Abstract
Background
The purpose of this study was to investigate the effects of Crocin on oxidative markers (GPx, SOD, MDA) in animal model of demyelination with Ethidium bromide (EB).
Methods
Female Wistar rats were assigned in to 4 groups; Sham, with no receiving any agent (Sham), Sham Operated group with injection of EB into the brain received no agent (SO), Sham Treatment group with injection of EB and receiving PBS as vehicle and Treatment group with injection of EB and receiving Crocin (100 mg/kg). Demyelination was induced by single dose injection of 10 μl of EB 0.1% into the Cisterna magna of the brain. Crocin was diluted and applied to each animal for 21 days, once per day gavage. The levels of oxidative markers (GPx, SOD and MDA) were measured by related standard kits. Data were analyzed by paired t-test and ANOVA with post hoc test.
Results
The results showed that crocin decreases the levels of GPx and SOD significantly as well as MDA level after 21 days (α ≤ 0.05). In addition, results showed that there were significant differences in the GPx, SOD and MDA levels between all groups at post treatment phase (α ≤ 0.05).
Conclusion
It can be concluded that crocin can moderate the level of oxidative markers after demyelination of the brain cells in MS cases. Due to this effect, crocin can be considered as an effective anti-oxidant in management of degenerative nervous system diseases.
Keyword: Neuroscience
1. Introduction
Multiple sclerosis is a chronic autoimmune disease associated with myelin degeneration and inflammation in central nervous system (CNS). The mechanisms of tissue injury are currently poorly understood, but recent data suggest that mitochondrial injury may play an important role in this process. Demyelinating diseases of the CNS are a heterogeneous group of chronic inflammatory disorders resulting in lesions along axons of nerve fibers in the brain, brain stem, spinal cord, and optic nerves which is loss of myelin sheath and nerve conduction deficits leading to motor and/or sensory dysfunction and are the leading cause of non traumatic neurological disability in young adults. Immunologists view multiple sclerosis as an autoimmune disease, in which T-lymphocytes specific for myelin antigens start an inflammatory reaction in the CNS, which finally leads to demyelination and ultimately axonal damages. Women are affected approximately twice as often as males who are more likely diagnosed later in life and have a progressive course of disease. MS affects various physical, mental, cognitive and … aspects in human [1, 2, 3, 4].
Unlike many other disorders that oxidative stress plays a secondary role in the occurrence and development of disease, experimental autoimmune encephalomyelitis (EAE) or multiple sclerosis develops directly synchronic with the appearance of oxidative and nitrotic injuries [5]. It has been revealed that oxidative stress markers are significantly higher in MS patients than healthy subjects [6]. It has also shown that reduction in the activity of the antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase, and glutathione, and also elevation in lipid proxidation products such as malondialdehyde (MDA) present in MS cases comparison with healthy subjects [7].
There are many methods have applied to treat, recover, modify or attenuate MS symptoms in these cases. Chemical drugs and natural agents are widely used to reduce oxidative stress and cognitive deficits in MS patients. One of the main natural agents which were widely studied to prove its anti-oxidant role in CNS impairments is Saffron [3].
Saffron was widely used in traditional medicine as an herbal medicine and was used as an analgesic, anti-depressant, antispasmodic, respiratory congestion enhancer, libido enhancer, sweating enhancer, treatment for menstrual disorders, sedation and tranquilization [4]. The four major bioactive compounds in saffron include Crocin, Croctein, Picocrocin and Safranal, which contribute to its medicinal and antioxidant properties [8]. Frequent studies have shown that crocin and croscetin as active ingredients of saffron are able to effect a variety of drug protections, such as protection against cardiovascular disease, inhibition of cancer cell proliferation, gastrointestinal protection, neural protection, liver cells and effects Anti-inflammatory and analgesic agents that are attributed to the antioxidant capacity of this compound [8, 9, 10].
Mohajeri et al showed that alcoholic extract of saffron and significantly decreased lipid peroxidation and increased antioxidant levels in dose-dependent manner [11, 12].
Zheng et al investigated the effect of crocin on oxidative and nitrate injuries due to reperfusion of cerebral microscopic vessels after general cerebral ischemia and showed that crocin inhibited the oxidative reaction and adjusted the structure of CMEC and phosphorylation of ERK1/2 and MMP-9 expression decreases in microscopic arteries [13].
Due to the lake of clear way to treatment this disorder, and regarding to positive effects of crocin on oxidative markers in MS, more and more studies are need to help or treat those whom suffer from MS. So, we investigated the effects of crocin on the level of anti-oxidants enzymes, lipid production, learning and memory deficits in animal model of demyelination with this hypothesis that crocin can improve the oxidative mechanism and impaired cognition behaviors in this case.
2. Material and methods
2.1. Animals
Female 4–6-month-old Wistar rats with a body weight of 250 ± 50 g were maintained on a 12 h light/12 h dark cycle, at a temperature of 25 °C, with free access to food and water were used to study. All procedures for this research were approved by AJUMS ethical committee (IR.AJUMS.REC.1395.110), in accordance with the internationally accepted principles for the care and use of laboratory animals.
2.2. Methods
In order to induce animal model of demyelination, direct injection of ethidium bromide into the cisterna point of the brain was applied. The animals were kept free accessing to the water and food in standard cages comply with standard laboratory conditions such as temperature, humidity, light cycle and easy access to food and water one week before starting experiments. In the current model of demyelination, the chemical ethidium bromide was used which is created by direct injection into the nervous system. Different methods are used to create the MS model with ethidium bromide. In single-stage models, higher doses of EB are used with a higher concentration of solution. In the present study, injections of 10 μl of EB 0.1% into the Cisterna magna was used to induce demyelination [14, 15]. For this method, at first, animals are initially anesthetized with ketamine (90 mg/kg) and xylazine (10 mg/kg) then placed in a stereotaxic device. The body temperature was maintained at about 35.5–39.8. Then, by creating a longitudinal gap in the posterior part of the head, the skulla surface appears with two points referring to Brigma and Lambda. After specifying Cisterna pontis, the spot was marked and cautiously with the help of a dental drill, a hole was drilled into the animal's skull to inject EB without any damage to the brain tissue. After creating a hole in the animal's skull, a 10 μl of ethidium bromide 0.1% was injected slowly into the Cisterna pontis using a Hamilton syringe. To use ethidium bromide in this method, it was diluted 10 mg/ml in phosphate saline buffer (PBS) [15]. After the solution is injected, the gum is sutured and the animal is transported to the separate cages. To evaluate the effects of EB and evaluation of demyelination after injection, the animals were evaluated daily while the demyelination symptoms were observed. After presentation of demyelination signs, the scoring was done according to the following 10 score system [16], 0, no clinical disease; 0.5, partial tail paralysis; 1.0, complete tail paralysis; 1.5, complete tail paralysis and discrete hind limb weakness; 2.0, complete tail paralysis and strong hind limb weakness; 2.5, unilateral hind limb paralysis; 3, complete hind limb paralysis; 3.5, hind limb paralysis and forelimb weakness; 4.0, complete paralysis (tetraplegia); 5.0: moribund or dead. In order to determine the severity of lesion, Cumulative Disease Index (CDI) score was determined as the average of the sum of daily clinical scores for each animal [16]. After these investigations, those who had score 2–4 were selected to experimental. Afterwards, animals were assigned in to 4 groups (n = 8 for each group): Sham: received no any agent or treatment administration (Sham), Sham operated, with injection of EB in cisterna magna and receiving no agent (SO), Treatment, with injection of EB and receiving crocin (Treat): Sham treatment, with injection of EB and receiving PBS as vehicle (SO). Administration of crocin and PBS (for treating approach) was done orally gavage of 100 mg/kg for each animal for 21 days. Injected EB and PBS for induction demyelination was performed one dose of 10 microliters for each animal. Data was recorded two times for each group, one time was done after demyelination (about 3 weeks after EB injection) as the pre treatment phase, and another time was done 21 days after onset of crocin administration as the post treatment phase. In the pre treatment phase, the whole tissue of each animal brain was ejected from the skulla and then was homogenized and kept in the -70° refrigerator to assay with post treatment data. Afterwards, data obtained from the groups was analyzed. To assay the level of GPx, MDA and SOD, standard kits according to their manufacturer's instructions (RANDOX RANSOD, United Kingdom) were used at two phases of experiments [17].
2.3. Statistical methods
All data are presented as Mean ± SEM. The mean of data for each group in any phase was analyzed. Data were analyzed using paired t-test, ANOVA followed by LSD post hoc test. Paired t-test was used for assessing the differences between pre-treatment and post-treatment of each group results. For analyzing the results of all groups at pre-treatment also at post-treatment, ANOVA test with LSD post hoc test was used. Values of p < 0.05 were considered significant. Statistical analyses were performed using the 19 release version of SPSS for Windows.
3. Results
To investigate the effect of crocin on experimental variables, paired t test was used to compare pre and post values for each group. Results are shown as following statements. Comparison of the pre and post values of GPx for each group is presented in Table 1.
Table 1.
Comparison of pre and post treatment values for GPx, MDA and SOD after crocin administration for 21 days in demyelinated rats. Data are presented as Mean ± SEM.
| Variable | Group | Pre | Post | Mean diff. | SEM | p-value |
|---|---|---|---|---|---|---|
| GPX | Sham | 4.06 | 4.10 | -0.045 | 0.919 | 0.963 |
| ST | 5.32 | 5.26 | 0.061 | 0.689 | 0.932 | |
| SO | 5.48 | 5.62 | -0.148 | 0.442 | 0.751 | |
| Treat | 5.55 | 3.75 | **1.79 | 0.346 | 0.004 | |
| MDA | Sham | 1.31 | 1.34 | -0.021 | 0.451 | 0.903 |
| ST | 4.20 | 4.05 | 0.147 | 0.531 | 0.792 | |
| SO | 4.41 | 4.44 | -0.029 | 0.419 | 0.946 | |
| Treat | 4.30 | 2.57 | *1.72 | 0.605 | 0.036 | |
| SOD | Sham | 6.65 | 6.82 | -0.162 | 0.650 | 0.813 |
| ST | 10.02 | 1.15 | -0.126 | 0.742 | 0.871 | |
| SO | 10.24 | 10.33 | -0.088 | 0.646 | 0.896 | |
| Treat | 10.80 | 7.55 | *3.25 | 1.06 | 0.028 |
GPx: Glutathione Proxidaze, MDA: Malondialdehyde, SOD: Superoxide dismutase, SEM: Standard error mean, SO: Sham Operated, Treat: Treatment, ST: Sham Treatment. **: P < 0.01, *: P < 0.05 between pre and post-treatment values.
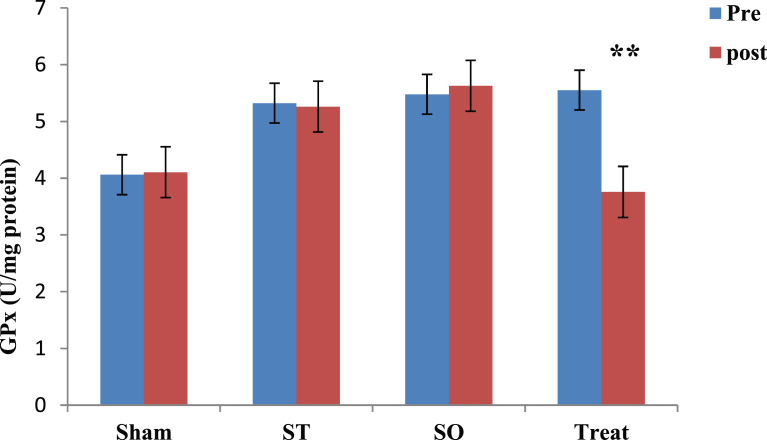
3.1. Effect of crocin on GPx concentration
Fig. 1 shows comparison of pre and post values of GPx in each group using paired t-test. Results show that 21 days orally gavage of crocin leads to significant decreasing (32%) in GPx level only in treatment (Treat) group. Other three groups showed no significant changes in GPx levels after crocin administration (p > .0.5). Paired t-test results showed that, there is a significant difference between pre and post values of GPx in Treat group (t = 5.185, **p = 0.004). There were no significant differences in GPx levels between pre and post phases in Sham, ST and SO groups (p > 0.05). The data for Sham ST and SO groups are respectively (t = 0.049, p = 0.963), (t = 0.089, p = 0.932) and (t = 0.442, p = 0.751) (See also Fig. 1).
Fig. 1.
Comparison of Glutathione Proxidase (GPx) between pre and post administration of Crocin in all groups in the whole brain tissue after 21 days with oral administration (100 mg/kg) in demelinated wistar rats. Data are presented as Mean ± SEM and analyzed by Paired t-test. GPx; Glutathione Proxidase, U/mg; Unit per milligram, SO; Sham operated, ST; Sham treatment, Treat; Treatment, SEM; Standard error mean, **; p < 0.01 compared to pre-test.
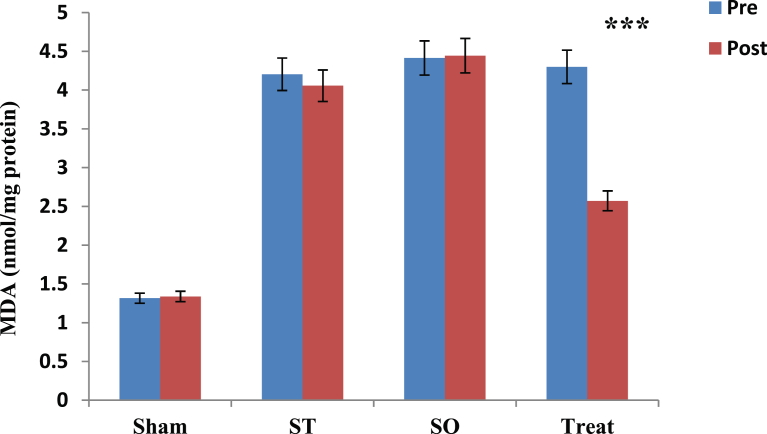
3.2. Effect of crocin on MDA concentration
Fig. 2 shows comparison of pre and post values of MDA in each group using paired t-test. Results showed that after 21 days of gavage of crocin leads to significant decreasing (40%) in MDA level only in treatment (Treat) group. Other three groups showed no significant changes in MDA levels after crocin administration. Paired t-test results showed that, there is a significant difference between pre and post values of MDA only in Treat group (t = 2.857, *p = 0.001). There were no significant differences in MDA levels between pre and post phases in Sham, ST and SO groups (p > 0.05). The data for Sham ST and SO groups are respectively (t = 0.048, p = 0.903), (t = 0.278, p = 0.792) and (t = 0.071, p = 0.946) (Table 1 & Fig. 2).
Fig. 2.
Comparison of Malondialdehyde (MDA) between pre and post administration of Crocin in all groups in the whole brain tissue after 21 days with oral administration (100 mg/kg) in demyelinated Wistar rats. Data are presented as Mean ± SEM and analyzed by Paired t test. MDA; Malondialdehyde, U/mg; Unit per milligram, SO; Sham operated, ST; Sham treatment, Treat; Treatment, SEM; Standard error mean,***; p < 0.001 compared to pre-test.
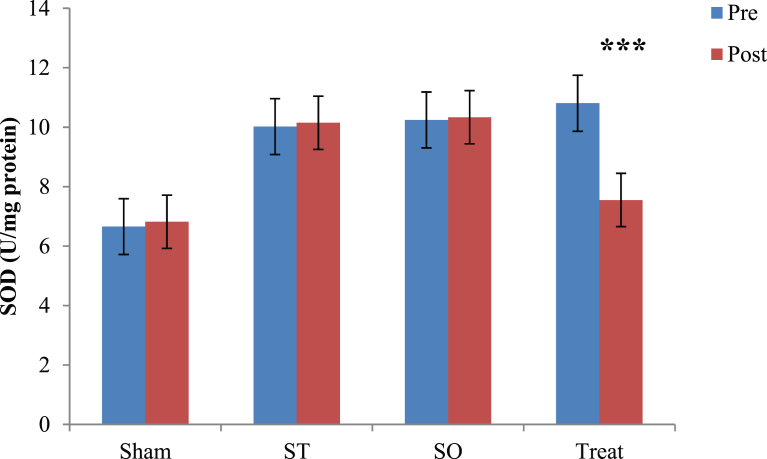
3.3. Effect of crocin on SOD
Fig. 3 shows comparison of pre and post values of SOD in each group using paired t test. Results showed that 21 days orally gavage of crocin leads to significant decreasing (30%) in SOD level only in treatment (Treat) group. Other three groups showed no significant changes in SOD levels after crocin administration Comparison of the pre and post values of SOD for each group is also presented in Table 1. Paired t-test results showed that there is a significant difference between pre and post values of SOD only in Treat group (t = 3.05, *p = 0.001). There were no significant differences in SOD levels between pre and post phases in Sham, ST and SO groups (p > 0.05). The data for Sham ST and SO groups are respectively (t = 0.250, p = 0.813), (t = 0.171, p = 0.871) and (t = 0.137, p = 0.896) (See also Table 1).
Fig. 3.
Comparison of Superoxidaze Dismutase (SOD) U/mg protein, between pre and post administration of Crocin in all groups in the whole brain tissue after 21 days with oral administration (100 mg/kg) in demyelinated Wistar rats. Data are presented as Mean ± SEM and analyzed by Paired t test. SOD; Superoxidaze Dismutase, U/mg; Unit per milligram, SO; Sham operated, ST; Sham treatment, Treat; Treatment, SEM; Standard error mean, ***; p < 0.001 compared to pre-test.
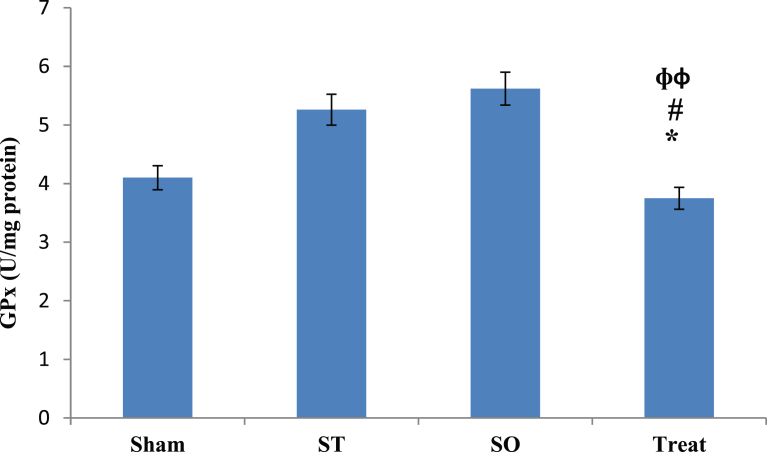
3.4. Comparison of post treatment values between four groups
Fig. 4 showed comparison of GPx levels at post treatment phase between all groups after 21 days administration of crocin. ANOVAs with post hoc test results showed statistically significant differences between 4 studied groups in post test phase (p = 0.01). Pairwise comparison results showed that, in comparison with Treat group, there was significant difference with Sham group (α ≤ 0.05, p = 0.040), significant difference with SO group (α ≤ 0.05, *p = 0.020), significant difference with ST group (α ≤ 0.05, *p = 0.003) in the GPx level at post treatment phase. There were no significant differences between other groups.
Fig. 4.
Effects of Crocin on Glutathione Proxidaze (GPx) in the whole brain tissue after 21 days with oral administration (100 mg/kg) in demyelinated Wistar rats. Data are presented as Mean ± SEM and analyzed by one way ANOVA with LSD's post hoc test. GPx; Glutathione Proxidaze, U/mg; Unit per milligram, Sham Operated, ST: Sham Treatment, Treat: Treatment, *: p < 0.05, vs. Sham group, #: p < 0.05, vs. ST group; ɸɸ: p < 0.01 vs. SO group.
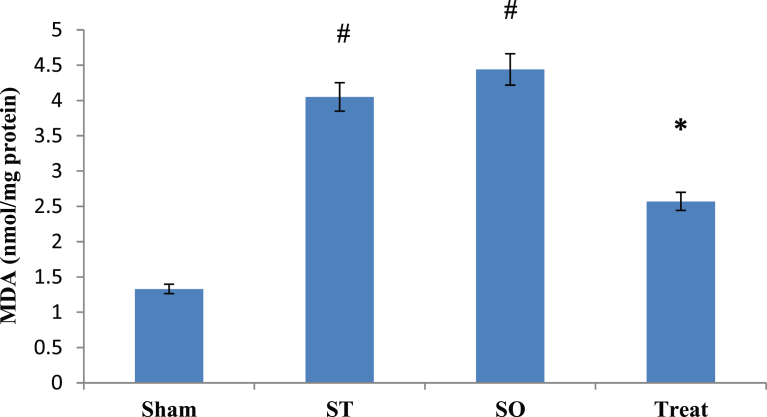
Fig. 5 showed comparison of MDA levels at post treatment phase between all groups after 21 days administration of crocin. ANOVAs with post hoc test results showed statistically significant differences between 4 studied groups in post test phase. Pairwise comparison results showed that, in comparison with Sham group, there was no significant difference with Treat group (α ≤ 0.05, p = 1.00), significant difference with ST group (α ≤ 0.05, *p = 0.042), significant difference with SO group (α ≤ 0.05, *p = 0.021) in the MDA level at post treatment phase. Compared to SO group, results showed significant difference with Treat group (α ≤ 0.05, *p = 0.014), no significant difference with ST group (α ≤ 0.05, p = 1.00).
Fig. 5.
Effects of Crocin on Malondialdehyde (MDA) in the whole brain tissue after 21 days with oral administration (100 mg/kg) in demyelinated Wistar rats. Data are presented as Mean ± SEM and analyzed by one way ANOVA and LSD's post hoc test. MDA; Malondialdehyde, U/mg; Unit per milligram, Sham Operated, ST: Sham Treatment, Treat: Treatment, *: p < 0.05, vs. ST group, #: p < 0.05, vs. Sham group.
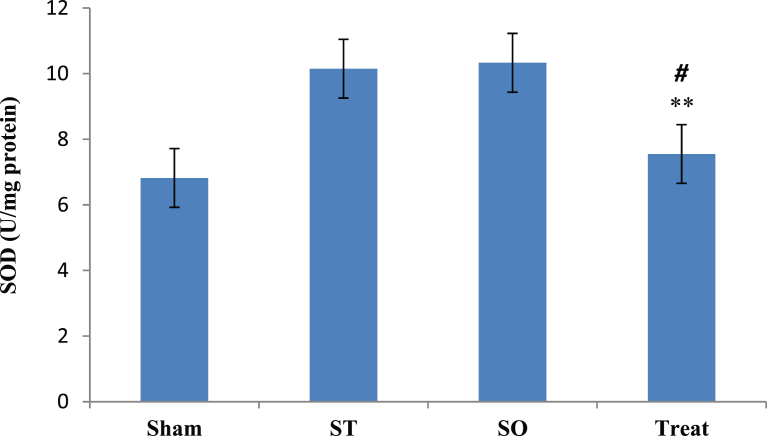
Fig. 6 showed comparison of SOD levels at post treatment phase between all groups after 21 days administration of crocin. ANOVAs with post hoc test results showed statistically significant differences between 4 studied groups in post test phase. Pairwise comparison results showed that, in comparison with Treat group, there was no significant difference with Sham group (α ≤ 0.05, p = 0.418), significant difference with ST group (α ≤ 0.05, *p = 0.013), significant difference with SO group (α ≤ 0.05, **p = 0.008) in the SOD level at post treatment phase. There were no significant differences between other groups.
Fig. 6.
Effects of Crocin on Superoxidaze Dismutase (SOD) in the whole brain tissue after 21 days with oral administration (100 mg/kg) in ddemyelinated Wistar rats. Data are presented as Mean ± SEM and analyzed by one way ANOVA and LSD's post hoc test. SOD; Superoxidaze Dismutase, U/mg; Unit per milligram, Sham Operated, ST: Sham Treatment, Treat: Treatment, **: p < 0.01 vs. SO group, #: p < 0.05, vs. ST group.
4. Discussion
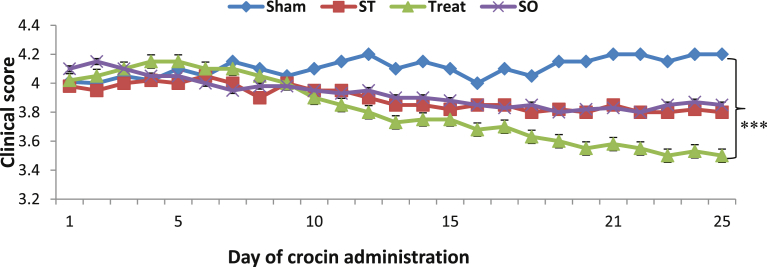
Our results showed that after 21 days, crocin (with dosage of 100 mg/kg) significantly reduced the activity of SOD and GPx enzymes and also reduced the concentration of MDA as a product of lipid proxdation in EAE animals. Reduction in anti-oxidant enzymes can be attributed to the reduced level of lipid proxidation products such as MDA. There are available some documents that demonstrate anti-oxidant role of crocin in brain after neuro damaging and inflammation [18, 19, 20, 21]; however, in the case of MS, studies about the role of the crocin are not widely studied yet and a leakage is observed in this scope. According to Fig. 7, crocin treatment made a significant decrease in clinical impairment scores, while control groups did not show any amelioration.
Fig. 7.
Comparison of daily clinical scores between all groups after 21 days of crocin with gavage administration in Wistar rats. Data are presented as Mean ± SEM and analyzed by ANOVA with LSD test. ST: Sham treatment, SO: Sham operated, Treat: Treatment, ***: Significant difference at p < 0.001, compared to Sham group (n=7).
Many studies have shown the effective role of saffron compounds (crocin) in improving neurological disorders, including pain relief [22], antioxidant, anti-inflammation and spasticity [8, 11, 23], recovery of ethanol-degraded memory [23], and Memory [13], improvement of EAE symptoms include oxidative stress, inhibition of leukocyte penetration into the brain [24, 25].
Oxidative stress and reactive oxygen species (ROS) contribute to the formation and remaining of MS lesions. Deep changes in the mitochondrial respiratory chain proteins and the removal of mitochondrial DNA in damaged neurons of MS lesions in advanced stages of disease are observed [26]. The first body defenses against oxidative stress include antioxidant enzymes such as glutathione peroxidase, superoxide dismutase and non-enzymatic antioxidants such as glutathione, ascorbic acid, bilirubin and others [27]. GPx, SOD and MDA provide a key role in the pathogenesis of MS through variable proteins against ROS. Crocin acts as a powerful antioxidant by removing free radicals and protects cells against oxidative damage [28]. The anti-inflammatory effects of saffron are due to its inhibitory effect on the expression of precursor-inflammatory gens such as cyclooxygenase-2 (Cox2) and reproductive nitric oxide synthesis [29].
According to the some studies, positive effect of crocin on the oxidative pathway may be related to its anxiolytic effect [30, 31, 32] activity. It has been shown that crocin inhibits microglial activation [33].
The metalloproteinase matrix plays an important role in the pathogenesis of EAE and MS through damages in CSF barrier. So the beneficial effects of crocin on the elevated levels of GPx and SOD in this study can also be due to a change in the performance of the metalloproteinases matrix [34].
Reduction in ROS levels in cellular environment can be also related to the antioxidant effect of crocin. Dose-dependent administration of crocin reduced ROS in cells, which can be proven by DCF measurements. Increased levels of DCF indicate DCFH oxidation by intracellular radicals that can be related to the crocin [19].
It has been shown that crocin enhances anti-inflammatory response by increasing in expression of heme oxygenase-1 (HO-1), inhibits expression of nitric oxide syntheses (iNOS) and nitric oxide production through downregulation of nuclear factor kappa B activity. Crocin also leads to intracellular Ca2+ mobilization and phosphorylation of Ca2+/calmodul independent protein kinase 4 (CAMK4). Crocin suppresses LPS-stimulated expression of iNOS via expression of HO-1 through Ca2+/calmodulin-CAMK4-PI3K/Akt-Nrf2 signaling cascades [35].
Mazumder et al. showed that pre-treatment administration of crocin has supportive effects on dopaminergic neurons in dark neurons in the hippocampal pyramidal layer and increased SOD activity and decreased ROS level as well as nuclear factor-κB (NF-κB) expression in the hippocampus in Parkinsonism model [36]. It has been shown that saffron and its active constitutions (crocin) can enhance maintenance and recall of information and antagonize impaired memory induced by ethanol in hippocampus region of the diabetic animals [37, 38], reduce streptozotocin-induced spatial memory deficit and oxidative stress [17, 39] and reduce the Bax/Bcl-2 ratio and inhibit apoptosis caused by beta amiloyide in Alzheimer's disease [40].
Rajaei et al. showed that crocin ameliorates Parkinson's disease by decreasing MDA and nitrite levels in the hippocampus, and improved aversive memory by modulating oxidative and inflammatory responses [41].
Naghizadeh et al. found that crocin prevented cognitive deficiency induced by ICV-STZ via decreasing MDA as well as increasing the levels of total and GPx activity [17].
Deslauriers et al. stated that neuroinflammation and endoplasmic reticulum (ER) stress are coregulated by crocin to prevent demyelination and neurodegeneration. They showed that daily treatment with crocin starting on day 7 post-EAE induction suppressed ER stress and inflammatory gene expression in spinal cords which was accompanied by preserved myelination and axonal density, together with reduced T cell infiltration and macrophage activation. EAE-associated neurobehavioral deficits were also ameliorated by crocin treatment [42].
The results indicated reduction of MDA after crocin administration, also reduction in GPx and SOD activity. MDA is a product of lipid proxidation, so, the reduced levels of GPx and SOD, as anti-oxidant enzymes, in the current study can be related to the decline in lipid proxidation levels such as MDA as it was occurred in our research. Investigation of other lipid proxidation products is required in further studies to prove this statement. Although, in consistent with previous findings, our results showed positive effects of crocin on brain oxidative markers profile in demyelinated rats, but the related mechanism is not cleared yet, so, one of the major limitation of the current study is the leakage of methods to determine the mechanism of crocin to increase the oxidative enzymes levels and behavior it is an important issue to be studied in future.
5. Conclusion
The present findings showed that administration of crocin for 21 days after demyelination led to significant decreasing in the brain level of GPx, MDA and SOD in toxic model of demyelination in animals. Although, the mechanism of this effect remains unknown, but it can be suggested that administration of crocin, as an effective ingredient in the reduction of free radicals and increased antioxidant activity, can be an effective way in the management of MS patients.
Declarations
Author contribution statement
Hadi Fathimoghaddam: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Yaghoub Farbod: Conceived and designed the experiments; Performed the experiments.
Ataallah Ghadiri: Performed the experiments; Analyzed and interpreted the data.
Rouholah Fatemi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Physiology Research Center (PRC), Ahvaz Jundishapur University of Medical Sciences with grant number of (IR.AJUMS.REC.1395.110).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Supplementary video 1. A sample of demyelinated Wistar rat with ethidium bromide. As observed in supplementary video 1, injection of ethidium bromide in to Cisterna magna led to mild to severe hind limb and forelimb paralysis and tail weakness in a Wistar rat at the first week of the study plan.
References
- 1.Haider L., Fischer M.T., Frischer J.M. Oxidative damage in multiple sclerosis lesions. Brain. 2011;134(7):1914–1924. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haider L., Zrzavy T., Hametner S. The topography of demyelination and neurodegeneration in the multiple sclerosis brain. Brain. 2016;139:807–815. doi: 10.1093/brain/awv398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hooshmandi Z., Rohani A.H., Eidi A., Fatahi Z., Golmanesh L., Sahraei H. Reduction of metabolic and behavioral signs of acute strss in male Wistar rats by saffron water extract and its constituent safranal. Pharm. Biol. 2011;49:947–954. doi: 10.3109/13880209.2011.558103. [DOI] [PubMed] [Google Scholar]
- 4.Barten L.J., Allington D.R., Procacci K.A., Rivey M.P. New approaches in the management of multiple sclerosis. Drug Des. Dev. Ther. 2010;4:343–366. doi: 10.2147/DDDT.S9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ljubisavljevic S., Stojanovic I., Pavlovic D., Sokolovic D., Stevanovic I. Aminoguanidine and N-acetyl-cysteine supress oxidative and nitrosative stress in EAE rat brains. Redox. For. Rep. 2011;16(4):166–172. doi: 10.1179/1351000211Y.0000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hritcu L., Stefan M., Brandsch R., Mihasan M. Enhanced behavioral response by decreasing brain oxidative stress to 6-hydroxy-l-nicotine in Alzheimer’s disease rat model. Neurosci. Lett. 2015;591:41–47. doi: 10.1016/j.neulet.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Wang S.W., Yang S.G., Liu W., Zhang Y.X., Xu P.X., Wang T. Alpha-tocopherol quinine ameliorates spatial memory deficits by reducing beta-amyloid oligomers, neuroinflammation and oxidative stress in transgenic mice with Alzheimer’s disease. Behav. Brain Res. 2016;296:109–117. doi: 10.1016/j.bbr.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Noorbakhsh F., Michalak M., Rakesh C.K., Acharjee S., Kristofor K., Ellestad A.M., Afkhami-Goli A., Amber M., Power P. Neuroinflammation and endoplasmic reticulum stress are coregulated by crocin to prevent demyelination and neurodegeneration. J. Immunol. 2011;187:4788–4799. doi: 10.4049/jimmunol.1004111. [DOI] [PubMed] [Google Scholar]
- 9.Khajuria D.K., Asad M., Asdaq S., Kumar P. The potency of Crocus sativus (Saffron) and its constituent crocin as an immunomodulator in animals, Latin. Am. J. Pharm. 2010 5;29 http://hdl.handle.net/10915/7972 [Google Scholar]
- 10.Mashmoul M., Azlan A., Khaza’ai H., Yusof B.N., Noor S.M. Effects of saffron extract and crocin on anthropometrical, nutritional and lipid profile parameters of rats fed a high fat diet. J. Funct. Food. 2014;8:180–187. [Google Scholar]
- 11.Mohajeri D., Mousavi Gh., Mesgari M., Doustar Y., Khayat Nouri M.H. Subacute toxicity of Crocus sativus L. (saffron) stigma ethanolic extract in rats. Am. J. Pharmacol. Toxicol. 2007;2:189–193. [Google Scholar]
- 12.Mohajeri S.A., Hosseinzadeh H., Keyhanfar F., Aghamohammadian J. Extraction of crocin from saffron (Crocus sativus) using molecularly imprinted polymer solid-phase extraction. J. Separ. Sci. 2010;33:2302–2309. doi: 10.1002/jssc.201000183. [DOI] [PubMed] [Google Scholar]
- 13.Zheng Y.Q., Liu J.X., Wang J.N., Xu L. Effects of crocin on reperfusioninduced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res. 2007;1138:86–94. doi: 10.1016/j.brainres.2006.12.064. [DOI] [PubMed] [Google Scholar]
- 14.Mazzanti C.M., Spanevello R.M., Obregon A., Pereira L.B., Streher C.A., Mushtaq A. Ethidium bromide inhibits rat brain acetyl cholinesterase activity in vitro. Chem. Biol. Interact. 2006;162:121–127. doi: 10.1016/j.cbi.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 15.Mazzanti C.M., Spanevello R.M., Morsch A., Zanin R., Battisti V., Mushtaq A. Previous treatment with ebselen and vitamin E alters adenine nucleotide hydrolysis in platelets from adult rats experimentally demyelinated with ethidium bromide. Life Sci. 2007;81:241–248. doi: 10.1016/j.lfs.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Naghashpour M., Amani R., Sarkaki A., Ghadiri A., Samarbafzadeh A., Jafarirad S., Saki Malehi A. Brain-derived neurotrophic and immunologic factors: beneficial effects of riboflavin on motor disability in murine model of multiple sclerosis. Iran. J. Basic. Med. Sci. 2016;19:439–448. PMCID: PMC4887718. [PMC free article] [PubMed] [Google Scholar]
- 17.Dianat M., Esmaeilizadeh M., Badavi M., Samarbaf-zadeh A.R., Naghizadeh B. Protective Effects of Crocin on Ischemia-reperfusion Induced Oxidative Stress in Comparison With Vitamin E in Isolated Rat Hearts. Jundishapur J. Nat. Pharm. Prod. 2014;9(2) doi: 10.17795/jjnpp-17187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christodoulou E., Kadoglou N.P., Kostomitsopoulos N., Valsami G. Saffron: a natural product with potential pharmaceutical applications. J. Pharm. Pharmacol. 2015;67(12):1634–1649. doi: 10.1111/jphp.12456. [DOI] [PubMed] [Google Scholar]
- 19.Shirali S., Bathaie S.Z., Nakhjavani M. Effect of crocin on the insulin resistance and lipid profile of streptozotocin-induced diabetic rats. Phytother Res. 2013;27(7):1042–1047. doi: 10.1002/ptr.4836. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava R., Ahmed H., Dharamveer R.K., Saraf S.A. Crocus sativus L: a comprehensive review. Pharmacogn. Rev. 2010;4(8):200–208. doi: 10.4103/0973-7847.70919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutheil W.G., Reed G., Ray A., Dhard A. Crocetin: an agent derived from saffron for prevention and therapyfor cancer. Curr. Pharm. Biotechnol. 2012;13(1):173–179. doi: 10.2174/138920112798868566. PMCID:PMC4461363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosseinzadeh H., Ziaei T. Effects of crocus sativus stigma extract and its constituents, crocin and safranal, on intact memory and scopolamine-induced learning deficits in rats performing the Morris water maze task. JMP. 2006;3(19):40–50. http://jmp.ir/article-1-663-en.html URL: [Google Scholar]
- 23.Alavizadeh S.H., Hosseinzadeh H. Bioactivity assessment and toxicity of crocin: a comprehensive review. Food Chem. Toxicol. 2014;64:65–80. doi: 10.1016/j.fct.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 24.Ghazavi A., Mosayebi G., Salehi H., Abtahi H. Effect of ethanol extract of saffron (Crocus sativus L.) on the inhibition of experimental autoimmune encephalomyelitis in C57bl/6 mice. Pak. J. Biol. Sci. 2009;12(9):690–695. doi: 10.3923/pjbs.2009.690.695. PMID: 19634472. [DOI] [PubMed] [Google Scholar]
- 25.Hosseinzadeh H., Sadeghnia H.R., Ghaeni F.A., Motamedshariaty V.S., Mohajeri S.A. Effects of saffron (Crocus sativus L.) and its active constituent, crocin, on recognition and spatial memory after chronic cerebral hypoperfusion in rats. Phytother Res. 2012;26:381–386. doi: 10.1002/ptr.3566. [DOI] [PubMed] [Google Scholar]
- 26.Schreibelt G., Van Horssen J., Van Rossum S., Dijkstra C.D., Drukarch B., De Vries H.E. Therapeutic potential and biological role of endogenous antioxidant enzymes in multiple sclerosis pathology. Brain Res. Rev. 2007;56(2):322–330. doi: 10.1016/j.brainresrev.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Tasset I., Agüera E., Sánchez-López F., Feijóo M., Giraldo A.I., Cruz A.H. Peripheral oxidative stress in relapsing remitting multiple sclerosis. Clin. Biochem. 2012;45(6):440–444. doi: 10.1016/j.clinbiochem.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Ghadrdoost B., Vafaei A.A., Rashidy-Pour A., Hajisoltani R., Bandegi A.R., Motamedi F. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur. J. Pharmacol. 2011;667(1):222–229. doi: 10.1016/j.ejphar.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Herold A., Cremer L., Călugaru A., Tamaş V., Ionescu F., Manea S. Hydroalcoholic plant extracts with anti-inflammatory activity. RAMI. 2003;62:117–129. PMID: 15493372. [PubMed] [Google Scholar]
- 30.Kamyar M., Razavi B.M., Hasani F.V., Mehri S., Foroutanfar A., Hosseinzadeh H. Crocin prevents haloperidol-induced orofacial dyskinesia: possible an antioxidant mechanism. Iran J. Basic. Med. Sci. 2016;19:1070–1079. PMCID: PMC5110655. [PMC free article] [PubMed] [Google Scholar]
- 31.Farkhondeh T., Samarghandian S., Shaterzadeh Yazdi H., Samini F. The protective effects of crocin in the management of neurodegenerative diseases: a review. Am. J. Neurodegener. Dis. 2018;7(1):1–10. PMID: 29531865. [PMC free article] [PubMed] [Google Scholar]
- 32.Pitsikas N., Sakellaridis N. Crocus sativus L. extracts antagonize memory impairments in different behavioural tasks in the rat. Behav. Brain Res. 2006;173:112–115. doi: 10.1016/j.bbr.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Lv B., Huo F., Zhu Z., Xu Z., Dang X., Chen T., Zhang T., Yang X. Crocin upregulates CX3CR1 expression by suppressing NF-κB/YY1 signaling and inhibiting lipopolysaccharide-induced microglial activation. Neurochem. Res. 2016;41:1949–1957. doi: 10.1007/s11064-016-1905-1. [DOI] [PubMed] [Google Scholar]
- 34.Stys P.K., Waxman S.G., Ransom B.R. Ionic mechanisms of anoxic injury in mammalian CNS white matter: role of Na+ channels and Na (+)- Ca2+ exchanger. J. Neurosci. 1992;12(2):430–439. doi: 10.1523/JNEUROSCI.12-02-00430.1992. PMID: 1311030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J.H., Park G.Y., Bang S.Y., Park S.Y., Bae S.K., Kim Y. Crocin suppresses LPS-stimulated expression of inducible nitric oxide synthase by upregulation of heme oxygenase-1 via calcium/calmodulin-dependent protein kinase 4. Mediators. Inflamm. 2014 doi: 10.1155/2014/728709. 728–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazumder A.G., Sharma P., Patial V., Singh D. Crocin attenuates kindling development and associated cognitive impairments in mice via inhibiting reactive oxygen species-mediated NF-κB activation. Basic Clin. Pharmacol. Toxicol. 2017;120:426–433. doi: 10.1111/bcpt.12694. [DOI] [PubMed] [Google Scholar]
- 37.Tamaddonfard E., Farshid A.A., Asri-Rezaee S., Javadi S., Khosravi V., Rahman B. Crocin improved learning and memory impairments in streptozotocin-induced diabetic rats. Iran. J. basic. Med. Sci. 2013;16(1):91–100. [PMC free article] [PubMed] [Google Scholar]
- 38.Abe K., Sugiura M., Shoyama Y., Saito H. Crocin antagonizes ethanol inhibition of NMDA receptor-mediated responses in rat hippocampal neurons. Brain Res. 1998;787(1):132–138. doi: 10.1016/s0006-8993(97)01505-9. [DOI] [PubMed] [Google Scholar]
- 39.Khalili M., Hamzeh F. Effects of active constituents of crocus sativus L., crocin on streptozocin-induced model of sporadic Alzheimer's disease male rats. Iran. Biomed. J. 2010;14 59–5. PMCID:PMC3878147. [PMC free article] [PubMed] [Google Scholar]
- 40.Asadi F., Jamshidi A.H., Khodagholi F., Yans A., Azimi L., Faizi M. Reversal effects of crocin on amyloid β-induced memory deficit: modification of autophagy or apoptosis markers. Pharmacol. Biochem. Behav. 2015;139(Pt A):47–58. doi: 10.1016/j.pbb.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Rajaei Z., Hosseini M., Alaei H. Effects of crocin on brain oxidative damage and aversive memory in a 6-OHDA model of Parkinson’s disease. Arq. Neuropsiquiatr. 2016;74:723–729. doi: 10.1590/0004-282X20160131. [DOI] [PubMed] [Google Scholar]
- 42.Deslauriers A.M., Afkhami-Goli A., Paul A.M., Bhat R.K., Acharjee S., Ellestad K.K., Noorbakhsh F. Neuroinflammation and endoplasmic reticulum stress are coregulated by crocin to prevent demyelination and neurodegeneration. J. Immunol. 2011;187(9) doi: 10.4049/jimmunol.1004111. 4788–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary video 1. A sample of demyelinated Wistar rat with ethidium bromide. As observed in supplementary video 1, injection of ethidium bromide in to Cisterna magna led to mild to severe hind limb and forelimb paralysis and tail weakness in a Wistar rat at the first week of the study plan.