Abstract
Bronchial occlusion with endobronchial Watanabe spigots (EWSs) can be an essential therapeutic measure for treating massive hemoptysis in intensive care patients when no other conventional options are available. A 68-year-old-man on mechanical ventilation and extracorporeal circulation after cardiovascular surgery presented massive hemoptysis. He was deemed unfit for bronchial artery embolization (BAE) and surgery while in the intensive care setting; thus, bronchial occlusion was performed using EWSs. His hemoptysis ceased, and he was successfully weaned from mechanical ventilation and extracorporeal circulation. Bronchial occlusion by EWSs may be considered an optimal, and at times, definitive treatment for obtaining hemostasis in these situations.
Keywords: bronchial occlusion, endobronchial Watanabe spigot, massive hemoptysis
Introduction
Life-threatening massive hemoptysis is one of the most challenging conditions in respiratory clinical practice and requires extremely prompt intervention. Bronchial artery embolization (BAE) or pulmonary lobectomy are often considered to be indicated in patients with massive hemoptysis; however, these measures are occasionally considered impossible due to the patient’s condition. Flexible bronchoscopy is useful for the localization of the bleeding site, and bronchial occlusion with endobronchial Watanabe spigots (EWSs) may be an effective option for the management of massive hemoptysis arising from peripheral lesions (1). We herein report the case of a critically ill patient with life-threatening hemoptysis who was salvaged by bronchial occlusion with EWSs.
Case Report
A 68-year-old-man with no known history of chronic lung disease was admitted to the cardiovascular surgery ward to undergo aortic valve replacement and coronary artery bypass grafting. His circulatory status was unstable during the operation and he continuously required mechanical ventilation and vasopressor treatment. Postoperatively, we diagnosed myocardial infarction, and an intra-aortic balloon pump (IABP) therapy, continuous hemodiafiltration (CHDF) and venoarterial extracorporeal membrane oxygenation (VA-ECMO) were performed due to multi-organ failure resulting from circulation insufficiency. On the seventh postoperative day, his organic function began to gradually improve and the cardiovascular surgeon started weaning the patient from VA-ECMO. However, he presented with sudden onset hemoptysis on the same day and his respiratory condition deteriorated. He was deemed unfit for BAE and surgery in intensive care. It seemed that the hemoptysis was associated with the hemorrhagic tendency due to anticoagulant treatment for extracorporeal circulation; however, anticoagulant therapy could not be discontinued to maintain extracorporeal circulation. Fresh blood from the endobronchial tube was noted, and we performed emergency bronchoscopy using a flexible bronchoscope (Olympus BF TYPE 1T260, Olympus, Tokyo, Japan). The site of origin was identified at the peripheral airway on the right B3, B4 and B5 (Fig. 1). Subsequently, the decision was made to occlude the bronchi using silicon spigots (Endobronchial Watanabe Spigot®, Novatech, La Ciotat, France). The EWS was grasped with the forceps using the side-grasping method (2), which we previously reported. Two 7-mm EWS were firmly inserted, one each into the right B3 and B5 segments, and one 6-mm EWS was inserted into the right B4 segment. After bronchial occlusion, there was no airway bleeding (Fig. 2). The total procedure took 20 minutes. Post-procedural chest X-ray visualized the three EWSs in optimal positions (Fig. 3). His respiratory condition was ameliorated and the next day, VA-ECMO and CHDF were discontinued. Two days later, we performed bronchoscopy again and all EWSs were successfully placed without the recurrence of hemoptysis. The patient was withdrawn from IABP and mechanical ventilation, and two weeks later, he was discharged from the cardiac care unit without the recurrence of bleeding. The spigots were removed 6 weeks later, and the patient’s condition has been stable.
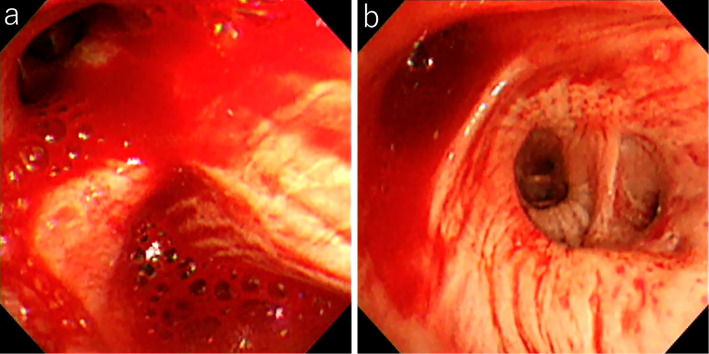
Figure 1.
The endoscopic findings. a: Bleeding from the right B3. b: Bleeding from the middle lobe bronchus.
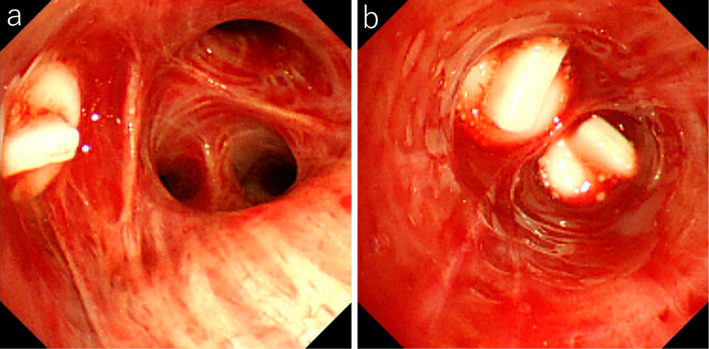
Figure 2.
The endoscopic findings after bronchial occlusion. a: No recurrent bleeding was seen from the right B3. b: No recurrent bleeding was seen from the middle lobe bronchus.
Figure 3.
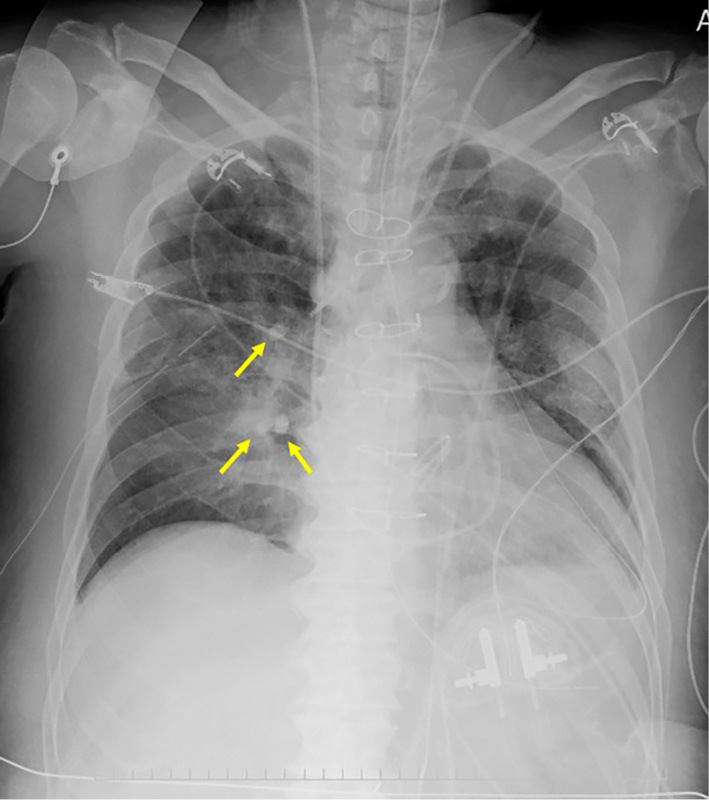
A chest radiograph taken after bronchial occlusion with EWSs. Three EWSs were inserted in optimal positions (arrows).
Discussion
A previous retrospective study reported that complications from massive hemoptysis occurred in 2.5% of patients on ECMO (3). The occurrence of massive hemoptysis in heparinized ECMO patients, although rare, is a serious and potentially lethal complication. From the standpoint of the complications associated with cardiac operations, the causes of perioperative life-threatening hemoptysis include catheter-induced pulmonary artery perfusion, surgical trauma, traumatic intubation, pulmonary edema or lung diseases that are present before the operation, such as neoplasia, which would be amplified by heparin and coagulopathy (4). In this case, we hypothesized that life-threatening hemoptysis developed in association with the use of anticoagulants for improving extracorporeal circulation, as well as thrombopenia and pulmonary edema due to congestive heart failure.
Conventional definitive treatments for massive hemoptysis due to peripheral lesions are BAE or surgery. However, sometimes patients who require intensive care with extracorporeal circulation are ineligible to undergo surgery or BAE due to hemodynamic instability and difficulties in transport. In the management of massive hemoptysis, it is difficult to control bleeding with measures, such as correcting coagulopathy, bronchoscopy with cold saline or diluted epinephrine lavage. Although bronchial blockade with bronchial blockers or balloon catheters is useful for isolating the bleeding lobe or lung and maintaining airway stabilization, these are temporary measures and it can be difficult to occlude two or more peripheral bleeding lesions with these methods. However, bronchial occlusion using EWSs is durable and can be used to treat patients with multiple lesions; thus, it has recently been reported as a definitive therapy for massive hemoptysis (5).
Bronchial occlusion using EWSs has been developed for the management of intractable pneumothorax and bronchial fistula (6), and the use of this technique has expanded to treat massive hemoptysis (1). Although flexible bronchoscopy is useful for the determination of the bleeding site and performing bronchial occlusion with EWSs, meticulous attention is required due to stimulation of the bronchus by the bronchoscopic procedure, which can be exacerbated by coughing.
We propose the following appropriate conditions for bronchial occlusion using EWSs for the management of hemoptysis. First, the origin of bleeding from the peripheral lesion is identified and is controlled by bronchial occlusion using several EWSs. Second, it is essential to maintain an acceptable visual field for the EWS procedure; and third, there are no other appropriate treatments than bronchial occlusion using EWSs. It is an uncommon situation for bronchial occlusion to be considered indicated.
In the present case, the patient was considered unfit for BAE and surgery because he was receiving intensive care. It seemed that it would not be possible to ameliorate the patient’s hemoptysis due to thrombopenia and the use of anticoagulants for extracorporeal circulation. In addition, bronchoscopy was less invasive because this patient had been intubated and was under sedation, and the origin of hemoptysis was identified from several peripheral airways. Given this potentially catastrophic situation, bronchial occlusion using EWSs to achieve for hemostasis was considered the optimal treatment. As developments in supportive equipment with extracorporeal circulation progress in intensive care medicine, similar situations will be increasingly encountered.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors thank Mr. Jason Tonge from St. Marianna University School of Medicine, and Professor Emeritus J. Patrick Barron of Tokyo Medical University for the linguistic review of this manuscript.
References
- 1. Dutau H, Palot A, Haas A, Decamps I, Durieux O. Endobronchial embolization with a silicone spigot as a temporary treatment for massive hemoptysis: a new bronchoscopic approach of the disease. Respiration 73: 830-832, 2006. [DOI] [PubMed] [Google Scholar]
- 2. Kida H, Muraoka H, Inoue T, Mineshita M, Kurimoto N, Miyazawa T. A novel technique for the placement of Endobronchial Watanabe Spigots into the bronchus: side-grasping method. J Bronchol Intervent Pulmonol 23: 71-75, 2016. [DOI] [PubMed] [Google Scholar]
- 3. Pitcher HT, Harrison MA, Shaw C, Cowan SW, Hirose H, Cavarocchi N. Management considerations of massive hemoptysis while on extracorporeal membrane oxygenation. Perfusion 31: 653-658, 2016. [DOI] [PubMed] [Google Scholar]
- 4. Fortin J, Vaillancourt R, Vigneault L, Laflamme M, Simon M, Bussières JS. Unusual cause of life-threatening hemoptysis during cardiac operation: surgical management revisited. Ann Thorac Surg 104: e251-e252, 2017. [DOI] [PubMed] [Google Scholar]
- 5. Adachi T, Oki M, Saka H. Management considerations for the treatment of idiopathic massive hemoptysis with endobronchial occlusion combined with bronchial artery embolization. Intern Med 55: 173-177, 2016. [DOI] [PubMed] [Google Scholar]
- 6. Watanabe Y, Matsuo K, Tamaoki A, Komoto R, Hiraki S. Bronchial occlusion with endobronchial Watanabe spigot. J Bronchol 10: 264-267, 2003. [Google Scholar]