Abstract
BACKGROUND
Thoracic aortic aneurysms progressively enlarge and predispose to acute aortic dissections. Up to 25% of individuals with thoracic aortic disease harbor an underlying Mendelian pathogenic variant. An evidence-based strategy for selection of genes to test in hereditary thoracic aortic aneurysm and dissection (HTAAD) helps inform family screening and intervention to prevent life-threatening thoracic aortic events.
OBJECTIVES
The purpose of this study was to accurately identify genes that predispose to HTAAD using the Clinical Genome Resource (ClinGen) framework.
METHODS
We applied the semiquantitative ClinGen framework to assess presumed gene-disease relationships between 53 candidate genes and HTAAD. Genes were classified as causative for HTAAD if they were associated with isolated thoracic aortic disease and were clinically actionable, triggering routine aortic surveillance, intervention, and family cascade screening. All gene-disease assertions were evaluated by a pre-defined curator-expert pair and subsequently discussed with an expert panel.
RESULTS
Genes were classified based on the strength of association with HTAAD into 5 categories: definitive (n = 9), strong (n = 2), moderate (n = 4), limited (n = 15), and no reported evidence (n = 23). They were further categorized by severity of associated aortic disease and risk of progression. Eleven genes in the definitive and strong groups were designated as “HTAAD genes” (category A). Eight genes were classified as unlikely to be progressive (category B) and 4 as low risk (category C). The remaining genes were recent genes with an uncertain classification or genes with no evidence of association with HTAAD.
CONCLUSIONS
The ClinGen framework is useful to semiquantitatively assess the strength of gene-disease relationships for HTAAD. Gene categories resulting from the curation may inform clinical laboratories in the development, interpretation, and subsequent clinical implications of genetic testing for patients with aortic disease.
Keywords: ClinGen, gene curation, gene-disease relationship, thoracic aortic aneurysm, thoracic aortic dissection
Thoracic aortic aneurysms asymptomatically enlarge over time and can result in acute aortic dissections (collectively designated as TAAD), which are life-threatening events that can cause premature death in ≥50% of affected individuals. Over the last 25 years, pathogenic variants in numerous genes have been identified that predispose to heritable presentations of thoracic aortic aneurysms and dissections, collectively termed HTAAD. HTAAD includes a clinically and genetically heterogeneous group of disorders. Identification of the underlying gene triggering HTAAD is powerful information that can be used not only to identify family members at risk for the disease, but also to inform thoracic aortic disease surveillance and management, including timing of surgical repair, risk for additional vascular diseases, and systemic complications. Additionally, disease gene discovery for HTAAD continues to improve our understanding of the underlying pathophysiology of the disease and aid in the development of new treatment strategies (1–3).
Improved technology and decreased costs for DNA sequencing have increased the use of genetic testing for HTAAD. With the increasing number of putative disease-associated genes, DNA diagnostic laboratories have steadily expanded the number of genes that are either on disease-specific gene panels or analyzed and reported from exome or genome sequencing. This rapid expansion of sequencing, however, has risked the inclusion of genes that may have less meaningful evidence to support their association with HTAAD. This in turn leads to a high burden of uncertainty, as the vast majority of variants in these genes would be properly classified as variants of uncertain significance (VUS), and therefore cannot meaningfully inform the management of patients or their families. Although highly gene-dependent, the overall data on the relative proportion of VUS compared with pathogenic variants in disease genes is almost 3:1 (4). Understanding the importance of these variants in the pathogenesis of HTAAD is hampered by a lack of validated functional tools and the limited number of rigorously interpreted variants in disease-associated genes in publicly available databases.
When selecting genes for inclusion on diagnostic panels or reporting from exome/genome sequencing, the clinical validity (i.e., the strength of evidence that variation in that gene predisposes to the disease) needs to be carefully considered. For many of the genes offered on diagnostic panels, the association of rare variants in the gene with the disease is unequivocal. For example, FBN1 is well established as the causal gene in individuals with Marfan syndrome, with occasional but well-established reports of thoracic aortic disease with subtle or absent manifestations of a systemic connective tissue disorder (5). In other cases, the strength of the data underlying the association between a given gene and thoracic aortic disease is limited, and further research is required to confirm or exclude a clinically relevant association.
There are also important questions about the clinical implications of pathogenic variants in certain genes. Some genes are associated with borderline enlargement of the aortic root or ascending aorta but without formal evidence that the enlargement progresses to dissection. An example is the FBN2 gene, underlying congenital contractural arachnodactyly. Mild, nonprogressive aortic dilatation has been reported in children harboring FBN2 pathogenic variants (6); a recent follow-up study did not reveal any evidence for progression to aortic dissection (B. Callewaert, personal communication, 2018). In contrast, other genes are associated with aortic dissection despite little to no preceding enlargement of the aorta; this is very well known for patients harboring COL3A1 pathogenic variants, underlying vascular Ehlers-Danlos syndrome (7).
We assembled an international panel of experts for the Aortopathy Working Group to curate a list of genes with putative association to HTAAD using a semiquantitative framework provided by the Clinical Genome Resource (ClinGen) (8). In this framework, the genes are classified into pre-specified tiers based on the clinical, genetic, and experimental evidence, along with discussion and consensus of the experts. These clinically validated genes can be used to prioritize genes for research, inform which genes should be included in aortopathy disease panels and results returned from exome/genome sequencing, and provide guidance for which genes to exclude from routine clinical reporting. For these purposes, HTAAD was defined as either thoracic aortic enlargement with evidence of progression to the life-threatening complication of acute aortic dissection or thoracic aortic dissection in the absence of aneurysm formation. The term “heritable” covers both isolated (but genetic and hence “heritable”) and familial cases of HTAAD. We also curated evidence for genes associated with thoracic aortic aneurysm with minimal evidence of progression to dissection, placing these in a separate category. For HTAAD, the underlying altered gene triggering disease can be used to identify family members at risk and inform clinical management. Thus, validating the HTAAD genes and pathogenic variants is critical to achieve the basic tenets of precision medicine for thoracic aortic disease.
METHODS
THE AORTOPATHY GROUP STRUCTURE AND WORKFLOW.
This work has been performed within the gene-disease validity framework of ClinGen (8). Launched in 2013 and supported by the National Institutes of Health, ClinGen is developing authoritative central resources that define the clinical relevance of genes and variants for use in precision medicine and research. The structure and main aims of ClinGen have been reported by Rehm et al. (4). Within the structure of ClinGen, several disease-specific Clinical Domain Working Groups are defined, and the aortopathy group was installed in 2015 as part of the Cardiovascular Disease Working Group. One of the goals of ClinGen is to implement evidence-based expert consensus for curating genes and for this purpose the ClinGen Gene Curation Working Group has developed a method that: 1) qualitatively defines gene-disease validity using a classification scheme based on the strength of evidence supporting the relationship; and 2) provides a standardized semiquantitative approach to evaluate available evidence and arrive at such a classification (8). Relevant clinical, genetic, and experimental evidence supporting or contradicting a gene-disease relationship is evaluated semiquantitatively by assigned biocurators, resulting in classifying the gene-disease pair into qualitative categories (“definitive,” “strong,” “moderate,” “limited,” “no reported evidence,” or “conflicting evidence”). Notably, experimental data alone cannot justify a clinical validity classification beyond “no reported evidence,” because at least 1 human variant in the gene with a plausible causal association must be present to attain “limited” classification. In a next step, classifications are reviewed by the disease experts who confirm or adjust the assignment based on clinical and scientific expertise.
For the Aortopathy Working Group, the 4 Cardiovascular Domain working group members (J.D.B., H.D., B.L., and D.M.) recruited 14 clinical domain experts in 5 different countries from academic centers with a major interest in genetic aortic disease. The group consisted of a mixture of clinical experts and molecular geneticists (Online Figure 1).
Three ClinGen biocurators and 1 domain expert curator (C.F.) were assigned to the group. A curator and 1 to 2 members of the Aortopathy Working group were assigned randomly for every gene curated. The matrix provided as an Excel sheet (Microsoft, Redmond, Washington) by ClinGen was used by the curators. This matrix has recently been structured into a web-based interface that can be used more easily, and the aortopathy curation data have been entered here. To verify consistency between the biocurators, the 5 initial genes on the list were done in duplicate (R.G. and C.F.). Biocurators applied the aforementioned rules for “clinical validity” using the matrices provided by ClinGen to assert the gene-disease association, and this information was then provided to the expert (both the extended Excel sheets as well as the summary matrix). The score obtained by the curators was considered provisional and could be modified by consensus of the clinical domain experts. Aortopathy Working Group monthly calls were organized to review the list of curated genes, come to a consensus on each individual score, and define discussion items.
SELECTION OF GENES TO BE CURATED.
The genes were selected based on the published data and genes tested on clinical aortopathy gene panels that are currently available. Two reviews formed the basis of the initial gene list (3,9). Six additional genes were added based on newly published data (B3GAT3, BGN, EMILIN1, FOXE3, HCN4, and SMAD2) (10–15), and another 7 were added because they are offered on diagnostic panels for aortic disease (COL9A1, COL9A2, COL11A1, COL18A1, MED12, UPF3B, and VCAN), providing a final list of 53 genes (Table 1 and Online Table 1). The majority of the curated genes are inherited in an autosomal dominant manner (n = 37). In addition, 4 genes with X-linked recessive inheritance (COL4A5, FLNA, MED12, and UPF3B), 1 with X-linked dominant inheritance (BGN), and 11 genes with autosomal recessive inheritance (ADAMTS10, B3GAT3, CBS, COL9A1, COL9A2, COL18A1, EFEMP2, GJA1, PLOD1, PLOD3, and SLC2A10) have been identified.
TABLE 1.
List of Category A Genes
| HGNC Gene Symbol |
Phenotype |
Aortic Disease Risk as Reported in the Published Data |
||
|---|---|---|---|---|
| OMIM ID | ORPHA ID | Reason for Listing | ||
| ACTA2 | Aortic aneurysm familial thoracic, 6 (#611788) | Familial thoracic aneurysm and dissection (91387); rare disease with thoracic aortic aneurysm and aortic dissection (285014) | Baseline publication | Multiple reported cases of TAA and AD |
| COL3A1 | Ehlers-Danlos syndrome type IV (#130050) | Ehlers-Danlos syndrome, vascular type (286); rare disease with thoracic aortic aneurysm and aortic dissection (285014) | Baseline publication | Multiple reported cases of AD |
| FBN1 | Marfan syndrome (#154700) | Familial thoracic aneurysm and dissection (91387); Marfan syndrome type 1 (284963); rare disease with thoracic aortic aneurysm and aortic dissection (285014) | Baseline publication | Multiple reported cases of TAA and AD |
| MYH11 | Aortic Aneurysm familial thoracic, 4 (#132900) | Familial thoracic aneurysm and dissection (91387); rare disease with thoracic aortic aneurysm and aortic dissection (285014) | Baseline publication | Multiple reported cases of TAA and AD |
| SMAD3 | Loeys-Dietz syndrome 3 (#613795) | Familial thoracic aneurysm and dissection (91387); aneurysm-osteoarthritis syndrome (284984) | Baseline publication | Multiple reported cases of TAA and AD |
| TGFB2 | Loeys-Dietz syndrome 4 (#614816) | Familial thoracic aneurysm and dissection (91387); rare disease with thoracic aortic aneurysm and aortic dissection (285014) | Baseline publication | Multiple reported cases of TAA and AD |
| TGFBR1 | Loeys-Dietz syndrome 1 (#609192) | Familial thoracic aneurysm and dissection (91387); rare disease with thoracic aortic aneurysm and aortic dissection (285014); Loeys-Dietz syndrome (60030) | Baseline publication | Multiple reported cases of TAA and AD |
| TGTBR2 | Loeys-Dietz syndrome 2 (#610168) | Familial thoracic aneurysm and dissection (91387); rare disease with thoracic aortic aneurysm and aortic dissection (285014); Loeys-Dietz syndrome (60030); Marfan syndrome type 2 (284973) | Baseline publication | Multiple reported cases of TAA and AD |
| MYLK | Aortic aneurysm familial thoracic, 7 (#613780) | Familial thoracic aneurysm and dissection (91387); rare disease with thoracic aortic aneurysm and aortic dissection (285014) | Baseline publication | Multiple reported cases of AD |
| LOX | Aortic aneurysm familial thoracic, 10 (#617168) | Familial thoracic aneurysm and dissection (91387); rare disease with thoracic aortic aneurysm and aortic dissection (285014) | Baseline publication | Multiple reported cases of TAA and AD |
| PRKG1 | Aortic aneurysm familial thoracic, 8 (#615436) | Familial thoracic aneurysm and dissection (91387); rare disease with thoracic aortic aneurysm and aortic dissection (285014) | Baseline publication | Multiple reported cases of TAA and AD |
Strong and definitive genes with their respective OMIM and ORPHA disease codes (if available), the reason for listing them, and relevant additional comments justifying their final categorization (listed in the rightmost column in italics).
AD = aortic dissection; TAA = thoracic aortic aneurysm.
DEFINING THE CLINICAL PHENOTYPE FOR CURATED HTAAD GENES.
Several factors were considered in defining the clinical phenotype associated with HTAAD.
First, HTAAD can occur either with or without additional systemic features (also termed syndromic and nonsyndromic, respectively). Although this curation effort was primarily intended to evaluate the gene-disease association for isolated TAAD, in practice, it proved very difficult in certain cases to separate the syndromic from the nonsyndromic presentations. To maximize the clinical utility of our findings, we took the pragmatic approach to include in our curation any probands in whom the presence of TAAD had been a key part of the presentation and had triggered genetic testing. For many HTAAD genes, the presence and severity of syndromic features predicts earlier onset of vascular manifestations, and later onset aortic disease can be associated with fewer or no syndromic features, in which case TAAD represents the key to the diagnosis. There are several examples of the spectrum of disease manifestations within 1 gene, for example, for the transforming growth factor-beta receptor (TGFBR)−1 and −2 genes as demonstrated by Teixido-Tura et al. (16). The authors nicely confirm the relationship between the craniofacial severity index and the age at the initial arterial event as had already been shown by Loeys et al. (17). Teixido-Tura et al. (16) also demonstrate that some mutation carriers have isolated aortic disease or do not even present any disease manifestation beyond the age of 60 years. Additional evidence supporting the relationship between syndromic features and severity of aortic disease was provided by Jondeau et al. (18).
Second, again aware of the need to maximize clinical utility of our findings, we specified that the genes designated as HTAAD genes needed evidence in the published data to support their clinical actionability. In other words, the associated thoracic aortic disease had to be clinically significant in terms of progressive aortic enlargement and/or dissection to trigger routine aortic imaging, medical and surgical management to prevent aortic dissections or other vascular catastrophies, and predictive genetic testing of at-risk family members.
Third, an additional difficulty was the absence of a validated code available for HTAAD in authoritative databases (neither in Orphanet nor in OMIM). To overcome this, lumping of different phenotypes was necessary in some cases to enable the curation work. The entities that were evaluated and the equivalent terms in OMIM and Orphanet are listed in Table 1 and Online Table 1. As the primary aim of the clinical validation of HTAAD genes was to confirm the association between specific genes and thoracic aortic disease, and not to focus on the association of genes with full syndromes, search terms used by the curators were strictly defined: “aortic dissection” OR “aortic aneurysm” OR “aortic dilatation” OR “aortic dilation” OR “aorta” OR “aortic.”
In addition to the standard ClinGen curation, and based on the considerations discussed in the previous text, we grouped genes into 4 distinct categories. The first category (categories A1 and A2) comprises genes that scored “definite” or “strong” after curation and are designated as “HTAAD genes.” The distinction between A1 and A2 is based on whether variants in the gene primarily cause isolated TAAD or whether some presentations are likely to include syndromic features. A second category (category B) comprises genes for which thoracic aortic enlargement can occur, but there is no evidence that the aortic enlargement progresses to dissection. The third category (category C) includes genes that cause conditions that are primarily diagnosed based on nonvascular features and have a low risk for thoracic aortic disease—in other words, variants in these genes might be considered to be “risk alleles” for thoracic aortic disease. The fourth category (category D) includes those genes for which some experimental data may suggest a link with thoracic aortic disease, but no clinical evidence is available.
GENETIC DATA.
To assess clinical validity, the ClinGen matrix was used (8). Points were attributed to causal genetic variants identified in the probands reported in the published data that fulfilled the clinical criteria as defined in the previous text. VUS were not considered. Strength of the genetic evidence was a function of mutation type, inheritance pattern, and segregation data.
EXPERIMENTAL/FUNCTIONAL DATA.
The ClinGen matrix for the assessment of functional data was applied for each gene. According to this matrix, parameters of “protein function,” “functional alteration,” and “models and rescue” were systematically scored. Protein function includes data on the interaction of the protein of interest with proteins known to be involved in the disease, expression in relevant tissues or altered expression in tissues from affected individuals, and biochemical function of the corresponding protein. The data used in this framework are consistent with those proposed by MacArthur et al. (19) to implicate a gene in disease.
The following issues arose and are specific to the functional data in the aortopathy curation process: 1) biocurators only included syndromes as additional search terms for the experimental data if thoracic aortic disease was 1 of the hallmarks of the syndrome; and 2) because TGF-β signaling is highly nonspecific and is activated in various processes, experts agreed that increased TGF-β signaling in aortic tissue was not sufficient as the sole functional evidence for the gene-disease association, except when the primary hit affects a gene in the canonical TGF-β signaling cascade.
Additional critical issues that were considered by the group when assessing evaluating animal models of disease included the following: 1) the influence of the genetic background of the mouse model on the disease phenotype; 2) incomplete description of possible aortic phenotype (not all tissues or organ systems are investigated for all animal models); 3) representative mutation type and mechanism (i.e., knockout animal models may not reflect the phenotype of missense mutations); 4) mouse models were only included if the mice demonstrated a thoracic aortic aneurysm phenotype (peripheral artery disease models and embryonic lethal models were excluded); and 5) morpholino-induced knockdown zebrafish model studies were only included when appropriate control studies were performed (rescue experiments, 2 morpholinos with different targets resulting in a same, specific phenotype [e.g., blocking translation or splicing], and sufficient controls were performed).
SCORING.
The sum of the genetic and experimental scores determined the classification of the gene, as per the ClinGen framework. Genes with a score of 1 to 7, 8 to 12, and 13 to 18 were classified as “limited,” “moderate,” and “strong,” respectively. A gene can only be assigned a “definitive” association with the disease when it is classified as strong and it is replicated over time (≥2 publications with convincing evidence in ≥3 years). Genes with low scores that are exclusively based on functional/experimental data with no evidence for a clinical association with the disease were categorized in the “no reported evidence” group.
RESULTS
A total of 53 HTAAD gene-disease pairs were curated by the randomly assigned curator-expert teams. An overview of the genes with their respective scores and curation outcome is provided in Online Table 2. A detailed list of all scoring matrices of the 53 curated genes is provided in Online Table 3. The scoring obtained by the biocurators remained largely unchanged after review by the experts on the Aortopathy Working Group for 48 of the 53 genes (90.5%). However, a change in final classification was obtained for 5 genes: B3GAT3, FOXE3, HCN4, MAT2A, and MYLK. MYLK moved from “moderate” to “strong” after expert review (see the following text). Upon critical review by the experts B3GAT3 changed from the “limited” to the “no evidence” category, because there was no published case with an isolated aortic aneurysmal phenotype. MAT2A, FOXE3, and HCN4 were moved from “moderate” to “limited” after expert review, because the data provided are limited to single supporting publications with few HTAAD families. Given the recent identification of the latter 3 genes, the classification may still change over time. The 90.5% agreement obtained is similar to the outcome of the ClinGen clinical validity group, where expert-curator concordance was obtained in 87.1% of the cases (8).
“HTAAD GENES” (CATEGORIES A1 AND A2: “DEFINITIVE” OR “STRONG” GENE-DISEASE ASSOCIATION).
The following genes were found to be definitively associated with HTAAD and are clinically actionable: ACTA2, COL3A1, FBN1, MYH11, MYLK, SMAD3, TGFB2, TGFBR1, and TGFBR2 (Table 1, Online Table 2). For genes in the A1 category, syndromic and nonsyndromic presentations were lumped for curation. All of these genes were identified more than 3 years ago, and their association with HTAAD is firmly established. Pathogenic variants in PRKG1 and LOX were more recently reported, but significant segregation data (i.e., high LOD score) and mouse models of variants, respectively, are available leading to high scores in the matrix and a strong association with HTAAD. With regard to COL3A1, although thoracic aortic disease is rare in vascular Ehlers-Danlos syndrome caused by pathogenic variants in this gene, affected individuals can present with acute aortic dissections, leading to the classification of COL3A1 in the “A1” category.
“POTENTIALLY DIAGNOSTIC GENES” THAT MAY ALLOW DIAGNOSIS OF THE CAUSE OF THORACIC AORTIC ENLARGEMENT, BUT WHICH ARE PRIMARILY ASSOCIATED WITH OTHER CLINICAL FEATURES AND WHICH DO NOT CARRY SIGNIFICANT RISKS OF PROGRESSION TO AORTIC DISSECTION (CATEGORY B: “MODERATE” OR “LIMITED” GENE-DISEASE ASSOCIATION).
Eight genes scored as moderate (EFEMP2) or limited (ELN, FBN2, FLNA, NOTCH1, SLC2A10, SMAD4, and SKI) for association with HTAAD. These genes are a heterogeneous group for which the evidence was often difficult to assess. Several genes, for example SKI and FLNA, are associated with syndromes in which the presentation is usually dominated by systemic features other than aortic disease. The evidence, therefore, for their association with a presentation of aortic dilatation and/or dissection is often rather lacking. The other common feature of these conditions is that there is no robust evidence of progression to aortic dissection. These genes were grouped in category B (Online Tables 1 and 2).
GENES WITH LIMITED EVIDENCE AS A MENDELIAN CAUSE OF HTAAD WHERE DIAGNOSIS IS PRIMARILY BASED ON NONVASCULAR FEATURES (CATEGORY C: “LIMITED” GENE-DISEASE ASSOCIATION).
COL4A5, CBS, PKD1, and PKD2 scored as limited genes for HTAAD, although well-described for other conditions, and have a very low incidence of thoracic aortic disease. Underlying disease (Alport syndrome for COL4A5 and polycystic kidney disease for PKD1 and −2) is primarily diagnosed based on renal abnormalities (category C) (Online Tables 1 and 2). For CBS, the primary disease (homocystinuria) is often diagnosed at newborn screening or in the pediatric setting due to systemic presentation of connective tissue disease or thromboembolism. The prevalence of TAAD in homocystinuria patients has been reported to be as high as 21% (20); however, there is, as yet, little evidence of a direct genetic cause and there is no evidence available about the progression of this dilatation or incidence of dissection. Variants in these genes may therefore be thought of as “risk alleles” for TAAD, possibly acting via classical cardiovascular risk factors such as hypertension.
NO (CLINICAL) EVIDENCE FOR HTAAD (CATEGORY D).
For 23 genes (ACVRL1, ADAMTS10, B3GAT3, COL1A1, COL1A2, COL4A1, COL5A1, COL5A2, COL9A1, COL9A2, COL11A1, COL18A1, EMILIN1, ENG, GATA5, GJA1, JAG1, MED12, PLOD1, PLOD3, SMAD6, UPF3B, and VCAN), the reported evidence for an association with HTAAD was exclusively based on experimental data with no documented evidence that variants in these genes lead to HTAAD in humans, or there was no evidence at all for an association with HTAAD. B3GAT3 was initially scored “limited” by the biocurator, but was downgraded to the “no evidence” category by the experts after detailed revision of the clinical and functional data provided.
RECENT GENES.
Recently identified genes (BGN, FOXE3, HCN4, MAT2A, MFAP5, SMAD2, and TGFB3) formed a special case. Even where fairly robust clinical data existed, the paucity of publications relating to variants in these genes has not yet allowed us to assign a meaningful curation classification. Under current classification guidelines, these genes achieved at most a limited or moderate score. For these genes, additional evidence (e.g., affected probands, clinical data in support of HTAAD, segregation of variants in HTAAD families, and/or functional studies) for their association (or lack thereof) with HTAAD is likely in the (near) future. We therefore assigned these genes a separate category, pending availability of further data (category “uncertain” in Online Tables 1 and 2).
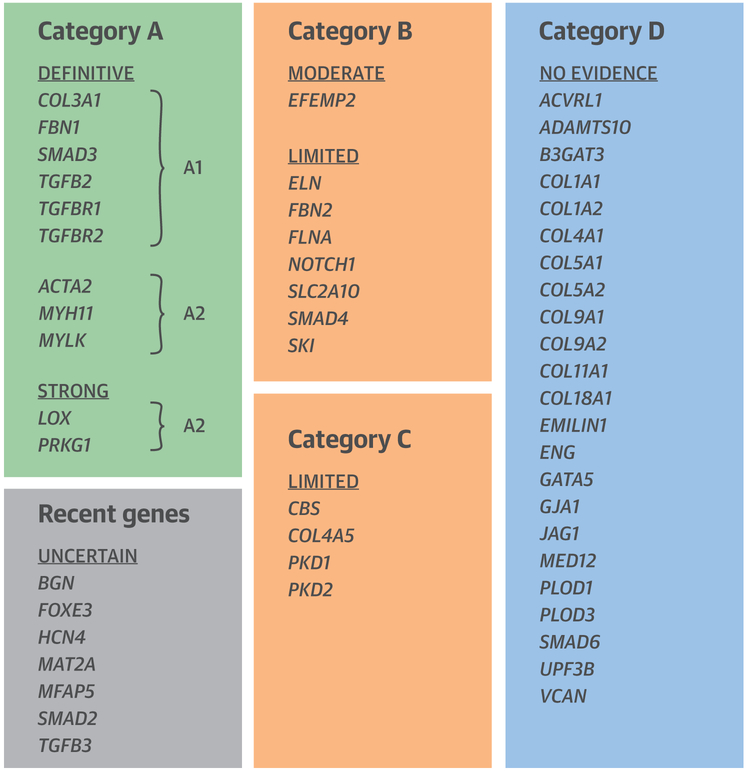
Online Figure 2 provides an overview of the flow of the gene curation process. The Central Illustration provides a schematic overview of the different gene categories.
CENTRAL ILLUSTRATION. Evaluation of the Clinical Validity of Genes for Heritable Thoracic Aortic Aneurysms and Dissections (HTAAD).
Renard, M. et al. J Am Coll Cardiol. 2018;72(6):605–15.
The semi-quantitative ClinGen framework was used to assess presumed gene-disease relationships between 53 candidate genes and HTAAD. Genes were classified based on the strength of association with HTAAD into 5 categories: definitive, strong, moderate, limited, and no reported evidence. They were further categorized by severity of associated aortic disease and risk of progression. Categories A1 and A2 comprise genes designated as “HTAAD genes” in A1 syndromic entities co-occur; these genes scored as “definitive” or “strong” in the curation process. Category B: “Potentially diagnostic genes,” which may allow diagnosis of the cause of thoracic aortic enlargement, but which are primarily associated with other clinical features and which do not carry significant risks of progression to aortic dissection; these genes scored as “moderate” or “limited” in the curation process. Category C: genes with limited evidence as a Mendelian cause of HTAAD where diagnosis is primarily based on nonvascular features; these genes scored as “limited” in the curation process. Category D: genes for which some experimental data may suggest a link with thoracic aortic disease but no clinical evidence is available. “Uncertain” is used for those genes for which the data are recent and preliminary and no accurate categorization is possible at present.
DISCUSSION
There is an increasing imperative to restrict diagnostic genetic testing to genes with sufficient evidence of an association with a specific disease to allow meaningful counseling and clinical decision making (21,22). A huge expansion of scientific published data about thoracic aortic aneurysm and dissection has led to an increase in numbers of putative disease-causing genes reported as part of disease-specific gene panels or from exome/genome sequencing. Although attractive for practical and commercial reasons, the larger gene panels increase the risk for ambiguous and even false-positive reports. A high probability of identifying VUS (21) can cause unnecessary distress for patients and family members and create diagnostic confusion, misallocation of resources for follow-up, and even unjustified genetic discrimination (23). The members of this working group believe that the quality of a diagnostic panel should be judged on the validity of the genes included in the test and the clinical value of the result, rather than simply the number of genes.
Clinical validity for a gene is defined as the accuracy for which identifying a pathogenic variant in the gene confers a predisposition for, or a diagnosis of, a specific clinical condition (24). Assessing clinical validity necessitates establishing standards based on the level of evidence supporting a causal relationship of a gene to a specific disease. Providing a tool for semiquantitative assessment of clinical validity of gene-disease pairs is one of the major objectives of ClinGen (8), and we have used this framework in the setting of thoracic aortic disease.
Based on the scores obtained with the curation and on careful clinical judgment of the aortic risk for each gene, we categorized 53 putative HTAAD genes into 4 major groups. Genes harboring pathogenic variants in the genes in category A, “HTAAD genes” scored “definitive” or “strong” using the framework and should trigger aortic imaging, medical therapy, and screening of family members for the variant with the goal of preventing acute aortic dissections. Variants in these genes, therefore, have a high diagnostic and clinical impact. Genes with moderate or limited association with HTAAD, in category B, could be sequenced for diagnosis in patients presenting clinical features matching a primary syndrome association. However, the diagnostic yield is likely to be low for patients presenting with isolated TAAD and no other suggestive clinical features. Identification of pathogenic variants in these category B genes, although potentially placing the patient into a lower-risk category for aortic events, will also have a less clear direct clinical impact in terms of informing the frequency of follow-up or timing of intervention than those in category A genes. Variants in category C genes should only be reported where there is a clinical suspicion of the primary renal disease association (for PKD1, PKD2, and COL4A5) or hyper-homocystinuria (for CBS). In terms of thoracic aortic disease, variants in these genes should be regarded as risk alleles and are not clinically actionable in isolation—and therefore, are not considered useful for reporting.
Seven genes recently reported as associated with thoracic aortic disease (BGN, FOXE3, HCN4, MAT2A, MFAP5, SMAD2, and TGFB3) currently have insufficient evidence to support a definitive association, as they are penalized by our scoring system on the basis of only having 1 or 2 supporting publications. Placement of these genes in the “limited” or “moderate” categories in which they fall under present classification may not adequately reflect their role in HTAAD, as these gene-disease associations are dynamic and may change over time—in both directions— with new evidence emerging. We therefore recommend continuing screening of these genes for pathogenic variants in “unsolved” thoracic aortic aneurysmal disease patients and on a research basis, and to monitor the scientific published data and open-access resources for further confirmation that these genes fulfill the ClinGen criteria to cause HTAAD. Input of newly identified variants in open-access interfaces such as ClinVar should be encouraged, and in this respect, continued funding of the ClinGen consortium is essential for proper and rigorous translation of genetic data into clinical care. Final classification will require further reports of segregation of variants in families and reports of additional affected individuals. Noteworthy, when screening of these genes results in the identification of a variant, a diagnostic report should only be provided to patients when the variant has been evaluated by (an) expert(s) in the field and is considered (likely) pathogenic according to the experts and American College of Medical Genetics and Genomics guidelines for variant classification. Variants that have not been assessed in detail or that are of uncertain significance should not be reported to the patient. Careful clinical follow-up and professional genetic counseling is, however, strongly recommended.
During curation, we also had to consider the type of variant and its location in the HTAAD genes, as in some cases, this is key to interpreting pathogenicity (Table 2). For example, the vast majority of pathogenic mutations in TGFBR1 and TGFBR2 are missense variants in the intracellular serine-threonine kinase domain of the receptors affecting their kinase activity (18) (B. Loeys and H. Dietz, personal communication, 2017). Care was taken to only score probands as having pathogenic variants based on the type and location of the variant and the established mechanisms of pathogenicity. Further work is planned to assist with the clinical interpretation of individual variants in the HTAAD genes. Notably, there is no correlation between the type of variant and its location in the HTAAD genes and syndromic versus nonsyndromic presentations of the disease. Two notable exceptions are: 1) the specific and recurrent Arg179 ACTA2 alteration causing smooth muscle dysfunction syndrome (25); and 2) the known association of so called “neonatal Marfan syndrome” with pathogenic variants located in the middle part of the FBN1 gene (sometimes called the neonatal region, exons 25 to 33). It is important to note, however, that: 1) pathogenic variants in the neonatal region of FBN1 also cause classical forms of the disease; and 2) VUS have been found within this region in HTAAD patients (5).
TABLE 2.
Type and Location of Pathogenic Variants in HTAAD Genes
| Gene | Pathogenic Variant Type | Protein Domain | Ref. # |
|---|---|---|---|
| ACT2 | Majority are missense variants | (26) | |
| COL3A1 | Two-thirds of pathogenic variants are Gly substitutions (Gly-X-Y) | Triple helical domain | (27) |
| FBN1 | DN and haploinsufficient variants | (5) | |
| LOX | Nonsense and missense variants with (predicted) LOF | Missense mutations are localized in conserved LOX catalytic domain | (28) |
| MYH11 | DN missense mutations and in-frame deletions | C-terminal coiled coil domain | (29) |
| MYLK | Nonsense and missense variants with (predicted) LOF | Kinase and calmodulin-binding domains | (30) |
| PRKG1 | Single gain-of-function variant c.530G>A;p (Arg177Gln) | cGMP-binding domain | (31) |
| SMAD3 | Nonsense and missense variants with (predicted) LOF | Majority in MH2 domain | (32) |
| TGFB2 | Nonsense and missense variants with (predicted) LOF | (33,34) | |
| TGFBR1 | Majority are missense variants that decrease kinase activity | Intracellular serine-threonine kinase domain | (35) |
| TGFBR2 | Majority are missense variants that decrease kinase activity | Intracellular serine-threonine kinase domain | (35) |
For those genes categorized in the definitive/strong groups (heritable thoracic aortic aneurysm and dissection [HTAAD] genes), relevant characteristics of pathogenic variants and their corresponding location in the protein are listed.
DN = dominant negative; LOF = loss of function.
Another of our key challenges was to establish a pragmatic but robust and replicable approach to deal with syndromic versus nonsyndromic presentations of thoracic aortic disease. We recognized that from a pragmatic point of view, syndromic presentations are not always easy to dissociate from isolated presentations of thoracic aortic disease. As with many genetic disorders, syndromic features may or may not be associated with pathogenic variants in predisposing genes. To that end, we included in our curation any patient where thoracic aortic aneurysm or dissection was one of the primary presenting features. This has enabled us to provide a more comprehensive categorization of HTAAD genes that can be used to inform the design of diagnostic gene panels for any patient presenting with TAAD as a primary feature, and where a genetic cause is suspected. It is important, therefore, to emphasize that these validated HTAAD genes, such as FBN1, TGFBR1, TGFBR2, and ACTA2, may predispose to thoracic aortic disease with or without syndromic features, and therefore, individuals without syndromic features should be assessed for mutations in all category A HTAAD genes. Also, there may be substantial clinical overlap between syndromes where aneurysms are known to be more aggressive and are hence a key feature of diagnosis and clinical management, such as Loeys-Dietz syndrome, and syndromes where aortic disease may be less severe, such as Shprintzen-Goldberg syndrome. Diagnosis of Loeys-Dietz syndrome versus Shprintzen-Goldberg syndrome is thus important for correct management and risk stratification, and supports the conclusion that the gene for Shprintzen-Goldberg syndrome, SKI, should be included in diagnostic panels for characteristic syndromic presentations, especially in the pediatric setting.
To improve future curation efforts for genes and gene variants, accurate and detailed reporting of the associated thoracic aortic disease needs to be improved and standardized. We recommend the reporting of standardized data for the cardiovascular manifestations but also for systemic features (Online Table 4). Aortic disease presentations (aneurysm, aneurysm surgery, type A or B dissection) and location and size of aortic aneurysms (root or ascending aorta, z-scores, or diameters with corresponding anthropometry and age information data) are minimal requirements for adequate assessment of the aortic disease phenotype. Our experience after the critical evaluation of the thoracic aortic disease reported in patients harboring pathogenic variants highlights the relevance of this issue. Aortic measurements and z-scores were not provided in some papers, and remeasurement of the aorta in the images provided sometimes revealed a z-score within normal limits for the case.
Finally, it is important to recognize that the gene classification suggested here does not reflect a formal assessment of penetrance of variants in a gene for HTAAD. The ClinGen gene-disease validity matrix is not designed to assess penetrance or expressivity, which will be addressed in a separate effort. As an example, the penetrance for HTAAD in COL3A1 mutation carriers is low (<5% [23]), but individuals with COL3A1 pathogenic variants can present with aortic dissections with minimal syndromic features, leading to the decision by the Aortopathy Working Group to include COL3A1 in the “definitive” category. The opposite holds true for SLC2A10, where there is a high prevalence of aortic enlargement, but the risk for dissection is low based on available data, leading to categorization of SLC2A10 in the additional category B of genes predictive of thoracic aortic enlargement without evidence of progression to aortic dissection.
STUDY LIMITATIONS.
First, the subjective interpretation of certain types of evidence may lead to variability between expert groups assessing evidence, although the high level of transparency of the evidence base, the incorporation of sufficient expert review, and the possibility to reassess classifications over time should limit this drawback.
Second, because the assessments provided in this paper were specifically directed at the disease phenotype of HTAAD, the “clinical validity” associations between some of these genes and other disease phenotypes may differ from what is reported here.
CONCLUSIONS
The ClinGen Aortopathy Working Group of experts in HTAAD has curated a list of genes, in which pathogenetic variation predisposes to thoracic aortic disease. The result of this gene curation effort may provide useful information to guide clinical laboratories in the development and interpretation of genetic testing for patients with aortic disease.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: A curated list of genes associated with HTAAD can identify patients and families at risk for thoracic aortic disease and reduce inconclusive diagnostic testing by limiting the scope of screened genes.
Based on current data using the HTAAD genes, the estimated mutation detection ratio of 15% to 20% is dependent on clinical selection criteria.
TRANSLATIONAL OUTLOOK: Future studies based on more complete and uniform clinical and genetic data could lead to improved genetic profiles with greater power to predict HTAAD.
ABBREVIATIONS AND ACRONYMS
- ClinGen
Clinical Genome Resource
- HTAAD
heritable thoracic aortic aneurysms and dissections
- TGF
transforming growth factor
- VUS
variant(s) of uncertain significance
REFERENCES
- 1.Milewicz DM, Regalado ES. Use of genetics for personalized management of heritable thoracic aortic disease: how do we get there? J Thorac Cardiovasc Surg 2015;149:S3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Backer J, Campens L, De Paepe A. Genes in thoracic aortic aneurysms/dissections—do they matter? Ann Cardiothorac Surg 2013;2:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature 2011;473:308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rehm HL, Berg JS, Brooks LD, et al. ClinGen— the Clinical Genome Resource. N Engl J Med 2015; 372:2235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Regalado ES, Guo DC, Santos-Cortez RL, et al. Pathogenic FBN1 variants in familial thoracic aortic aneurysms and dissections. Clin Genet 2016;89: 719–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callewaert BL, Loeys BL, Ficcadenti A, et al. Comprehensive clinical and molecular assessment of 32 probands with congenital contractural arachnodactyly: report of 14 novel mutations and review of the literature. Hum Mutat 2009;30: 334–41. [DOI] [PubMed] [Google Scholar]
- 7.Pepin M, Schwarze U, Superti-Furga A, Byers PH. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med 2000;342:673–80. [DOI] [PubMed] [Google Scholar]
- 8.Strande NT, Riggs ER, Buchanan AH, et al. Evaluating the clinical validity of gene-disease associations: an evidence-based framework developed by the clinical genome resource. Am J Hum Genet 2017;100:895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arslan-Kirchner M, Arbustini E, Boileau C, et al. Clinical utility gene card for: hereditary thoracic aortic aneurysm and dissection including next-generation sequencing-based approaches. Eur J Hum Genet 2016;24:e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baasanjav S, Al-Gazali L, Hashiguchi T, et al. Faulty initiation of proteoglycan synthesis causes cardiac and joint defects. Am J Hum Genet 2011; 89:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meester JA, Vandeweyer G, Pintelon I, et al. Loss-of-function mutations in the X-linked biglycan gene cause a severe syndromic form of thoracic aortic aneurysms and dissections. Genet Med 2017;19:386–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capuano A, Bucciotti F, Farwell KD, et al. Diagnostic exome sequencing identifies a novel gene, EMILIN1, associated with autosomal-dominant hereditary connective tissue disease. Hum Mutat 2016;37:84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuang SQ, Medina-Martinez O, Guo DC, et al. FOXE3 mutations predispose to thoracic aortic aneurysms and dissections. J CLin Invest 2016;126: 948–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vermeer AM, Lodder EM, Thomas D, et al. Dilation of the aorta ascendens forms part of the clinical spectrum of HCN4 mutations. J Am Coll Cardiol 2016;67:2313–5. [DOI] [PubMed] [Google Scholar]
- 15.Micha D, Guo DC, Hilhorst-Hofstee Y, et al. SMAD2 mutations are associated with arterial aneurysms and dissections. Hum Mutat 2015;36:1145–9. [DOI] [PubMed] [Google Scholar]
- 16.Teixido-Tura G, Franken R, Galuppo V, et al. Heterogeneity of aortic disease severity in patients with Loeys-Dietz syndrome.Heart 2016;102:626–32. [DOI] [PubMed] [Google Scholar]
- 17.Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006; 355:788–98. [DOI] [PubMed] [Google Scholar]
- 18.Jondeau G, Ropers J, Regalado E, et al. International registry of patients carrying TGFBR1 or TGFBR2 Mutations: results of the MAC (Montalcino Aortic Consortium). Circ Cardiovasc Genet 2016;9:548–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacArthur DG, Manolio TA, Dimmock DP, et al. Guidelines for investigating causality of sequence variants in human disease. Nature 2014;508: 469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorenzini M, Guha N, Davison JE, et al. Isolated aortic root dilation in homocystinuria. J Inherit Metab Dis 2018;41:109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pugh TJ, Kelly MA, Gowrisankar S, et al. The landscape of genetic variation in dilated cardiomyopathy as surveyed by clinical DNA sequencing. Genet Med 2014;16:601–8. [DOI] [PubMed] [Google Scholar]
- 22.Rehm HL. Disease-targeted sequencing: a cornerstone in the clinic. Nat Rev Genet 2013;14: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohammed S, Lim Z, Dean PH, et al. Genetic insurance discrimination in sudden arrhythmia death syndromes: empirical evidence from a cross-sectional survey in North America. Circ Cardiovasc Genet 2017;10:e001442. [DOI] [PubMed] [Google Scholar]
- 24.Holtzman NA. Promoting safe and effective genetic tests in the United States: work of the Task Force on Genetic Testing. Clin Chem 1999;45: 732–8. [PubMed] [Google Scholar]
- 25.Milewicz DM, Ostergaard JR, Ala-Kokko LM, et al. De novo ACTA2 mutation causes a novel syndrome of multisystemic smooth muscle dysfunction. Am J Med Genet A 2010;152A: 2437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Regalado ES, Guo DC, Prakash S, et al. Aortic disease presentation and outcome associated with ACTA2 mutations. Circ Cardiovasc Genet 2015;8: 457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Superti-Furga A, Steinmann B, Ramirez F, Byers PH. Molecular defects of type III procollagen in Ehlers-Danlos syndrome type IV. Hum Genet 1989;82:104–8. [DOI] [PubMed] [Google Scholar]
- 28.Guo DC, Regalado ES, Gong L, et al. LOX mutations predispose to thoracic aortic aneurysms and dissections. Circ Res 2016;118: 928–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu L, Vranckx R, Khau Van Kien P, et al. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet 2006;38:343–9. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Guo DC, Cao J, et al. Mutations in myosin light chain kinase cause familial aortic dissections. Am J Hum Genet 2010;87:701–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo DC, Regalado E, Casteel DE, et al. Recurrent gain-of-function mutation in PRKG1 causes thoracic aortic aneurysms and acute aortic dissections. Am J Hum Genet 2013;93:398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Regalado ES, Guo DC, Villamizar C, et al. Exome sequencing identifies SMAD3 mutations as a cause of familial thoracic aortic aneurysm and dissection with intracranial and other arterial aneurysms. Circ Res 2011;109: 680–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boileau C, Guo DC, Hanna N, et al. TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat Genet 2012;44:916–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindsay ME, Schepers D, Bolar NA, et al. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat Genet 2012;44:922–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loeys BL, Chen J, Neptune ER, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 2005;37:275–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.