Abstract
Purpose: Although efforts have been made to identify neurobiological characteristic of major depressive disorder (MDD) in recent years, trait- and state-related biological characteristics of MDD still remains unclear. Using functional magnetic resonance imaging (fMRI), the aim of this study was to explore whether altered spontaneous neural activities in MDD are trait- or state- related.
Materials and Methods: Resting-state fMRI data were analyzed for 72 current MDD (cMDD) patients (first-episode, medication-naïve), 49 remitted MDD (rMDD) patients, and 78 age- and sex- matched healthy control (HC) subjects. The values of amplitude of low-frequency fluctuation (ALFF) were compared between groups.
Results: Compared with the cMDD group, the rMDD group had increased ALFF values in the left middle occipital gyrus, left middle temporal gyrus and right cerebellum anterior lobe. Besides, compared with the HC group, the cMDD group had decreased ALFF values in the left middle occipital gyrus. Further analysis explored that the mean ALFF values in the left middle occipital gyrus, left middle temporal gyrus and right cerebellum anterior lobe were correlated positively with BDI scores in rMDD patients.
Conclusion: Abnormal activity in the left middle occipital gyrus, left middle temporal gyrus and right cerebellum anterior lobe may be state-specific in current (first-episode, medication-naïve) and remitted (medication-naïve) depression patients. Furthermore, the state-related compensatory effect was found in these brain areas.
Keywords: major depressive disorder, remission, trait-related, state-related, resting-state fMRI, amplitude of low-frequency fluctuation
Introduction
Major depressive disorder (MDD) is a high-recurrence psychiatric condition. which more than half of patients seen for first-episode MDD in China experience a recurrence of MDD symptoms within 5 years after the initial depression onset (Ji et al., 2001). Moreover, clinical factors, including the number of previous episodes and subclinical residual symptoms, appear to be the most important predictors of recurrence (Hardeveld et al., 2010). Therefore, distinguishing the correlation between altered brain activity and different clinical states may lead to a better understanding of the neurobiological mechanisms underlying MDD pathogenesis and recurrence, which, in turn, may improve clinical diagnosis and prognostic evaluation of MDD.
Some neuroimaging studies, primarily using task-related functional magnetic resonance imaging (fMRI) and structural magnetic resonance imaging (sMRI) methods, have examined brain activation and structural changes in different clinical states, including current MDD (cMDD) and remitted MDD (rMDD). These researches have explored several abnormal alterations of brain region in patients, which could be trait-related or state-related markers of depression. However, a number of brain regions, including regions within the orbitofrontal cortex, insular cortex, posterior cingulate cortex (PCC), amygdala, hippocampus, and prefrontal cortex, have been dually implicated as potential trait-related and state-related biomarkers of depression (Caetano et al., 2004; Wang et al., 2008; Lorenzetti et al., 2010; van Eijndhoven et al., 2013; Liu et al., 2014; Ming Q. et al., 2017). Inconsistency across studies might be partially related to different task paradigms or analytical methods, including stress task or source recollection paradigm, cortical thickness or brain region volume analysis.
Resting-state fMRI studies, wherein subjects are not performing an explicit task during the scan, can complement task fMRI studies (Su et al., 2010; Biswal, 2012). Resting-state data can be analyzed by multifarious approaches, such as seed-based approaches, independent component analysis, graph methods, clustering algorithms, neural networks, and pattern classifier (Lee et al., 2013; Dong et al., 2018). In recent years, a resting-state fMRI analytical method named amplitude of low-frequency fluctuation (ALFF) was developed to assess the spontaneous low frequency (0.01–0.08 Hz) fluctuations (LFF) in the BOLD fMRI signal at rest, which could reflect the intensity of brain regional spontaneous neural activity (Zang et al., 2007). Besides, some articles indicated that the ALFF was associated with the neuronal glucose metabolism and local field potential activity (Logothetis et al., 2001; Tomasi et al., 2013). Therefore ALFF analysis has been widely used to explore potentially related cerebral biomarkers in mental disorders, which was shown to be a reliable and sensitive approach (Zhang et al., 2014; Fan et al., 2017).
To our knowledge, only one resting-state fMRI study investigating state- and trait-related functional alterations in cMDD and rMDD have been published. Jing reported that abnormal activity of the putamen may be a potential trait-related marker of MDD (Jing et al., 2013). However, Jing’s study included only female patients and did not exclude the effects of comorbidities, treatment condition, or the number of prior episodes. Some depression researches indicated that the comorbidities, treatment condition, and the number of prior episodes would make a difference in the activities of brain areas, such as cingulate cortex, prefrontal lobe, striatum, temporal lobe and insula (Schaefer et al., 2006; Takami et al., 2007; Waugh et al., 2012). Thus the effects of comorbidities, treatment condition, the number of prior episodes should be considered in the depression study.
Notably, Mayberg’s classical neurobiological model implied that the MDD patient has fronto-limbic dysfunctions, including the prefrontal cortex, cingulate cortex, amygdala and striatum, which would account for the dysregulation of the affective and cognitive behavior in patients (Mayberg, 1997, 2003). Moreover, some meta-analysis articles of resting-state fMRI suggested that the dysfunctions of the fronto-limbic circuit, default mode network (DMN) and cerebellum, which play an important role in the cognitive processing and affective regulation, would be the biomarkers of drug-naive MDD patients (Xue et al., 2016; Ming Z.M. et al., 2017).
The purpose of this study was to investigate MDD state-related and trait-related neuroimaging alterations of spontaneous neural activity by resting-state fMRI. To exclude the potential influence of comorbidities, treatment condition, and the number of prior episodes, we enrolled a large sample, including 72 first-episode, medication-naive MDD patients (cMDD), 49 remitted MDD patients (rMDD), and 78 age- and sex- matched healthy controls (HCs). The participants were subjected to resting-state fMRI with ALFF analysis. In the context of classical neurobiological model and the studies mentioned above, we hypothesized that the state-related or trait-related characteristic of MDD would be explored in the brain areas of fronto-limbic circuit, default mode network and cerebellum.
Materials and Methods
Participants
The cMDD and rMDD participants were recruited from the psychology clinic at Second Xiangya Hospital affiliated with Central South University in Changsha, Hunan, China. With advertisements and posters, age-, sex-matched HCs were recruited from two colleges and a local community in Changsha. The sample of all the depression patents and normal people was registered from 2014 to 2017.
The clinical states of the patients, including cMDD and rMDD groups, were evaluated independently by two psychiatrists using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Patient Edition (First et al., 2002) and 17-item Hamilton Depression Rating Scale (Hamilton, 1960). At the same time, demographic data and clinical variables information were collected by interview. Patients, who met the DSM-IV-TR criteria for MDD and were in their first MDD, were included in the cMDD group. Inclusion criteria for the rMDD patients were as follows: having at least one episode of MDD in the past 10 years; not meeting the DSM-IV-TR criteria for MDD more than 30 days before the scan; a 17-item HAM-D score ≤7 on the scan day. In this study the remitted MDD was not described as clinical outcome of recovery but a remitted state, which was not shown currently presenting symptoms of MDD. Moreover, this criterion has been used in previous studies of remitted depression (Goulden et al., 2012). The HC group was required to have no history of any prior DSM-IV-TR Axis I disorder. The exclusion criteria for all three groups were: a history of alcohol/substance abuse; use of antidepressants or undergoing psychotherapy or psychotropic medications; having other major psychiatric disorder; a neurological disorder diagnosis; structural brain abnormalities; and any MRI contraindication.
All participants were informed of the study’s purpose and signed informed consent forms. This study was approved by the Ethics Committee of the Second Xiangya Hospital of Central South University.
Psychological Measures
All participants filled out a Beck Depression Inventory-II (Beck et al., 1996) composed of 21 self-report items, which proved to be a validated depressive symptoms scale. This multiple-choice inventory could evaluate the MDD symptoms including irritability, feelings of guilt, suicidal ideation, fatigue, and weight loss (Ayloo et al., 2015). In our current sample, Cronbach’s alphas for cMDD, rMDD, and HC groups were 0.850, 0.858, and 0.823, respectively.
Image Acquisition
Magnetic resonance imaging (MRI) scans were performed on a 3.0-T Siemens Magnetom Skyra scanner (Siemens Healthineers, Erlangen, Germany). During scanning, all participants were instructed to remain motionless with their eyes closed, and to think of nothing in particular but to not fall asleep. To reduce patient head movements and noise, the subjects were fit with foam pads about the head and earplugs. The acquisition parameters were as follows: 32 axial slices, 4-mm slice thickness with a 1-mm gap, 2000-ms repetition time, 30-ms echo time, 80° flip angle, 256 × 256-mm field of view, 64 × 64 data matrix. Three-dimensional T1-weighted magnetization-prepared rapid gradient echo sagittal images were also acquired with the following parameters: 176 axial slices with no gap, 1900-ms repetition time, 2.01-ms echo time, 1-mm3 voxel size, 9° flip angle, 256 × 256-mm field of view, 256 × 256 data matrix.
Data Processing
Resting-state fMRI data preprocessing was conducted in Data Processing Assistant for Resting-State fMRI software (DPARSF V2.3, Yan and Zang, 20101). The first 10 volumes of the functional time series were removed to ensure stable magnetization and adaptation of participants to scanning noise. Subsequently, slice timing and head motion correction were performed. Data were discarded from 30 subjects (10 cMDD, 9 rMDD, and 4 HC) due to translation >2 mm in any direction or rotation >2° around any axis in any of six head motion parameters. Besides, regression of the Friston 24 motion parameters was conducted to control the potential influence of head motion (Satterthwaite et al., 2013). After the correction mentioned above, the EPI template of standard Montreal Neurological Institute (MNI) was used for spatial normalization with a resampling voxel size of 3 × 3 × 3 mm3. The preprocessing image was spatially smoothed with an 8 × 8 × 8 mm full width at half-maximum Gaussian kernel.
To discard biases from low-frequency drift and high-frequency noise, detrending and band-pass (0.01–0.08 Hz) filtering were conducted. After that, the time series of each voxel was converted into the frequency domain, of which power spectrum was calculated. The square root was calculated at each frequency of the power spectrum. Finally, the average square root was then obtained at each voxel across the frequency range of 0.01–0.08 Hz, which was obtained to take as ALFF to reflect absolute intensity of brain spontaneous neural activity (Zang et al., 2007).
After the data processing, the final analysis included 72 medication-naive, first-episode cMDD patients (39 females), 49 rMDD patients (26 females), and 78 HC subjects (43 females).
ALFF Data Statistical Analysis
Amplitude of low-frequency fluctuations maps were processed using the Resting-State fMRI Data Analysis Toolkit (REST V1.8, Song et al., 2011; see text footnote1). These maps were then exported to SPM12 (Wellcome Trust Center for Neuroimaging, London, United Kingdom2) for statistical analyses. An analysis of covariance (ANCOVA) was used to detect between group differences on ALFF maps, including levels of education as a covariate of no interest. An initial voxelwise threshold of p < 0.001 uncorrected was used, to which a clusterwise correction (FDR) for multiple comparison was applied.
Independently of the result, the map from the uncorrected main effect of group from the ANCOVA was used to mask the exploratory between group comparisons among the three different groups (Henson et al., 2002; Fan et al., 2017). For these exploratory analyses, an initial voxelwise threshold of p < 0.001 uncorrected was used, to which a clusterwise correction for multiple comparison (FDR) was applied. An additional Bonferroni correction for multiple comparisons was applied on the cluster p-value to account for the number of tests performed (6 comparisons: cMDD > rMDD, cMDD < rMDD, cMDD > HC, cMDD < HC, rMDD > HC, rMDD < HC). A cluster with a p-value of 0.008 or below would therefore be significant (p = 0.05/6 = 0.008).
To further examining the connection between the abnormal regional brain activity and depression symptom severity, correlation analyses were conducted between psychometric (HAM-D and BDI) scores and mean ALFF values obtained in brain regions with abnormal activity in the cMDD and rMDD groups, separately.
Results
Cohort Characteristics
The demographic and clinical data are summarized in Table 1. The groups’ characteristics did not differ in terms of sex or age, though the HC group’s education level was, on average, higher than that the other two groups [HC > cMDD = rMDD, F(2,196) = 12.023, p < 0.001]. Mean HAMD scores were greater in the cMDD group than in the rMDD group (t = 14.410, df = 119, p < 0.001). A one-way ANOVA revealed a main effect of group on BDI scores [F(2,196) = 310.668, p < 0.001] and post hoc analysis showed that each group’s BDI scores differed from those of the other two (cMDD > rMDD > HC, all post hoc p-values < 0.005).
Table 1.
Demographic and clinical characteristics of cMDD, rMDD, and HC groupsa.
| Characteristic | cMDD (N = 72) | rMDD (N = 49) | HC (N = 78) | F/t | P | / Cohen’s d |
|---|---|---|---|---|---|---|
| Age, years | 22.38 (5.67) | 22.57 (6.41) | 22.19 (3.52) | 0.083 | 0.921 | <0.001 |
| Sex, N females (%) | 39 (54.2) | 26 (53.1) | 43 (55.1) | 0.049 | 0.952 | <0.001 |
| Education, years | 13.28 (2.51) | 13.87 (2.48) | 15.10 (1.98) | 12.023 | <0.001 | 0.123 |
| Illness duration, years | 0.85 (0.83) | 1.22 (0.92) | – | 1.571 | 0.102 | 0.42 |
| Remission duration, years | – | 0.51 (0.30) | – | – | – | – |
| HAM-D score | 22.25 (6.00) | 6.45 (5.81) | – | 14.410 | <0.001 | 2.68 |
| BDI score | 29.46 (9.32) | 6.91 (5.87) | 3.23 (3.99) | 310.668 | <0.001 | 3.17 |
Means are shown with standard deviations in parentheses, unless otherwise specified. acMDD, current major depressive disorder; rMDD, remitted major depressive disorder; HC, healthy control; BDI, Beck Depression Inventory; HAM-D, 17-item Hamilton Depression Rating Scale.
ALFF Differences
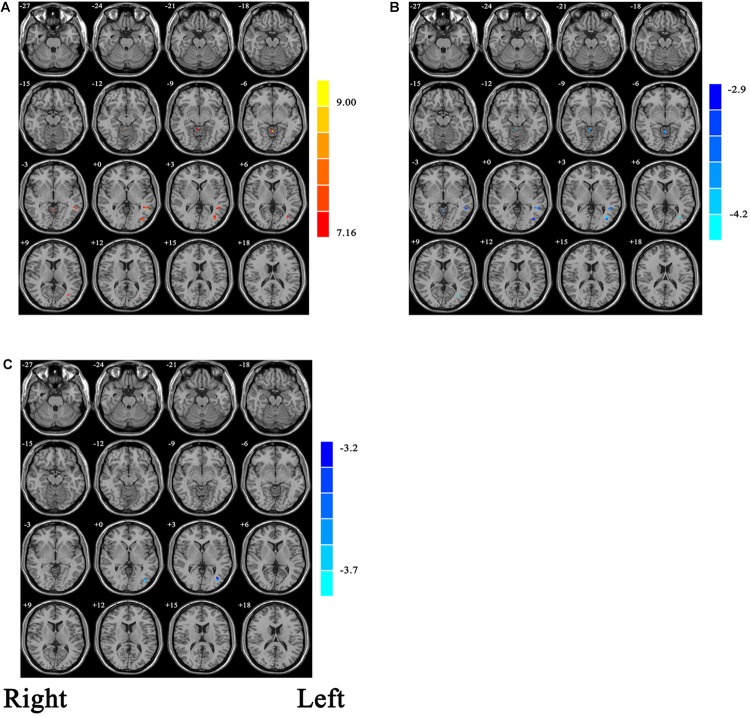
With the one-way ANCOVA analysis, main effects of group on ALFF values were identified in the brain areas including the occipital and temporal cortices, as well as in the cerebellum (p = 0.001, uncorrected; see Figure 1A).
FIGURE 1.
(A) Statistic maps showing ANOVA results of ALFF differences among current major depression disorder (cMDD), remitted major depression disorder (rMDD), and healthy control (HC) groups (p < 0.001, uncorrected). (B) Brain regions showing ALFF differences between cMDD and rMDD [p < 0.008, false discovery rate (FDR) corrected]. (C) Brain regions showing ALFF differences between cMDD and HC (p < 0.008, FDR corrected). ANOVA and post hoc t-tests were conducted using years of education as covariates of no interest. Two-sample t-test results are expressed within a mask showing significant group differences from the ANOVA. Red and blue denote ALFF increases and decreases, respectively; color bars indicate t-values.
Within the mask of these significant group differences, post hoc t-tests showed that, compared with the cMDD group, rMDD group had increased ALFF values in the left middle occipital gyrus, left middle temporal gyrus and right cerebellum anterior lobe (Table 2 and Figure 1B). Besides, compared with the HC group, the cMDD group had decreased ALFF values in the left middle occipital gyrus (Table 2 and Figure 1C). Furthermore, all p-values in the post hoc t-tests were corrected with false discovery rate (FDR) method (all p-values < 0.008; see Table 2).
Table 2.
Brain regions with significantly different ALFF values among the cMDD, rMDD, and HC groups.
| Brain regions | Voxels | Peak coordinates (MNI) |
Peak T-values | P uncorrected | P correcteda | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| cMDD < rMDD | |||||||
| Left middle occipital gyrus (BA 19) | 17 | −36 | −72 | 6 | −4.5577 | 0.001 | 0.004 |
| Left middle temporal gyrus | 15 | −45 | −45 | 3 | −3.8307 | 0.001 | 0.004 |
| Right cerebellum anterior lobe | 11 | 6 | −45 | −12 | −4.2424 | 0.001 | 0.004 |
| cMDD < HC | |||||||
| Left middle occipital gyrus (BA 19) | 12 | −39 | −78 | 0 | −3.8093 | 0.001 | 0.002 |
ALFF, amplitude of low frequency fluctuations; BA, Brodmann area; x, y, z, coordinates of peak locations in the Montreal Neurological Institute (MNI) space. aFalse discovery rate (FDR) corrected (p < 0.008).
Correlations Between ALFF Values and Clinical Variables
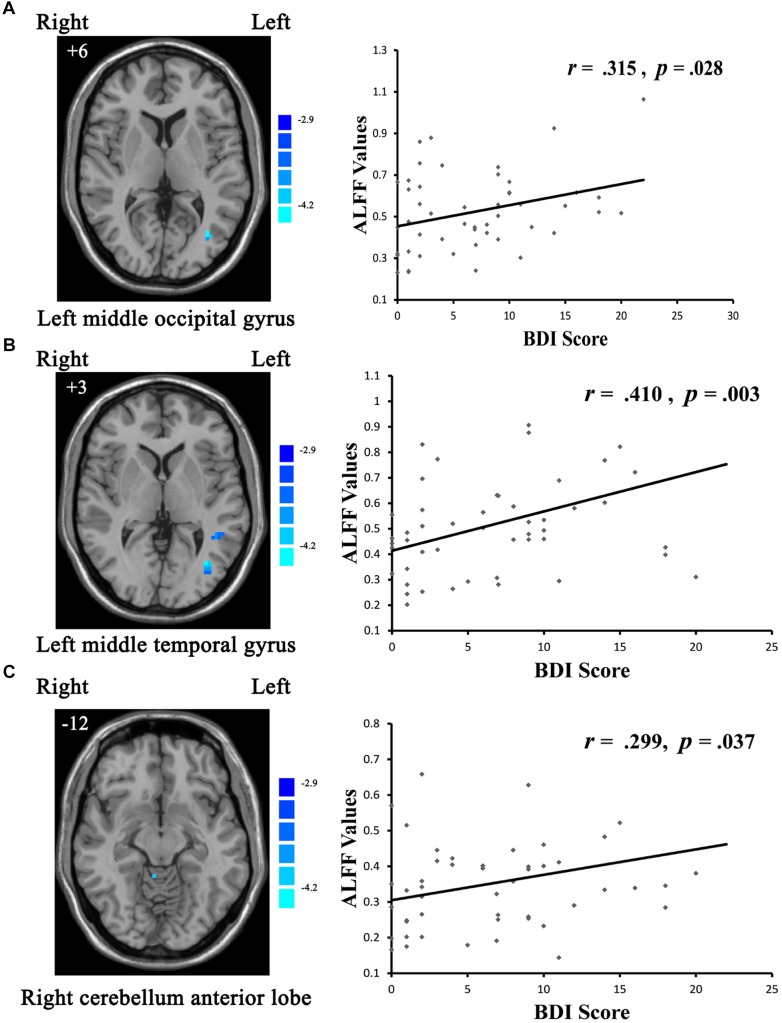
With respect to potential relationships between regional brain activity and clinical variables, mean ALFF values in the cMDD and rMDD groups, were separately obtained in the brain regions showing differences with the one-way ANCOVA analysis. The correlations between the mean ALFF values and BDI, HAMD scores were examined. Eventually, only the BDI scores in the rMDD group were correlated positively with ALFF values in the left middle occipital gyrus (r = 0.315, uncorrected p = 0.028; see Figure 2A), left middle temporal gyrus (r = 0.410, uncorrected p = 0.003; significant with Bonferroni correction [0.05/6]; see Figure 2B), right cerebellum anterior lobe (r = 0.299, uncorrected p = 0.037; see Figure 2C).
FIGURE 2.
Scatter plots showing significant positive correlations between BDI scores and regional ALFF values in the (A) left middle occipital gyrus (uncorrected p = 0.028), (B) left middle temporal gyrus (uncorrected p = 0.003; significant with Bonferroni correction), (C) right cerebellum anterior lobe (uncorrected p = 0.037).
Discussion
The major finding of the current study was that the rMDD group showed increased hyperactivities in the left middle occipital gyrus, left middle temporal gyrus and right cerebellum anterior lobe when compared with the cMDD group by resting-state fMRI. Compared with the HC group, the cMDD group demonstrated decreased hypoactivity in the left middle occipital gyrus. The spontaneous neural activities in the left middle occipital gyrus, left middle temporal gyrus and right cerebellum anterior lobe were positively relevant with clinical symptom. These results suggested that brain activities in the left middle temporal gyrus, left middle occipital gyrus, and right cerebellum anterior lobe may serve as state-related biological characteristics of MDD.
The middle temporal gyrus, which is located between the superior temporal gyrus and inferior temporal gyrus, plays a vital role in the cognitive processing, such as language, memory and object vision processing (Onitsuka, 2004). Colombo and Gross (1994) reported that deficit in the middle temporal gyrus in monkeys would cause a poor performance in the cognitive task which requires visual object discrimination and recognition. A structural MRI study reported gray matter reduction in the middle temporal gyrus in treatment-resistant depression patients when compared with healthy subjects (Ma et al., 2012). Besides, the middle temporal gyrus is involved in the DMN (Zhou and Yu, 2010; Xue et al., 2016). Abnormal DMN functional alterations have been found closely bound up with rumination and autobiographical memory impairment in depression patients (Sumner et al., 2010; Paul et al., 2015).
The present study also found more hyperactivity in the middle temporal gyrus in the rMDD group compared with the cMDD group. This result and the relation between clinical symptom and brain activity in rMDD group may indicate the state-related functional compensation in the middle temporal gyrus. The functional compensation implies that the individuals suffered from damage of the central nervous system (CNS) would trigger the residual structures to achieve recovery, including behavioral, physical, or cognitive strategies (Tanaka, 2001). Furthermore, the temporal regions were deeply involved in social cognitive and affective processing (Wang et al., 2012). Therefore, we tentatively put forward that the state-related hyperactivity in the middle temporal gyrus may be involved in a compensatory mechanism, which is consistent with Goetz’s study that the compensatory effect has been found in the same brain region among the depression patients during a cognitive reappraisal task (Goetz et al., 2018).
Consistent with previous studies (Guo et al., 2013; Liang et al., 2013), the hyperactivity in the left middle occipital gyrus was found in MDD patients when compared with HC. The brain activity level in the left middle occipital gyrus was positively relevant with clinical symptom. The depressed patients with occipital brain activity abnormalities have shown a disproportionate attentional preference toward negative visual information (Guo et al., 2013). Depression-associated abnormalities of the middle occipital gyrus were related with abnormal neuropsychological activity, leading to a motor block and lowered attention (Yu et al., 2017). As described previously, this increased brain activity may be due to the state-related compensatory effect in the left middle occipital gyrus, which plays an active role in cognitive processing, including visual information processing and verbal episodic memory (Li et al., 2016).
The cerebellum, a structure used to be most appreciated for its important role in motor coordination and behavior (Stein, 1986), was reported taking part in emotional and cognitive processing in recent depression studies (Schmahmann, 2000; Lekeu et al., 2002; Lin et al., 2012). Our study found increased activities in the anterior lobe of cerebellum in the rMDD group than in the cMDD group. Besides, the activity level in this area was correlated with depressive symptom in the rMDD group, which may imply that the state-related hyperactivities in the anterior lobe of cerebellum found in the rMDD patients are involved in a compensatory mechanism. The relation between cerebellum brain activity and depressive symptom has been reported by fMRI and sMRI findings (Zeng et al., 2012; Xu et al., 2017). This association may be illustrated by the cerebellar connections with limbic regions, brainstem, temporal lobe, prefrontal lobe, and cingulate gyrus, areas shown to have profound influence on cognitive processing and emotional regulation (Haines et al., 1984; Middleton and Strick, 2001; Turner et al., 2007). A growing number of studies have paid attention to the role of cerebellum in depression (Lin et al., 2012; Phillips et al., 2015; Ming Z.M. et al., 2017), and our research implied that the anterior cerebellum may be related with the depression remitted process.
Limitations
Our study has some limitations that need to be addressed in further studies. Firstly, we used a cross-sectional study design. Longitudinal studies are required to clarify how neural brain activities evolve from depression onset to remission, and from remission to recurrence. Second, to exclude potential confounders, we excluded patients with comorbidities. However, more than three-quarters of MDD patients have comorbidities (Kessler et al., 2003). Thus, it is not known whether the state-related features found in this study would be detectable in MDD patients with comorbidities. Third, in our study, the ANCOVA anlysis result of ALFF values was uncorrected and voxels cluster size was small for a clusterwise correction. Therefore, our findings, as an exploring research, may only imply a trend of the abnormal activity brain area in cMDD, rMDD and HC groups. Longitudinal studies across different clinical states would be required in the future exploration.
Conclusion
To our knowledge, this is the first study to explore state-related alterations of spontaneous neural activity in medication-naïve current and remitted depression. Consistent with the research hypothesis, state-related abnormal spontaneous neural activities were observed in the DMN and cerebellum, including the middle temporal gyrus, cerebellum anterior lobe. Furthermore, the state-related compensatory effect was found in the middle occipital gyrus, middle temporal gyrus and cerebellum anterior lobe. Although these findings remain to be confirmed, our study provides a fresh perspective for elucidating the neurobiology of MDD development, maintenance and recovery. The state-related characteristic of MDD suggested by our study may be useful for improving clinical diagnosis and prognostic evaluation of MDD, planning of targeted interventions, and monitoring of therapeutic efficacy.
Author Contributions
SY supervised the study. CC performed the analysis and wrote paper. DD, YJ, and QM contributed to the analysis. XZ, XS, GX, and YG collected the data. All co-authors revised and approved the version to be published.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding. This research was supported by the National Natural Science Foundation of China (Grant Number 81471384).
References
- Ayloo S., Thompson K., Choudhury N., Sheriffdeen R. (2015). Correlation between the beck depression inventory and bariatric surgical procedures. Surg. Obesity Relat. Dis. 11 637–642. 10.1016/j.soard.2014.11.005 [DOI] [PubMed] [Google Scholar]
- Beck A. T., Steer R. A., Brown G. K. (1996). Manual for the Beck Depression Inventory-II. Washington, DC: American University, 21. [Google Scholar]
- Biswal B. B. (2012). Resting state fMRI: a personal history. Neuroimage 62 938–944. 10.1016/j.neuroimage.2012.01.090 [DOI] [PubMed] [Google Scholar]
- Caetano S. C., Hatch J. P., Brambilla P., Sassi R. B., Nicoletti M., Mallinger A. G., et al. (2004). Anatomical MRI study of hippocampus and amygdala in patients with current and remitted major depression. Psychiatry Res. 132 141–147. 10.1016/j.pscychresns.2004.08.002 [DOI] [PubMed] [Google Scholar]
- Colombo M., Gross C. G. (1994). Responses of inferior temporal cortex and hippocampal neurons during delayed matching-to-sample in monkeys (Macaca fascicularis). Behav. Neurosci. 108 443–455. 10.1037/0735-7044.108.3.443 [DOI] [PubMed] [Google Scholar]
- Dong D., Ming Q., Zhong X., Pu W., Zhang X., Jiang Y., et al. (2018). State-independent alterations of intrinsic brain network in current and remitted depression. Prog. Neuro Psychopharmacol. Biol. Psychiatry 89 475–480. 10.1016/j.pnpbp.2018.08.031 [DOI] [PubMed] [Google Scholar]
- Fan J., Zhong M., Gan J., Liu W., Niu C., Liao H., et al. (2017). Spontaneous neural activity in the right superior temporal gyrus and left middle temporal gyrus is associated with insight level in obsessive-compulsive disorder. J. Affect. Disord. 207 203–211. 10.1016/j.jad.2016.08.027 [DOI] [PubMed] [Google Scholar]
- First M., Spitzer R., Gibbon M., Williams J. (2002). Structured Clinical Interview for DSM-IV-TR Axis I Disorders–Patient Edtion (SCID-I/P, 11/2002 revision). New York, NY: New York State Psychiatric Institute. [Google Scholar]
- Goetz D. S. E., Foland-Ross L. C., Gotlib I. H. (2018). Neural correlates of top-down regulation and generation of negative affect in major depressive disorder. Psychiatry Res. Neuroimag. 276 1–8. 10.1016/j.pscychresns.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulden N., Mckie S., Thomas E. J., Downey D., Juhasz G., Williams S. R., et al. (2012). Reversed frontotemporal connectivity during emotional face processing in remitted depression. Biol. Psychiatry 72 604–611. 10.1016/j.biopsych.2012.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W. B., Liu F., Xun G. L., Hu M. R., Guo X. F., Xiao C. Q., et al. (2013). Reversal alterations of amplitude of low-frequency fluctuations in early and late onset, first-episode, drug-naive depression. Prog. Neuro Psychopharmacol. Biol. Psychiatry 40 153–159. 10.1016/j.pnpbp.2012.08.014 [DOI] [PubMed] [Google Scholar]
- Haines D. E., Dietrichs E., Sowa T. E. (1984). Hypothalamo-cerebellar and cerebello-hypothalamic pathways: a review and hypothesis concerning cerebellar circuits which may influence autonomic centers and affective behavior (part 1 of 2). Brain Behav. Evol. 24 198–209. 10.1159/000121317 [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1960). A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23 56–62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardeveld F., Spijker J., Graaf R. D., Nolen W. A., Beekman A. T. F. (2010). Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr. Scand. 122 184–191. 10.1111/j.1600-0447.2009.01519.x [DOI] [PubMed] [Google Scholar]
- Henson R. N. A., Shallice T., Gorno-Tempini M. L., Dolan R. J. (2002). Face repetition effects in implicit and explicit memory tests as measured by fMRI. Cereb. Cortex 12 178–186. 10.1093/cercor/12.2.178 [DOI] [PubMed] [Google Scholar]
- Ji J., Kleinman A., Becker A. E. (2001). Suicide in contemporary China: a review of China’s distinctive suicide demographics in their sociocultural context. Harvard Rev. Psychiatry 9 1–12. 10.1080/10673220127875 [DOI] [PubMed] [Google Scholar]
- Jing B., Liu C. H., Ma X., Yan H. G., Zhuo Z. Z., Zhang Y., et al. (2013). Difference in amplitude of low-frequency fluctuation between currently depressed and remitted females with major depressive disorder. Brain Res. 1540 74–83. 10.1016/j.brainres.2013.09.039 [DOI] [PubMed] [Google Scholar]
- Kessler R. C., Berglund P., Demler O., Jin R., Koretz D., Merikangas K. R., et al. (2003). The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication. J. Am. Med. Assoc. 289 3095–3105. 10.1001/jama.289.23.3095 [DOI] [PubMed] [Google Scholar]
- Lee M. H., Smyser C. D., Shimony J. S. (2013). Resting state fMRI: a review of methods and clinical applications. Am. J. Neuroradiol. 34 1866–1872. 10.3174/ajnr.A3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekeu F., Marczewski P., Van Der Linden M., Collette F., Degueldre C., Del Fiore G., et al. (2002). Effects of incidental and intentional feature binding on recognition: a behavioral and PET activation study. Neuropsychologia 40 131–144. 10.1016/S0028-3932(01)00088-4 [DOI] [PubMed] [Google Scholar]
- Li G., Ma X., Bian H., Sun X., Zhai N., Yao M., et al. (2016). A pilot fMRI study of the effect of stressful factors on the onset of depression in female patients. Brain Imag. Behav. 10 195–202. 10.1007/s11682-015-9382-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M. J., Zhou Q., Yang K. R., Yang X. L., Fang J., Chen W. L., et al. (2013). Identify changes of brain regional homogeneity in bipolar disorder and unipolar depression using resting-state FMRI. PLoS One 8:e79999. 10.1371/journal.pone.0079999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. C., Chou K. H., Chen H. L., Huang C. C., Lu C. H., Li S. H., et al. (2012). Structural deficits in the emotion circuit and cerebellum are associated with depression, anxiety and cognitive dysfunction in methadone maintenance patients: a voxel-based morphometric study. Psychiatry Res. 201 89–97. 10.1016/j.pscychresns.2011.05.009 [DOI] [PubMed] [Google Scholar]
- Liu C. H., Jing B., Ma X., Xu P. F., Zhang Y., Li F., et al. (2014). Voxel-based morphometry study of the insular cortex in female patients with current and remitted depression. Neuroscience 262 190–199. 10.1016/j.neuroscience.2013.12.058 [DOI] [PubMed] [Google Scholar]
- Logothetis N. K., Pauls J., Augath M., Trinath T., Oeltermann A. (2001). Neurophysiological investigation of the basis of the fMRI signal. Nature 412 150–157. 10.1038/35084005 [DOI] [PubMed] [Google Scholar]
- Lorenzetti V., Allen N. B., Whittle S., Yucel M. (2010). Amygdala volumes in a sample of current depressed and remitted depressed patients and healthy controls. J. Affect. Disord. 120 112–119. 10.1016/j.jad.2009.04.021 [DOI] [PubMed] [Google Scholar]
- Ma C., Ding J., Li J., Guo W., Long Z., Liu F., et al. (2012). Resting-state functional connectivity bias of middle temporal gyrus and caudate with altered gray matter volume in major depression. PLoS One 7:e45263. 10.1371/journal.pone.0045263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg H. S. (1997). Limbic-cortical dysregulation: a proposed model of depression. J. Neuropsychiatry Clin. Neurosci. 9 471–481. 10.1176/jnp.9.3.471 [DOI] [PubMed] [Google Scholar]
- Mayberg H. S. (2003). Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br. Med. Bull. 65 193–207. 10.1093/bmb/65.1.193 [DOI] [PubMed] [Google Scholar]
- Middleton F. A., Strick P. L. (2001). Cerebellar projections to the prefrontal cortex of the primate. J. Neurosci. 21 700–712. 10.1523/JNEUROSCI.21-02-00700.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming Q., Zhong X., Zhang X., Pu W., Dong D., Jiang Y., et al. (2017). State-independent and dependent neural responses to psychosocial stress in current and remitted depression. Am. J. Psychiatry 174 971–979. 10.1176/appi.ajp.2017.16080974 [DOI] [PubMed] [Google Scholar]
- Ming Z. M., Hu X., Lu L. M., Liangqing Zhang M. M., Chen L., Gong Q., et al. (2017). Intrinsic cerebral activity at resting state in adults with major depressive disorder: a meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 75 157–164. 10.1016/j.pnpbp.2017.02.001 [DOI] [PubMed] [Google Scholar]
- Onitsuka T. (2004). Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: an MRI study. Am. J. Psychiatry 161 2103–2110. 10.1176/appi.ajp.161.9.1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul H. J., Madison F., Phoebe F., Gotlib I. H. (2015). Depressive rumination, the default-mode network, and the dark matter of clinical neuroscience. Biol. Psychiatry 78 224–230. 10.1016/j.biopsych.2015.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. R., Hewedi D. H., Eissa A. M., Moustafa A. A. (2015). The cerebellum and psychiatric disorders. Front. Public Health 3:66. 10.3389/fpubh.2015.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite T. D., Elliott M. A., Gerraty R. T., Ruparel K., Loughead J., Calkins M. E., et al. (2013). An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage 64 240–256. 10.1016/j.neuroimage.2012.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer H. S., Putnam K. M., Benca R. M., Davidson R. J. (2006). Event-related functional magnetic resonance imaging measures of neural activity to positive social stimuli in pre- and post-treatment depression. Biol. Psychiatry 60 974–986. 10.1016/j.biopsych.2006.03.024 [DOI] [PubMed] [Google Scholar]
- Schmahmann J. D. (2000). The role of the cerebellum in affect and psychosis. J. Neurolinguist. 13 189–214. 10.1016/S0911-6044(00)00011-7 [DOI] [Google Scholar]
- Song X. W., Dong Z. Y., Long X. Y., Li S. F., Zuo X. N., Zhu C. Z., et al. (2011). REST: a toolkit for resting-state functional magnetic resonance imagingdata processing. PLoS One 6:e25031. 10.1371/journal.pone.0025031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J. F. (1986). Role of the cerebellum in the visual guidance of movement. Nature 323 217–221. 10.1038/323217a0 [DOI] [PubMed] [Google Scholar]
- Su L., Li T., Deng W., Jiang L., Wu Q., Tang H., et al. (2010). Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch. Gen. Psychiatry 67 783–792. 10.1001/archgenpsychiatry.2010.84 [DOI] [PubMed] [Google Scholar]
- Sumner J. A., Griffith J. W., Susan M. (2010). Overgeneral autobiographical memory as a predictor of the course of depression: a meta-analysis. Behav. Res. Ther. 48 614–625. 10.1016/j.brat.2010.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takami H., Okamoto Y., Yamashita H., Okada G., Yamawaki S. (2007). Attenuated anterior cingulate activation during a verbal fluency task in elderly patients with a history of multiple-episode depression. Am. J. Geriatr. Psychiatry 15 594–603. 10.1097/01.JGP.0b013e31802ea919 [DOI] [PubMed] [Google Scholar]
- Tanaka K. (2001). Temporal lobe. Int. Encycloped. Soc. Behav. Sci. 28 15595–15599. 10.1016/B0-08-043076-7/03469-0 [DOI] [Google Scholar]
- Tomasi D., Wang G. J., Volkow N. D. (2013). Energetic cost of brain functional connectivity. Proc. Natl. Acad. Sci. U.S.A. 110 13642–13647. 10.1073/pnas.1303346110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner B. M., Paradiso S., Marvel C. L., Pierson R., Boles Ponto L. L., Hichwa R. D., et al. (2007). The cerebellum and emotional experience. Neuropsychologia 45 1331–1341. 10.1016/j.neuropsychologia.2006.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eijndhoven P., Van Wingen G., Katzenbauer M., Groen W., Tepest R., Fernandez G., et al. (2013). Paralimbic cortical thickness in first-episode depression: evidence for trait-related differences in mood regulation. Am. J. Psychiatry 170 1477–1486. 10.1176/appi.ajp.2013.12121504 [DOI] [PubMed] [Google Scholar]
- Wang L., Dai W., Su Y., Wang G., Tan Y., Jin Z., et al. (2012). Amplitude of low-frequency oscillations in first-episode, treatment-naive patients with major depressive disorder: a resting-state functional mri study. PLoS One 7:e48658. 10.1371/journal.pone.0048658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Krishnan K. R., Steffens D. C., Potter G. G., Dolcos F., Mccarthy G. (2008). Depressive state- and disease-related alterations in neural responses to affective and executive challenges in geriatric depression. Am. J. Psychiatry 165 863–871. 10.1176/appi.ajp.2008.07101590 [DOI] [PubMed] [Google Scholar]
- Waugh C. E., Hamilton J. P., Chen M. C., Joormann J., Gotlib I. H. (2012). Neural temporal dynamics of stress in comorbid major depressive disorder and social anxiety disorder. Biol. Mood Anxiety Disord. 2 1–15. 10.1186/2045-5380-2-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L. Y., Xu F. C., Liu C., Ji Y. F., Wu J. M., Wang Y., et al. (2017). Relationship between cerebellar structure and emotional memory in depression. Brain Behav. 7:e00738. 10.1002/brb3.738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Z., Pu W., Yao S. (2016). Functional alterations of fronto-limbic circuit and default mode network systems in first-episode, drug-naïve patients with major depressive disorder: a meta-analysis of resting-state fMRI data. J. Affect. Disord. 206 280–286. 10.1016/j.jad.2016.09.005 [DOI] [PubMed] [Google Scholar]
- Yan C. G., Zang Y. F. (2010). DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 4:13 10.3389/fnsys.2010.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H. L., Liu W. B., Wang T., Huang P. Y., Jie L. Y., Sun J. Z., et al. (2017). Difference in resting-state fractional amplitude of low-frequency fluctuation between bipolar depression and unipolar depression patients. Eur. Rev. Med. Pharmacol. Sci. 21 1541–1550. [PubMed] [Google Scholar]
- Zang Y. F., He Y., Zhu C. Z., Cao Q. J., Sui M. Q., Liang M., et al. (2007). Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 29 83–91. 10.1016/j.braindev.2006.07.002 [DOI] [PubMed] [Google Scholar]
- Zeng L. L., Shen H., Liu L., Wang L., Li B., Fang P., et al. (2012). Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain 135 1498–1507. 10.1093/brain/aws059 [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhu X., Wang X., Zhu X., Zhong M., Yi J., et al. (2014). First-episode medication-naive major depressive disorder is associated with altered resting brain function in the affective network. PLoS One 9:e85241. 10.1371/journal.pone.0085241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Yu C. (2010). Increased neural resources recruitment in the intrinsic organization in major depression. J. Affect. Disord. 121 220–230. 10.1016/j.jad.2009.05.029 [DOI] [PubMed] [Google Scholar]