Abstract
We recently explored the expression of CXCR5 on T and B cells from peripheral blood of patients with primary Sjögren’s syndrome (SS). Here we investigated the frequency of CD25+FoxP3+CD4+ regulatory T cells (Tregs) among CXCR5+CD4+ follicular cells in the same cohort of patients. We confirm that the frequency of Tregs among follicular T cells is increased in SS patients and also provide novel data showing an increased frequency of PD‐1 expressing cells among CXCR5+FoxP3+CD4+ T cells.

Response to Letter to the Editor:
Contribution of FoxP3+ Tfr cells to overall human blood CXCR5+ T cells, by Válter R. Fonseca and Luis Graca.
We thank Válter R. Fonseca and Luis Graca for their interest in our study: ‘Diminished CXCR5 expression in peripheral blood of patients with Sjögren’s syndrome may relate to both genotype and salivary gland homing’ 1, which was undertaken to explore CXCR5 expression in relation to CXCR5 genotype and clinical features of patients with Sjögren’s syndrome (SS).
Importantly, we showed that the frequency of all CXCR5+ B cell subsets is decreased in peripheral blood of patients with SS when compared to age‐ and gender‐matched healthy donors (HD). We further observed an accumulation of CXCR5+ cells in salivary glands of SS patients, suggesting that these cells migrate to the salivary glands as the site of autoimmune inflammation. However, we did not find a decrease in the total frequency of CXCR5+CD4+ T cells in peripheral blood of SS patients, which was also confirmed by Fonseca et al. 2. Notably, however, a subgroup of SS patients presented an increase in CXCR5+ programmed death 1 (PD‐1)+CD4+ T cells, findings corroborated by Fonseca et al. 2 and Verstappen et al. 3. Finally, we observed a significant decrease of CXCR5+CCR6+ [T helper type 17 (Th17)‐like] cells in the peripheral blood of SS patients 1. These data highlight the importance of subphenotyping T cell effector functions in autoimmune disorders such as SS.
We read with great interest the study by Fonseca et al., in which they describe an increased frequency of follicular regulatory T cells (Tfr) [CXCR5+forkhead box protein 3 (FoxP3+)] in SS patients 4. We therefore examined the frequency of this subset in the same cohort of primary SS patients as described in 1. In accordance with our previous data, the frequency of CXCR5+CD4+ T cells did not differ between SS patients and controls, and this was also true when specifically analyzing the FoxP3‐negative fraction of CXCR5+CD4+ T cells (Fig. 1a). The frequency of FoxP3+CD25+ T cells tended to be higher in SS patients, but without reaching statistical significance (Fig. 1b). This was accompanied by an increase in the FoxP3lowCD45RAneg (Fraction III) population in SS patients (Fig. 1b, lower panel).
Figure 1.
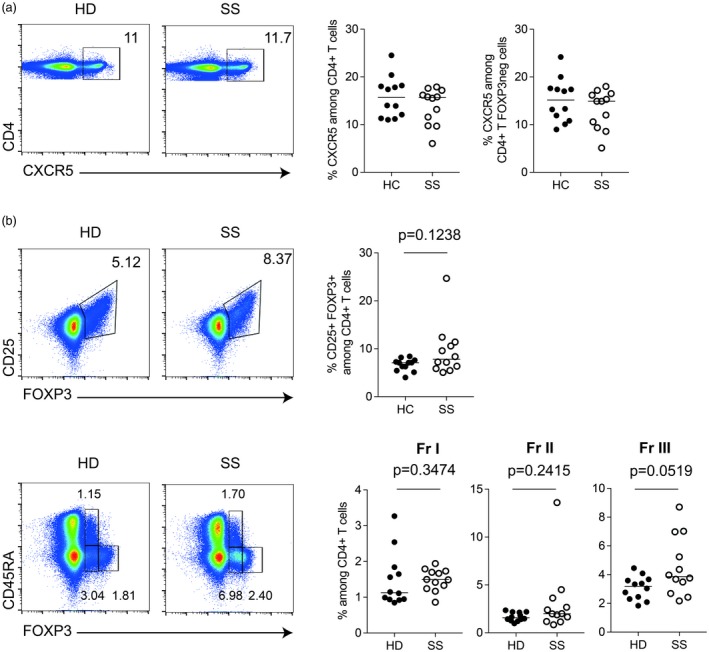
Frequency of CXCR5+CD4+ and forkhead box protein 3 (FoxP3)+CD4+ T cell subsets in peripheral blood mononuclear cells (PBMCs) from patients with Sjögren’s syndrome and healthy donors. (a) Gating strategy to identify Tfh (CXCR5+) CD4+ T cells (upper panel). (b) Gating strategy to identify CD25+FoxP3+CD4+ T cells (upper panel) regulatory CD4+ T cell subsets: Fraction I (CD45RA+FOXP3low), Fraction II (CD45RA+CD25high) and Fraction III (CD45RA–Fox3low) (lower panel), quantified in PBMC samples from patients with SS (n = 12) and healthy donors (HD) (n = 12) matched by age and sex. (b) Black dots indicate healthy donor (HD) samples and white dots indicate Sjögren’s syndrome (SS) samples; the line indicates the median. Gating strategy included lymphocyte gating (based on forward‐ and side‐scatter) followed by doublets, CD14+ and dead cell exclusion. CD4+ T cells were then selected. Mann–Whitney U‐test, P‐values less than 0·05 were considered statistically significant.
Further, the frequency of FoxP3+CD25+ among CXCR5+CD4+ T cells was increased in SS patients (Fig. 2a, middle panel), and the frequency of FoxP3+CXCR5+ T cells showed the same trend (Fig. 2a, lower panel). Of note, the frequency of CXCR5+ among FoxP3+CD25+CD4+ T cells was similar in SS patients and HD donors (Fig. 2a, upper panel). The ratio between Tfr (CD4+FoxP3+CXCR5+) and Tfh (CD4+CXCR5+) cells was increased in SS patients compared to HD (Fig. 2a). Hence, we confirm the studies by Fonseca et al. 2, 4, demonstrating that the ratio between Tfr and Tfh is increased in SS patients.
Figure 2.
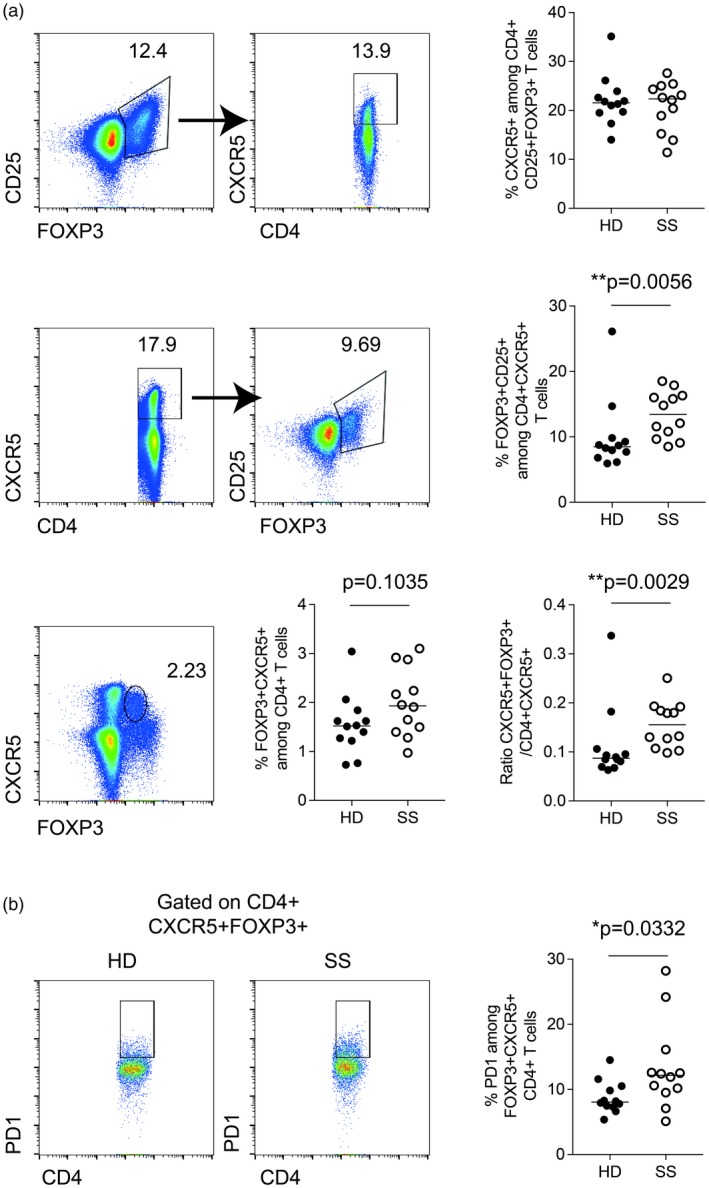
Frequency of CXCR5+forkhead box protein 3 (FoxP3)+CD4+ T cell subsets in peripheral blood mononuclear cells (PBMCs) from patients with Sjögren’s syndrome (SS) and healthy donors. (a) Gating strategy to identify follicular regulatory T cells (Tfr) (FoxP3+CXCR5+) CD4+ T cells. Gating strategy included lymphocyte gating (based on forward‐ and side‐scatter) followed by doublets, CD14+ and dead cell exclusion. CD4+FoxP3+CD25+ T cells (upper panel) or CD4+CXCR5+ (middle panel) were then selected. The ratio between Tfr (CD4+FoxP3+CXCR5+) and Tfh (CD4+CXCR5+) is represented in the lower panel. (b) Representative gating strategy to assess programmed death‐1 (PD‐1)+ frequency among FoxP3+CXCR5+CD4+ T cells. Quantified in PBMC samples from patients with SS (n = 12) and healthy donors (HD) (n = 12) matched by age and sex. Black dots indicate healthy donor (HD) samples and white dots indicate SS samples; the line indicates the median. Mann–Whitney U‐test, P‐values less than 0·05 were considered statistically significant.
Previous reports have identified that a subset of circulating Tfh cells express PD‐1 5, 6, the frequency of which correlates with disease activity scores and serum autoantibodies in systemic lupus erythematosus (SLE) 6. We have previously shown that circulating Tfh PD‐1+ cell frequencies are increased in a subgroup of SS patients 1. The relative count of these cells correlated to autoantibody titers and EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) scores in the study of Fonseca et al. 2. Here, we evaluated the expression of PD‐1 on CXCR5+FoxP3+ cells and found that PD‐1 expression was significantly more common among this subset in SS patients (Fig. 2b). Previous studies have shown that Tfh FoxP3+ T cells is not a homogeneous population, but contains both naive (CD45RA+) and memory T cells (CD45RA–) separating into Fraction I and Fraction III, respectively 4, 7, 8. Here we confirm this finding (Fig. 3), and show that the composition of FoxP3‐positive Tfh cells is not different in SS patients when compared to HD. Our findings highlight the heterogeneity of Tfr, cells as illustrated by their increased expression of PD‐1 in SS patients (Fig. 2b). Hence, functional studies including in‐vitro suppressive assays or FoxP3 Treg‐specific demethylated region methylation assays should take the heterogeneity of follicular regulatory T cells into account to clearly demonstrate their regulatory capacity.
Figure 3.
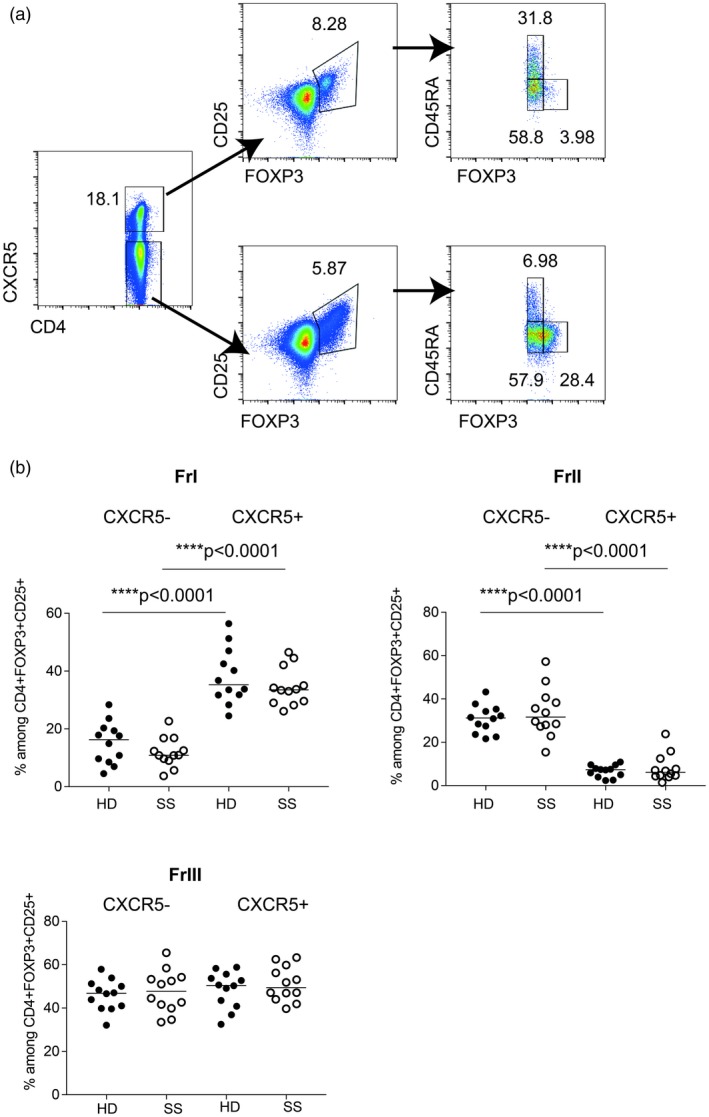
Frequency of regulatory T cell subsets within the follicular regulatory T cells (Tfr) [CD4+forkhead box protein 3 (FoxP3)+CD25+] population in peripheral blood mononuclear cells (PBMCs) from patients with Sjögren’s syndrome (SS) and healthy donors. (a) Representative gating strategy to identify regulatory CD4+ T cell subsets: Fraction I (CD45RA+FoxP3low), Fraction II (CD45RA+CD25high), Fraction III (CD45RA–Fox3low) within CD4+CXCR5+CD25+FoxP3+ T cells (upper panel) and CD4+CXCR5−CD25+FoxP3+ T cells (lower panel) and quantified in PBMC samples from patients with SS (n = 12) and healthy donors (HD) (n = 12) matched by age and sex. (b) Black dots indicate healthy donor (HD) samples and white dots indicate SS samples; the line indicates the median. Gating strategy included lymphocyte gating (based on forward‐ and side‐scatter) followed by doublets, CD14+ and dead cell exclusion. CD4+ T cells were then selected. Mann–Whitney U‐test, P‐values less than 0·05 were considered significant.
In conclusion, we confirm the findings of Fonseca and colleagues and agree that subphenotyping of Tfh and Tfr cells is an important topic in autoantibody‐positive autoimmune diseases. In this letter, we also provide novel data regarding the increased expression of PD‐1 on Tfr in SS patients, of importance in view of the autoimmune side effects of cancer check‐point inhibitor therapies targeting the PD‐1 pathway. The functional relevance of Tfr cells in peripheral blood of SS patients is still not completely understood, but is certainly a mirror of ongoing autoimmune humoral responses at the site of inflammation, and may constitute an important population of cells to consider in therapeutic attempts to rebalance the immune system in this rheumatic disease.
Disclosures
The authors declare no financial or commercial conflicts of interest.
This is a response to the Letter to the Editor submitted by Fonseca, V. R. and Graca, L. Contribution of FoxP300+ Tfr cells to overall human blood CXCR5+ T cells. Clin Exp Immunol 2019; 195:302–04. doi: 10.1111/cei.13245
These letters discuss Aqrawi, L. A., Ivanchenko, M., Björk, A., Ramírez Sepúlveda, J. I., Imgenberg‐Kreuz, J., Kvarnström, M., Haselmayer, P., Jensen, J. L., Nordmark, G., Chemin, K., Skarstein, K. and Wahren‐Herlenius, M. Diminished CXCR5 expression in peripheral blood of patients with Sjögren’s syndrome may relate to both genotype and salivary gland homing. Clin Exp Immunol 2018; 192:259‐270. doi: 10.1111/cei.13118
References
- 1. Aqrawi LA, Ivanchenko M, Bjork A et al Diminished CXCR1 expression in peripheral blood of patients with Sjogren’s syndrome may relate to both genotype and salivary gland homing. Clin Exp Immunol 2018;192:259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fonseca VR, Romao VC, Agua‐Doce A et al The ratio of blood T follicular regulatory cells to T follicular helper cells marks ectopic lymphoid structure formation while activated follicular helper T cells indicate disease activity in primary Sjogren’s syndrome. Arthritis Rheumatol 2018;70:774–84. [DOI] [PubMed] [Google Scholar]
- 3. Verstappen GMNU, Mossel E, Haacke EA et al Is the T follicular regulatory: follicular helper T cell ratio in blood a biomarker for ectopic lymphoid structure formation in Sjögren’s syndrome? Comment on the article by Fonseca et al . Arthritis Rheumatol 2018;70:1354–5. [DOI] [PubMed] [Google Scholar]
- 4. Fonseca VR, Agua‐Doce A, Maceiras AR et al Human blood Tfr cells are indicators of ongoing humoral activity not fully licensed with suppressive function. Sci Immunol 2017;2:eaan 1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Locci M, Havenar‐Daughton C, Landais E et al Human circulating PD‐1+CXCR5–CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 2013;39:758–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. He J, Tsai LM, Leong YA et al Circulating precursor CCR6(lo)PD‐1(hi) CXCR6(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity 2013;39:770–81. [DOI] [PubMed] [Google Scholar]
- 7. Lim HW, Broxmeyer HE, Kim CH. Regulation of trafficking receptor expression in human forkhead box P3+ regulatory T cells. J Immunol 2006;177:840–51. [DOI] [PubMed] [Google Scholar]
- 8. Miyara M, Yoshioka Y, Kitoh A et al Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009;30:899–911. [DOI] [PubMed] [Google Scholar]


