Abstract
BACKGROUND:
Depression is highly prevalent in lung cancer. Although there is a known association between inflammation and depression, this relationship has not been examined in patients with lung cancer who undergo treatment with immune and other targeted drug therapies. Peripheral blood C-reactive protein (CRP), a marker of systemic inflammation, may help identify metastatic lung cancer patients with inflammation-associated depression.
METHOD:
Patients with metastatic lung cancer undergoing treatment were evaluated for depression using the Hospital Anxiety and Depression Scale (HADS). Inflammation (CRP and CRP cutoffs ≥1 and ≥3 mg/mL) and demographic and treatment variables were analyzed for association with depression.
RESULTS:
One hundred nine consecutive participants exhibited an average plasma CRP concentration of 1.79 mg/mL (median, 0.75 mg/mL [standard deviation, 2.5 mg/mL), and 20.7% had a CRP concentration of ≥3.0 mg/mL; 23.9% met depression screening criteria (HADS ≥8). A log transformation of CRP was significantly correlated with depression severity (r = 0.47, P < .001). CRP was the only covariate to predict depression severity (P = .008) in a multivariate model including lung cancer disease subtype and type of systemic treatment. Receiver operating characteristic analysis indicated that CRP had moderate predictive accuracy in identifying elevated depression (area under the curve = 0.74). A cutoff of CRP ≥3.0 generated high specificity (88%) but identified only 50% of those with elevated depression.
CONCLUSION:
Elevated CRP is associated with depression in patients with metastatic lung cancer. Thus, CRP may identify a subset of lung cancer patients with inflammation-induced depression and may be useful in predicting response to treatments that target inflammation or its downstream mediators on the brain.
Keywords: lung cancer, depression, C-reactive protein, inflammation, acute phase reactant, hospital anxiety and depression scale
INTRODUCTION
Lung cancer has one of the highest rates of comorbid depression among all cancer types.1,2 Unfortunately, the highest rates of depression are in patients whose cancers have the poorest survival rate.3 Based on a large cohort of studies, the prevalence of depression in lung cancer ranges from 16% to 29%,4,5 rates that exceed the average prevalence of major depression in cancer more generally, which is approximately 15%.2 A contributing factor for the high rate of depression in patients with lung cancer may be inflammation, because inflammation is elevated in both conditions. As such, lung cancer provides an environment enhanced with patients who are living with chronic inflammation. Moreover, inflammation has been associated with depression in multiple clinical settings, including medically healthy and chronically ill patients.6–8 Indeed, increased peripheral biomarkers of inflammation have been found in a subset of patients with depression and in the development and perpetuation of depression in population-based and case-controlled studies.9–14 Medical illnesses, including lung cancer, are also associated with both higher rates of inflammation and a higher prevalence of depression including treatment refractory depression.15,16
Tumor necrosis factor α, interleukin-6, and interleukin-1β are proinflammatory cytokines that are most commonly and consistently associated with depression.17–19 In lung cancer, biomarkers such as interleukin-6 and tumor necrosis factor α are elevated in patients with depressed states.20 Nevertheless, the clinical usefulness of this association is complicated by a number of factors. Concentrations of these cytokines are not routinely measured in hospital or clinic laboratories, and diurnal and other physiologic variations in cytokines whose half-lives are relatively short make single time point sampling logistically complicated. Therefore, the association between depression and specific inflammatory cytokines has not led to practice-changing knowledge in the treatment of depression in the setting of chronic inflammation.
C-reactive protein (CRP) is an acute phase reactant and biomarker produced by the liver in response to multiple proinflammatory cytokines, especially interleukin-6, and increases by at least 25% with general inflammation.21,22 Peripheral blood CRP has been associated with depression and other measures of psychological distress in both medically healthy and chronically ill populations.17,23 Of note, peripheral blood CRP has also recently been shown to correlate strongly with central (ie, cerebrospinal fluid [CSF]) CRP, which has in turn been correlated with inflammatory markers in CSF.24 Cutoff points have been established for the delineation of inflammation, with CRP >1 mg/L and >3 mg/L indicating moderate and high inflammation, respectively.25,26 CRP has a relatively long half-life and is therefore less susceptible to diurnal/circadian fluctuations.27 In addition, CRP is easily obtained by routine laboratory testing and may be followed in a primary medical setting as a part of routine laboratory work. Because the CRP laboratory test is a standard laboratory value that exhibits less fluctuation than proinflammatory cytokines, it has distinct clinical advantages as a general measure of inflammation over cytokine assays that are subject to greater variation and are not immediately available in clinical practice.28 Given its ability to measure chronic inflammation, CRP has been studied in the lung cancer context as both a measurement of lung cancer risk and as a prognostic marker of disease activity once lung cancer has been diagnosed.29,30 However, it has not been studied in relation to depression in lung cancer or while undergoing anticancer treatments. Understanding its role as a marker of inflammation that may be associated with the presence of depression in patients with metastatic lung cancer has important and specific treatment implications. Inflammation has been linked to poor recovery from depression and appears to make certain antidepressant medications and psychotherapies less effective.31–33
At present, the selection of depression treatments in the lung cancer setting remains a trial-and-error endeavor. The identification of an inflammatory phenotype of depression that responds preferentially to a specific antidepressant, anti-inflammatory medication, and/or psychotherapy would begin to transform the management of depression into an individualized and tailored approach. In addition, the identification of demographic and treatment factors that predict depression would also be helpful to stratify lung cancer patients who are at risk for depression associated with inflammation. Therefore, we conducted a cross-sectional study of CRP with demographic and treatment variables in patients with stage IV lung cancer who were receiving anticancer treatment. The goal of this study was to establish an investigational foundation to understand the relationship between inflammation and depression in the setting of ongoing anticancer treatments in patients with metastatic lung cancer.
PATIENTS AND METHODS
The Memorial Sloan Kettering Cancer Center Institutional Review Board (IRB) approved this study in May 2018. Surveys and routine blood work (CRP) were collected as standard of care practice from May 2017 to November 2017.
Patients
Men and women with histologically confirmed stage IV lung cancer who were undergoing active treatment, spoke English, and had a performance status of ECOG 2 or greater were included. Participants with other cancers or not undergoing treatment for stage IV lung cancer were excluded. Participants had to be on active treatment for at least 1 month and had to be more than 1 month from receiving the diagnosis of lung cancer to be included.
Procedure
Patients were asked to complete a one-time survey by a treating staff member (eg, nurse practitioner, medical oncologist). They filled out the questionnaire containing standardized survey questions, and CRP laboratory values were obtained the same day that the questionnaires were completed. Available psychological services were provided in the survey, and patients were asked to raise any concerns with clinic staff and, in particular, to tell a staff member if they felt significantly depressed or had suicidal ideation. The surveys were collected as routine standard of care screening as per distress screening guidelines.
Measures
Patient demographic and medical characteristics
Patient demographic information was obtained from the medical record and included age, race/ethnicity, sex, marital status, body mass index, length of time since diagnosis, type of treatment (eg, chemotherapy, immunotherapy, targeted therapy), line of treatment (ie, first, second, third or beyond), and whether they were taking an antidepressant medication.
Biological characteristics (CRP)
CRP values were obtained by way of turbidimetric immunoassay in a Clinical Laboratory Improvement Amendments (CLIA)-certified lab.34 The inter- and intra-assay coefficient of variation was reliably less than 5%. For some analyses, CRP cut-points of 1 mg/mL and 3 mg/mL were used. A cut-point of 1 mg/mL was chosen because of its use in evaluating antidepressant responses in recent studies.25,26 A cut-point of 3 mg/mL was chosen on the basis of functional magnetic resonance imaging data showing depletion of dopa-mine in participants with CRP elevations above this threshold.10
Depression
Depression severity was measured by the Hospital Anxiety and Depression Scale (HADS), which has been validated in the lung cancer setting.35,36 The HADS is a 14-item symptom rating scale that was developed to identify clinically significant cases of anxiety and depressive disorders among medically ill patients.35 Unlike most symptom rating scales, physical symptoms are excluded from the HADS due to the potential confounding effects of illness on symptoms such as sleep, appetite disturbance, and fatigue. The HADS is divided into an anxiety subscale (HADS-A) and a depression subscale (HADS-D). Responses are rated 0 to 3 points such that total scores on the HADS-A and HADS-D may range from 0 to 21 points. A cutoff of 8 on the HADS-D subscale is most commonly used to identify clinically significant depression, with an average sensitivity and specificity of 0.80.35,37 Because research on inflammation has focused primarily on depression, not anxiety, only HADS-D data were analyzed in this study.
Statistical Analysis
The primary outcome of this study was severity of depressive symptoms, and in particular, its association with CRP in patients with metastatic lung cancer. Because CRP data are not normally distributed (see below), CRP values were log-transformed before data analysis; however, untransformed values are also reported for ease of interpretation. Univariate associations between patient demographic and medical characteristics with depression (HADS-D) and CRP were assessed with Spearman correlation coefficients (rank-order correlations for age, body mass index, time since diagnosis), independent t tests (dichotomous variables of race/ethnicity, marital status, antidepressant use), and analysis of variance (categorical variables of type of treatment, disease status). Statistically significant covariates were selected for inclusion in multiple regression analysis. For multilevel categorical variables, dummy coding was used to assess the contribution of treatment type (ie, immunotherapy and targeted therapies; chemotherapy was set as the reference variable) and disease type (ie, squamous cell and small cell lung cancer; adenocarcinoma was set as the reference variable). Finally, to assess the predictive accuracy of CRP in identifying clinically significant depressive symptoms, receiver operating characteristic curve analysis was used, with the area under the curve statistic used to quantify sensitivity and specific across a range of possible CRP cut-scores. Statistical procedures were performed using SPSS version 24 software (SPSS, Chicago, Illinois), and all statistical tests were 2-tailed with a 5% significance level.
RESULTS
Out of 140 potential participants, 109 returned survey information (77.9% response rate). Sample characteristics are presented in Table 1. The average age was 65.9 years, and the majority of patients were women (62.4%), white (85.2%), and married (69.7%). Most patients had adenocarcinoma non–small cell lung cancer (71.8%) and were receiving chemotherapy (45.2%), followed by immunotherapy (33.7%) and targeted biologic therapy (21.2%). Twenty-six of the 109 participants had clinically significant symptoms of depression (23.9%) (HADS-D ≥8).
TABLE 1.
Clinical and Demographic Characteristics of the Patients (n = 109)
| Characteristic | Values |
|---|---|
| Categorical variables, mean (SD) | |
| Age, y | 65.9 (9.3) |
| Body mass index, kg/m2 | 26.1 (5.0) |
| Time with disease, mo | 15.4 (17.3) |
| CRP, mg/mL | 1.79 (2.5) |
| HADS-D scorea | 4.9 (3.7) |
| Continuous variables, n (%) | |
| Met criteria screen for depressionb | 26 (23.9) |
| CRP cutoff | |
| ≥1.0 mg/mL | 49 (45.3) |
| ≥3.0 mg/mL | 23 (20.7) |
| Sex | |
| Men | 41 (37.6) |
| Women | 68 (62.4) |
| Disease type | |
| Adenocarcinoma | 79 (71.8) |
| Squamous cell carcinoma | 7 (6.4) |
| Small cell lung cancer | 18 (16.5) |
| Unspecified | 5 (4.6) |
| Treatment type | |
| Chemotherapy | 47 (45.2) |
| Immunotherapy | 35 (33.7) |
| Targeted therapy | 22 (21.2) |
| Missing | 6 (5.5) |
| Line of treatment | |
| First | 56 (53.3) |
| Second | 34 (32.4) |
| Third or beyond | 15 (14.3) |
| Missing | 5 (4.5) |
| Race/Ethnicity | |
| Black | 7 (6.4) |
| White | 93 (85.2) |
| Latino | 7 (6.4) |
| Asian | 2 (1.8) |
| Married | |
| Yes | 76 (69.7) |
| No | 33 (30.3) |
| Antidepressant | |
| Yes | 18 (16.5) |
| No | 91 (83.5) |
Abbreviations: CRP, C-reactive protein; HADS-D, Hospital Anxiety and Depression Scale–Depression Subscale; SD, standard deviation.
Range: 0–21.
HADS-D ≥8.
The mean CRP concentration of the sample was 1.79 mg/mL (median, 0.75 mg/mL [SD, 2.5 mg/mL]) with a range of <0.05 to 18.51 mg/mL. As expected, CRP values were highly skewed and kurtotic (skew = 2.45, kurtosis = 6.78); the distribution approximated a normal distribution following log transformation (skew = −0.78, kurtosis = 0.85). The CRP cutoff of ≥1 mg/mL was met by 45.3% of the sample and the cutoff of ≥3 mg/mL was met by 20.7%.
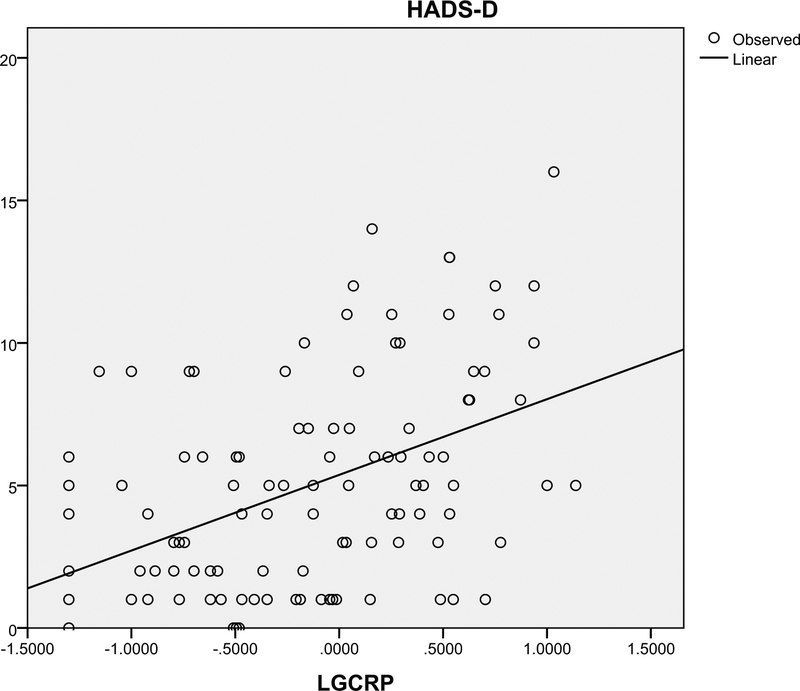
Depression was significantly associated with CRP (P < .001), receiving chemotherapy (P = .006), and small cell lung cancer subtype (P = .021) (Table 2). However, when these variables were included in a multiple regression analysis predicting depression, only CRP (log-transformed) was significantly associated with depression (adjusted R2 = 0.23, P = .001) (Table 3). Linear regression revealed that CRP was a predictor for approximately 20% of depression variability (adjusted R2 0.2, P = .001) (Fig. 1). In addition, patients who had clinically significant depression scores (HADS-D≥8) had higher median CRP levels (3.4 mg/mL versus 1.3 mg/mL) (P = .003) and were more likely to be receiving advanced lines of treatment (P = .24).
TABLE 2.
Univariate Analyses of Demographic, Physiologic and Treatment Characteristics Related to Depression as Measured Using the Hospital Anxiety and Depression Scale–Depression Subscale
| Univariate Analysis | ||
|---|---|---|
| Variable | r | P |
| Age | 0.12 | .38 |
| BMI | 0.08 | .32 |
| Time with disease | −0.06 | .75 |
| CRP | 0.47 | <.001 |
| F | P | |
| Line of treatmenta | 2.18 | .12 |
| Treatment typeb | 5.42 | .006 |
| Disease typec | 3.37 | .021 |
| Race | 0.23 | .88 |
| t | P | |
| Sex | −1.60 | .11 |
| Marital status | 0.90 | .37 |
| Treatment with antidepressants | −1.08 | .28 |
Abbreviations: BMI, body mass index; CRP, C-reactive protein; HADS-D, Hospital Anxiety and Depression Scale–Depression Subscale.
First, second, third and beyond.
Chemotherapy, immunotherapy, targeted therapy.
Adenocarcinoma, squamous cell carcinoma, small cell carcinoma.
TABLE 3.
Multivariate Regression of Significant Demographic and Treatment Variables on Depression as Measured Using the Hospital Anxiety and Depression Scale
| Depression (HADS-D) | |||
|---|---|---|---|
| Variable | Regression Coefficient | t | P |
| CRP (log transformed) | 0.42 (0.6) | 4.232 | <.001 |
| Treatment type | |||
| Chemotherapy | Reference | ||
| Immunotherapy | −0.05 (0.8) | −0.49 | .63 |
| Targeted therapy | −0.09 (1.0) | −0.81 | .42 |
| Disease type | |||
| Adenocarcinoma | Reference | ||
| Squamous cell carcinoma | 0.05 (1.3) | 0.49 | .62 |
| Small cell carcinoma | 0.19 (1.0) | 1.97 | .051 |
Abbreviations: CRP, C-reactive protein; HADS-D, Hospital Anxiety and Depression Scale–Depression Subscale.
F = 6.607; adjusted R2 = 0.226.
Figure 1.
Curve estimation of C-reactive protein (CRP) and depression as measured by the Hospital Anxiety and Depression Scale–Depression Subscale (HADS-D). CRP has been log-transformed given its skewed distribution (LGCRP). Adjusted R2 = 0.2.
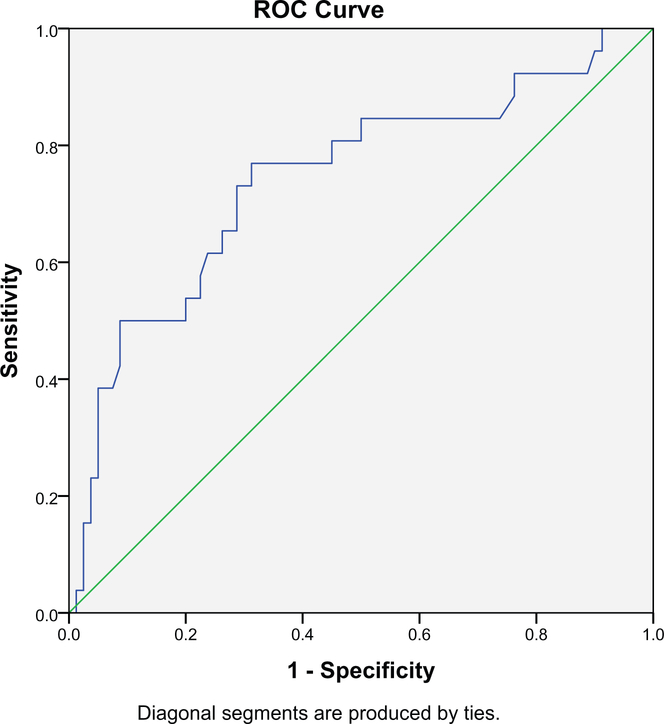
Receiver operating characteristic curve analysis indicated a moderate level of predictive accuracy for CRP values (area under the curve = 0.743, P < .001) (Fig. 2). When examining previously established CRP cut-points, both the ≥1 mg/mL and ≥3 mg/mL CRP cutoffs were associated with clinically significant depression symptoms (P < .001) (Table 4). Of those meeting depression screening criteria (ie, sensitivity), 76.9% had a CRP level ≥1 mg/mL and 50% had a CRP level ≥3 mg/mL. Specificity was 65% for CRP ≥1 mg/mL and 88% for CRP ≥3 mg/mL.
Figure 2.
Receiver operator characteristic (ROC) curve for C-reactive protein and its identification of clinically significant depression as determined by the Hospital Anxiety and Depression Scale–Depression Subscale (HADS-D ≥8).
TABLE 4.
Association Between C-Reactive Protein Inflammation Cutoff Scores and Clinically Significant Depression as Determined Using the Hospital Anxiety and Depression Scale (HADS ≥8)
| Sensitivity and Specificity Analyses | Depression (HADS-D ≥8) | |||
|---|---|---|---|---|
| Yes | No | Total | χ2 | |
| CRP level, n (%) | Missing = 2 (1.8%) | |||
| ≥3.0 mg/mL | 13 (50.0) | 9 (11.3) | 22 (20.8) | |
| 1–2.99 mg/mL | 7 (26.9) | 18 (22.5) | 25 (23.6) | |
| <1.0 mg/mL | 6 (23.1) | 53 (66.2) | 59 (55.6) | |
| Total, n (%) | 26 (24.5) | 80 (75.5) | 106 (100) | 20.93, P<.001 |
Abbreviations: CRP, C-reactive protein; HADS-D, Hospital Anxiety and Depression Scale–Depression Subscale.
Eighteen (16.5%) participants reported that they were taking antidepressant medication, including only 7 of the 26 participants (26.9%) with clinically significant depression. As shown in Table 2, there was no univariate association between antidepressant use and severity of depressive symptoms. Moreover, when antidepressant use was included as an interaction term in a regression model predicting depression severity, there was no evidence that this variable mediated or moderated the effect of inflammation on depression (P = .28).
DISCUSSION
Inflammation in lung cancer is associated with depression both in terms of severity and clinical significance. Both inflammation and depression were elevated and significantly associated in this population of lung cancer patients actively undergoing anticancer treatments. Although small cell lung cancer, advanced lines of treatment, and receiving chemotherapy were also associated with depression, inflammation as reflected by CRP was the strongest predictor of depression. The rate of depression was consistent with other cohorts of lung cancer patients using various measures of depression.2,38 The level of inflammation as measured by CRP was higher on average than medically healthy populations.39,40 However, the distribution revealed that approximately one fifth of patients had significantly elevated CRP (≥3 mg/mL), which seems to be a representative rate among medically ill patients from various medical conditions associated with inflammation.41 In addition, patients with moderate or high inflammation were more likely to have clinically significant depression. This distribution suggests that patients with metastatic lung cancer receiving anticancer treatments are enhanced with for inflammation and that this population would represent an ideal cohort in which to study the treatment of inflammation-associated depression. Preliminary work suggests that CRP may predict checkpoint inhibitor response and therefore could be used more routinely in the future with increasing use of immunotherapy.42 It should be noted that inflammation is not present in all cases of depression in the lung cancer setting and may not represent a causal relationship, given the cross-sectional nature of this study.
Nevertheless, the question of addressing the inflammatory component associated with this type of depression is compelling.43 Successful identification of preferential response to treatment would escalate the selection of antidepressant pharmacotherapy from a trial-and-error endeavor to a practice that uses a predictive biomarker in its treatment of depression. Enhanced depression treatment would be especially helpful in the lung cancer setting, where the prevalence of depression is the highest of all cancer subtypes, and its treatment could fulfill this unmet need.44
The pharmacologic treatment of inflammation associated depression has focused on either reducing inflammation or increasing dopamine, which has been shown to be depleted in the setting of inflammation.45 Although there are data regarding the efficacy of using anti-inflammatory agents to treat depression,43 the use of antidepressant drugs that facilitate the availability of dopamine are already in clinical use for depression and therefore hold more immediate clinical relevance as a feasible option for a depressed patient who is medically ill.
Studies have shown that exposure to chronic inflammation leads to decreased dopamine in the brain of nonhuman primates.46 Moreover, CRP levels ≥3 mg/mL were associated with decreased connectivity among dopamine-rich reward circuitry in depressed patients as revealed by functional magnetic resonance imaging.10,45,47 While serotonin is typically thought of as the neurotransmitter most commonly associated with depression, the absence of dopamine and its metabolites led to the depressive symptoms of anhedonia, fatigue, and psychomotor retardation.45 This combination of symptoms is often referred to as “sickness behavior” and appears to reflect an inflammation-based depressive syndrome.48 In this way, dopamine and neurons involved in dopamine synthesis play a pivotal role in modulating depressive symptoms.49,50 A lack of dopamine due to inflammation may explain treatment refractoriness, particularly in the medical setting, where inflammation is more common—that is, treatment refractoriness is more likely in patients with inflammation who are treated with selective serotonin reuptake inhibitors (SSRIs) that only target serotonin and not the reuptake of dopamine.33
Two primary studies have evaluated CRP as a predictive marker to direct antidepressant treatment in patients with elevated CRP levels. The retrospective study by Jha et al25 revealed that depressed patients who also had inflammation (CRP ≥1 mg/L) responded to the addition of buproprion, an atypical antidepressant that increases dopamine, over monotherapy with the typical SSRI, escitalopram. Uher et al26 prospectively found that CRP (using a cutoff of CRP ≥1) predicted treatment outcome with depressed inflamed patients who experienced less depression after using nortiptyline, a tricyclic anti-depressant that increases catecholamines in neurotransmitters over escitalipram, a standard SSRI that does not increase catecholamines or dopamine. These studies were performed in noncancer populations that were not necessarily enhanced with for inflammation.
Depression treatment trials—especially those examining psychopharmacologic treatments—for patients with cancer are lacking, and selective or targeted depression treatments are virtually nonexistent. CRP is a potential predictive biomarker for differential response to antidepressant treatments that would be particularly useful for patients with lung cancer who also exhibit high levels of inflammation. A feasibility study evaluating the effectiveness of antidepressants that upregulate dopamine over SSRIs without dopamine enhancement in patients with lung cancer and inflammatory depression is clearly warranted. CRP biomarker cutoffs have been used previously and could be validated in this population.
The strengths of this study are its homogenous clinical population, the preclinical understanding of inflammation on antidepressant treatments, and the use of an easy-to-obtain biomarker of inflammation, which makes the data relevant for busy clinicians who need to identify lung cancer patients with depression and inflammation. The weaknesses of this study are its cross-sectional design, lack of other measures of inflammation that have been studied in the lung cancer context previously, lack of other psychological measures of depression, and a relatively small number of patients. Other variables, such as smoking status, receipt of nonsteroidal anti-inflammatory drugs or glucocorticoids, or the presence of active infection or other comorbidities that may affect CRP levels were not addressed. Antidepressant effect on both inflammation and depression were evaluated and found to not contribute significantly as an interaction term. However, an interaction effect may not have been appreciated, because only a relatively small number of patients were taking antidepressants.
It should be noted that many participants had a greater than expected time with disease (mean, 17.8 months [standard deviation, 19.1 months]), younger age, and a higher than expected prevalence of adenocarcinoma, which may be reflective of participants who self-select to be treated at an academic institution with access to clinical trials and expertise in molecular-based therapies. Although the demographics of lung cancer are changing, this sample may not accurately reflect community-dwelling lung cancer patients.51 In addition, the study sampled patients before the adaptation of front-line chemoimmunotherapy, which is why patients were analyzed by treatment types that did not account for chemo-immunotherapy.52
Although inflammation was a stronger predictor of depression than illness-related variables, the relationship between small cell lung cancer, receiving chemo-therapy, and a greater number of lines of treatment and depression should be noted. Perhaps the symptom and treatment burden associated with small cell lung cancer, chemotherapy, and later lines of treatment lead to greater depression symptoms. There may also be a selection bias based on the timing of treatments. For example, chemo-therapy remained the most common first-line treatment during this period of treatment. Therefore, patients receiving chemotherapy were more often at the beginning of their treatment trajectory. Naturally, this cohort included more patients who had rapidly advancing disease that precluded them from ever receiving second-line treatments, immunotherapy, or targeted therapies—that is, patients already on second-line immunotherapy would have a better disease prognosis because they made it to a second- or even third-line treatment. Therefore, the association between chemotherapy and depression may represent an association between depression and worse prognosis rather than chemotherapy independent of inflammation. Alternatively, less depression seen with immunotherapy and targeted therapies may be reflective of better prognosis of non–small cell lung cancer and not an inherent property in the treatment itself.
In conclusion, although studies have begun to evaluate the efficacy of using an easily obtained biomarker such as CRP to select antidepressant treatment, further studies are needed, and lung cancer may provide a unique setting that enhances for both inflammation and depression.53 The appropriate selection of effective antidepressant medication or therapy in the inflamed, depressed lung cancer setting would reduce the overall burden of depression in lung cancer patients.
Acknowledgments
FUNDING SUPPORT
This study was supported by the National Cancer Institute Cancer Center Support Grant P30 CA008748 (Principal Investigator: Craig Thompson). Kelly Shaffer was supported by National Cancer Institute Grant T32 CA009461 (Principal Investigator: Jamie Ostroff).
Footnotes
This study was presented in abstract form at the American Psychosocial Oncology Society 2018 annual conference.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Krebber AM, Buffart LM, Kleijn G, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psycho-oncology. 2014;23:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell AJ, Chan M, Bhatti H, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. 2011;12:160–174. [DOI] [PubMed] [Google Scholar]
- 3.Pirl WF, Temel JS, Billings A, et al. Depression after diagnosis of advanced non-small cell lung cancer and survival: a pilot study. Psychosomatics. 2008;49:218–224. [DOI] [PubMed] [Google Scholar]
- 4.Hopwood P, Stephens RJ. Depression in patients with lung cancer: prevalence and risk factors derived from quality-of-life data. J Clin Oncol. 2000;18:893–903. [DOI] [PubMed] [Google Scholar]
- 5.Walker MS, Zona DM, Fisher EB. Depressive symptoms after lung cancer surgery: their relation to coping style and social support. Psycho-oncology. 2006;15:684–693. [DOI] [PubMed] [Google Scholar]
- 6.Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2013;150:736–744. [DOI] [PubMed] [Google Scholar]
- 7.Smith KJ, Au B, Ollis L, Schmitz N. The association between C-reactive protein, interleukin-6 and depression among older adults in the community: a systematic review and meta-analysis. Exp Gerontol. 2018;102:109–132. [DOI] [PubMed] [Google Scholar]
- 8.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimaki M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 2015;49:206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith RS. The macrophage theory of depression. Med Hypotheses. 1991;35:298–306. [DOI] [PubMed] [Google Scholar]
- 10.Felger JC, Li Z, Haroon E, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2016;21:1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. [DOI] [PubMed] [Google Scholar]
- 12.Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7:942–947. [DOI] [PubMed] [Google Scholar]
- 13.Bonaccorso S, Marino V, Biondi M, Grimaldi F, Ippoliti F, Maes M. Depression induced by treatment with interferon-alpha in patients affected by hepatitis C virus. J Affect Disord. 2002;72:237–241. [DOI] [PubMed] [Google Scholar]
- 14.Capuron L, Hauser P, Hinze-Selch D, Miller AH, Neveu PJ. Treatment of cytokine-induced depression. Brain behav Immun. 2002;16:575–580. [DOI] [PubMed] [Google Scholar]
- 15.Watson J, Round A, Hamilton W. Raised inflammatory markers. BMJ. March 2012;344:e454. [DOI] [PubMed] [Google Scholar]
- 16.Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, Cleare AJ. Inflammation and clinical response to treatment in depression: a meta-analysis. Eur Neuropsychopharmacol. 2015;25:1532–1543. [DOI] [PubMed] [Google Scholar]
- 17.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. [DOI] [PubMed] [Google Scholar]
- 18.Misiak B, Beszlej JA, Kotowicz K, et al. Cytokine alterations and cognitive impairment in major depressive disorder: from putative mechanisms to novel treatment targets. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80:177–188. [DOI] [PubMed] [Google Scholar]
- 19.Wium-Andersen MK, Orsted DD, Nielsen SF, Nordestgaard BG. Elevated C-reactive protein levels, psychological distress, and depression in 73, 131 individuals. JAMA Psychiatry. 2013;70:176–184. [DOI] [PubMed] [Google Scholar]
- 20.Du YJ, Zhang HY, Li B, et al. Sputum interleukin-6, tumor necrosis factor-alpha and salivary cortisol as new biomarkers of depression in lung cancer patients. Prog Neuropsychopharmacol Biol Psychiatry. 2013;47:69–76. [DOI] [PubMed] [Google Scholar]
- 21.Moshage HJ, Janssen JA, Franssen JH, Hafkenscheid JC, Yap SH. Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation. J Clin Invest. 1987;79:1635–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steel DM, Whitehead AS. The major acute phase reactants: C-reactive protein, serum amyloid P component and serum amyloid A protein. Immunol Today. 1994;15:81–88. [DOI] [PubMed] [Google Scholar]
- 23.Rashmi N, Galhotra V, Goel P, Rajguru JP, Jha SK, Kulkarni K. Assessment of C-reactive proteins, cytokines, and plasma protein levels in hypertensive patients with apical periodontitis. J Contemp Dent Pract. 2017;18:516–521. [DOI] [PubMed] [Google Scholar]
- 24.Felger JC, Haroon E, Patel TA, et al. What does plasma CRP tell us about peripheral and central inflammation in depression? [published online ahead of print June 12, 2018] Mol Psychiatry. 10.1038/s41380-018-0096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jha MK, Minhajuddin A, Gadad BS, et al. Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology. 2017;78:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uher R, Tansey KE, Dew T, et al. An inflammatory biomarker as a differential predictor of outcome of depression treatment with escitalopram and nortriptyline. Am J Psychiatry. 2014;171:1278–1286. [DOI] [PubMed] [Google Scholar]
- 27.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jing X, Huang C, Zhou H, et al. Association between serum C-reactive protein value and prognosis of patients with non-small cell lung cancer: a meta-analysis. Int J Clin Exp Med. 2015;8:10633–10639. [PMC free article] [PubMed] [Google Scholar]
- 30.Shiels MS, Katki HA, Hildesheim A, et al. Circulating inflammation markers, risk of lung cancer, and utility for risk stratification. J Natl Cancer Inst. 2015;107:djv199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harley J, Luty S, Carter J, Mulder R, Joyce P. Elevated C-reactive protein in depression: a predictor of good long-term outcome with antidepressants and poor outcome with psychotherapy. J Psychopharmacol. 2010;24:625–626. [DOI] [PubMed] [Google Scholar]
- 33.Haroon E, Daguanno AW, Woolwine BJ, et al. Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology. 2018;95:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pineiro M, Pato R, Soler L, et al. A new automated turbidimetric immunoassay for the measurement of canine C-reactive protein. Vet Clin Pathol. 2018;47:130–137. [DOI] [PubMed] [Google Scholar]
- 35.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69–77. [DOI] [PubMed] [Google Scholar]
- 36.Schellekens MPJ, van den Hurk DGM, Prins JB, Molema J, van der Drift MA, Speckens AEM. The suitability of the Hospital Anxiety and Depression Scale, Distress Thermometer and other instruments to screen for psychiatric disorders in both lung cancer patients and their partners. J Affect Disord. 2016;203:176–183. [DOI] [PubMed] [Google Scholar]
- 37.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. [DOI] [PubMed] [Google Scholar]
- 38.Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psycho-oncology. 2001;10:19–28. [DOI] [PubMed] [Google Scholar]
- 39.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43:52–58. [PubMed] [Google Scholar]
- 40.Rifai N, Ridker PM. Population distributions of C-reactive protein in apparently healthy men and women in the United States: implication for clinical interpretation. Clin Chem. 2003;49:666–669. [DOI] [PubMed] [Google Scholar]
- 41.Landry A, Docherty P, Ouellette S, Cartier LJ. Causes and outcomes of markedly elevated C-reactive protein levels. Can Fam Physician. 2017;63:e316–e323. [PMC free article] [PubMed] [Google Scholar]
- 42.Simeone E, Gentilcore G, Giannarelli D, et al. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol Immunother. 2014;63:675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry. 2018;23:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walker J, Hansen CH, Martin P, et al. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: a cross-sectional analysis of routinely collected clinical data. Lancet Psychiatry. 2014;1:343–350. [DOI] [PubMed] [Google Scholar]
- 45.Felger JC, Treadway MT. Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacology. 2017;42:216–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Felger JC. Imaging the role of inflammation in mood and anxiety-related disorders. Curr Neuropharmacol. 2018;16:533–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol. 2012;33:315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tobias K, Rosenfeld B, Pessin H, Breitbart W. Measuring sickness behavior in the context of pancreatic cancer. Med Hypotheses. 2015;84:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tye KM, Mirzabekov JJ, Warden MR, et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lortet-Tieulent J, Soerjomataram I, Ferlay J, Rutherford M, Weiderpass E, Bray F. International trends in lung cancer incidence by histological subtype: adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer. 2014;84:13–22. [DOI] [PubMed] [Google Scholar]
- 52.Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. [DOI] [PubMed] [Google Scholar]
- 53.Miller AH, Trivedi MH, Jha MK. Is C-reactive protein ready for prime time in the selection of antidepressant medications? Psychoneuroendocrinology. 2017;84:206. [DOI] [PubMed] [Google Scholar]