Abstract
Takayasu arteritis (TAK) is a large-vessel vasculitis affecting the aorta and its main branches. Hemoptysis can be experienced as the respiratory manifestation, but origination from a bronchial artery is rare. Ulcerative colitis (UC) shares genetic similarities with TAK; HLA-B52*01 is associated with TAK and UC. We herein report a patient who presented with hemoptysis from the right bronchial artery and was diagnosed with TAK during the follow-up of UC. Transcatheter embolization was performed, and prednisolone and tocilizumab induced remission. Complication of TAK should be considered in the clinical course of HLA-B52-positive UC patients, and tocilizumab may be a treatment option.
Keywords: Takayasu arteritis, ulcerative colitis, hemoptysis, bronchial arteries, tocilizumab
Introduction
Takayasu arteritis (TAK) is a rare, systemic vasculitis, mainly involving the aorta and its branches. The causes of TAK are still unknown, but HLA-B52*01 is often associated with this disease (1). Pulmonary artery involvement is a late manifestation of the disease and leads to secondary bronchial artery involvement. Hemoptysis is a rare manifestation, mostly originating from the pulmonary artery but rarely from the bronchial artery (2, 3). TAK is often refractory to concomitant treatment with glucocorticoids and immunosuppressant, but a recent, randomized controlled trial showed the efficacy of tocilizumab (TCZ), an anti-interleukin (IL)-6 receptor antibody, for refractory TAK (4).
Ulcerative colitis (UC) is an inflammatory bowel disease and autoimmune disorder affecting the rectum and extending to the proximal colon. TAK and UC share genetic similarities, and HLA-B52*01 plays an important role in both (5). Therefore, the prevalence of UC in TAK (6.4%) and the prevalence of TAK in UC (0.21%) are much higher than in the general population in Japan (5, 6).
We herein report a patient with UC and hemoptysis, who was ultimately diagnosed with TAK.
Case Report
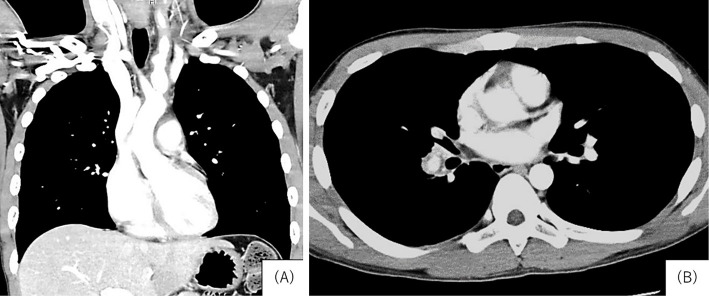
A 22-year-old Japanese man presented to the local hospital emergency room with the sudden onset of hemoptysis in November 2017. Enhanced computed tomography (CT) revealed spindle-shaped enlargement and irregular wall thickness of the left carotid artery along with wall thickening of the ascending aorta and right pulmonary artery (Fig. 1). Bronchoscopy suggested active bleeding from Segment 10 of the right lower lobe. The patient was therefore transferred to our hospital.
Figure 1.
Enhanced chest computed tomography. A: Spindle-shaped enlargement and irregular wall thickness of the left carotid artery were observed. B: Wall thickening of the right pulmonary artery was observed.
From 14 years of age, he had been treated for UC with mesalamine and salazosulfapyridine (SASP). He had also been diagnosed with congenital cataracts. Because remission of UC was maintained, drug treatments were discontinued one year before the current presentation. He experienced relapse three months later but improved after the re-administration of SASP. Four months prior to the current presentation, he developed gradual onset of carotidynia. The level of C-reactive protein (CRP) was 1.94 mg/dL in July 2017.
On admission, he was alert and oriented. His vital signs included body temperature 37.9℃, blood pressure 126/56 mmHg and equal bilaterally, pulse rate 99/min, and O2 saturation 97% in room air. A left carotid bruit was noted, with no other significant findings in the chest or abdomen. The white blood cell count was 10,490/μL (with 74.8% neutrophils), red blood cells 429×104/μL, hemoglobin 9.6 g/dL, hematocrit 32.4%, platelets 55×104/μL, CRP 7.75 mg/dL, proteinase 3-antineutrophil cytoplasmic antibody 3.24 IU/mL (normal range <3.5 IU/mL), and IL-6 34.4 pg/mL. HLA-B52 was positive. The urinalysis findings were normal, and transthoracic echocardiography showed no findings of valvular dysfunction or pulmonary hypertension (tricuspid regurgitation peak gradient: 22 mmHg). Fluoro-D-glucose positron emission tomography-CT showed a mild uptake in the ascending aorta, the left carotid artery, and the right pulmonary artery. (Fig. 2). Based on these results, he was diagnosed with TAK complicated by preceding UC.
Figure 2.
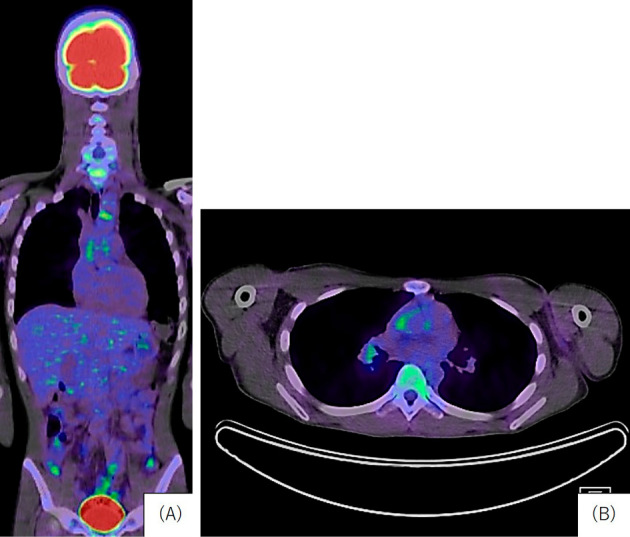
>Fluoro-D-glucose positron emission tomography-computed tomography. A: A mild uptake was observed in the ascending aorta and left common carotid artery (coronal). B: A mild uptake in the right pulmonary artery and left common carotid artery (axial).
Angiography showed active bleeding from a right bronchial artery (Fig. 3), and transcatheter right bronchial artery embolization was performed. Colonoscopy indicated that the UC activity was well controlled. After the procedure, a daily dose of prednisolone 30 mg and TCZ 162 mg/week was initiated to induce remission. At 2 months after treatment initiation, the dose of prednisolone was reduced to 15 mg/day without constitutional, respiratory, or gastrointestinal symptoms.
Figure 3.
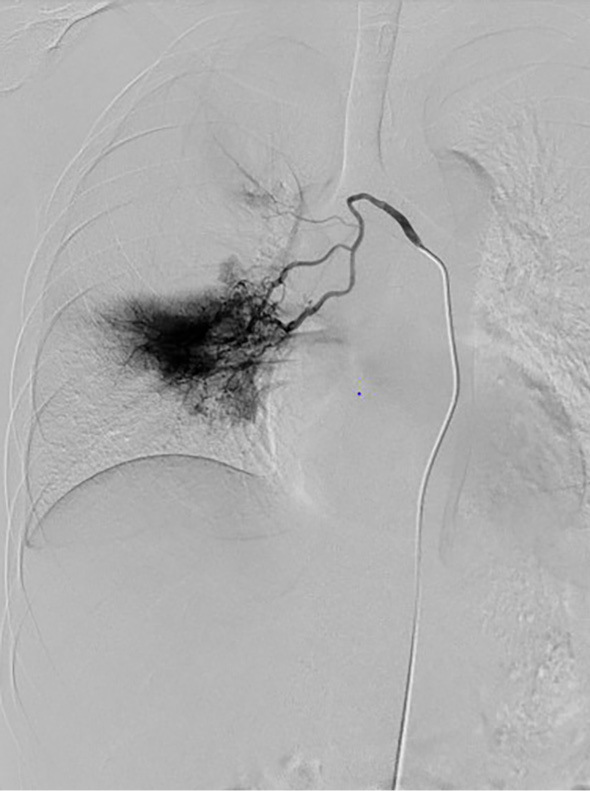
Right bronchial angiography. Bleeding originating from the right bronchial artery was observed.
Discussion
TAK is a rare, systemic arteritis of unknown etiology that involves the aorta and its main branches. The prevalence of TAK is reportedly much higher in Japan (about 40 cases per million) than in the USA (2.6 cases per million) (7, 8). The prevalence of TAK in patients with UC is particularly high, at 0.21% (6), and UC was found in 6.4% of patients with TAK (5). HLA-B*52:01 plays an important role in the co-occurrence of UC and TAK (5). Although it might not be practical to survey all UC patients for large-vessel vasculitis, HLA typing can be performed to determine the risk of the future development of TAK in UC patients, as the prevalence of TAK is much higher in these patients than in the general population.
Pulmonary involvement in TAK includes pulmonary hypertension, thrombosis, infarctions, and pleuritis. These pulmonary manifestations can be caused by pulmonary or bronchial arteritis (9). Hemoptysis is a rare symptom in patients with TAK and is mainly caused by pulmonary arteritis and/or pulmonary hypertension (2). In the present case, hemoptysis originated from the bronchial artery. Bronchial artery involvement was followed by pulmonary artery involvement and might have developed long after the onset of TAK.
Although glucocorticoids (GCs) are used as the first-line treatment for TAK, patients with TAK often experience relapse and develop adverse effects from GCs. Other immunosuppressive agents, such as methotrexate, azathioprine, or mycophenolate mofetil, are used for refractory or relapsing cases while still continuing GC therapy (5, 10, 11); however, these agents may not show sufficient clinical benefit. Anti-tumor necrosis factor (TNF) agents also have insufficient efficacy for TAK, and several reports have shown that patients with inflammatory bowel diseases treated with anti-TNF agents still developed TAK (12, 13). Of note, a recent randomized controlled trial demonstrated the efficacy and safety of TCZ in patients with refractory TAK (4), and some case reports have shown the effectiveness of TCZ in patients with rheumatoid arthritis associated with UC. Therefore, TCZ may be an option for treating active TAK with quiescent UC.
In conclusion, the complication of TAK should be considered in the clinical course of HLA-B52-positive patients with UC to avoid the development of a severe condition. TCZ may be a treatment option for patients with TAK and UC.
Written informed consent for this case report was obtained from the patient.
Author's disclosure of potential Conflicts of Interest (COI).
Ken-Ei Sada: Honoraria, Chugai Pharma. Jun Wada: Honoraria, Daiichi Sankyo, MSD, Tanabe Mitsubishi and Taisho Toyama; Research funding, Baxter, Dainippon Sumitomo, Ono and Teijin Pharma.
Financial Support
This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (nannti-ippann-018), and the Japan Agency for Medical Research and Development (17ek0109104 and 17ek0109121).
References
- 1. Terao C, Yoshifuji H, Kimura A, et al. Two susceptibility loci to Takayasu arteritis reveal a synergistic role of the IL12B and HLA-B regions in a Japanese population. Am J Hum Genet 93: 289-297, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Toledano K, Guralnik L, Lorber A, et al. Pulmonary arteries involvement in Takayasu's arteritis: two cases and literature review. Semin Arthritis Rheum 41: 461-470, 2011. [DOI] [PubMed] [Google Scholar]
- 3. Lopez AJ, Brady AJ, Jackson JE. Case report: therapeutic bronchial artery embolization in a case of Takayasu's arteritis. Clin Radiol 45: 415-417, 1992. [DOI] [PubMed] [Google Scholar]
- 4. Nakaoka Y, Isobe M, Takei S, et al. Efficacy and safety of tocilizumab in patients with refractory Takayasu arteritis: results from a randomised, double-blind, placebo-controlled, phase 3 trial in Japan (the TAKT study). Ann Rheum Dis 77: 348-354, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Terao C, Matsumura T, Yoshifuji H, et al. Takayasu arteritis and ulcerative colitis: high rate of co-occurrence and genetic overlap. Arthritis Rheumatol 67: 2226-2232, 2015. [DOI] [PubMed] [Google Scholar]
- 6. Oshitani N, Watanabe K, Nakamura S, Higuchi K, Arakawa T. [Extraintestinal complications in patients with ulcerative colitis]. Nihon Rinsho (Jpn J Clin Med) 63: 874-878, 2005(in Japanese, Abstract in Englsh). [PubMed] [Google Scholar]
- 7. Terao C, Yoshifuji H, Mimori T. Recent advances in Takayasu arteritis. Int J Rheum Dis 17: 238-247, 2014. [DOI] [PubMed] [Google Scholar]
- 8. Hall S, Barr W, Lie JT, Stanson AW, Kazmier FJ, Hunder GG. Takayasu arteritis. A study of 32 North American patients. Medicine (Baltimore) 64: 89-99, 1985. [PubMed] [Google Scholar]
- 9. Gothi D, Joshi JM. A 16-year-old girl with hemoptysis, intermittent loss of vision, and a carotid bruit. Chest 133: 300-304, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Wen D, Du X, Ma CS. Takayasu arteritis: diagnosis, treatment and prognosis. Int Rev Immunol 31: 462-473, 2012. [DOI] [PubMed] [Google Scholar]
- 11. Mason JC. Takayasu arteritis--advances in diagnosis and management. Nat Rev Rheumatol 6: 406-415, 2010. [DOI] [PubMed] [Google Scholar]
- 12. Enrico T, Stefano F, Maurizio P, Maria GS, Elena B. Treatment of refractory Takayasu arteritis with Tocilizumab: 7 Italian patients from a single referral center. J Rheumatol 40: 2047-2051, 2013. [DOI] [PubMed] [Google Scholar]
- 13. Sy A, Khalidi N, Dehghan N, et al. Vasculitis in patients with inflammatory bowel diseases: a study of 32 patients and systematic review of the literature. Semin Arthritis Rheum 45: 475-482, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]