Abstract
Identifying modifiable factors that contribute to preeclampsia risk associated with assisted reproduction can improve maternal health. Vascular dysfunction predates clinical presentation of preeclampsia. Therefore, we examined if a non-physiologic hormonal milieu, a modifiable state, affects maternal vascular health in early pregnancy. Blood pressure, endothelial function, circulating endothelial progenitor cell numbers (CPC), lipid levels, and corpus luteum (CL) hormones were compared in a prospective cohort of women with infertility history based on number of CL: 0 CL (programmed frozen embryo transfer (FET), N=18); 1 CL (spontaneous conception [N=16] and natural cycle FET [N=12]); or > 3 CL associated with in-vitro fertilization [N=11]. Women with 0 or > 3 CL lacked the drop in mean arterial blood pressure compared to those with 1 CL (both P=0.05). Reactive Hyperemia Index (RHI) was impaired in women with 0 CL compared to 1 CL (P=0.04) while baseline pulse wave amplitude was higher with > 3 CL compared to 1 CL (P=0.01) or 0 CL (P=0.01). Comparing only FET cycles, a lower RHI and a higher augmentation index is noted in FETs with suppressed CL compared to FETs in a natural cycle (both P=0.03). The number of angiogenic and non-angiogenic CPCs was lower in the absence of a CL in FETs (P=0.01 and P=0.03). Vascular health in early pregnancy is altered in women with aberrant numbers of CL (0 or >3), and might represent insufficient cardiovascular adaptation contributing to an increased risk of preeclampsia.
Keywords: infertility, in vitro fertilization, pregnancy, corpus luteum, endothelial function, hypertensive disease, endothelial progenitor cells
Introduction
Early pregnancy is a critical time that lays the foundation for a healthy pregnancy or one that results in complications, yet our understanding of the mechanisms underlying maternal adaptations to pregnancy is incomplete. Multiple studies have shown that pregnancies after ART have an increased risk for developing hypertensive diseases, e.g. gestational hypertension and preeclampsia 1, 2, 3. The pathogenesis of preeclampsia in many women especially in early-onset preeclampsia involves impaired placentation in early pregnancy provoking an abnormal maternal response that manifests as endothelial dysfunction with the clinical signs of new-onset hypertension and proteinuria, or impaired function of other organs. Women who conceive after ART share risk factors with women who conceive naturally and go on to develop preeclampsia, including higher maternal age, body mass index (BMI), multifetal pregnancies, and preexisting maternal conditions (e.g. hypertension, diabetes, insulin resistance)1. However, several studies have shown that these factors alone do not account for all the observed increased risk 3, 4. Additional parameters specific to fertility treatments may add to the well-described increased risk for preeclampsia 4–7, including the type of ART procedure (invasive vs. non-invasive), manipulation of oocytes and sperm (e.g. IVF vs. ICSI), gonadotropin dose, or use of donor eggs 3, 5–9. An aspect that has received less attention is the impact of corpus luteum (CL) number on the hormonal environment in early pregnancy.
Pregnancies achieved with fertility treatments start for the most part in a non-physiologic maternal endocrine environment 10. There are two main fertility treatment methods used which impact the number of CL in opposite directions. In contrast to spontaneous singleton pregnancies, which occur in the presence of one CL, in-vitro fertilization (IVF) with transfer of fresh embryos results in a supraphysiologic number of CL. In the majority of frozen-thawed embryo transfer (FET) protocols, the pituitary-ovarian axis is suppressed by estradiol supplementation in the context of a programmed cycle, resulting in the absence of a CL. Recently, a Cochrane systematic review and a large database study laid out additional evidence showing an even higher risk for the development of preeclampsia in women following a FET compared to a fresh embryo transfer during the stimulation cycle 4, 6. This association has been demonstrated even in the same women that underwent pregnancies after both fresh embryo transfer and FET 7. However, those studies did not report the type of protocol used for FETs, which is important when considering that a current randomized trial where 75% of the FETs were performed in a natural, ovulatory cycle, found no increased risk of preeclampsia compared to those with fresh embryo transfers 11. Therefore, the increased risk of preeclampsia with ART may not be due to frozen vs. fresh embryo transfer but driven by the CL number.
In spontaneous pregnancies, the CL plays an important role by producing crucial hormones (e.g. estradiol and progesterone) for implantation, placentation, and pregnancy maintenance. These hormones are provided to support pregnancy in several methods of ART. Interestingly, animal models have demonstrated that relaxin, a potent vasodilator also secreted by the CL, contributes to cardiovascular and renal adaptations seen in pregnancy 12–14. In humans a study of women with ovarian failure who conceived through ART with CL suppression suggested that relaxin is also involved in renal adaptation in human pregnancy 15.
Normal human pregnancy is characterized by dramatic changes in the cardiovascular system, including a decrease in mean arterial pressure (MAP) accompanied by an increase in cardiac output and a decrease in systemic vascular resistance even before the complete establishment of the maternal-fetal-placental unit- as early as 6 weeks’ gestation 16. Data on adaptation to human pregnancy after ART are limited and primarily examined renal adaptation 15. The aim of the present study is to investigate whether the number of CL and the mode of conception impact maternal vascular health in the first trimester of pregnancy. Blood pressure changes, vascular reactivity, circulating angiogenic and non-angiogenic endothelial progenitor cell numbers were evaluated, taking into account lipid and CL hormones levels, at 11-14 weeks’ gestation in a cohort of women with history of infertility grouped by CL number and mode of conception.
Methods
Study design and population
The data that support the findings of this study are available from the corresponding author upon reasonable request. The longitudinal cohort study “Pregnancy Outcomes Following Infertility and Endothelial Cell Health (POFIECH)” approved by the Institutional Review Board (IRB) at Stanford University, adheres to the principles of the Declaration of Helsinki and Title 45, U.S. Code of Federal Regulations and followed procedures in accordance with institutional guidelines. POFIECH is a subset of participants of the prospective cohort study “Pregnancy Outcomes Following Infertility (POFI)” at Stanford. Patients with viable, sonographically confirmed, singleton intrauterine pregnancies were recruited at 6-8 weeks’ gestation between June 2015 and December 2017 at the Stanford University School of Medicine, Division of Reproductive Endocrinology and Infertility. All ultrasound examinations were performed at the academic fertility clinic by reproductive endocrinologists.
All participants gave written informed consent for the measurement of vascular reactivity, collection of blood and urine samples, the use of their demographic and clinical data collected as part of the POFI study. Exclusion criteria for the POFIECH study were age >50 years, BMI >30 kg/m2, latex allergy, known vascular disease (i.e., chronic hypertension, lupus erythematosus, rheumatoid disease, etc.), steroid or heparin intake, donor egg transfer, or surrogate pregnancies. The POFIECH study is designed to have a total of three study visits performed in the first trimester (11-14 weeks), third trimester (35-37 weeks), and postpartum (6-12 weeks). This manuscript presents the data from the first study visit.
Participants were grouped according to their CL status at conception (S1: please see http://hyper.ahajournals.org): (1) 0 CL from FET in a programmed cycle (N=18). Those participants were administered oral estradiol until 10 weeks gestation; (2) 1 CL FET in a natural cycle (N=12) or from spontaneous conception after infertility (N=16); or (3) > 3 CL associated with IVF and fresh embryo transfer (N=11). Number of retrieved eggs ranged from 5 to 16. IVF patients received follicle-stimulating hormone and human menopausal gonadotropin for ovarian stimulation and a GnRH-antagonist (N=9), or microdose leuprolide acetate (N=2) for ovulation suppression. Progesterone was administered vaginally (fresh embryo transfer: N=10; FET: N=21) or intramuscularly (fresh embryo transfer: N=1; FET: N=9) and discontinued at 10 weeks of gestation per routine, which is before the first research visit.
The primary outcome of interest was impact on maternal vascular health. Primary analysis involved the comparison of outcomes by CL status regardless of mode of conception. The 1 CL group served as the control and 0 CL and > 3 CL as exposure groups. Secondary analysis included a) the comparison of FET protocols with natural cycle FET as control and FET in a programmed cycle as comparison groups and b) the effect of embryo treatment in culture and cryopreservation with spontaneous conceptions as control and natural cycle FET as comparison groups.
Measurement of Endothelial Function
The participant’s weight and height were measured on testing day (Scale-Tronix scale, White Plains, NY; Seca stadiometer, Columbia, MD). Four sets of resting blood pressures were taken on the testing arm in a sitting position after at least five minutes of rest using the oscillometric method (Connex Vital Signs Monitor, Welch Allyn, Beaverton, OR) by a trained technician. The average of the last three blood pressure measurements was used as per laboratory protocol. The preconception blood pressures and BMI were obtained from the medical record.
Vascular endothelial function was determined non-invasively using the EndoPAT 2000 device (Itamar Medical, Caesarea, Israel) in accordance with the manufacturer’s recommendations and in line with the published literature 17–20. As outcome variables, the device measures 1) peripheral endothelial function using the reactive hyperemia index (RHI, arbitrary units), 2) baseline pulse wave amplitude (BPWA) to reflect arterial tone, and 3) augmentation index (AI) normalized to heart rate of 75 (AI75) to reflect arterial stiffness. MAP was calculated using the following formula: (MAP= [systolic blood pressure + 2× diastolic blood pressure] / 3). The relative delta between preconception and first trimester blood pressure values were calculated as percent change ([preconception value− study visit value])/preconception value ×100).
Circulating progenitor cells characterization
Peripheral blood was collected into sodium-heparin blood vacutainer tubes and mononuclear cells (MNCs) were isolated using density-gradient centrifugation as previously described 21. 0.5-1.0× 106 MNCs were analyzed by polychromatic flow cytometry after staining with antibodies to CD31, CD34, CD45, AC133, glycophorin-A (erythrocyte exclusion), CD14 (macrophage exclusion), and LIVE/DEAD fixable Aqua amine reactive dye. To facilitate accurate compensation and gating, compensation beads, fluorescence minus-one controls and bi-exponential gating were utilized 22. Angiogenic (CD45-dim CD34+ CD31+ AC133+) and non-angiogenic (same but CD133−) circulating progenitor cells (CPCs) were reported as % of total lymphocytes.
Quantification of lipid and hormone levels
Plasma fasting levels of total cholesterol, triglycerides, LDL, HDL, and non-HDL cholesterol were measured using automated methods in the clinical laboratory at Stanford Medical Center at time of blood draw. Estradiol and progesterone levels were determined with a Roche Cobas e-411 at the IVF laboratory at Stanford on first thaw serum (previously stored at −80°C). Serum levels of relaxin-2, the human equivalent of relaxin in other species, were measured using an updated, validated immunosorbent assay (R&D Quantikine ELISA) with a detection range of 7.8 pg/ml to 500 pg/ml according to the manufacturer’s recommendations.
Statistical Analysis
A sample size calculation was performed using published data on differences in RHI with a goal of 12-15 per group and initial enrollment target of 50 assuming a certain withdraw/drop-out rate. However, given a higher than expected withdrawal and drop-out rate we had to enroll greater than 75 subjects and consequently did not hit target for all groups. Study data were collected and managed using the REDCap electronic data capture tool hosted at Stanford 23. Statistical analyses were performed using the JMP Pro 13 software (SAS, Cary, NC, USA). Normality of the data distribution was evaluated by the Shapiro–Wilk test. Categorical variables are expressed as frequencies and percentages. Continuous variables are summarized as mean and standard deviation (SD) for normally distributed data or median and interquartile range (IQR) for non-normally distributed data. Vascular reactivity and lipid concentrations were compared among the three CL groups using one-way ANOVA to compare means or the Kruskal-Wallis rank sum test to compare medians. Γ2 statistics tested differences in proportions between groups. Secondary analyses were carried out based on whether a FET in a natural cycle (1 CL) or a programmed cycle (0 CL) was performed or whether conception occurred spontaneously (1 CL) or in a natural cycle FET (1CL) using the two-sample student’s t-test or the Mann-Whitney U test. Correlations between the vascular reactivity parameters and other items were investigated using the Spearman’s correlation coefficient ρ. The type I error was controlled two-sided at a 0.05 level.
Results
Demographics
A total of 85 patients were recruited of whom some miscarried (N=5) or withdrew (N=23) mainly due to time concerns before the research study visit. Demographic data of the 57 women who participated in the first research visit are shown in Table 1A and B. CL groups were comparable in regard to participant age, gestational age at study visit, ethnicity, smoker (ever), gravidity or parity although distribution of White and Asian race varied (Table 1A). As would be expected, there was a higher rate of aspirin intake until 10 weeks’ gestation and a higher rate of male factor as a reason for the infertility in the 0 and > 3 CL groups. Women with programmed FETs had similar demographic and clinical characteristics before conception compared to women with FETs in a natural cycle but had a higher rate of aspirin exposure (Table 1B). Women with conceptions after a natural cycle FET compared to spontaneous conceptions were more likely to take aspirin, have male factor infertility or a single-gene disorder but less likely to have diminished ovarian reserve.
Table 1.
General characteristics of the study population by corpus luteum (CL) number (A) or mode of conception (B).
| A Corpus luteum number | |||
|---|---|---|---|
| Characteristic | 0 CL (n=18) | 1 CL (n=28) | > 3 CL (n=11) |
| Participant age (years) * | 35.1 ± 3.8 | 34.5 ± 3.3 | 35.7 ± 3.6 |
| Gestational age (days) † | 86.0 (82.8 - 96.3) | 91.5 (85.0 - 95.0) | 92.0 (86.0 - 95.0) |
| Race ‡ | |||
| Asian | 11 (61.1) | 13 (46.4) | 4 (36.4) |
| White | 6 (33.3) | 13 (46.4) | 7 (63.6) |
| African American | 0 | 0 | 0 |
| Other | 1 (5.6) | 2 (7.1) | 0 |
| Ethnicity ‡ | |||
| Hispanic/Latino | 0 | 2 (7.1) | 1 (91.0) |
| Non-Hispanic/Non-Latino | 18 (100) | 26 (92.9) | 10 (90.9) |
| Smoker (ever) ‡ | 1 (5.6) | 1 (3.7) | 0 |
| Gravidity † | 2 (0-2) | 1 (0-2) | 1 (0-3) |
| Parity † | 0 (0-0.3) | 0 (0-0) | 0 (0-1) |
| Aspirin ≤ 10 weeks ‡ | 17 (94.4) | 11 (39.3) | 11 (100) |
| Reason for infertility ‡ | |||
| Age | 0 | 0 | 0 |
| Diminished ovarian reserve | 6 (33.3) | 4 (14.3) | 5 (45.5) |
| Male factor | 11 (61.1) | 5 (17.9) | 5 (45.5) |
| PCOS | 1 (5.6) | 1 (3.6) | 1 (9.1) |
| Other ovulatory disorder | 1 (5.6) | 1 (3.6) | 0 |
| Tubal | 1 (5.6) | 4 (14.3) | 1 (9.1) |
| Uterine | 2 (11.1) | 4 (14.3) | 1 (9.1) |
| Endometriosis | 1 (5.6) | 3 (10.7) | 1 (9.1) |
| Recurrent pregnancy loss | 2 (11.1) | 6 (21.4) | 2 (18.2) |
| Single gene disorder | 2 (11.1) | 3 (10.7) | 0 |
| Same sex partner | 0 | 1 (3.6) | 0 |
| Unexplained | 1 (5.6) | 4 (14.3) | 2 18.2) |
| Other | 0 | 1 (3.6) | 1 (9.1) |
| B Mode of conception | |||
| Characteristic | Programmed cycle FET (n=18) | Natural cycle FET (n=12) | Spontaneous conception (n=16) |
| Participant age (years) * | 35.1 ± 3.8 | 33.6 ± 3.4 | 35.2 ± 3.3 |
| Gestational age (days) * | 88.4 ± 6.6 | 88.2 ± 5.3 | 88.6 ± 15.6 |
| Race ‡ | |||
| Asian | 11 (61.1) | 7 (58.3) | 6 (37.5) |
| White | 6 (33.3) | 4 (33.3) | 9 (56.3) |
| African American | 0 | 0 | 0 |
| Other | 1 (5.6) | 1 (8.3) | 1 (6.2) |
| Ethnicity ‡ | |||
| Hispanic/Latino | 0 | 1 (8.3) | 1 (6.2) |
| Non-Hispanic/Non-Latino | 18 (100) | 11 (91.7) | 15 (93.8) |
| Smoker (ever) ‡ | 1 (5.6) | 0 | 1 (6.2) |
| Gravidity † | 2 (0 - 2) | 1 (0 - 2) | 1 (0.8 - 25) |
| Parity † | 0 (0 - 0.3) | 0 (0 - 0) | 0 (0 - 0) |
| Aspirin ≤ 10 weeks ‡ | 17 (94.4) | 8 (66.7) | 3 (18.6) |
| Reason for infertility ‡ | |||
| Age | 0 | 0 | 0 |
| Diminished ovarian reserve | 6 (33.3) | 1 (8.3) | 3 (18.7) |
| Male factor | 11 (61.1) | 5 (41.7) | 0 |
| PCOS | 1 (5.6) | 0 | 1 (6.2) |
| Other ovulatory disorder | 1 (5.6) | 0 | 3 (18.7) |
| Tubal | 1 (5.6) | 1 (8.3) | 1 (6.2) |
| Uterine | 2 (11.1) | 2 (16.7) | 2 (12.5) |
| Endometriosis | 1 (5.6) | 2 (16.7) | 1 (6.2) |
| Recurrent pregnancy loss | 2 (11.1) | 1 (8.3) | 5 (31.3) |
| Single gene disorder | 2 (11.1) | 2 (16.7) | 1 (6.2) |
| Same sex partner | 0 | 1 (8.3) | 0 |
| Unexplained | 1 (5.6) | 2 (6.7) | 2 (12.5) |
| Other | 0 | 0 | 1 (6.2) |
PCOS, polycystic ovary syndrome
Mean value ± SD are reported.
Median value (interquartile range) are shown.
Number (% of total) are shown.
Clinical characteristics
BMI before conception and at the first trimester research visit was lower in the 0 CL compared to the > 3 CL group (Table 2A). No differences were found in systolic, diastolic, and mean arterial pressures (MAP) between CL groups at baseline before conception. There was a trend towards higher systolic (P=0.06) and diastolic (P=0.07) blood pressures, and MAP (P=0.07) in patients at 11-14 weeks’ gestation with 0 vs 1 CL (Table 2A). The percent change drop in systolic and diastolic blood pressures in the first trimester was lower in participants with 0 (P=0.03 and P=0.06, respectively) or > 3 CL compared to 1 CL (P=0.06 and P=0.06, respectively). While MAP dropped as expected in first trimester for women with 1 CL, MAP increased in participants with 0 (P=0.05) or > 3 CL (P=0.04).
Table 2.
Blood pressure and Body Mass Index of the study population by corpus luteum (CL) number (A) or mode of conception (B).
| A Corpus luteum number | |||||
|---|---|---|---|---|---|
| Characteristic | 0 CL (n=18) | 1 CL (n=28) | > 3 CL (n=11) | P-value 1 CL vs. 0 CL | P-value 1 CL vs. > 3 CL |
| Preconception | |||||
| BMI (kg/m2) * | 22.1 ± 2.7 | 23.3 ± 3.0 | 24.2 ± 2.0 | 0.15 | 0.18 |
| SBP (mmHg) † | 106.5 (101.5 - 117.3) | 110.0 (102.0 - 120.8) | 102.0 (102.0 - 113.0) | 0.35 | 0.27 |
| DBP (mmHg) * | 69.2 ± 8.3 | 70.7 ± 7.7 | 66.5 ± 10.8 | 0.46 | 0.19 |
| MAP (mmHg) * | 82.7 ± 8.4 | 84.5 ± 7.6 | 79.9 ± 9.4 | 0.34 | 0.11 |
| Study visit (11-14 weeks) | |||||
| BMI (kg/m2) * | 22.5 ± 2.8 | 23.8 ± 3.3 | 24.8 ± 3.0 | 0.18 | 0.24 |
| SBP (mmHg) † | 108.5 (103.5 - 115) | 103.5 (99.3 - 110.8) | 106.0 (102 - 113) | 0.06 | 0.26 |
| DBP (mmHg) * | 72.9 ± 6.3 | 69.4 ± 5.5 | 69.5 ± 4.4 | 0.07 | 0.68 |
| MAP (mmHg) † | 84.67 (79.2 - 90) | 80.3 (76.3 - 86.1) | 79.3 (78.3 - 87.3) | 0.07 | 0.40 |
| Percent change over first trimester | |||||
| BMI (kg/m2) * | 1.6 ± 3.9 | 1.9 ± 3.8 | 1.9 ± 5.03 | 0.78 | 0.66 |
| SBP * | −0.4 ± 7.9 | −7.1 ± 11.3 | 0.01 ± 4.9 | 0.03 | 0.06 |
| DBP * | 4.9 ± 9.7 | −2.4 ± 12.2 | 4.7 ± 12.2 | 0.06 | 0.06 |
| MAP * | 2.6 ± 8.4 | −4.4 ± 10.6 | 2.6 ± 8.5 | 0.05 | 0.04 |
| B Mode of conception | |||||
| Characteristic | Programmed cycle FET (n=18) | Natural cycle FET (n=12) | Spontaneous conception (n=16) | P-value natural cycle vs. programmed FET | P-value natural cycle FET vs. spontaneous conception |
| Preconception | |||||
| BMI (kg/m2) * | 22.1 ± 2.7 | 23.3 ± 3.1 | 23.2 ± 2.9 | 0.30 | 0.96 |
| SBP (mmHg) * | 109.7 ± 10.6 | 106.3 ± 8.6 | 116.6 ± 11.1 | 0.55 | 0.03 |
| DBP (mmHg) * | 69.2 ± 8.3 | 69.6 ± 6.7 | 71.6 ± 8.5 | 0.38 | 0.50 |
| MAP (mmHg) * | 82.7 ± 8.4 | 81.8 ± 6.9 | 86.6 ± 7.6 | 0.30 | 0.13 |
| Study visit (11-14 weeks) | |||||
| BMI (kg/m2) * | 22.5 ± 2.8 | 23.9 ± 3.4 | 23.7 ± 3.47 | 0.22 | 0.76 |
| SBP (mmHg) † | 108.5 (103.5 - 115) | 103.5 (99.3 - 105) | 103.5 (99.3 - 111) | 0.06 | 0.76 |
| DBP (mmHg) * | 72.9 ± 6.3 | 68.5 ± 5 | 70.0 ± 5.9 | 0.02 | 0.55 |
| MAP (mmHg) * | 85.1 ± 6.7 | 80.4 ± 5 | 80.8 ± 6.2 | 0.02 | 0.53 |
| Percent change over first trimester | |||||
| BMI * | 1.6 ± 3.9 | 2.6 ± 2.9 | 1.4 ± 4.4 | 0.44 | 0.37 |
| SBP * | 0.2 ± 7.4 | −1.6 ± 9.1 | −8.6 ± 9.9 | 0.10 | 0.05 |
| DBP * | 6.1 ± 10.9 | −0.8 ± 11 | −1.1 ± 12.7 | 0.19 | 0.66 |
| MAP * | 3.4 ± 8.9 | −1.2 ± 9.5 | −4.8 ± 9.9 | 0.57 | 0.24 |
BMI, body mass index; DBP, diastolic blood pressure; MAP, mean arterial pressure; SBP, systolic blood pressure;
Mean ± SD are reported, and ANOVA or t-test are performed.
Median with interquartile range are reported and Kruskal-Wallis or Mann-Whitney U test is performed.
Systolic, diastolic, and mean arterial blood pressures were not different before conception, but were higher in the first trimester in women with programmed cycle compared to women with natural cycle FETs (Table 2B). Systolic blood pressures before spontaneous conception and the percent change to first trimester systolic blood pressure were higher compared to women with FETs in a natural cycle. There was no difference in diastolic or MAP before and after conception between spontaneous conceptions and FETs in a natural cycle.
Endothelial function
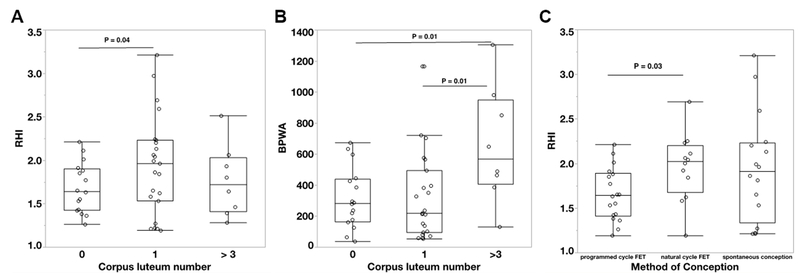
Compared to women with one CL, women who conceived without a CL had impaired endothelial function, reflected in a significantly lower RHI (P=0.04), (Figure 1A: please see http://hyper.ahajournals.org). RHI was also lower in participants with > 3 CL after IVF but not statistically different from participants with 1 CL. There was no difference in AI75 between the CL groups. BPWA was higher in women with >3 CL compared to participants with 0 or 1 (Figure 1B).
Figure 1:
Vascular endothelial function measured as reactive hyperemia index (RHI; A, C) and baseline pulse wave amplitude (BPWA, B) in first trimester comparing different numbers of corpora lutea (0 CL [N=18], 1 CL [N=28] or > 3 CL [N=11]; A&B) and different modes of conception (programmed cycle frozen-thawed embryo transfer [FET, N=18], natural cycle FET [N=12] or spontaneous conception [N=16], C). Box plots represent median, 10th, 25th, 75th and 90th percentile.
Women with suppression of CL formation in a programmed FET had a significantly lower RHI and lnRHI as well as a higher AI75 compared to women who conceived after FET in a natural cycle (all P=0.03), (Figure 1C: please see http://hyper.ahajournals.org). There were no differences in vascular reactivity between participants with spontaneous conceptions and conceptions after natural cycle FETs (Figure 1C: please see http://hyper.ahajournals.org).
Circulating progenitor cell numbers
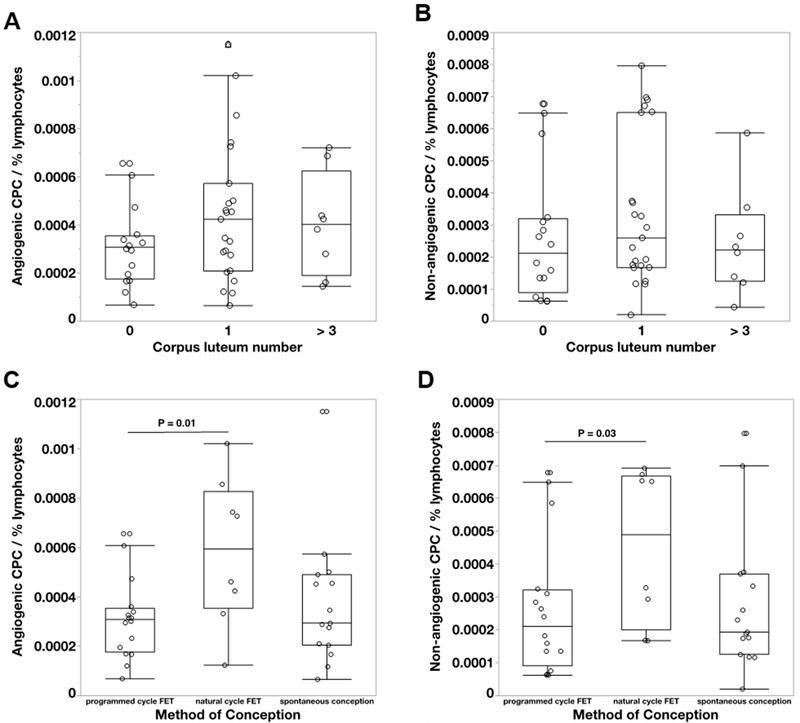
Polychromatic flow cytometry was performed on maternal blood of 45 of the 57 participants. Eleven participants were excluded due to low cell numbers after PBMC isolation, fluorochrome oversaturation, staining problems, or refusal of blood draw. There was a non-significant trend towards lower angiogenic and non-angiogenic CPCs in cycles without a CL compared to conceptions in the presence of 1 CL (P=0.19 and P=0.2, respectively), (Figure 2A and B: please see http://hyper.ahajournals.org). Both angiogenic CPCs (P=0.01) and non-angiogenic CPCs (P=0.03) were significantly lower in programmed FET cycles compared to FETs in a natural cycle (Figure 2C and D: please see http://hyper.ahajournals.org). There was no difference in angiogenic and non-angiogenic CPC numbers between conceptions, which were spontaneous vs in a natural cycle FET (Figure 2C and D: please see http://hyper.ahajournals.org).
Figure 2:
Comparison of angiogenic (A, C) and non-angiogenic (B, D) circulating progenitor cell (CPC) numbers in first trimester of women conceived with different numbers of corpora lutea (0 CL [N=17], 1 CL [N=23] or > 3 CL [N=8]; A&B) and different modes of conception (programmed cycle frozen-thawed embryo transfer [FET, N=17], natural cycle FET [N=8] or spontaneous conception [N=16], C&D). Box plots represent median, 10th, 25th, 75th and 90th percentile.
Lipid and hormone levels
Serum plasma concentrations of lipids (N=56) and pregnancy hormones (N=55) are presented in Table 3A and 3B. Note: one participant refused the blood draw and from a second participant hormone levels are not available. Total cholesterol was higher in the absence compared to the presence of 1 CL (but was not different in FET cycles). Non-HDL and LDL cholesterol were higher in participants with a FET in a natural cycle compared to spontaneous conception. Triglycerides, cholesterol/HDL and LDL/HDL were not different among any groups.
Table 3:
Lipid and hormone characteristics of the study population by corpus luteum (CL) number (A) or mode of conception (B).
| A Corpus luteum number | |||||
|---|---|---|---|---|---|
| Characteristic | 0 CL (n=17) ‡, § | 1 CL (n=28) | > 3 CL (n=10) § | P-value 1 CL vs. 0 CL | P-value 1 CL vs. > 3 CL |
| Total cholesterol (mmol/L) * | 5.1 ± 0.8 | 4.6 ± 0.7 | 4.7 ± 1.1 | 0.04 | 0.79 |
| Triglycerides (mmol/L) † | 0.9 (0.6 – 1.1) | 0.9 (0.6 – 1.2) | 0.9 (0.5 – 1.4) | 0.78 | 0.67 |
| HDL cholesterol (mmol/L) * | 2.2 ± 0.5 | 2.0 ± 0.3 | 1.8 ± 0.3 | 0.20 | 0.13 |
| Non-HDL cholesterol (mmol/L) * | 2.9 ± 0.6 | 2.6 ± 0.6 | 3.0 ± 1.0 | 0.12 | 0.31 |
| LDL cholesterol (mmol/L) * | 2.5 ± 0.5 | 2.1 ± 0.6 | 2.5 ± 0.9 | 0.07 | 0.35 |
| Cholesterol/HDL † | 2.3 (2.1 - 2.9) | 2.3 (2.1 - 2.6) | 2.6 (2.3 t- 3.4) | 0.97 | 0.15 |
| LDL/HDL * | 1.2 ± 0.4 | 1.1 ± 0.4 | 1.4 ± 0.6 | 0.63 | 0.14 |
| Relaxin (pg/mL) † | 13.0 (12.0 - 15.5) | 517.0 (293.6 - 625.2) | 2016.3 (745.2 - 4397.9) | <0.0001 | 0.0003 |
| Estradiol (pmol/L) † | 8869.1 (6114.1 – 11477.4) | 9504.2 (8077.3 – 11826.1) | 7151.1 (5230.4 – 10722.6) | 0.81 | 0.04 |
| Progesterone (nmol/L) † | 108.4 (94.5 – 134.8) | 107.2 (86.5 – 134.8) | 170.7 (110.0 – 190.8) | 0.36 | 0.06 |
| B Mode of conception | |||||
| Characteristic | Programmed cycle FET (n=17) ‡, § | Natural cycle FET (n=12) | Spontaneous conception (n=16) | P-value natural cycle vs. programmed FET | P-value natural cycle FET vs. spontaneous conception |
| Total cholesterol (mg/dl) * | 5.1 ± 0.8 | 4.8 ± 0.8 | 4.4 ± 0.7 | 0.46 | 0.10 |
| Triglycerides (mg/dl) † | 0.9 (0.6 – 1.1) | 0.9 (0.7 – 1.1) | 0.9 (0.6 – 1.3) | 0.95 | 1.0 |
| HDL-cholesterol (mg/dl) * | 2.2 ± 0.5 | 1.9 ± 0.3 | 2.0 ± 0.3 | 0.35 | 0.61 |
| Non-HDL-cholesterol (mg/dl) * | 2.9 ± 0.6 | 2.9 ± 0.6 | 2.4 ± 0.6 | 0.89 | 0.03 |
| LDL-cholesterol (mg/dl) * | 2.5 ± 0.5 | 2.4 ± 0.6 | 1.9 ± 0.5 | 0.84 | 0.01 |
| Cholesterol/HDL * | 2.4 ± 0.5 | 2.5 ± 0.4 | 2.2 ± 0.4 | 0.33 | 0.12 |
| LDL/HDL * | 1.2 ± 0.4 | 1.3 ± 0.3 | 1.1 ± 0.4 | 0.59 | 0.12 |
| Relaxin (pg/ml) † | 13.0 (12.0 - 15.5) | 489.7 (251.5 - 864.2) | 517.0 (293.6 - 607.1) | <0.0001 | 0.98 |
| Estradiol (pmol/L) † | 8869.1 (6114.1 - 11477.4) | 9439.9 (8077.1 - 10774.4) | 9553.8 (8024.8 - 12637.4) | 0.45 | 0.95 |
| Progesterone (nmol/L) † | 108.4 (94.5 - 134.8) | 113.2 (85.5 - 113.2) | 106.4 (88.7 - 133.6) | 0.76 | 0.80 |
Mean values ± SD are reported, and ANOVA or t-test are performed.
Median values with interquartile range are reported and Kruskal-Wallis test or Mann-Whitney U test is performed.
1 patient refused blood draw.
2 patients total without hormone levels
As expected, serum relaxin concentrations were markedly less and at the lower range of detectability in the 0 vs 1 CL and > 3 CL groups and lower in the 1 CL group compared to >3 CL. Relaxin levels were also lower in programmed compared to natural cycle FETs but not different between natural cycle FET and spontaneous conception. Women with >3 CL after IVF had higher progesterone concentrations compared to women that conceived with 0 or 1 CL. Estradiol and progesterone concentrations were not different among FETs in a programmed or natural cycle. These steroid hormone concentrations were similar between spontaneous and natural cycle FET conceptions.
Association between endothelial reactivity and maternal factors
Several maternal factors were assessed for a correlation with endothelial function, including BMI, blood pressure, lipid and hormone concentrations. Relaxin concentrations weakly correlated negatively with AI75 (ρ=−0.35; P=0.009) and the number of angiogenic CPCs (ρ=0.31; P=0.03) in the whole population. A weak negative correlation was detected for RHI and change in diastolic (ρ=−0.32; P=0.02) and mean arterial blood pressure (ρ=−0.29; P=0.03) over the first trimester and a weak positive correlation for BPWA and BMI (ρ=0.28; P=0.04) in the whole population. In participants with FETs, relaxin and estradiol concentrations weakly correlated with RHI (ρ=0.38; P=0.045 and ρ=0.57; P=0.001). In participants with FETs, only relaxin weakly correlated with angiogenic (ρ=0.41; P=0.03) and non-angiogenic CPC numbers (ρ=0.42; P=0.03), and negatively with AI75 (ρ=−0.43; P=0.02). None of the other parameters (lipids, progesterone) showed a significant correlation to endothelial reactivity measures; therefore, these variables were not regarded as potential confounders for the observed differences (please see http://hyper.ahajournals.org).
Summary of results
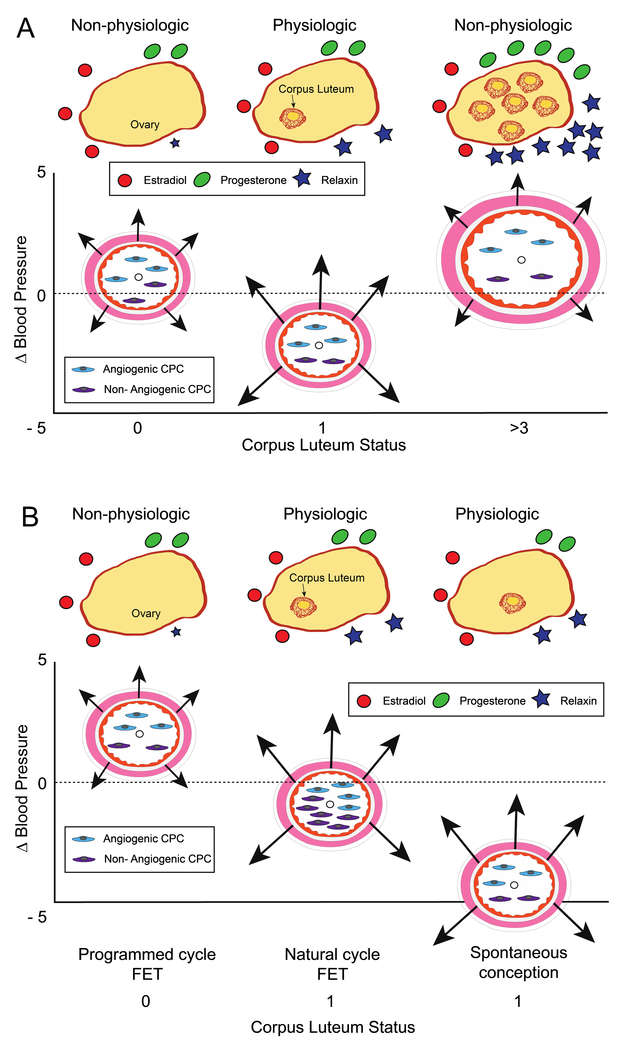
Figure 3 represents a graphical summary of the results of the entire cohort (panel A) including hormone levels (top of panel), changes in mean arterial pressure, vascular reactivity assessment and circulating progenitor cell numbers (bottom of panel). Comparison among programmed FET, natural cycle FET and spontaneous conceptions are presented in panel B.
Figure 3:
Summary of the maternal endocrine milieu in first trimester of pregnancy and pregnancy adaptation and vascular health. (A) Comparison among groups by CL number. CL number within yellow ovary and CL hormonal products depicted by symbols at the top of the figure. Vascular parameters for each CL group represented below. Arteries (pink smooth muscle and orange endothelial lining) are centered on y-axis based on change in MAP at first trimester visit compared to preconception (small black circle in lumen), arrows (length) represent reactive hyperemia index (RHI), a measure of endothelial reactivity, and artery diameters represent baseline pulse wave amplitude (BPWA), a measure of arterial tone. Circulating progenitor cell number represented by cells in lumen. Relaxin, a vasodilator secreted by the corpus luteum (CL), and BPWA increased with CL number. RHI was highest in women with 1 CL. Women with aberrant numbers of CL (0 or > 3) had higher blood pressure levels and lacked the typical drop in mean arterial pressure (y-axis). (B). Comparison between FET groups compared with spontaneous conception to note differences based on absence of CL and /or natural vs IVF embryo. Suppression of CL development (CL=0) in frozen embryo transfer (FET) cycles was associated with a lower RHI compared with FETs in a natural cycle (CL=1), as well as lower numbers of angiogenic and non-angiogenic circulating progenitor cells (CPCs). MAP decline was greater if CL=1 and CPCs were most numerous in the natural cycle FET.
Discussion
In this study, we report differences in vascular health in the first trimester of pregnancy in women with differing numbers of CL and modes of conception. Our results demonstrate that among conceptions achieved with zero or >3 CL, the natural decline of MAP is absent and vascular endothelial function is impaired, albeit in different ways. Women had a lower RHI in the absence of a CL and higher BPWA in the presence of multiple CL. When we compared infertile women with natural cycle FETs to those who conceived spontaneously after a period of infertility (all with one CL), we didn’t find any significant differences in clinical, vascular reactivity or hormonal parameters, suggesting comparable vascular adaptation in early pregnancy with FET in a natural cycle. In contrast, women that conceived after a FET in a programmed cycle (CL=0) compared to in a natural cycle (CL=1), a comparison that controls for any impact from the use of a frozen embryo, blood pressure, vascular reactivity, relaxin levels and circulating progenitor cell numbers were significantly different. These findings imply that the hormonal environment [whether exogenous (programmed cycle treatments) or endogenous (CL number)] and not IVF or cryopreservation has the more significant impact on vascular changes in early pregnancy.
Of note in the FET pregnancies, relaxin concentrations correlated with aberrant vascular reactivity measures suggesting contribution to the changes in cardiovascular adaptation in early pregnancy. One might argue against this conclusion due to the lack of a simple dose response for relaxin, with the > 3 CL group more closely resembling the 0 than the 1 CL group in terms of blood pressure and RHI. However, our observation is consistent with data showing a biphasic effect of relaxin on glomerular filtration rate and effective renal plasma flow in conscious, non-pregnant rats in the context of both too little or too much relaxin 24, 25. Additional supporting data that relaxin does not necessarily follow a “traditional dose response” comes from the work of Sarwar et al. 26. Specifically, relaxin signaling through its receptor dependently increased cAMP and cGMP levels in human umbilical artery vascular smooth muscle cells in a sigmoidal-shaped manner, while cAMP and cGMP signaling to increasing doses of relaxin followed a bell shape curve in human umbilical vein endothelial and vascular smooth muscle cells. Therefore, certain vascular responses to relaxin, or other CL factors, may require an optimal intermediate concentration that results in healthy pregnancy.
Vascular dysfunction predates clinical presentation of preeclampsia and is considered to be part of the pathogenesis. In a recent study MAP measured between 11 and 14 weeks’ gestation was a strong predictor for the development of gestational hypertension and preeclampsia 27. Endothelial dysfunction demonstrated by a lower RHI was also found in a cohort of 105 preeclamptic women compared to 110 normotensive, pregnant controls at 30-32 weeks’ gestation. Several studies assessing flow-mediated dilatation showed worse vascular function already in the second trimester in women who subsequently developed preeclampsia 28, 29. Therefore, in the current study the early pregnancy vascular changes related to CL number may indeed reflect an increased risk of preeclampsia. It will be important for future studies to consider treatment protocols used for FET (e.g., transfer in a natural, ovulatory cycle vs. in an artificial, programmed cycle) when determining risk of preeclampsia.
When comparing programmed (CL=0) vs. natural cycle (CL=1) FETs not only is there a difference in blood pressure but also fewer CPCs, cells thought to be important in maintaining a healthy endothelium. Relaxin, a 6 kD circulating peptide, in animal studies induces systemic and renal vasodilation at least in part due to nitric oxide (NO) production 12, 14, 15. One mechanism of action for higher CPC in the CL 1 group may involve NO release and stimulation of migration of human endothelial progenitor cells 30. Reduced number and functions of CPCs and late-outgrowth endothelial progenitor cells have previously been shown in maternal and cord blood of women with preeclampsia 31–34. This highlights a potential connection between endovascular state and endothelial progenitor cell numbers.
While circulating relaxin is produced predominately, if not exclusively by the CL in human pregnancy, the other CL hormones, estradiol and progesterone, are also produced by the developing placenta. Both steroid hormones are important players in implantation and pregnancy maintenance. Interestingly, in this study progesterone didn’t correlate with any of the vascular or clinical parameters, while estradiol correlated with RHI only in the FET population. Therefore, given the strongest correlations with relaxin, it is more likely a contributing mediator for the differences observed. If relaxin is indeed a major mediator of cardiovascular adaptation and vascular health in early human pregnancy, supplementation of physiologic relaxin levels in fertility treatment protocols, could improve vascular function. Whether this would impact later obstetrical outcomes is not currently known. The current study supports and expands upon the previously articulated theory that, relaxin deficiency in early pregnancy compromises maternal vascular adaptations to pregnancy affecting both renal and placental function 35.
Given the relatively small sample sizes in this prospective cohort study, our findings should be interpreted accordingly. Some true associations may not have reached statistical significance because of lack of power or vice-versa from type 1 error. Also, we were not able to control for potential confounders or perform vascular health assessments before conception and therefore, there is a chance women that conceived with aberrant CL numbers could have been different at baseline. Nevertheless, a major advantage of the study is the design with inclusion of different CL groups and treatment protocols along with the broad registration of clinical characteristics, endothelial evaluation, and hormone patterns. Although a higher incidence of male factor infertility was detected in the groups with multiple CL after IVF and programmed FETs, one would expect overall healthier women with better vascular health in both groups. The opposite was the case, underscoring the observed effects and our findings. In addition, more women took aspirin until 10 weeks of gestation as part of the treatment protocol in those two groups. One would expect a protective effect of aspirin on vascular health given its effective use in the prevention of both cardiovascular disease and preeclampsia 36–38. Again, a positive effect on vascular health couldn’t be confirmed but might be due to the discontinuation of aspirin before the vascular health assessment. While we observed differences in the first trimester these may not persist throughout pregnancy. Further evaluation is required to determine if they reflect a first sign of impaired cardiovascular adaption related to altered CL number, thereby contributing to the increased risk of hypertensive disease of pregnancy. Along with any direct impact on maternal vasculature by the early hormone milieu arising from the CL, there may also be an impact on placentation, that contributes to vascular adaptation and the later development of preeclampsia 35. As this analysis focused on first trimester effects, additional data from second and third trimester pregnancies along with placental biopsies will assist in evaluating these possibilities.
Conclusion
We found that blood pressure, endothelial function, and the number of circulating endothelial progenitor cells were affected in treatment protocols leading to aberrant CL numbers. Specifically, the findings of this study support that conception following a FET in a programmed cycle (CL=0) has a negative influence on maternal vascular health in early pregnancy compared to pregnancies conceived following FET in a natural cycle (CL=1) or spontaneous conception (CL=1).
Perspectives
In light of increasing utilization of FETs predominantly in programmed cycles in the absence of a CL, along with a higher risk for the development of preeclampsia, more studies examining the effect of fertility treatment on maternal physiology in pregnancy are needed. If the findings of our study can be confirmed in larger cohort or randomized control trials, support with CL products such as relaxin, or the transfer of embryos in a natural cycle could improve maternal and pregnancy health.
Supplementary Material
Novelty and Significance.
1. What Is New?
Both a lack of or supraphysiologic numbers of CL at conception are accompanied by subtle yet distinct vascular abnormalities in early pregnancy.
Impaired vascular reactivity, lack of blood pressure declines, and lower CPC numbers are noted in women who had FETs in a programmed cycle compared to a natural cycle.
Spontaneous conceptions and natural cycle FETs have comparable vascular health parameters.
2. What Is Relevant?
Impaired vascular health in early pregnancy, as observed in women with a lack of CL at conception, might contribute to the risk of preeclampsia later in pregnancy.
Women with FETs in a natural cycle have favorable vascular parameters.
3. Summary
If findings are confirmed, FETs in a natural cycle would be preferred to FETs in a programmed cycle.
We speculate that supplementation of CL products in early pregnancy, e.g. relaxin, might improve vascular adaptation and pregnancy outcome for women undergoing FET in a programmed cycle.
Acknowledgments
The authors would like to thank all participants that enabled the collection of these data, Raquel R. Fleischmann D.V.M. C.C.R.P. for recruiting participants and collecting data, Mariam Stephen for scheduling and coordination of research visits, Angela Chen for performing the EndoPAT measurements, the staff of Stanford Clinical and Transitional Research Unit for sample processing, Dr. Yasser El-Sayed for program support and Elizabeth Seckel for copy editing.
Sources of Funding
This study was funded by P01 HD 065647-01A1 from the National Institute of Child Health and Human Development (VLB, KPC), Stanford Department of Obstetrics and Gynecology Division of Maternal-Fetal Medicine and Obstetrics Research Funds and supported by the Stanford Child Health Research Institute. Frauke von Versen-Höynck is funded by the German Research Foundation (VE490/8-1) and Virginia D. Winn is funded as a Arline and Pete Harman Faculty Scholar through Stanford Child Health Research Institute and a H&H Evergreen Faculty Scholar. The use of REDCap was supported by Stanford CTSA award number UL1 TR001085 from NIH/NCRR.
Footnotes
Conflicts of Interest/Disclosures
KPC discloses use patents for relaxin. All other authors have no conflict of interest to declare.
References
- 1.Bartsch E, Medcalf KE, Park AL, Ray JG. Clinical risk factors for pre-eclampsia determined in early pregnancy: Systematic review and meta-analysis of large cohort studies. Bmj. 2016;353:i1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomopoulos C, Tsioufis C, Michalopoulou H, Makris T, Papademetriou V, Stefanadis C. Assisted reproductive technology and pregnancy-related hypertensive complications: A systematic review. J Hum Hypertens. 2013;27:148–157 [DOI] [PubMed] [Google Scholar]
- 3.Zhu L, Zhang Y, Liu Y, Zhang R, Wu Y, Huang Y, Liu F, Li M, Sun S, Xing L, Zhu Y, Chen Y, Xu L, Zhou L, Huang H, Zhang D. Maternal and live-birth outcomes of pregnancies following assisted reproductive technology: A retrospective cohort study. Scientific reports. 2016;6:35141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sites CK, Wilson D, Barsky M, Bernson D, Bernstein IM, Boulet S, Zhang Y. Embryo cryopreservation and preeclampsia risk. Fertil Steril. 2017;108:784–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Storgaard M, Loft A, Bergh C, Wennerholm UB, Soderstrom-Anttila V, Romundstad LB, Aittomaki K, Oldereid N, Forman J, Pinborg A. Obstetric and neonatal complications in pregnancies conceived after oocyte donation: A systematic review and meta-analysis. Bjog. 2017;124:561–572 [DOI] [PubMed] [Google Scholar]
- 6.Wong KM, van Wely M, Mol F, Repping S, Mastenbroek S. Fresh versus frozen embryo transfers in assisted reproduction. Cochrane Database Syst Rev. 2017;3:Cd011184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Opdahl S, Henningsen AA, Tiitinen A, Bergh C, Pinborg A, Romundstad PR, Wennerholm UB, Gissler M, Skjaerven R, Romundstad LB. Risk of hypertensive disorders in pregnancies following assisted reproductive technology: A cohort study from the conartas group. Hum Reprod. 2015;30:1724–1731 [DOI] [PubMed] [Google Scholar]
- 8.Thomopoulos C, Salamalekis G, Kintis K, Andrianopoulou I, Michalopoulou H, Skalis G, Archontakis S, Argyri O, Tsioufis C, Makris TK, Salamalekis E. Risk of hypertensive disorders in pregnancy following assisted reproductive technology: Overview and meta-analysis. J Clin Hypertens (Greenwich). 2017;19:173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jie Z, Yiling D, Ling Y. Association of assisted reproductive technology with adverse pregnancy outcomes. Iranian journal of reproductive medicine. 2015;13:169–180 [PMC free article] [PubMed] [Google Scholar]
- 10.Conrad KP, Baker VL. Corpus luteal contribution to maternal pregnancy physiology and outcomes in assisted reproductive technologies. Am J Physiol Regul Integr Comp Physiol. 2013;304:R69–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi Y, Sun Y, Hao C, Zhang H, Wei D, Zhang Y, Zhu Y, Deng X, Qi X, Li H, Ma X, Ren H, Wang Y, Zhang D, Wang B, Liu F, Wu Q, Wang Z, Bai H, Li Y, Zhou Y, Sun M, Liu H, Li J, Zhang L, Chen X, Zhang S, Sun X, Legro RS, Chen ZJ. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med. 2018;378:126–136 [DOI] [PubMed] [Google Scholar]
- 12.Debrah DO, Novak J, Matthews JE, Ramirez RJ, Shroff SG, Conrad KP. Relaxin is essential for systemic vasodilation and increased global arterial compliance during early pregnancy in conscious rats. Endocrinology. 2006;147:5126–5131 [DOI] [PubMed] [Google Scholar]
- 13.Novak J, Danielson LA, Kerchner LJ, Sherwood OD, Ramirez RJ, Moalli PA, Conrad KP. Relaxin is essential for renal vasodilation during pregnancy in conscious rats. J Clin Invest. 2001;107:1469–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conrad KP. Emerging role of relaxin in the maternal adaptations to normal pregnancy: Implications for preeclampsia. Semin Nephrol. 2011;31:15–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith MC, Murdoch AP, Danielson LA, Conrad KP, Davison JM. Relaxin has a role in establishing a renal response in pregnancy. Fertil Steril. 2006;86:253–255 [DOI] [PubMed] [Google Scholar]
- 16.Chapman AB, Abraham WT, Zamudio S, Coffin C, Merouani A, Young D, Johnson A, Osorio F, Goldberg C, Moore LG, Dahms T, Schrier RW. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 1998;54:2056–2063 [DOI] [PubMed] [Google Scholar]
- 17.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr., Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141 [DOI] [PubMed] [Google Scholar]
- 18.Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the framingham heart study. Circulation. 2008;117:2467–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kvehaugen AS, Dechend R, Ramstad HB, Troisi R, Fugelseth D, Staff AC. Endothelial function and circulating biomarkers are disturbed in women and children after preeclampsia. Hypertension. 2011;58:63–69 [DOI] [PubMed] [Google Scholar]
- 20.Selamet Tierney ES, Newburger JW, Gauvreau K, Geva J, Coogan E, Colan SD, de Ferranti SD. Endothelial pulse amplitude testing: Feasibility and reproducibility in adolescents. J Pediatr. 2009;154:901–905 [DOI] [PubMed] [Google Scholar]
- 21.Grundmann M, Haidar M, Placzko S, Niendorf R, Darashchonak N, Hubel CA, von Versen-Höynck F. Vitamin d improves the angiogenic properties of endothelial progenitor cells. Am J Physiol Cell Physiol. 2012;303:C954–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Estes ML, Mund JA, Ingram DA, Case J. Identification of endothelial cells and progenitor cell subsets in human peripheral blood. Curr Protoc Cytom. 2010;Chapter 9:Unit 9 33 31–11 [DOI] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (redcap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danielson LA, Conrad KP. Time course and dose response of relaxin-mediated renal vasodilation, hyperfiltration, and changes in plasma osmolality in conscious rats. Journal of applied physiology (Bethesda, Md. : 1985). 2003;95:1509–1514 [DOI] [PubMed] [Google Scholar]
- 25.Debrah DO, Conrad KP, Danielson LA, Shroff SG. Effects of relaxin on systemic arterial hemodynamics and mechanical properties in conscious rats: Sex dependency and dose response. Journal of applied physiology (Bethesda, Md. : 1985). 2005;98:1013–1020 [DOI] [PubMed] [Google Scholar]
- 26.Sarwar M, Samuel CS, Bathgate RA, Stewart DR, Summers RJ. Serelaxin-mediated signal transduction in human vascular cells: Bell-shaped concentration-response curves reflect differential coupling to g proteins. Br J Pharmacol. 2015;172:1005–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gasse C, Boutin A, Cote M, Chaillet N, Bujold E, Demers S. First-trimester mean arterial blood pressure and the risk of preeclampsia: The great obstetrical syndromes (gos) study. Pregnancy Hypertens. 2018;12:178–182 [DOI] [PubMed] [Google Scholar]
- 28.Meeme A, Buga GA, Mammen M, Namugowa A. Endothelial dysfunction and arterial stiffness in pre-eclampsia demonstrated by the endopat method. Cardiovascular journal of Africa. 2017;28:23–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weissgerber TL, Milic NM, Milin-Lazovic JS, Garovic VD. Impaired flow-mediated dilation before, during, and after preeclampsia: A systematic review and meta-analysis. Hypertension. 2016;67:415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Segal MS, Sautina L, Li S, Diao Y, Agoulnik AI, Kielczewski J, McGuane JT, Grant MB, Conrad KP. Relaxin increases human endothelial progenitor cell no and migration and vasculogenesis in mice. Blood. 2012;119:629–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Versen-Höynck F, Brodowski L, Dechend R, Myerski AC, Hubel CA. Vitamin d antagonizes negative effects of preeclampsia on fetal endothelial colony forming cell number and function. PLoS One. 2014;9:e98990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munoz-Hernandez R, Miranda ML, Stiefel P, Lin RZ, Praena-Fernandez JM, Dominguez-Simeon MJ, Villar J, Moreno-Luna R, Melero-Martin JM. Decreased level of cord blood circulating endothelial colony-forming cells in preeclampsia. Hypertension. 2014;64:165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gumina DL, Black CP, Balasubramaniam V, Winn VD, Baker CD. Umbilical cord blood circulating progenitor cells and endothelial colony-forming cells are decreased in preeclampsia. Reprod Sci. 2017;24:1088–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luppi P, Powers RW, Verma V, Edmunds L, Plymire D, Hubel CA. Maternal circulating cd34+vegfr-2+ and cd133+vegfr-2+ progenitor cells increase during normal pregnancy but are reduced in women with preeclampsia. Reprod Sci. 2010;17:643–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conrad KP. G-protein-coupled receptors as potential drug candidates in preeclampsia: Targeting the relaxin/insulin-like family peptide receptor 1 for treatment and prevention. Hum Reprod Update. 2016;22:647–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: Systematic review and metaanalysis. Am J Obstet Gynecol. 2017;218:287–293.e281 [DOI] [PubMed] [Google Scholar]
- 37.Raju N, Sobieraj-Teague M, Bosch J, Eikelboom JW. Updated meta-analysis of aspirin in primary prevention of cardiovascular disease. Am J Med. 2016;129:e35–36 [DOI] [PubMed] [Google Scholar]
- 38.Poon LC, Wright D, Rolnik DL, Syngelaki A, Delgado JL, Tsokaki T, Leipold G, Akolekar R, Shearing S, De Stefani L, Jani JC, Plasencia W, Evangelinakis N, Gonzalez-Vanegas O, Persico N, Nicolaides KH. Aspirin for evidence-based preeclampsia prevention trial: Effect of aspirin in prevention of preterm preeclampsia in subgroups of women according to their characteristics and medical and obstetrical history. Am J Obstet Gynecol. 2017;217:585e581–585.e585 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.