Abstract
Background:
Cognitive dysfunction is common in psychotic disorders, and may reflect underlying pathophysiology. However, substantial cognitive heterogeneity exists both within and between diagnostic categories, creating challenges for studying the neurobiology of cognitive dysfunction in patients. The aim of this study was to identify patients with psychosis with intact versus impaired cognitive profiles, and to examine resting state functional connectivity between patient groups and compared to healthy controls to determine the extent to which patterns of connectivity are overlapping or distinct.
Methods:
Participants with affective or non-affective psychosis (n=120) and healthy controls (n=31) were administered the MATRICS Consensus Cognitive Battery, clinical and community functioning assessments, and an fMRI scan to measure resting state functional connectivity (RSFC). Cognitive composite scores were used to identify groups of patients with and without cognitive dysfunction. RSFC was compared between groups of patients and healthy controls, controlling for demographic and clinical variables.
Results:
Both cognitively intact and cognitively impaired patients showed decreased intrinsic connectivity compared to controls in frontoparietal control (FPN) and motor networks. Patients with cognitive impairment showed additional reductions in FPN connectivity compared to patients with intact cognition, particularly in subnetwork A.
Conclusions:
We leveraged the heterogeneity in cognitive ability among patients with psychosis to disentangle the relative contributions of cognitive dysfunction and presence of an underlying psychotic illness using resting state functional connectivity. These findings suggest at least partially separable effects of presence of a psychotic disorder and neurocognitive impairment contributing to network dysconnectivity in psychosis.
Keywords: cognition, functional connectivity, network, schizophrenia, bipolar disorder, psychosis
1. Introduction
Cognitive dysfunction is common in psychotic disorders, and may be reflective of underlying liability or disease progression. However, substantial cognitive heterogeneity exists both within and between diagnostic categories, creating challenges for studying the pathophysiology of cognitive dysfunction in psychosis. Some patients exhibit selective deficits, others exhibit significant global cognitive dysfunction, and a subset of patients do not exhibit any substantial neurocognitive impairment (Goldstein and Shemansky, 1995; Heinrichs and Awad, 1993; Hill et al., 2002; Lewandowski et al., 2014; Palmer et al., 1997; Seaton et al., 2001; Van Rheenen et al., 2017b). Moreover, patients with shared diagnostic and clinical features may exhibit different cognitive abnormalities, while patients across diagnoses may share similar cognitive deficits. This raises the possibility that patients with psychotic disorders with and without cognitive deficits may represent distinct subtypes of illness reflective of unique biological substrates. Despite the fact that cognitive status is linked to long-term functional outcomes, the biology of cognitive dysfunction in psychosis remains poorly understood and treatment options are limited.
Identification of biological differences between patients with psychosis with and without cognitive deficits may help clarify the extent to which cognitive grouping strategies reflect homogeneous neurobiological underpinnings, which may be related to pathophysiological factors and disease course. If cognitive symptoms reflect underlying pathophysiology, patients grouped by neurocognitive profile should show differential associations with neurobiological markers based on cognitive phenotype. Several studies have probed this question using structural MRI, and have found structural brain abnormalities associated with psychosis itself (i.e. contrast between cognitively intact patients and controls), and additional abnormalities related to neurocognitive impairment (i.e. contrasts between patients with and without cognitive impairment). The latter comparisons have revealed reduced gray and white matter volume (Van Rheenen et al., 2017a; Woodward and Heckers, 2015), reduced white matter and enlarged lateral ventricle volumes (Wexler et al., 2009), and widespread cortical thinning (Cobia et al., 2011) in patients with cognitive impairment compared to patients who were neuropsychologically “near normal.”
Disruption to large-scale distributed neural networks has been implicated in the cognitive dysfunction present in psychotic disorders using both task-based (Fornito et al., 2011) and resting state (Lynall et al., 2010; Sheffield et al., 2017) neuroimaging paradigms. However, disentangling the effects of cognition and psychosis on network function has been experimentally challenging, since overlapping sets of brain regions are likely involved. One network of particular interest is the frontoparietal control network (FPN), which is associated with cognitive control and executive processes (Buckner et al., 2011; Choi et al., 2012; Vincent et al., 2008) and includes the rostrolateral prefontal cortex (riPFC), insula, dorsal anterior cingulate cortex (dACC), precuneus, dorsolateral prefrontal cortex (dlPFC), and inferior parietal lobule. Patients with psychosis exhibit reduced FPN connectivity compared to controls (Baker et al., 2014; Favre et al., 2014), reduced activation of FPN nodes during tasks of attentional control (e.g. (Sepede et al., 2014)), and association between FPN functional connectivity and neurocognitive performance on the MATRICS battery (Argyelan et al., 2014). The Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study used a combination of cognitive and electrophysiological measures to identify “biotypes,” or cross-diagnostic groups that shared similar characteristics believed to be closer to the illness than broad diagnostic categories, and found differences amongst biotypes in resting state connectivity in a large sample of patients and relatives (Meda et al., 2016). This study suggests that grouping strategies along symptom dimensions may be more closely associated with resting state network connectivity than is diagnosis. However, the extent to which network abnormalities are specifically associated with cognition or other aspects of the psychosis syndrome remains unclear (Jalbrzikowski and Bearden, 2016). To our knowledge, no studies to date have examined whether cognitive profiles per se are reflective of differential network connectivity changes.
The present study aimed to identify groups of patients with psychosis with intact or impaired cognition, and to examine resting state functional connectivity (RSFC) between patient groups and compared to healthy controls. By comparing patients with and without cognitive impairment to controls, we aimed to examine network connectivity in association with cognitive dysfunction and with psychosis separately. Specifically, we aimed to group patients into “neuropsychologically intact” and “neuropsychologically impaired” groups based on scores on a standardized cognitive test battery, and examine RSFC by group. We hypothesized that 1) all patients would demonstrate decreased RSFC compared to healthy controls, and 2) patients with poor cognitive functioning would show specific reductions in resting state connectivity in networks associated with cognitive functioning including the FPN compared to both healthy controls and neuropsychologically normal patients.
2. Materials and Materials
2.1. Participants
Participants with diagnoses of affective or non-affective psychosis (n=120) and healthy controls (n=31) were recruited through the Schizophrenia and Bipolar Disorder Program (SBDP) and via fliers posted at McLean Hospital. Participants were recruited in the context of several separate but related studies: 1) cognitive remediation in SZ or Bipolar Disorder (BD) (n= 42), 2) neuroimaging (n=33), or 3) clinical characterization of psychosis (n=76). For subjects who participated in one of the cognitive remediation intervention studies, baseline cognitive and imaging data were used. Inclusion criteria included a DSM-IV diagnosis of SZ, Schizoaffective Disorder (SZA), Schizophreniform Disorder, Psychosis NOS, BD I with psychosis, or MDD with psychosis, ages 18 to 65. Exclusion criteria for all participants included history of head trauma with loss of consciousness, history of seizure, and current substance abuse or dependence. Healthy controls had no personal or first-degree family history of a psychiatric diagnosis, and no history of substance abuse or dependence. All procedures were approved by the McLean Hospital IRB.
2.2. Materials
Diagnosis was determined using the Structured Clinical Interview for DSM-IV (SCID-IV-TR) through patient interview, medical record review, and consultation with the participants’ treatment providers. Clinical assessment included the Young Mania Rating Scale (YMRS; (Young et al., 1978)), the Montgomery-Asberg Depression Rating Scale (MADRS; (Montgomery and Asberg, 1979)), and the Positive and Negative Syndrome Scale (PANSS; (Kay et al., 1987)). Community functioning was measured using an abbreviated version of the Multnomah Community Ability Scale (MCAS; (Barker et al., 1994)), as described by Lewandowski et al. (Lewandowski et al., 2013). Premorbid IQ was measured with the North American Adult Reading Test (NAART; (Uttl, 2002)).
Cognition was measured using the MATRICS Consensus Cognitive Battery (MCCB; (Nuechterlein et al., 2008)). The MCCB is comprised of ten subtests that make up seven domain scores and a composite. Domains include: Speed of Processing (Trail Making Test A; Brief Assessment of Cognition in Schizophrenia: Symbol Coding; Category Fluency); Attention/Vigilance (Continuous Performance Test: Identical Pairs); Working Memory (Wechsler Memory Scale Spatial Span; Letter Number Span); Visual Learning (Brief Visuospatial Memory Test); Verbal Learning (Hopkins Verbal Learning Test); Reasoning/Problem Solving (Neuropsychological Assessment Battery: Mazes); and Social Cognition (Mayer-Salovey-Caruso Emotional Intelligence Test: Managing Emotions). Subtest, domain, and composite scores are converted to T-scores based on MCCB age and gender adjusted norms. The MCCB takes approximately 60-90 minutes to complete.
2.3. Image Acquisition
Details of image acquisition for the majority of participants have been described previously (Baker et al., 2014). Briefly, data were acquired using a Siemens 3T Tim Trio scanner with a 12-channel phased-array head coil. High-resolution, T1-weighted, multiecho, magnetization-prepared, gradient-echo structural images were collected (van der Kouwe et al., 2008). Functional data were collected with a gradient-echo echo planar imaging sequence to detect blood oxygenation level-dependent (BOLD) contrast. BOLD acquisition parameters for the three protocols combined in this analysis were as follows: (1) TR = 3000ms, TE = 30ms, flip angle = 85°, 3×3×3mm voxels, 72×72 matrix, FOV = 216mm, 47 interleaved axial slices with no gap, and duration = 6.2 minutes (124 time points); (2) TR = 2500ms, TE = 24ms; flip angle = 82°, 3.5×3.5×3.5mm voxels, 64×64 matrix, FOV = 224mm, 42 axial slices, and duration = 10 minutes (240 time points); and (3) TR = 2500ms, TE = 30ms; flip angle = 82°, 3.5×3.5×3.5mm voxels, 64×64 matrix, FOV = 224mm, 41 axial slices, and duration = 6.67 minutes (160 time points). An automated alignment procedure aligning whole brain coverage to the anterior commissure-posterior commissure was used for consistency across participants. One to two functional scans were acquired per participant, and we assessed mean estimates across scans in the case of two acquisitions.
2.4. Procedure
Neuropsychological, neuroimaging, and diagnostic data were collected in 2–3 sessions. Patient-reported information regarding medication and dose was collected, and chlorpromazine equivalents (CPZ) were calculated using guidelines described by Baldessarini (Baldessarini, 2012). During resting-state functional scans, participants were told to stay awake, remain still, keep their eyes open, and think of nothing in particular; no fixation marker was used. Participants were monitored with eye tracking to ensure that eyes remained open during the functional scan.
2.5. Statistical Approach
Cognitive groupings were determined using MCCB Composite scores, using a cutoff of MCCB Composite <−1.0SD to define a group characterized by impaired neuropsychological functioning (Impaired), and MCCB Composite scores of >/=−.5SD to define a group characterized by intact neuropsychological functioning (Intact). Similar strategies have been used in previous reports to identify more homogeneous groups of patients based on cognition (Bryson et al., 1993; Gold, 2008; Heinrichs and Awad, 1993; Palmer et al., 1997; Rojo et al., 2010). This approach was selected a) to create groups that were distinct from each other, and b) because cognitive scores within .5SD of the mean are generally considered to be within the normal range whereas scores <1.0SD below the mean are generally considered impaired, thereby creating conceptually meaningful groupings. Groups were compared on all MCCB domains scores using ANOVA; T-scores were reported for all MCCB variables. Groups were also compared on demographic and clinical variables using ANOVA or Chi2 as appropriate. Post-hoc paired t-tests were conducted for all significant ANOVA effects. All group comparisons of behavioral data were conducted using Bonferroni correction for multiple comparisons.
The neuroimaging analytic approach has been described previously (Baker et al., 2014). Briefly, resting state fMRI data were preprocessed for fcMRI analysis (Biswal et al., 1995; Van Dijk et al., 2010; Vincent et al., 2006) and temporally filtered to retain frequencies below 0.08 Hz. We regressed 1) six parameters obtained by correction for rigid body head motion (Jenkinson et al., 2002; Smith et al., 2009), 2) the signal averaged over the whole brain, 3) the signal averaged over the ventricles, and 4) the signal averaged over the deep cerebral white matter. BOLD data were projected to the FreeSurfer (4.5.0) cortical ribbon of each participant, and then assessed for correlation structure between 122 surface-based regions of interest (sROIs, 61 per hemisphere) derived from the Yeo et al. (Yeo et al., 2011)17-network parcellation, which is publicly available for download (https://surfer.nmr.mgh.harvard.edu/fswiki/CorticalParcellation_Yeo2011). Specifically, we computed Pearson correlation coefficients between each regional fMRI time course, averaged across all vertices within the region, and the mean fMRI time course for every other region. The regional correlation values were z-transformed to approximate normality and then compared across the groups using ANOVA after linear regression of age, sex, and race. All tests were corrected for multiple comparisons using a false discovery rate (FDR) of q < 0.05, corresponding in this data set to − log P =1.411 FDR corrected.
3. Results
3.1. Cognitive Characteristics by Group
Patients were divided into Intact and Impaired groups as described above. Based on the grouping method, data from 17 participants whose Composite MCCB scores fell between −1.0 and −.5 SD were excluded. Neurocognitive profiles by cognitive group were examined using the seven MCCB domain scores and the composite (Figure 1). Groups differed significantly on all cognitive variables (p<.0001). Pairwise comparisons with Bonferroni correction showed that the Intact group (n=50) demonstrated a cognitive profile in which all scores were at the normative mean; this group did not differ from the control group on any cognitive domain score except processing speed (driven by higher than average scores in the control group). In contrast, the Impaired group (n=53) performed significantly worse than the Intact patients and healthy controls on all cognitive domains and the composite (p<.001 for all comparisons) (Table 1).
Figure 1. Cognitive Characteristics by Group.
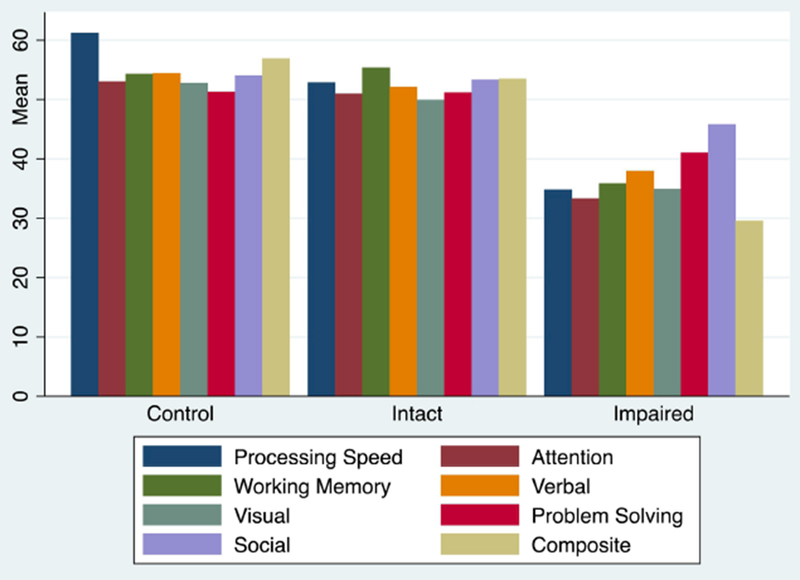
Age and gender normed MCCB domain and Composite scores by group. Groups differed significantly on all cognitive variables (p<0001).
Table 1.
Cognitive Domain Scores by Group
| Control (n=31) | Intact (n=50) | Impaired (n=53) | F-statistic df (2, 131) | Post-hoc t-test^ | |
|---|---|---|---|---|---|
| Processing Speed | 59.6 (10.5) | 52.4 (8.3) | 34.3 (9.5) | 82.16**** | 2<1<0 |
| Attention/Vigilance | 53.0 (9.0) | 51.0 (8.9) | 33.0 (10.6) | 61.21**** | 2<1,0 |
| Working Memory | 53.4 (10.3) | 55.3 (7.5) | 35.6 (10.7) | 63.03**** | 2<1,0 |
| Verbal Learning | 53.7 (10.1) | 52.4 (9.8) | 37.7 (6.5) | 49.12**** | 2<1,0 |
| Visual Learning | 52.2 (8.5) | 49.4 (9.0) | 35.0 (10.7) | 42.18**** | 2<1,0 |
| Problem Solving | 49.9 (8.7) | 51.1 (9.1) | 40.5 (8.6) | 21.20**** | 2<1,0 |
| Social Cognition | 53.6 (10.7) | 53.4 (9.6) | 44.0 (11.3) | 12.84**** | 2<1,0 |
| COMPOSITE | 55.7 (9.6) | 53.4 (5.2) | 28.8 (9.0) | 162.53**** | 2<1,0 |
All reported post-hoc comparisons were significant after Bonferroni correction.
p<.0001
3.2. Demographic and Clinical Characteristics by Group
Participants did not differ on most demographic variables, but did differ on education and premorbid IQ as measured by the NAART (Table 2). Pairwise comparisons showed that Impaired patients differed significantly from both Intact patients and healthy controls on educational attainment (p<.001). Neither patient group differed significantly from controls on NAART; however, the patient groups differed from each other (p<.001). The two patient groups differed from each other on symptoms of mania and psychosis (p<.05 and p<.01, respectively) and CPZ equivalents (p<.001), but did not differ in terms of depression symptoms. Groups differed on community functioning (F=20.33, p<.0001; Control>Intact>Impaired). Both cognitive groups included patients with primary and affective psychoses, although diagnoses were not distributed evenly (Chi2(2) =11.88; p< 01), Patients with SZ were slightly overrepresented in the Impaired group, whereas patients with BD were slightly overrepresented in the Intact group; frequencies are similar to those of previous reports, with 30% of patients with primary psychosis (SZ, SZA) characterized as Intact and 64% of the BD group characterized as Intact (see Table 3).
Table 2.
Demographic and Clinical Characteristics by Group
| Control (n=31) | Intact (n=50) | Impaired (n=53) | F-statistic df (2, 131) | |
|---|---|---|---|---|
| Age, years | 33.1 (10.9) | 31.7 (10.1) | 31.4 (12.2) | 0.24ns |
| Education (years) | 16.1 (1.9) | 15.9 (1.7) | 13.7 (1.8) | 26.38**** |
| Sex, % female | 45% | 43% | 26% | Chi2=4.09ns |
| Race, % Caucasian | 81% | 92% | 79% | Chi2=4.16ns |
| NAART^ | 114.2 (5.5) | 116.8 (8.0) | 108.8 (10.0) | 9.51*** |
| YMRS | -- | 4.8 (5.2) | 7.8 (7.0) | 5.67 * |
| MADRS | -- | 11.3 (9.7) | 10.5 (9.0) | 0.18ns |
| PANSS positive | -- | 10.1 (3.8) | 13.1 (5.9) | 8.83** |
| PANSS negative | -- | 11.0 (4.0) | 14.2 (6.4) | 8.98** |
| PANSS general | -- | 25.1 (6.6) | 26.5 (7.6) | 0.96ns |
| CPZ Equivalent | -- | 152.4 (181.4) | 328.2 (313.9) | 14.25*** |
| MCAS | 54.6 (1.2) | 48.1 (4.6) | 43.4 (7.3) | 20.33**** |
NAART: North American Adult Reading Test
p<.05
p<.01
p<.001
p<.000l
Table 3.
Diagnostic Distribution by Cognitive Group
| Intact | Impaired | Chi2* | |
|---|---|---|---|
| SZ | 6 | 17 | 23 |
| 11.0 | 12.0 | 23.0 | |
| 2.3 | 2.1 | 4.4 | |
| SZA | 8 | 16 | 24 |
| 11.5 | 12.5 | 24.0 | |
| 1.1 | 1.0 | 2.1 | |
| BD | 35 | 20 | 55 |
| 26.4 | 28.6 | 55.0 | |
| 2.8 | 2.6 | 5.4 | |
Chi2 = 11.88 p=.003
Distribution of diagnoses by cognitive group. Actual frequency; Expected frequency; Chi2 Contribution
3.3. Neuroimaging Results by Cluster
Neuroimaging data from eight participants were excluded from the imaging analyses due to poor data quality (three from the Intact group, five from the Impaired group) for a final imaging sample of 31 Controls, 47 Intact patients, and 48 Impaired patients. Scan quality was determined using a signal-to-noise ratio (SNR), derived as the mean/SD of the mean slice intensity time series from the participant’s BOLD T2* image series. We conducted a regression analysis to compare z-transformed Pearson correlations across pairwise regional interactions controlling for the effects of age, sex, and race. Three primary comparisons were conducted: 1) controls vs. Intact patients, which isolated the effect of the presence of a psychotic disorder on interregional correlations by matching controls and patients on cognitive profiles, 2) Intact vs. Impaired patient groups, which matched groups based on presence of a psychotic disorder to isolate the effects of cognition, and 3) controls vs. the Impaired patient group, examining the effects of both psychotic disorder and cognitive dysfunction on connectivity measures (Figure 2).
Figure 2. Effects of Patient Status and Cognition on Functional Connectivity in Frontoparietal Control and Motor Networks.
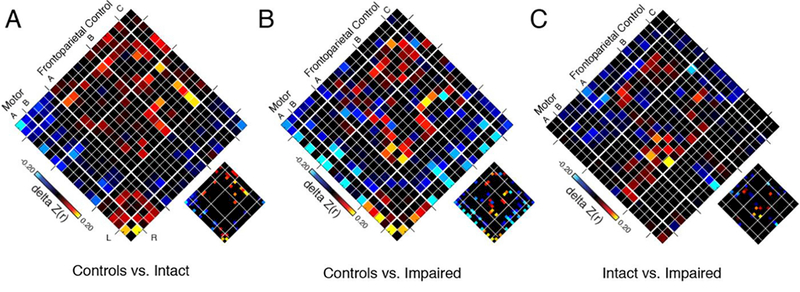
Each panel shows a 19×19 adjacency matrix corresponding to group differences in interregional connectivity among nodes of the frontoparietal control and motor networks based on (A) diagnosis alone, (B) diagnosis and cognition, and (C) cognition alone. Specifically, leftmost panel (A) shows the difference in connectivity (delta Z[r]) when comparing Intact patients (n=47) to Control participants (n=31), with hotter colors representing interactions that were lower in patients than controls. Middle panel (B) shows differences in connectivity comparing globally Impaired patients (n=48) with Controls (n=31), with hotter colors representing interactions that were lower in patients than Controls. Rightmost panel (C) shows connectivity differences when comparing Intact (n=47) and Impaired (n=48) patients, with hotter colors representing interactions that were lower in more impaired patients. Large matrices show unthresholded maps; sub-images show false discovery rate-thresholded significant effects (FDR, q<0.05). Left and right hemisphere nodes are shown on left and right sides of each diamond, respectively to highlight the symmetry of the findings; within-network interactions are shown along the central diagonal. The FPN subnetwork A includes 7 regions: intraparietal sulcus (IPS), lateral anterior prefrontal cortex (PFCla), posterior temporal, dorsal prefrontal cortex (PFCd), cingulate, medial posterior prefrontal cortex (PFCmp), and orbitofrontal cortex (OFC). The FPN subnetwork B includes 5 regions: lateral posterior prefrontal cortex (PFClp), lateral anterior prefrontal cortex (PFCla), inferior parietal lobule (IPL), temporal, and PFCmp. The FPN subnetwork C includes 2 regions: precuneus and the cingulate gyrus C component. The motor network includes 2 subnetworks: A (2 regions) and B (4 regions).
Compared to controls, Intact patients showed reduced within-network correlations in the Visual and Motor networks and the FPN, particularly in the FPN-B subnetwork, including areas of the inferior parietal and temporal lobules; patients also showed reduced correlations within the motor network (Figure 2A). Comparison of Impaired patients to controls found that patients with impaired cognitive profiles showed reduced correlations in several networks including Ventral Attention, Default, and FPN across both FPN-A and FPN-B subnetworks, which includes the intraparietal sulcus (IPS), lateral anterior prefrontal cortex (PFCla), posterior temporal, dorsal prefrontal cortex (PFCd), as well as in the motor network (Figure 2B). Comparison of the two patient groups revealed that Impaired patients showed reduced correlations localized primarily in the FPN compared to Intact patients, specifically in the FPN-A subnetwork (Figure 2C). There were no significant differences between patient groups in motor network or Default Network correlations. Because patient groups differed significantly on several clinical measures including psychosis and mania symptoms and CPZ, we added these measures as covariates to the analyses. After including CPZ, PANSS total score, YMRS, and scan type as covariates in the patient-only comparisons1 all findings remained unchanged. To examine the possibility that a more continuous approach to the data better explained our findings we examined correlations between FPN networks and Composite MCCB scores across the patient sample. The correlation of mean FPN connectivity (average of FPN subnetworks) with MCCB Composite was non-significant (r=.04, p=.64). Additionally, neither FPN subnetwork A nor FPN subnetwork B were significantly correlated with MCCB composite across the patient sample (r=.10, p=.32 and r=.13, p=.16, respectively).
Based on our above findings we conducted a post-hoc analysis of correlations in FPN subnetworks A and B. An average connectivity index within the FPN subnetwork A (FPN-A) and FPN subnetwork B (FPN-B) was extracted and compared across groups using ANOVA. We found a significant effect of group on correlations in FPN-A (F(2, 123)=5.74, p=.004) and FPN-B (F(2, 123)=5.16, p=.007). Pairwise comparisons indicated that controls had higher correlations than Impaired and Intact patients in both FPN-A (t(77)=3.56, p<001 and (t(76)=2.18, p<.05, respectively) and FPN-B (t(77)=2.38, p<.05 and (t(76)=2.38,p<05, respectively). The two patient groups did not differ significantly from each other (t(93)=1.23, p=. 11); however, calculation of effect size of differences between groups (i.e. Intact-Impaired, Control-Intact, and Control-Impaired) showed greater magnitude of difference from Controls in both FPN subnetworks in Impaired patients than in Intact patients (Figure 3), with large effects of cognitive impairment on both FPN A and B in the Impaired group; Intact patients showed small effects in FPN A and medium effects of FPN B compared to controls.
Figure 3. Post Hoc Comparisons of Average Connectivity by Group: Frontoparietal Control Networks A and B.
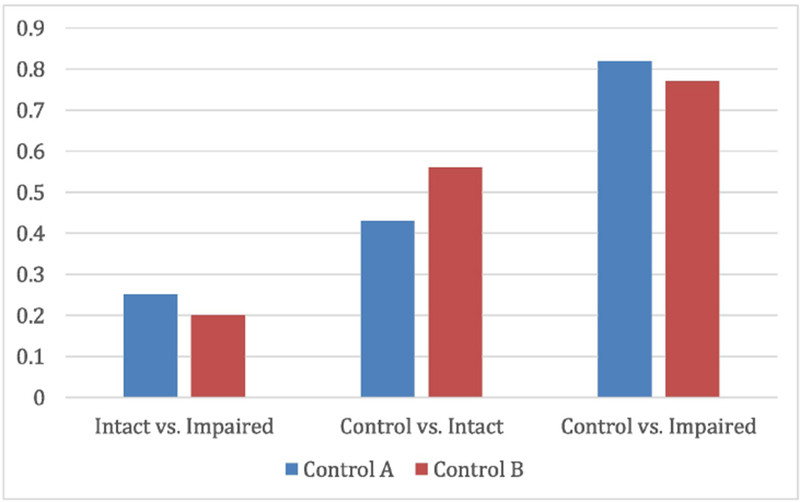
Effects sizes (Cohen’s d) of the pairwise contrasts in subnetwork connectivity in FPN subnetwork A (“Control A”) and FPN subnetwork B (“Control B”). Mean within-subnetwork connectivity was calculated for each group; pairwise effect sizes (Intact vs. Impaired; Control vs. Intact; Control vs. Impaired) were then calculated to reflect the magnitude of within network connectivity in the less impaired group relative to the more impaired group, or the patient groups compared to the control group.
4. Discussion
The present study examined functional connectivity in patients with psychosis with and without cognitive impairment, and in healthy controls. We found evidence of reduced connectivity in both patient groups in FPN and motor networks, with additional dysconnectivity associated with cognitive dysfunction in FPN, particularly subnetwork A. Previous work has indicated that the FPN shows pronounced reductions in functional connectivity in patients with SZ or BD compared to healthy controls (Baker et al., 2014; Littow et al., 2015; Woodward et al., 2011). Although an earlier study did not report a relationship between FPN connectivity and a measure of IQ (Baker et al., 2014), other groups have found associations between FPN and higher order cognition (Badre and D’Esposito, 2007; Cole et al., 2011), suggesting that differences in cognitive profiles between patients and controls and within patient groups might explain some of the observed connectivity differences. Here, our study design allowed us to examine the unique contributions of presence of a psychotic disorder and cognitive impairment to network connectivity differences. Our results demonstrated a robust pattern of dysconnectivity in the FPN and motor network associated with diagnosis of a psychotic disorder (Figure 2A, 2B) even when groups were matched for cognitive performance (Figure 2A), and large effects of cognitive impairment on functional connectivity in the FPN amongst patients, even after accounting for presence of a psychotic disorder, state symptom severity and antipsychotic load (Figure 2C). This is the first study we are aware of to examine functional connectivity among cortical brain networks in patients with psychosis grouped based on cognitive impairment. Of note, lack of correlation between FPN and cognition across the patient sample suggests that associations between cognitive impairment and functional connectivity may be more reflective of discreet phenotypes than a continuum of impairment.
Intriguingly, while we saw medium and medium-large effects in Intact and Impaired patients respectively compared to controls in FPN subnetwork B, we saw a particularly large effect of cognition in the FPN subnetwork A; comparisons against the control group revealed large effects in the Impaired group (d=.82), with small to medium effects in the Intact group (d=.43). These findings (1) support the hypothesis that the FPN can be divided into multiple functionally distinct subnetworks and (2) provide new evidence that cognitive functioning and psychosis may impinge to different degrees upon different parts of this network. Moreover, this biological segregation supports clinical and neuropsychological evidence for distinct subtypes of psychotic illness – or potentially, dimensions of impairment – with varying loads of cognitive dysfunction contributing to the illness syndrome. While it is possible that these findings reflect degrees of overall illness severity, which may be more moderate in “intact” patients and more severe in “impaired” patients, differences in FPN subnetworks between patient groups remained after controlling for state clinical symptoms and antipsychotic burden, suggesting that the additional FPN dysconnectivity in the Impaired group is not simply a reflection of general illness severity. These subnetwork analyses were post-hoc and should therefore be interpreted with caution until findings can be replicated.
It should be noted that the present grouping strategy is not meant to imply that the Intact group is altogether cognitively unaffected, but rather to examine the extent to which heterogeneity along this important symptom dimension is associated with biological correlates that would be expected to underpin cognition. Indeed, it is possible that patients in both groups have experienced cognitive effects of illness starting from different cognitive “baselines,” as has been shown in previous work with twin pairs (Goldberg et al., 1995). Nevertheless, by parsing variability along the cognitive dimension we found network alterations that are associated with cognitive impairment specifically.
The present study has several limitations. First, data were assembled from several separate but related studies, with slightly different study criteria. Additionally, image acquisition parameters differed slightly amongst studies, as noted above. However, scan type was distributed across groups (Chi2= 6.97, ns), and inclusion of scan type as a covariate did not change our findings. Considerable cognitive heterogeneity exists in our cognitively impaired group, limiting our ability to study specific associations between separable cognitive domains and network connectivity. Large samples will be needed to examine these associations at an even more granular level, and would provide key insights into the relationships between impairment in specific cognitive domains and resting state connectivity in patients with psychosis. Lastly, our group and others have found that patients with psychosis may be characterized by more than two dichotomous cognitive groupings; however, our sample was underpowered to evaluate effects of multiple cognitive groups on connectivity measures. Again, larger samples may clarify associations between more nuanced cognitive profiles and network connectivity.
4.1. Conclusions
Functional connectivity of higher order cortical association networks has consistently been shown to be reduced in patients with psychosis, indicating it may reflect a core endophenotype. However, an overlapping set of cortical territories is also known to participate in cognition, which is disrupted in a significant portion of people with psychotic illnesses. In the present study, we leveraged the heterogeneity in cognitive ability among patients with psychosis to disentangle the relative contributions of cognitive dysfunction and presence of an underlying psychotic illness using fMRI-measured functional connectivity. Previous work suggests that more homogeneous grouping of patients along the cognitive symptom dimension may be more reflective of specific neurobiological mechanisms (Clementz et al., 2016; Woodward and Heckers, 2015). Indeed, we found unique contributions of illness and cognitive dysfunction to network connectivity, even after controlling for illness severity, suggesting that grouping patients along the cognitive dimension effectively permits examination of unique aspects of illness and their association with neurobiology.
Acknowledgments
Funding Body Agreements and Policies
This work was supported by: NIMH grant R21MH110699 (KEL); NIMH grant K24MH104449 (DO); NIMH grant K23MH104515 (JTB), NIDA grant T32DA015036-15 (JMM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure
Conflicts of Interest
All authors KEL, JMM, DO, LAN, GZL, RJJ, DO, and JTB have no financial or commercial disclosures to report in relation to this work.
Five participants were not included in the covariate analysis due to missing medication data.
REFERENCES
- Argyelan M, Ikuta T, DeRosse P, Braga RJ, Burdick KE, John M, Kingsley PB, Malhotra AK, Szeszko PR, 2014. Resting-state fMRI connectivity impairment in schizophrenia and bipolar disorder. Schizophr. Bull 40(1) 100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, D’Esposito M, 2007. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J. Cogn. Neurosci 19(12) 2082–2099. [DOI] [PubMed] [Google Scholar]
- Baker JT, Holmes AJ, Masters GA, Yeo BT, Krienen F, Buckner RL, Öngür D, 2014. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 71(2) 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldessarini RJ (2012). Chemotherapy in Psychiatry. New York: Springer Verlag. [Google Scholar]
- Barker S, Barron N, McFarland BH, Bigelow DA, 1994. A community ability scale for chronically mentally ill consumers: Part I. Reliability and validity. Community Ment. Health J 30(4) 363–383. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS, 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34(4) 537–541. [DOI] [PubMed] [Google Scholar]
- Bryson GJ, Silverstein ML, Nathan A, Stephen L, 1993. Differential rate of neuropsychological dysfunction in psychiatric disorders: comparison between the Halstead-Reitan and Luria-Nebraska batteries. Percept. Mot. Skills. 76(1) 305–306. [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT, 2011. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 106(5)2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EY, Yeo BT, Buckner RL, 2012. The organization of the human striatum estimated by intrinsic functional connectivity. J. Neurophysiol 108(8) 2242–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, Keshavan MS, Tamminga CA, 2016. Identification of Distinct Psychosis Biotypes Using Brain-Based Biomarkers. Am. J. Psychiatry 173(4) 373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobia DJ, Csernansky JG, Wang L, 2011. Cortical thickness in neuropsychologically near-normal schizophrenia. Schizophr. Res 133(1-3) 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Anticevic A, Repovs G, Barch D, 2011. Variable global dysconnectivity and individual differences in schizophrenia. Biol. Psychiatry. 70(1) 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre P, Baciu M, Pichat C, Bougerol T, Polosan M, 2014. fMRI evidence for abnormal resting-state functional connectivity in euthymic bipolar patients. J. Affect. Disord 165 182–189. [DOI] [PubMed] [Google Scholar]
- Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS, 2011. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biol. Psychiatry. 70(1) 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, 2008. Is cognitive impairment in schizophrenia ready for diagnostic prime time. World Psychiatry. 7(1) 32–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Torrey EF, Gold JM, Bigelow LB, Ragland RD, Taylor E, Weinberger DR 1995. Genetic risk of neuropsychological impairment in schizophrenia: a study of monozygotic twins discordant and concordant for the disorder. Schizophr. Res 17(1) 77–84. [DOI] [PubMed] [Google Scholar]
- Goldstein G, Shemansky WJ, 1995. Influences on cognitive heterogeneity in schizophrenia. Schizophr. Res 18(1) 59–69. [DOI] [PubMed] [Google Scholar]
- Heinrichs RW, Awad AG, 1993. Neurocognitive subtypes of chronic schizophrenia. Schizophr. Res. 9(1) 49–58. [DOI] [PubMed] [Google Scholar]
- Hill SK, Ragland JD, Gur RC, Gur RE, 2002. Neuropsychological profiles delineate distinct profiles of schizophrenia, an interaction between memory and executive function, and uneven distribution of clinical subtypes. J. Clin. Exp. Neuropsychol 24(6) 765–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbrzikowski M, Bearden CE, Biotypes: The tip of the research domain criteria iceberg. Biol. Psychiatry: Cognitive Neuroscience and Neuroimaging. 1(6) 486–487. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S, 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 17(2) 825–841. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA, 1987. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull 13(2) 261–276. [DOI] [PubMed] [Google Scholar]
- Lewandowski KE, Cohen BM, Keshavan MS, Sperry SH, Ongur D, 2013. Neuropsychological functioning predicts community outcomes in affective and non-affective psychoses: a 6-month follow-up. Schizophr. Res 148(1-3) 34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski KE, Sperry SH, Cohen BM, Ongur D, 2014. Cognitive variability in psychotic disorders: a cross-diagnostic cluster analysis. Psychol. Med 44(15) 3239–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littow H, Huossa V, Karjalainen S, Jääskeläinen E, Haapea M, Miettunen J, Tervonen O, Isohanni M, Nikkinen J, Veijola J, Murray G, Kiviniemi VJ, 2015. Aberrant Functional Connectivity in the Default Mode and Central Executive Networks in Subjects with Schizophrenia - A Whole-Brain Resting-State ICA Study. Front. Psychiatry. 6 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, Bullmore E, 2010. Functional connectivity and brain networks in schizophrenia. J. Neurosci 30(28) 9477–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda SA, Clementz BA, Sweeney JA, Keshavan MS, Tamminga CA, Ivleva EI, Pearlson GD, 2016. Examining functional resting-state connectivity in psychosis and its subgroups in the bipolar-schizophrenia network on intermediate phenotypes cohort. Biol. Psychiatry: Cognitive Neuroscience and Neuroimaging. 1(6) 488–297. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M, 1979. A new depression scale designed to be sensitive to change. Br. J. Psychiatry. 134 382–389. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR, 2008. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am. J. Psychiatry. 165(2) 203–213. [DOI] [PubMed] [Google Scholar]
- Palmer BW, Heaton RK, Paulsen JS, Kuck J, Braff D, Harris MJ, Zisook S, Jeste DV, 1997. Is it possible to be schizophrenic yet neuropsychologically normal. Neuropsychology. 11(3) 437–446. [DOI] [PubMed] [Google Scholar]
- Rojo E, Pino O, Guilera G, Gómez-Benito J, Purdon SE, Crespo-Facorro B, Cuesta MJ, Franco M, Martínez-Arán A, Segarra N, Tabarés-Seisdedos R, Vieta E, Bernardo M, Mesa F, Rejas J, Spanish WGICF, 2010. Neurocognitive diagnosis and cut-off scores of the Screen for Cognitive Impairment in Psychiatry (SCIP-S). Schizophr. Res. 116(2–3) 243–251. [DOI] [PubMed] [Google Scholar]
- Seaton BE, Goldstein G, Allen DN, 2001. Sources of heterogeneity in schizophrenia: the role of neuropsychological functioning. Neuropsychol. Rev. 11(1) 45–67. [DOI] [PubMed] [Google Scholar]
- Sepede G, Spano MC, Lorusso M, De Berardis D, Salerno RM, Di Giannantonio M, Gambi F, 2014. Sustained attention in psychosis: Neuroimaging findings. World J. Radiol 6 261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield JM, Kandala S, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA, Clementz BA, Lerman-Sinkoff DB, Hill SK, Barch DM, 2017. Transdiagnostic Associations Between Functional Brain Network Integrity and Cognition. JAMA Psychiatry. 74(6) 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF, 2009. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. USA. 106(31) 13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttl B, 2002. North American Adult Reading Test: age norms, reliability, and validity. J Clin Exp. Neuropsychol. 24(8) 1123–1137. [DOI] [PubMed] [Google Scholar]
- van der Kouwe AJW, Benner T, Salat DH, Fischl B, 2008. Brain morphometry with multiecho MPRAGE. Neuroimage. 40(2) 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL, 2010. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J. Neurophysiol. 103(1) 297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rheenen TE, Cropley V, Zalesky A, Bousman C, Wells R, Bruggemann J, Sundram S, Weinberg D, Lenroot RK, Pereira A, Shannon Weickert C, Weickert TW, Pantelis C, 2017a. Widespread Volumetric Reductions in Schizophrenia and Schizoaffective Patients Displaying Compromised Cognitive Abilities. Schizophr. Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rheenen TE, Lewandowski KE, Tan EJ, Ospina LH, Ongur D, Neill E, Gurvich C, Pantelis C, Malhotra AK, Rossell SL, Burdick KE, 2017b. Characterizing cognitive heterogeneity on the schizophrenia-bipolar disorder spectrum. Psychol. Med. 47(10) 1848–1864. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL, 2008. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 100(6) 3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL, 2006. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J. Neurophysiol. 96(6) 3517–3531. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Zhu H, Bell MD, Nicholls SS, Fulbright RK, Gore JC, Colibazzi T, Amat J, Bansal R, Peterson BS, 2009. Neuropsychological near normality and brain structure abnormality in schizophrenia. Am. J. Psychiatry. 166(2) 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Heckers S, 2015. Brain Structure in Neuropsychologically Defined Subgroups of Schizophrenia and Psychotic Bipolar Disorder. Schizophr. Bull. 41(6) 1349–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Rogers B, Heckers S, 2011. Functional resting-state networks are differentially affected in schizophrenia. Schizophr. Res. 130(1–3) 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL, 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106(3) 1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA, 1978. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry. 133 429–435. [DOI] [PubMed] [Google Scholar]


