atorvastatin given one week before and one week after pul monary resection found a 50% reduction in the incidence of POAF, but the trial was terminated early due to difficulty enrolling statin naïve patients [126]. A second study loaded patients on chronic statin therapy with atorvastatin prior to emergency non-cardiac surgery and found a POAF incidence of 6.8% in the treatment arm and 17% in the placebo arm. Although both of these trials are small, and in different populations, they suggest that there may be a benefit of statin therapy for preventing non-cardiac surgery on POAF and a larger randomized trial would help clarify the effect.
While the studies above suggest a benefit for statin treatment of AF, many of the studies are small and conflicting, which makes drawing any definitive conclusion difficult.
4.4. Ventricular Tachyarrhythmia
Sudden cardiac death (SCD) is due to coronary heart disease in 75% of cases, often due to ventricular arrhythmias (VA), and accounts for 15–20% of all death [127]. Studies of the general population with SCD have shown the majority of patients who undergo angiography after SCD have an acute occlusion [128]. It has also been shown that after MI, SCD is commonly due to repeat MI in the first month, but after 3 months, it is more commonly due to VA [129]. VA’s can be separated into monomorphic ventricular tachycardia (VT) and polymorphic VT. Monomorphic VT is most commonly due to reentry related to scar from an old MI, but can also be seen in other etiologies of cardiomyopathy and with idiopathic etiology. However, acute myocardial ischemia can modify autonomic tone and lead to increased monomorphic VT. Polymorphic VT and ventricular fibrillation can be seen in the setting of acute MI, electrolyte abnormalities, drug toxicities, and inherited channelopathies. Given the diverse mechanisms and etiologies for VA, it is unlikely that statins will reduce all VAs. Additionally, hyperlipidemia has not been identified as a consistent risk factor for SCD in epidemiologic studies of SCD and those with ventricular fibrillation may have less hyperlipidemia then those without ventricular fibrillation [130–132]. It is difficult to separate the CAD risk factors from the presence of CAD in population based studies, since CAD is the most common etiology of SCD. While much of the effects of statins on VA and SCD may be related to reductions in CAD, it has been suggested that statins have other antiarrhythmic activity that reduce VA. Data has demonstrated that CRP is elevated in those at risk for SCD, which indicates that the anti-inflammatory properties may play a role in SCD reduction [133].
A mouse model showed that statins decrease caveolin-1 expression and promote eNOS while improving heart rate variability, a marker for autonomic function [34]. Statins have also been shown to improve low heart rate variability in humans [134]. Prolonged QTc interval predisposes to VA, primarily polymorphic VT, and statins have been shown to decrease the QTc interval in a small randomized controlled trial in patients with heart failure [135]. Ventricular late potentials represent damaged myocardium that is a substrate for VA [136]. Early statin administration after MI lead to decreased ventricular late potentials and in hospital incidence of VA, but these effects may be due primarily to reduced myocardial ischemia and may not be independent antiar-rhythmic effects [137].
Small observational studies of patients with CAD and implantable cardioverter defibrillators (ICD) found a reduced incidence of VA requiring ICD intervention in those treated with statins [138, 139]. Statin use reduced both monomorphic VT and polymorphic VT, either because statins had an effect on the re-entry circuit, or because they reduced ischemic triggers of both polymorphic and monomorphic VT. The effect was also seen in the MADIT-II trial which enrolled patients with a prior MI, and where statin users had a hazard ratio of 0.65 for VA compared to those not on statins, although the study did not separate monomorphic and polymorphic VT [140]. The same effect was seen amongst patients with non-ischemic cardiomyopathy in the MADITCRT trial, with a 77% reduction in the risk of fast VA or death [141]. In MADIT-CRT there was a decrease in VT cycle length as well a significant reduction in polymorphic VT, and although there was also a reduction in monomorphic VT, the trend did not reach statistical significance. Given that the reduction of VA is seen both in patients with CAD and patients without, this suggests a clinical effect of statins beyond coronary plaque stability and reduction of myocar-dial ischemia, possibly through autonomic modulation and reduction in inflammation.
Unfortunately, information on VA and SCD is not reliably recorded in many of the statin randomized controlled trials and the trials were not powered appropriately to detect a difference in the incidence of SCD and VA. A meta-analysis was therefore done for both the primary and secondary prevention statin trials that reported the outcome and included unpublished data from the trials [142]. The meta-analysis found no reduction of VA (OR 1.02, 95% CI 0.84–1.25, p=0.87), cardiac arrest (OR 1.05, 95% CI 0.76–1.45, p=0.84), but did show a reduction in SCD (OR .90, 95% CI 0.82–0.97, p=.01) with statin therapy [142]. The meta-analysis was unable to determine whether the VAs were polymorphic or monomorphic VT. While the meta-analysis did not show a reduction in VA with statin therapy, VA reporting was heterogeneous and there is likely to be significant under reporting of VAs in the statin trials, which may have reduced the power to detect an effect. Whether or not statins truly have benefit on VA reduction can only be assessed by a large randomized trial.
CONCLUSION
The tremendous clinical benefits of statins in reduction of CAD has been demonstrated in multiple clinical trials and led to the hypothesis that statins have pleiotropic benefits beyond LDL-C lowering. Data has shown that statins have benefits on the endothelium, vascular smooth muscle, platelets, myocardium, and immune system that cannot be explained by LDL-C reduction alone. Much of this benefit is due to inhibition of FPP and GGPP synthesis and the downstream effects on Ras, Rho, Rac, eNOS, and NADPH oxidase. While statins reduce CRP, it has been difficult to conclusively demonstrate pleiotropic statin effects in clinical trials due since cholesterol biosynthesis, FPP, and GGPP are inhibited by statins concurrently. Statins have demonstrated benefit in diseases, such as stroke, which are not clearly linked to LDL-C, but a similar benefit has been seen with the non-statin medications ezetimibe and the PCSK9i. While statins have been postulated to have anti-arrhythmic benefits on AF and VA that are not related to LDL-C reduction, those benefits have not been conclusively demonstrated in all studies. Regardless, statins will remain first line medication for cholesterol reduction and secondary prevention of MI.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- LDL-C
Low Density Lipoprotein Cholesterol
- HMG-CoA
3-Hydroxy-3-Methyl-Glutaryl-Coenzyme
- ACAD
Coronary Artery Disease
- PCSK9i
Proprotein Convertase Subtilisin Kexin 9 Inhibitors
- AF
Atrial Fibrillation
- FPP
Farnesylpyrophosphate
- GGPP
Geranylgeranylpyrophosphate
- ROCK
Rho Kinase
- NADPH
Nicotinamide Adenine Di-Nucleotide Phosphate
- ROS
Reactive Oxygen Species
- LVH
Left Ventricular Hypertrophy
- PPAR-γ
Proliferator Activated Receptor γ
- NO
Nitric Oxidee
- NOS
Endothelial NO Synthase
- ECs
Endothelial Cells
- SMC
Smooth Muscle Cells
- IL
interleukin
- CRP
C-Reactive Protein
- MI
Myocardial Infarction
- POAF
Post-operative AF
- OR
Odds Ratio
- CI
Confidence Interval
- SCD
Sudden Cardiac Death
- VA
Ventricular Arrhythmias
- ICD
Implantable Cardioverter Defibrillators
Footnotes
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- [1].Mendis S, Davis S, Norrving B. Organizational update: the world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke. 2015;46(5):e121–122. [DOI] [PubMed] [Google Scholar]
- [2].Ference BA, Majeed F, Penumetcha R, Flack JM, Brook RD. Effect of naturally random allocation to lower low-density lipoprotein cholesterol on the risk of coronary heart disease mediated by polymorphisms in NPC1L1, HMGCR, or both: a 2 × 2 factorial Mendelian randomization study. J Am Coll Cardiol. 2015;65(15):1552–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science. 2001;292(5519):1160–1164. [DOI] [PubMed] [Google Scholar]
- [4].Sacks FM, Pfeffer MA, Moye LA, et al. The Effect of Pravastatin on Coronary Events after Myocardial Infarction in Patients with Average Cholesterol Levels. N Engl J Med. 1996;335(14):1001–1009. [DOI] [PubMed] [Google Scholar]
- [5].Shepherd J, Cobbe SM, Ford I, et al. Prevention of Coronary Heart Disease with Pravastatin in Men with Hypercholesterolemia. N Engl J Med. 1995;333(20):1301–1308. [DOI] [PubMed] [Google Scholar]
- [6].Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: Results of afcaps/texcaps. JAMA. 1998;279(20):1615–1622. [DOI] [PubMed] [Google Scholar]
- [7].Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus Moderate Lipid Lowering with Statins after Acute Coronary Syndromes. N Engl J Med. 2004;350(15):1495–1504. [DOI] [PubMed] [Google Scholar]
- [8].Oesterle A, Laufs U, Liao JK. Pleiotropic Effects of Statins on the Cardiovascular System. Circ Res. 2017;120(1):229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med. 2015;372(25):2387–2397. [DOI] [PubMed] [Google Scholar]
- [10].Nicholls SJ, Puri R, Anderson T, et al. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. JAMA. 2016;316(22):2373–2384. [DOI] [PubMed] [Google Scholar]
- [11].Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017. [DOI] [PubMed] [Google Scholar]
- [12].Ridker PM, Revkin J, Amarenco P, et al. Cardiovascular Efficacy and Safety of Bococizumab in High-Risk Patients. N Engl J Med. 2017. [DOI] [PubMed] [Google Scholar]
- [13].Sirtori CR. The pharmacology of statins. Pharmacol Res. 2014;88:3–11. [DOI] [PubMed] [Google Scholar]
- [14].Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343(6257):425–430. [DOI] [PubMed] [Google Scholar]
- [15].Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11(18):2295–2322. [DOI] [PubMed] [Google Scholar]
- [16].Cho KJ, Hill MM, Chigurupati S, Du G, Parton RG, Hancock JF. Therapeutic levels of the hydroxmethylglutaryl-coenzyme A reductase inhibitor lovastatin activate ras signaling via phospholipase D2. Mol Cell Biol. 2011;31(6):1110–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273(37):24266–24271. [DOI] [PubMed] [Google Scholar]
- [18].Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97(12):1129–1135. [DOI] [PubMed] [Google Scholar]
- [19].Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol. 2005;19(1):117–125. [DOI] [PubMed] [Google Scholar]
- [20].Kivisto KT, Niemi M. Influence of drug transporter polymorphisms on pravastatin pharmacokinetics in humans. Pharm Res. 2007;24(2):239–247. [DOI] [PubMed] [Google Scholar]
- [21].Kitamura S, Maeda K, Wang Y, Sugiyama Y. Involvement of multiple transporters in the hepatobiliary transport of rosuvastatin. Drug Metab Dispos. 2008;36(10):2014–2023. [DOI] [PubMed] [Google Scholar]
- [22].Eto M, Kozai T, Cosentino F, Joch H, Luscher TF. Statin prevents tissue factor expression in human endothelial cells: role of Rho/Rho-kinase and Akt pathways. Circulation. 2002;105(15):1756–1759. [DOI] [PubMed] [Google Scholar]
- [23].Shimizu T, Liao JK. Rho Kinases and Cardiac Remodeling. Circ J. 2016;80(7):1491–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame: a decade of hypertrophic signaling hits. Circ Res. 2006;98(6):730–742. [DOI] [PubMed] [Google Scholar]
- [25].Takemoto M, Node K, Nakagami H, et al. Statins as antioxidant therapy for preventing cardiac myocyte hypertrophy. J Clin Invest. 2001;108(10):1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Miller YI, Choi SH, Fang L, Tsimikas S. Lipoprotein modification and macrophage uptake: role of pathologic cholesterol transport in atherogenesis. Subcell Biochem. 2010;51:229–251. [DOI] [PubMed] [Google Scholar]
- [27].Yano M, Matsumura T, Senokuchi T, et al. Statins activate peroxisome proliferator-activated receptor gamma through extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase-dependent cyclooxygenase-2 expression in macrophages. Circ Res. 2007;100(10):1442–1451. [DOI] [PubMed] [Google Scholar]
- [28].Chen M, Li H, Wang G, Shen X, Zhao S, Su W. Atorvastatin prevents advanced glycation end products (AGEs)-induced cardiac fibrosis via activating peroxisome proliferator-activated receptor gamma (PPAR-gamma). Metabolism. 2016;65(4):441–453. [DOI] [PubMed] [Google Scholar]
- [29].Gauthier TW, Scalia R, Murohara T, Guo JP, Lefer AM. Nitric oxide protects against leukocyte-endothelium interactions in the early stages of hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1995;15(10):1652–1659. [DOI] [PubMed] [Google Scholar]
- [30].Laufs U, Fata VL, Liao JK. Inhibition of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase blocks hypoxia-mediated down-regulation of endothelial nitric oxide synthase. J Biol Chem. 1997;272(50):31725–31729. [DOI] [PubMed] [Google Scholar]
- [31].Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106(1):57–62. [DOI] [PubMed] [Google Scholar]
- [32].Rikitake Y, Kim HH, Huang Z, et al. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005;36(10):2251–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399(6736):601–605. [DOI] [PubMed] [Google Scholar]
- [34].Pelat M, Dessy C, Massion P, Desager JP, Feron O, Balligand JL. Rosuvastatin decreases caveolin-1 and improves nitric oxide-dependent heart rate and blood pressure variability in apolipoprotein E−/− mice in vivo. Circulation. 2003;107(19):2480–2486. [DOI] [PubMed] [Google Scholar]
- [35].Sen-Banerjee S, Mir S, Lin Z, et al. Kruppel-like factor 2 as a novel mediator of statin effects in endothelial cells. Circulation. 2005;112(5):720–726. [DOI] [PubMed] [Google Scholar]
- [36].Vasa M, Fichtlscherer S, Adler K, et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103(24):2885–2890. [DOI] [PubMed] [Google Scholar]
- [37].Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105(6):739–745. [DOI] [PubMed] [Google Scholar]
- [38].Braun-Dullaeus RC, Mann MJ, Dzau VJ. Cell cycle progression: new therapeutic target for vascular proliferative disease. Circulation. 1998;98(1):82–89. [DOI] [PubMed] [Google Scholar]
- [39].Kobashigawa JA, Katznelson S, Laks H, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333(10):621–627. [DOI] [PubMed] [Google Scholar]
- [40].Chen Z, Fukutomi T, Zago AC, et al. Simvastatin reduces neointimal thickening in low-density lipoprotein receptor-deficient mice after experimental angioplasty without changing plasma lipids. Circulation. 2002;106(1):20–23. [DOI] [PubMed] [Google Scholar]
- [41].Chandrasekar B, Mummidi S, Mahimainathan L, et al. Interleukin-18-induced human coronary artery smooth muscle cell migration is dependent on NF-kappaB- and AP-1-mediated matrix metalloproteinase-9 expression and is inhibited by atorvastatin. J Biol Chem. 2006;281(22):15099–15109. [DOI] [PubMed] [Google Scholar]
- [42].Li M, Liu Y, Dutt P, Fanburg BL, Toksoz D. Inhibition of serotonin-induced mitogenesis, migration, and ERK MAPK nuclear translocation in vascular smooth muscle cells by atorvastatin. Am J Physiol Lung Cell Mol Physiol. 2007;293(2):L463–471. [DOI] [PubMed] [Google Scholar]
- [43].Wang SM, Tsai YJ, Jiang MJ, Tseng YZ. Studies on the function of rho A protein in cardiac myofibrillogenesis. J Cell Biochem. 1997;66(1):43–53. [PubMed] [Google Scholar]
- [44].Satoh M, Ogita H, Takeshita K, Mukai Y, Kwiatkowski DJ, Liao JK. Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci U S A. 2006;103(19):7432–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nagase M, Fujita T. Role of Rac1-mineralocorticoid-receptor signalling in renal and cardiac disease. Nature reviews Nephrology. 2013;9(2):86–98. [DOI] [PubMed] [Google Scholar]
- [46].Ma J, Wang Y, Zheng D, Wei M, Xu H, Peng T. Rac1 signalling mediates doxorubicin-induced cardiotoxicity through both reactive oxygen species-dependent and -independent pathways. Cardiovasc Res. 2013;97(1):77–87. [DOI] [PubMed] [Google Scholar]
- [47].Tanaka S, Fukumoto Y, Nochioka K, et al. Statins exert the pleiotropic effects through small GTP-binding protein dissociation stimulator upregulation with a resultant Rac1 degradation. Arterioscler Thromb Vasc Biol. 2013;33(7):1591–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Antoniades C, Demosthenous M, Reilly S, et al. Myocardial redox state predicts in-hospital clinical outcome after cardiac surgery effects of short-term pre-operative statin treatment. J Am Coll Cardiol. 2012;59(1):60–70. [DOI] [PubMed] [Google Scholar]
- [49].Del Re DP, Miyamoto S, Brown JH. RhoA/Rho kinase up-regulate Bax to activate a mitochondrial death pathway and induce cardiomyocyte apoptosis. J Biol Chem. 2007;282(11):8069–8078. [DOI] [PubMed] [Google Scholar]
- [50].Haudek SB, Gupta D, Dewald O, et al. Rho kinase-1 mediates cardiac fibrosis by regulating fibroblast precursor cell differentiation. Cardiovasc Res. 2009;83(3):511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Okamoto R, Li Y, Noma K, et al. FHL2 prevents cardiac hypertrophy in mice with cardiac-specific deletion of ROCK2. FASEB J. 2013;27(4):1439–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gabrielli L, Winter JL, Godoy I, et al. Increased rho-kinase activity in hypertensive patients with left ventricular hypertrophy. Am J Hypertens. 2014;27(6):838–845. [DOI] [PubMed] [Google Scholar]
- [53].Calo LA, Vertolli U, Pagnin E, et al. Increased rho kinase activity in mononuclear cells of dialysis and stage 3–4 chronic kidney disease patients with left ventricular hypertrophy: Cardiovascular risk implications. Life Sci. 2016;148:80–85. [DOI] [PubMed] [Google Scholar]
- [54].Jones SP, Teshima Y, Akao M, Marban E. Simvastatin attenuates oxidant-induced mitochondrial dysfunction in cardiac myocytes. Circ Res. 2003;93(8):697–699. [DOI] [PubMed] [Google Scholar]
- [55].Node K, Fujita M, Kitakaze M, Hori M, Liao JK. Short-term statin therapy improves cardiac function and symptoms in patients with idiopathic dilated cardiomyopathy. Circulation. 2003;108(7):839–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Horwich TB, MacLellan WR, Fonarow GC. Statin therapy is associated with improved survival in ischemic and non-ischemic heart failure. J Am Coll Cardiol. 2004;43(4):642–648. [DOI] [PubMed] [Google Scholar]
- [57].Kjekshus J, Apetrei E, Barrios V, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357(22):2248–2261. [DOI] [PubMed] [Google Scholar]
- [58].Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9645):1231–1239. [DOI] [PubMed] [Google Scholar]
- [59].Aoki I, Aoki N, Kawano K, et al. Platelet-dependent thrombin generation in patients with hyperlipidemia. J Am Coll Cardiol. 1997;30(1):91–96. [DOI] [PubMed] [Google Scholar]
- [60].Laufs U, Gertz K, Huang P, et al. Atorvastatin upregulates type III nitric oxide synthase in thrombocytes, decreases platelet activation, and protects from cerebral ischemia in normocholesterolemic mice. Stroke. 2000;31(10):2442–2449. [DOI] [PubMed] [Google Scholar]
- [61].Haramaki N, Ikeda H, Takenaka K, et al. Fluvastatin alters platelet aggregability in patients with hypercholesterolemia: possible improvement of intraplatelet redox imbalance via HMG-CoA reductase. Arterioscler Thromb Vasc Biol. 2007;27(6):1471–1477. [DOI] [PubMed] [Google Scholar]
- [62].Ali FY, Armstrong PC, Dhanji AR, et al. Antiplatelet actions of statins and fibrates are mediated by PPARs. Arterioscler Thromb Vasc Biol. 2009;29(5):706–711. [DOI] [PubMed] [Google Scholar]
- [63].Pignatelli P, Carnevale R, Pastori D, et al. Immediate antioxidant and antiplatelet effect of atorvastatin via inhibition of Nox2. Circulation. 2012;126(1):92–103. [DOI] [PubMed] [Google Scholar]
- [64].Glynn RJ, Danielson E, Fonseca FA, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360(18):1851–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Libby P, Tabas I, Fredman G, Fisher EA. Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res. 2014;114(12):1867–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Dichtl W, Dulak J, Frick M, et al. HMG-CoA reductase inhibitors regulate inflammatory transcription factors in human endothelial and vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23(1):58–63. [DOI] [PubMed] [Google Scholar]
- [67].Rezaie-Majd A, Maca T, Bucek RA, et al. Simvastatin reduces expression of cytokines interleukin-6, interleukin-8, and monocyte chemoattractant protein-1 in circulating monocytes from hypercholesterolemic patients. Arterioscler Thromb Vasc Biol. 2002;22(7):1194–1199. [DOI] [PubMed] [Google Scholar]
- [68].Jougasaki M, Ichiki T, Takenoshita Y, Setoguchi M. Statins suppress interleukin-6-induced monocyte chemo-attractant protein-1 by inhibiting Janus kinase/signal transducers and activators of transcription pathways in human vascular endothelial cells. Br J Pharmacol. 2010;159(6):1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Luan Z, Chase AJ, Newby AC. Statins inhibit secretion of metalloproteinases-1, −2, −3, and −9 from vascular smooth muscle cells and macrophages. Arterioscler Thromb Vasc Biol. 2003;23(5):769–775. [DOI] [PubMed] [Google Scholar]
- [70].Kagami S, Owada T, Kanari H, et al. Protein geranylgeranylation regulates the balance between Th17 cells and Foxp3+ regulatory T cells. Int Immunol. 2009;21(6):679–689. [DOI] [PubMed] [Google Scholar]
- [71].Kim YC, Kim KK, Shevach EM. Simvastatin induces Foxp3+ T regulatory cells by modulation of transforming growth factor-beta signal transduction. Immunology. 2010;130(4):484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Sato K, Nuki T, Gomita K, Weyand CM, Hagiwara N. Statins reduce endothelial cell apoptosis via inhibition of TRAIL expres sion on activated CD4 T cells in acute coronary syndrome. Atherosclerosis. 2010;213(1):33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].De Caterina R, Libby P, Peng HB, et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96(1):60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wojciak-Stothard B, Williams L, Ridley AJ. Monocyte adhesion and spreading on human endothelial cells is dependent on Rho-regulated receptor clustering. J Cell Biol. 1999;145(6):1293–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wei H, Fang L, Song J, Chatterjee S. Statin-inhibited endothelial permeability could be associated with its effect on PECAM-1 in endothelial cells. FEBS Lett. 2005;579(5):1272–1278. [DOI] [PubMed] [Google Scholar]
- [76].Ridker PM, Danielson E, Fonseca FAH, et al. Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein. N Engl J Med. 2008;359(21):2195–2207. [DOI] [PubMed] [Google Scholar]
- [77].Yusuf S, Bosch J, Dagenais G, et al. Cholesterol Lowering in Intermediate-Risk Persons without Cardiovascular Disease. N Engl J Med. 2016;374(21):2021–2031. [DOI] [PubMed] [Google Scholar]
- [78].de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292(11):1307–1316. [DOI] [PubMed] [Google Scholar]
- [79].Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285(13):1711–1718. [DOI] [PubMed] [Google Scholar]
- [80].Kinlay S, Schwartz GG, Olsson AG, et al. High-dose atorvastatin enhances the decline in inflammatory markers in patients with acute coronary syndromes in the MIRACL study. Circulation. 2003;108(13):1560–1566. [DOI] [PubMed] [Google Scholar]
- [81].Liu PY, Liu YW, Lin LJ, Chen JH, Liao JK. Evidence for statin pleiotropy in humans: differential effects of statins and ezetimibe on rho-associated coiled-coil containing protein kinase activity, endothelial function, and inflammation. Circulation. 2009;119(1):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Landmesser U, Bahlmann F, Mueller M, et al. Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation. 2005;111(18):2356–2363. [DOI] [PubMed] [Google Scholar]
- [83].Fichtlscherer S, Schmidt-Lucke C, Bojunga S, et al. Differential effects of short-term lipid lowering with ezetimibe and statins on endothelial function in patients with CAD: clinical evidence for ‘pleiotropic’ functions of statin therapy. Eur Heart J. 2006;27(10):1182–1190. [DOI] [PubMed] [Google Scholar]
- [84].Rudofsky G, Reismann P, Groener JB, et al. Identical LDL-cholesterol lowering but non-identical effects on NF-kappaB activity: High dose simvastatin vs combination therapy with ezetimibe. Atherosclerosis. 2012;223(1):190–196. [DOI] [PubMed] [Google Scholar]
- [85].Matsue Y, Matsumura A, Suzuki M, Hashimoto Y, Yoshida M. Differences in action of atorvastatin and ezetimibe in lowering low-density lipoprotein cholesterol and effect on endothelial function: randomized controlled trial. Circ J. 2013;77(7):1791–1798. [DOI] [PubMed] [Google Scholar]
- [86].Kawagoe Y, Hattori Y, Nakano A, et al. Comparative study between high-dose fluvastatin and low-dose fluvastatin and ezetimibe with regard to the effect on endothelial function in diabetic patients. Endocr J. 2011;58(3):171–175. [DOI] [PubMed] [Google Scholar]
- [87].Pesaro AE, Serrano CV Jr., Fernandes JL, et al. Pleiotropic effects of ezetimibe/simvastatin vs. high dose simvastatin. Int J Cardiol. 2012;158(3):400–404. [DOI] [PubMed] [Google Scholar]
- [88].Blom DJ, Hala T, Bolognese M, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370(19):1809–1819. [DOI] [PubMed] [Google Scholar]
- [89].Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489–1499. [DOI] [PubMed] [Google Scholar]
- [90].Sahebkar A, Di Giosia P, Stamerra CA, et al. Effect of monoclonal antibodies to PCSK9 on high-sensitivity C-reactive protein levels: a meta-analysis of 16 randomized controlled treatment arms. Br J Clin Pharmacol. 2016;81(6):1175–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377(12):1119–1131. [DOI] [PubMed] [Google Scholar]
- [92].Yaghi S, Elkind MS. Lipids and Cerebrovascular Disease: Research and Practice. Stroke. 2015;46(11):3322–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo controlled trial. The Lancet.360(9326):7–22. [DOI] [PubMed] [Google Scholar]
- [94].LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352(14):1425–1435. [DOI] [PubMed] [Google Scholar]
- [95].Everett BM, Glynn RJ, MacFadyen JG, Ridker PM. Rosuvastatin in the prevention of stroke among men and women with elevated levels of C-reactive protein: justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER). Circulation. 2010;121(1):143–150. [DOI] [PubMed] [Google Scholar]
- [96].Amarenco P, Bogousslavsky J, Callahan A 3rd, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549–559. [DOI] [PubMed] [Google Scholar]
- [97].Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114(9):1453–1468. [DOI] [PubMed] [Google Scholar]
- [98].Muhlhauser U, Zolk O, Rau T, Munzel F, Wieland T, Eschenhagen T. Atorvastatin desensitizes beta-adrenergic signaling in cardiac myocytes via reduced isoprenylation of G-protein gamma-subunits. FASEB J. 2006;20(6):785–787. [DOI] [PubMed] [Google Scholar]
- [99].Kumagai K, Nakashima H, Saku K. The HMG-CoA reductase inhibitor atorvastatin prevents atrial fibrillation by inhibiting inflammation in a canine sterile pericarditis model. Cardiovasc Res. 2004;62(1):105–111. [DOI] [PubMed] [Google Scholar]
- [100].Dudley SC Jr., Hoch NE, McCann LA, et al. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation. 2005;112(9):1266–1273. [DOI] [PubMed] [Google Scholar]
- [101].Adam O, Frost G, Custodis F, et al. Role of Rac1 GTPase activation in atrial fibrillation. J Am Coll Cardiol. 2007;50(4):359–367. [DOI] [PubMed] [Google Scholar]
- [102].Pena JM, MacFadyen J, Glynn RJ, Ridker PM. High-sensitivity C-reactive protein, statin therapy, and risks of atrial fibrillation: an exploratory analysis of the JUPITER trial. Eur Heart J. 2012;33(4):531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Maggioni AP, Fabbri G, Lucci D, et al. Effects of rosuvastatin on atrial fibrillation occurrence: ancillary results of the GISSI-HF trial. Eur Heart J. 2009;30(19):2327–2336. [DOI] [PubMed] [Google Scholar]
- [104].Macfarlane PW, Norrie J. The value of the electrocardiogram in risk assessment in primary prevention: experience from the West of Scotland Coronary Prevention Study. J Electrocardiol. 2007;40(1):101–109. [DOI] [PubMed] [Google Scholar]
- [105].Macfarlane PW, Murray H, Sattar N, et al. The incidence and risk factors for new onset atrial fibrillation in the PROSPER study. Europace. 2011;13(5):634–639. [DOI] [PubMed] [Google Scholar]
- [106].Haywood LJ, Ford CE, Crow RS, et al. Atrial fibrillation at baseline and during follow-up in ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial). J Am Coll Cardiol. 2009;54(22):2023–2031. [DOI] [PubMed] [Google Scholar]
- [107].Schwartz GG, Chaitman BR, Goldberger JJ, Messig M. High-dose atorvastatin and risk of atrial fibrillation in patients with prior stroke or transient ischemic attack: analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Am Heart J. 2011;161(5):993–999. [DOI] [PubMed] [Google Scholar]
- [108].Fauchier L, Clementy N, Babuty D. Statin therapy and atrial fibrillation: systematic review and updated meta-analysis of published randomized controlled trials. Curr Opin Cardiol. 2013;28(1):7–18. [DOI] [PubMed] [Google Scholar]
- [109].Fang WT, Li HJ, Zhang H, Jiang S. The role of statin therapy in the prevention of atrial fibrillation: a meta-analysis of randomized controlled trials. Br J Clin Pharmacol. 2012;74(5):744–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478–2486. [DOI] [PubMed] [Google Scholar]
- [111].Dernellis J, Panaretou M. Effect of C-reactive protein reduction on paroxysmal atrial fibrillation. Am Heart J. 2005;150(5):1064. [DOI] [PubMed] [Google Scholar]
- [112].Ogawa S, Yamashita T, Yamazaki T, et al. Optimal treatment strategy for patients with paroxysmal atrial fibrillation: J-RHYTHM Study. Circ J. 2009;73(2):242–248. [DOI] [PubMed] [Google Scholar]
- [113].Watanabe E, Yamashita T, Suzuki S, et al. Statin treatment for patients with paroxysmal atrial fibrillation. Int Heart J. 2011;52(2):103–106. [DOI] [PubMed] [Google Scholar]
- [114].Xia W, Yin Z, Li J, Song Y, Qu X. Effects of rosuvastatin on asymmetric dimethylarginine levels and early atrial fibrillation recurrence after electrical cardioversion. Pacing Clin Electrophysiol. 2009;32(12):1562–1566. [DOI] [PubMed] [Google Scholar]
- [115].Ozaydin M, Varol E, Aslan SM, et al. Effect of atorvastatin on the recurrence rates of atrial fibrillation after electrical cardioversion. Am J Cardiol. 2006;97(10):1490–1493. [DOI] [PubMed] [Google Scholar]
- [116].Tveit A, Grundtvig M, Gundersen T, et al. Analysis of pravastatin to prevent recurrence of atrial fibrillation after electrical cardioversion. Am J Cardiol. 2004;93(6):780–782. [DOI] [PubMed] [Google Scholar]
- [117].Almroth H, Hoglund N, Boman K, et al. Atorvastatin and persistent atrial fibrillation following cardioversion: a randomized placebo-controlled multicentre study. Eur Heart J. 2009;30(7):827–833. [DOI] [PubMed] [Google Scholar]
- [118].Negi S, Shukrullah I, Veledar E, Bloom HL, Jones DP, Dudley SC. Statin therapy for the prevention of atrial fibrillation trial (SToP AF trial). J Cardiovasc Electrophysiol. 2011;22(4):414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Demir K, Can I, Koc F, et al. Atorvastatin given prior to electrical cardioversion does not affect the recurrence of atrial fibrillation in patients with persistent atrial fibrillation who are on antiarrhythmic therapy. Med Princ Pract. 2011;20(5):464–469. [DOI] [PubMed] [Google Scholar]
- [120].Suleiman M, Koestler C, Lerman A, et al. Atorvastatin for prevention of atrial fibrillation recurrence following pulmonary vein isolation: a double-blind, placebo-controlled, randomized trial. Heart Rhythm. 2012;9(2):172–178. [DOI] [PubMed] [Google Scholar]
- [121].Yan P, Dong P, Li Z, Cheng J. Statin therapy decreased the recurrence frequency of atrial fibrillation after electrical cardioversion: a meta-analysis. Med Sci Monit. 2014;20:2753–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60(22):2263–2270. [DOI] [PubMed] [Google Scholar]
- [123].Patti G, Chello M, Candura D, et al. Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: results of the ARMYDA-3 (Atorvastatin for Reduction of MYocardial Dysrhythmia After cardiac surgery) study. Circulation. 2006;114(14):1455–1461. [DOI] [PubMed] [Google Scholar]
- [124].Zheng Z, Jayaram R, Jiang L, et al. Perioperative Rosuvastatin in Cardiac Surgery. N Engl J Med. 2016;374(18):1744–1753. [DOI] [PubMed] [Google Scholar]
- [125].Bhave PD, Goldman LE, Vittinghoff E, Maselli JH, Auerbach A. Statin use and postoperative atrial fibrillation after major noncardiac surgery. Heart Rhythm. 2012;9(2):163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Amar D, Park B, Zhang H, et al. Beneficial effects of perioperative statins for major pulmonary resection. J Thorac Cardiovasc Surg. 2015;149(6):1532–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Hayashi M, Shimizu W, Albert CM. The spectrum of epidemiology underlying sudden cardiac death. Circ Res. 2015;116(12):1887–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Redfors B, Ramunddal T, Angeras O, et al. Angiographic findings and survival in patients undergoing coronary angiography due to sudden cardiac arrest in western Sweden. Resuscitation. 2015;90:13–20. [DOI] [PubMed] [Google Scholar]
- [129].Pouleur AC, Barkoudah E, Uno H, et al. Pathogenesis of sudden unexpected death in a clinical trial of patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. Circulation. 2010;122(6):597–602. [DOI] [PubMed] [Google Scholar]
- [130].Stecker EC, Vickers C, Waltz J, et al. Population-based analysis of sudden cardiac death with and without left ventricular systolic dys-function: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47(6):1161–1166. [DOI] [PubMed] [Google Scholar]
- [131].Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336(18):1276–1282. [DOI] [PubMed] [Google Scholar]
- [132].Dekker LR, Bezzina CR, Henriques JP, et al. Familial sudden death is an important risk factor for primary ventricular fibrillation: a case-control study in acute myocardial infarction patients. Circulation. 2006;114(11):1140–1145. [DOI] [PubMed] [Google Scholar]
- [133].Albert CM, Ma J, Rifai N, Stampfer MJ, Ridker PM. Prospective study of C-reactive protein, homocysteine, and plasma lipid levels as predictors of sudden cardiac death. Circulation. 2002;105(22):2595–2599. [DOI] [PubMed] [Google Scholar]
- [134].Pehlivanidis AN, Athyros VG, Demitriadis DS, Papageorgiou AA, Bouloukos VJ, Kontopoulos AG. Heart rate variability after long-term treatment with atorvastatin in hypercholesterolaemic patients with or without coronary artery disease. Atherosclerosis. 2001;157(2):463–469. [DOI] [PubMed] [Google Scholar]
- [135].Vrtovec B, Okrajsek R, Golicnik A, Ferjan M, Starc V, Radovancevic B. Atorvastatin therapy increases heart rate variability, decreases QT variability, and shortens QTc interval duration in patients with advanced chronic heart failure. J Card Fail. 2005;11(9):684–690. [DOI] [PubMed] [Google Scholar]
- [136].Kostapanos MS, Liberopoulos EN, Goudevenos JA, Mikhailidis DP, Elisaf MS. Do statins have an antiarrhythmic activity? Cardiovasc Res. 2007;75(1):10–20. [DOI] [PubMed] [Google Scholar]
- [137].Kayikcioglu M, Can L, Evrengul H, Payzin S, Kultursay H. The effect of statin therapy on ventricular late potentials in acute myocardial infarction. Int J Cardiol. 2003;90(1):63–72. [DOI] [PubMed] [Google Scholar]
- [138].De Sutter J, Tavernier R, De Buyzere M, Jordaens L, De Backer G. Lipid lowering drugs and recurrences of life-threatening ventricular arrhythmias in high-risk patients. J Am Coll Cardiol. 2000;36(3):766–772. [DOI] [PubMed] [Google Scholar]
- [139].Chiu JH, Abdelhadi RH, Chung MK, et al. Effect of statin therapy on risk of ventricular arrhythmia among patients with coronary artery disease and an implantable cardioverter-defibrillator. Am J Cardiol. 2005;95(4):490–491. [DOI] [PubMed] [Google Scholar]
- [140].Vyas AK, Guo H, Moss AJ, et al. Reduction in ventricular tachyarrhythmias with statins in the Multicenter Automatic Defibrillator Implantation Trial (MADIT)-II. J Am Coll Cardiol. 2006;47(4):769–773. [DOI] [PubMed] [Google Scholar]
- [141].Buber J, Goldenberg I, Moss AJ, et al. Reduction in life-threatening ventricular tachyarrhythmias in statin-treated patients with nonischemic cardiomyopathy enrolled in the MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy). J Am Coll Cardiol. 2012;60(8):749–755. [DOI] [PubMed] [Google Scholar]
- [142].Rahimi K, Majoni W, Merhi A, Emberson J. Effect of statins on ventricular tachyarrhythmia, cardiac arrest, and sudden cardiac death: a meta-analysis of published and unpublished evidence from randomized trials. Eur Heart J. 2012;33(13):1571–1581. [DOI] [PubMed] [Google Scholar]