Abstract
Tosufloxacin, which is not used to treat Mycobacterium tuberculosis, is a fluoroquinolone recommended for pneumonia when the possibility of tuberculosis infection cannot be excluded. In the present case, symptoms and chest infiltrative shadow initially improved by tosufloxacin. Therefore, we regarded this patient as having general pneumonia and did not perform follow-up chest X-ray until the infiltrates had completely disappeared. However, a few weeks later, the symptoms and the infiltrates had worsened, so M. tuberculosis was isolated from the patient’s sputum. This case suggests that patients suspected of having pulmonary tuberculosis should be monitored carefully, even if antibiotics without antituberculous activity are initially effective.
Keywords: tuberculosis, tosufloxacin, pneumonia, elderly
Introduction
Fluoroquinolones have been increasingly used as broad-spectrum antibiotics for the treatment of pneumonia. Most fluoroquinolones have anti-tuberculosis activity, and the use of these antibiotics before a diagnosis of tuberculosis has been excluded increases the mortality in patients with tuberculosis due to a delay in the detection of Mycobacterium tuberculsosis (1, 2). Tosufloxacin (TFLX) has no antibacterial activity against M. tuberculosis in vitro and is therefore recommended as a treatment for community-acquired pneumonia when the possibility of pulmonary tuberculosis infection cannot be excluded (3, 4).
We herein report the case of a patient treated with TFLX for community-acquired pneumonia because the possibility of M. tuberculosis co-infection could not be excluded, although the diagnosis of tuberculosis was still delayed. The present case highlights a pitfall associated with using TFLX to treat pneumonia that might actually be lung tuberculosis.
Case Report
An 85-year-old man without a history of tuberculosis visited our clinic with a 3-day history of a fever and anorexia. He suffered from Alzheimer's disease and lived in a long-term care facility. A physical examination revealed a body temperature of 36.6°C, a percutaneous oxygen saturation (SpO2) of 95% (room air), a respiratory rate (RR) of 20 pm, a blood pressure of 135/78 mmHg, and a heart rate (HR) of 71 bpm. Laboratory tests revealed a decreased leukocyte count (3,100 cells/μL) and elevated serum levels of C-reactive protein (CRP; 5.2 mg/dL). Chest X-ray taken at the first visit (Fig. 1A) showed infiltrates in both lung fields but predominantly on the right. We did not perform chest CT at that time. Although a sputum-acid-fast smear test was negative, we were unable to exclude the possibility of pulmonary tuberculosis because of multiple patchy infiltrates on chest X-ray; we therefore prescribed TFLX, which does not cover M. tuberculosis. A Gram stain analysis is not routinely performed at our clinic. Methicillin-resistant Staphylococcus aureus (MRSA) was isolated from the sputum culture, but we initially regarded this as colonization. Rapid urine antigen test for Streptococcus pneumoniae and Legionella pneumophila were both negative.
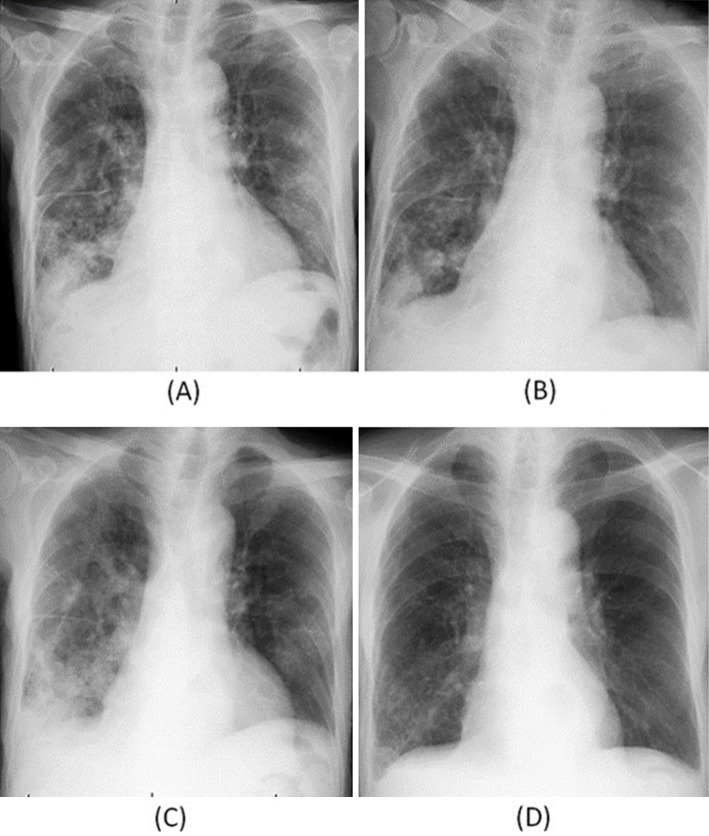
Figure 1.
Chest X-ray findings at the first visit (A), seven days after the start of the tosufloxacin administration (B), three weeks after the end of the administration of tosufloxacin (C) and two months after the start of treatment with multi-antituberculous drugs (D).
The patient's symptoms gradually disappeared after a few days. Because chest X-ray taken seven days from the start of TFLX administration revealed improvement (Fig. 1B), we diagnosed him with general bacterial pneumonia and ended TFLX after 14 days. Therefore, we did not follow up his chest X-ray until the infiltrates completely disappeared. However, at three weeks after the end of TFLX administration, the fever and anorexia relapsed, and he returned to the hospital. A physical examination revealed a body temperature of 37.4℃, an RR of 24 pm, a SpO2 of 95% (room air), a blood pressure of 164/86 mmHg, and a HR of 88 bpm. Laboratory tests revealed a normal leukocyte count (4,150 cells/μL) and elevated serum levels of CRP (4.8 mg/dL). Chest X-ray (Fig. 1C) revealed expanded infiltrates in both lung fields, and chest CT showed consolidation with a tree-in-bud appearance (Fig. 2). A sputum-acid-fast smear test was positive, and M. tuberculosis was isolated from the culture. After starting treatment with multi-antituberculous drugs including ethambutol, isoniazid and rifampin, his symptoms, pulmonary infiltrates on X-ray and CRP levels gradually improved (Fig. 1D).
Figure 2.
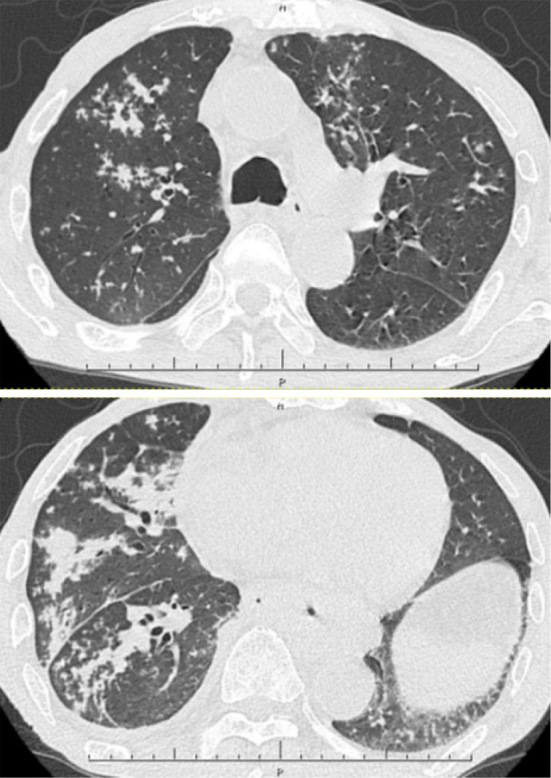
Chest CT findings at three weeks after the end of the administration of tosufloxacin at the same time as in Fig. 1 (C).
Discussion
This case was initially suspected of being community-acquired pneumonia based on the findings of chest radiographs and blood tests. Initial treatment was started with TFLX, which does not have anti-bacterial activities for M. tuberculosis, because the possibility of tuberculosis infection could not be excluded. The fever and symptoms improved on treatment with TFLX, so he was diagnosed with general bacterial pneumonia and not pulmonary tuberculosis. Therefore, we did not carefully his chest X-ray findings until the infiltrates had completely disappeared, which caused a delay in his tuberculosis diagnosis.
Most fluoroquinolones used in clinical practice have antibacterial activity against M. tuberculosis (3). Indeed, Kelly et al. reported that treatment with fluoroquinolone (Levofloxacin 11/16, Gatifloxacin 1/16, Trovafloxacin 2/16, Ciprofloxacin 1/16 or Ciprofloxacin followed by Trovafloxacin 1/16) before a diagnosis of tuberculosis was confirmed or denied was associated with a 21-day delay in treatment after the diagnosis (2). Wang et al. reported that 79 (14.4%) of 548 tuberculosis patients received a fluoroquinolone before the diagnosis of tuberculosis (Ciprofloxacin 42/79, Levofloxacin 21/79, Moxifloxacin 16/79), and 52 (65.8%) experienced clinical improvement after fluoroquinolone use. The median interval from the initial visit to starting anti-tuberculous treatment in the fluoroquinolone-administered group was 42 days, which was significantly longer than that in the non-fluoroquinolone-administered group (34 days) (5). A systematic review also demonstrated that fluoroquinolones increase the quinolone resistance of M. tuberculosis 2.7-fold (1). In recent studies, multiple fluoroquinolone prescriptions and the use of a fluoroquinolone for >10 days have been associated with fluoroquinolone-resistant tuberculosis (6, 7). Fluoroquinolones are used as second-line pulmonary tuberculosis therapy. The use of a fluoroquinolone before the diagnosis of tuberculosis has been confirmed or denied is not recommended in consideration of both a delayed diagnosis and drug resistance.
TFLX has no antibacterial activity against M. tuberculosis in vitro (3). For this reason, TFLX is recommended for the treatment of pneumonia when fluoroquinolone is required as a broad-spectrum antibiotic for respiratory infections. In the present case, the diagnosis of tuberculosis was delayed despite TFLX being used for the first treatment. We stopped following the patient when the symptoms and infiltrates on chest X-ray improved after TFLX administration, which resulted in the delay in his tuberculosis detection. This case suggests the possible co-existence of tuberculosis and general bacterial infections in patients diagnosed with lung tuberculosis. Indeed, it was reported that 24.3% (37/152) of patients had mixed infections in a study that assessed the isolation rate of general bacteria in patients with lung tuberculosis (8). Methicillin-sensitive S. aureus (28%, 14/50), Klebsiella pneumoniae (14%, 7/50), MRSA (10%, 5/50) and Streptococcus pneumoniae (8%, 4/50) were isolated as causes of mixed infections with M. tuberculosis. In another study, 29.7% (54/182) of the patients diagnosed with pulmonary tuberculosis were reported to have a combined infection with Mycoplasma pneumoniae (18.2%, 12/66), K. pneumoniae (16.6%, 11/66), Leginonella pneumophila (9%, 2/66), S. pneumoniae (9%, 6/66), S. aureus (9%, 6/66) or Haemophilus influenzae (9%, 6/66) (9).
While TFLX reportedly has no antibacterial activity against M. tuberculosis in vitro (3, 4, 10), there is no evidence that TFLX has a favorable clinical effect on M. tuberculosis infection. Although we cannot completely exclude the possibility that TFLX partially killed M. tuberculosis in this case, implying no co-infection with general bacteria, this is unlikely, as the peak blood concentration of TFLX (0.66±0.16 μg/mL) in humans is known to be much lower than the minimum inhibitory concentration of TFLX (from 5 to ≥100 μg/mL) against M. tuberculosis (4, 10).
In the present case, MRSA was isolated from the sputum, but the clinical symptoms improved by treatment with TFLX. Other bacteria aside from MRSA may have been co-infected along with M. tuberculosis. TFLX is a fluoroquinolone recommended for the treatment of community-acquired pneumonia when the possibility of tuberculosis infection cannot be excluded. However, physicians need to keep in mind the possibility of tuberculosis infection combined with a general bacterial infection. We believe that TFLX is still a reasonable choice for the treatment of community-acquired pneumonia or nursing and healthcare-associated pneumonia as well as penicillin with beta-lactamase inhibitor or macrolides. An accurate diagnosis of pneumonia should be made by carefully ruling out any M. tuberculosis infection with repeated sputum acid-fast bacilli smear tests, and patients suspected of having pulmonary tuberculosis should be monitored until the lung involvements have completely resolved, even if antibiotics without antibacterial activity to M. tuberculosis are initially effective.
The authors state that they have no Conflict of Interest (COI).
Nobuhiro Fujishima and Kosaku Komiya are members of Oita prefectural tuberculosis control project 2017-2020.
References
- 1. Chen TC, Lu PL, Lin CY, Lin WR, Chen YH. Fluoroquinolones are associated with delayed treatment and resistance in tuberculosis: a systematic review and meta-analysis. Int J Infect Dis 15: e211-e216, 2011. [DOI] [PubMed] [Google Scholar]
- 2. Dooley KE, Golub J, Goes FS, Merz WG, Sterling TR. Empiric treatment of community-acquired pneumonia with fluoroquinolones, and delays in the treatment of tuberculosis. Clin Infect Dis 34: 1607-1612, 2002. [DOI] [PubMed] [Google Scholar]
- 3. Aubry A, Pan XS, Fisher LM, Jarlier V, Cambau E. Mycobacterium tuberculosis DNA gyrase: interaction with quinolones and correlation with antimycobacterial drug activity. Antimicrob Agents Chemother 48: 1281-1288, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kawahara S, Tada A, Nagare H. [Clinical evaluation of new quinolones as antituberculosis drugs]. Kekkaku 74: 71-75, 1999(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 5. Wang JY, Hsueh PR, Jan IS, et al. Empirical treatment with a fluoroquinolone delays the treatment for tuberculosis and is associated with a poor prognosis in endemic areas. Thorax 61: 903-908, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Long R, Chong H, Hoeppner V, et al. Empirical treatment of community-acquired pneumonia and the development of fluoroquinolone-resistant tuberculosis. Clin Infect Dis 48: 1354-1360, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Devasia RA, Blackman A, Gebretsadik T, et al. Fluoroquinolone resistance in Mycobacterium tuberculosis: the effect of duration and timing of fluoroquinolone exposure. Am J Respir Crit Care Med 180: 365-370, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Okimoto N, Honda Y, Asaoka N, et al. [Bacteria with Mycobacteruim tuberculosis detected by sputum culture]. Kansenshogaku Zasshi 75: 1062-1063, 2001(in Japanese). [DOI] [PubMed] [Google Scholar]
- 9. Lin GM, Chang FY, Chou CH, Lin YP, Ku CH. Characteristics and outcome of patients with dual pulmonary tuberculosis and non-mycobacterial respiratory infections. J Clin Med Res 3: 309-318, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kawahara S, Tada A, Takeuchi M, Kamisaka K, Okada C. In vitro antimycobacterial activity of tosfloxacin. IRYO 47: 942-946, 1993. [Google Scholar]