Summary
Thorough understanding of the complex pathophysiology of osteoarthritis (OA) is necessary in order to open new avenues for treatment. The aim of this study was to characterize the CD4+ T cell population and evaluate their activation and polarization status in OA joints. Fifty‐five patients with end‐stage knee OA (Kellgren–Lawrence grades III–IV) who underwent surgery for total knee arthroplasty (TKA) were enrolled into this study. Matched samples of synovial membrane (SM), synovial fluid (SF) and peripheral blood (PB) were analysed for CD3+CD4+CD8– T cell subsets [T helper type 1 (Th1), Th2, Th17, regulatory T cells] and activation status (CD25, CD69, CD45RO, CD45RA, CD62L) by flow cytometry. Subset‐specific cytokines were analysed by cytometric bead array (CBA). SM and SF samples showed a distinct infiltration pattern of CD4+ T cells. In comparison to PB, a higher amount of joint‐derived T cells was polarized into CD3+CD4+CD8– T cell subsets, with the most significant increase for proinflammatory Th1 cells in SF. CBA analysis revealed significantly increased immunomodulating cytokines [interferon (IFN)‐γ, interleukin (IL)‐2 and IL‐10] in SF compared to PB. Whereas in PB only a small proportion of CD4+ T cells were activated, the majority of joint‐derived CD4+ T cells can be characterized as activated effector memory cells (CD69+CD45RO+CD62L–). End‐stage OA knees are characterized by an increased CD4+ T cell polarization towards activated Th1 cells and cytokine secretion compared to PB. This local inflammation may contribute to disease aggravation and eventually perpetuate the disease process.
Keywords: bi‐compartmental knee osteoarthritis, synovial fluid, synovial membrane, T cell polarization, T helper cells
Introduction
Osteoarthritis (OA) is the most prevalent joint disease worldwide. It is the predominant cause for chronic pain and immobility leading to a significant impairment of quality of life years 1. There are currently no therapies to halt disease progression and therapeutic strategies are limited to treating major symptoms during onset and progression of the disease. Total knee arthroplasty (TKA) is the treatment of choice in end‐stage disease, and due to the increasing life expectancy, numbers of TKA procedures have been rising continually 2, 3. Socio‐economic costs of OA comprising direct and indirect costs are inevitably increasing worldwide 4, 5, 6, 7.
It is now accepted that OA pathophysiology cannot be sufficiently described simply by wear and tear. OA is a heterogeneous articular disease, leading to chondrodegeneration, inflammation and periarticular destruction. Reflecting recent literature, a central theory postulates that OA is a result of misbalance between anabolic and catabolic mechanisms, which are mediated via proinflammatory cytokines and cellular pathways 8. There is compelling evidence that synovial inflammation is not only responsible for the main clinical symptoms in OA, but also contributes significantly to disease progression 9. Magnetic resonance imaging (MRI) studies have emphasized the value of synovitis as a prognostic factor for disease progression 10. Immunohistological studies have confirmed infiltration of activated inflammatory cells and production of proinflammatory cytokines in the synovium, with CD4+ T cells being the major constituent 11, 12, 13, 14. Flow cytometry analysis by our group has rendered similar results, with CD4+ T cells being one of the most prevalent mononuclear cells in end‐stage OA 15. Further, recent animal studies have unveiled the therapeutic potential of altering the immune response as a treatment option in OA 16.
However, before this approach can be applied to human disease a much better understanding of the complex cellular and cytokine interactions is necessary. Thus, the aim of this study was to further characterize the CD4+ T cell population and evaluate their activation and polarization status in OA joints. This knowledge is pivotal for evaluation whether immunomodulatory therapy in OA joints could provide an alternative treatment option for OA.
Materials and methods
Study population
A total of 55 patients with primary knee OA (38 women, 17 men) were enrolled into this study with a mean age of 68·4 ± 9·9 years; 26 of those were analysed for intracellular markers and cytokine secretion and 29 were analysed for cell surface markers. OA was defined according to the American College of Rheumatology criteria. All patients had Kellgren–Lawrence (K&L) grades III–IV 17 and were scheduled for total knee replacement surgery at the University Hospital of Heidelberg. None of the patients had a history of underlying inflammatory pathology, intake of disease‐modifying anti‐rheumatic drugs (DMARD) or intra‐articular injection of corticosteroids or hyaluronic acid. Systemic inflammatory parameters [C‐reactive protein (CRP), erythrocyte sedimentation rate (ESR) and white blood cells (WBC)] were within the physiological range at the time of surgery. Only few patients reported occasional intake of non‐steroidal anti‐inflammatory drugs (NSAID) [5], none of them on a daily basis. No significant differences regarding demographic parameters were found between male and female study participants. The local ethics committee approved this study (S‐333/2007). Informed consent from all patients were obtained prior to study enrolment.
Sample collection
Synovial membrane (SM), synovial fluid (SF) and peripheral blood (PB) were harvested at the time of surgery. SF was removed prior to arthrotomy by needle aspiration into sterile tubes and further processed as described below. SM was taken from the suprapatellar pouch intra‐operatively. Heparinized PB samples were taken concurrently during the operation.
Sample preparation
SF samples were treated with bovine testicular hyaluronidase (10 mg/ml; Sigma‐Aldrich, Darmstadt, Germany) for 30 min at 37°C and washed twice with phosphate‐buffered saline (PBS). SM samples were rinsed twice with PBS, minced finely with sterilized scissors and digested with collagenase B (1 mg/ml; Roche, Tucson, AZ, USA) and bovine testicular hyaluronidase (2 mg/ml) at 37°C for 2 h in RPMI‐1640 culture medium supplemented with 10 μg/ml penicillin–streptomycin (Invitrogen, Carlsbad, CA, USA) and 5% fetal calf serum (FCS) (Biochrom AG, Berlin, Germany). The cell suspension was filtered through a 100‐μm (BD Biosciences, San Jose, CA, USA) and a 40‐μm pore‐size cell strainer (EMD Millipore, Temecula, CA, USA) to remove any undigested tissue. The filtered cell suspension was washed twice with PBS. These SF and SM cell suspensions and the heparin anti‐coagulated whole blood were treated with Ficoll‐PaqueTM PLUS (GE Healthcare, Chicago, IL, USA) density gradient centrifugation in order to isolate mononuclear cells. Further, T cells were isolated from mononuclear cells by CD3 magnetic‐activated cell sorting (MACS) bead separation (Miltenyi Biotec, Bergisch Gladbach, Germany), based on a previously described protocol 18.
Flow cytometry analysis of cell surface markers and intracellular staining
Multi‐colour flow cytometry was used to analyse activation status and to identify CD3+CD4+CD8– T cell subsets by their distinct expression of surface and intracellular markers. In brief, for staining of regulatory T cell (Treg)‐specific surface markers, CD3+ MACS‐isolated T cells from PB, SF and SM were washed twice in staining buffer, blocked with FcR blocking reagent (Miltenyi Biotec) and then stained for 30 min at 4°C with fluorescein isothiocyanate (FITC)‐labelled monoclonal antibody (mAb) against CD4 (clone RPA‐T4; BD Biosciences), phycoerythrin (PE)‐labelled mAb against CD25 (clone M‐A251; BD Biosciences) and peridinin‐chlorophyll‐cyanin 5.5 (PerCP‐Cy5.5)‐labelled mAb against CD127 (clone RDR5; eBioscience, San Diego, CA, USA).
For staining of intracellular markers, MACS CD3 isolated T cells from PB, SF and SM were taken into culture at a final density of 106 T cells/ml and incubated for 10 h at 37°C/5% CO2. Cell cultures were stimulated with phorbol–myristate–acetate (PMA) (50 ng/ml) and ionomycin (1 μg/ml; BD Biosciences). After 4 h of activation 50 µl supernatant was harvested for cytometric bead array (CBA) analysis (see below) and brefeldin A (5 μg/ml; Sigma‐Aldrich) was added for another 6 h to prevent secretion and allow intracellular detection of cytokines. After a total of 10 h of activation, T cells were collected, washed twice in fluorescence‐activated cell sorter (FACS buffer) and blocked with FCS blocking reagent.
For surface marker expression, PE‐Cy7 conjugated mAb against CD3 (clone SK7, BD Biosciences), allophycocyanin (APC)‐Cy7 conjugated mAb against CD4 (clone RPA‐T4; BD Biosciences) and VioBlue‐labelled mAb against CD8 (clone BW135/80; Miltenyi Biotec) were used and staining was performed at 4°C for 30 min. Following surface staining, T cells were fixed and permeabilized using cytofix/cytoperm reagent (BD Biosciences) and then stained with APC‐labelled anti‐interferon (IFN)‐γ (clone B27; isotype control clone MOPC‐21; BD Biosciences), FITC‐labelled anti‐IL‐4 (clone MP4‐2502; isotype control clone R3‐34; BD Biosciences) and PE‐labelled anti‐IL‐17A (clone N49‐653; isotype control clone MOPC‐21; BD Biosciences). Cytokine values for CD3+CD4+CD8– T cells were measured by flow cytometry. CD3+CD4+CD8– T cell subsets were defined by intracellular cytokine staining and surface marker expression, respectively, and described as: IFN‐γ [T helper type 1 (Th1)], IL‐4 (Th2), IL‐17A (Th17) and CD25+/highCD127low/– (Treg). Cut‐off was defined based on isotype controls.
Analysis of the activation markers was performed with FITC‐labelled mAb against CD4 (clone RPA‐T4; BD Biosciences) and PE‐labelled mAb specific for one of the activity markers CD45RA (clone HI100; BioLegend, San Diego, CA, USA), CD45RO (clone UCHL1; BioLegend), CD69 (clone FN50; Miltenyi Biotec) and CD62L/L‐selectin (clone DREG56; BD Biosciences). After staining the cells were washed again and taken into a final volume of 200 µl MACS staining buffer. Before flow cytometric detection, 0·5 μg/ml 7‐aminoactinomycin D (7‐AAD) (eBioscience) was added to the cell suspensions to exclude cell debris and dead cells. Flow analysis was performed using a MACSQuant Analyser (Miltenyi Biotec). Data analysis was performed using FlowJo version 9.6 (Treestar, Ashland, OH, USA). The cut‐off for all cell surface markers was defined based on fluorescence minus one (FMO) controls, as described previously 19.
Cytometric bead array
Concentrations of multiple cytokines from supernatants of MACS isolated and stimulated CD3+ T cell cultures from PB, SF and SM (see above) were determined using the human Th1/Th2/Th17 CBA kit (BD Biosciences). This allowed the simultaneous detection of IFN‐γ, IL‐2, IL‐4, IL‐6, IL‐10, IL‐17A and tumour necrosis factor (TNF)‐α. CBA analysis was performed according to the manufacturer’s instructions. In brief, a total sample volume of 50 µl supernatants of activated T cell culture (see above) from PB, SF and SM was mixed with 50 µl of antibody‐coated capture beads and 50 µl of PE‐conjugated detection antibodies. This mixture was incubated for 3 h in the dark at room temperature. The samples were washed with 1 ml buffer at 1100 rpm for 5 min and resuspended in 300 µl wash buffer. Data acquisition by flow cytometry was performed using a MACSQuant Analyser (Miltenyi Biotec).
Statistical analysis
Data are presented as arithmetic mean ± standard error of the mean (s.e.m.), if not stated otherwise. Gaussian distribution was assessed using the Kolmogorov–Smirnov test. To compare concurrent SM, SF and PB samples, we used one‐way analysis of variance (anova) followed by Bonferroni correction or Kruskal–Wallis test followed by Dunn’s post‐hoc test, as appropriate. P‐values < 0·05 were considered significant. Statistical analysis was performed using spss version 22.0 (IBM SPSS Inc., Armonk, NY, USA).
Results
Description of the study population
The study population consisted of 69·1% females with an average body mass index (BMI) of 30·2 ± 6·3 kg/m2 (Table 1). The analysis of peripheral blood inflammatory parameters confirmed that patients had no signs of systemic inflammation. Statistical analysis showed no significant correlation of BMI or age with inflammatory parameters (leucocytes, CRP, ESR; data not shown). Two representative plane radiographs confirmed that patients had advanced OA and presented with K&L grade III (20%) and K&L grade IV (80%).
Table 1.
Characteristics of the study population
| Total study population | Male | Female | P‐values | |
|---|---|---|---|---|
| Number of patients, n | 55 | 17 (30·9%) | 38 (69·1%) | |
| Age at surgery, years | 68·4 ± 9·9 | 70·2 ± 8·7 | 67·7 ± 10·5 | 0·390 |
| Mean ± s.d. (range) | (40–87) | (56–87) | (40–83) | |
| BMI, kg/m2 | 30·2 ± 6·3 | 29·4 ± 5·0 | 30·2 ± 6·8 | 0·910 |
| Mean ± s.d. (range) | (19·5–50·1) | (22·7–40·8) | (19·5–50·1) | |
| Leucocytes/nl | 6·7 ± 1·8 | 6·5 ± 1·6 | 6·8 ± 1·9 | 0·552 |
| Mean ± s.d. (range) | (3·0–12·1) | (4·1–9·4) | (3·0–12·1) | |
| C‐reactive protein, mg/dl | 4·1 ± 3·6 | 3·34 ± 2·5 | 4·4 ± 4·1 | 0·327 |
| Mean ± s.d. (range) | (1·1–20·8) | (1·1–10·2) | (1·9–20·8) | |
| K&L score, n (%) | ||||
| III | 11 (20%) | 3 (17·6%) | 8 (21·1 %) | 0·775 |
| IV | 44 (80%) | 14 (82·4%) | 30 (78·9 %) | |
| Medication | ||||
| DMARD | 0 (0%) | 0 (0%) | 0 (0%) | |
| NSAID (occasional) | 5 (9·1%) | 2 (11.8%) | 3 (7.8%) | |
| NSAID (regular) | 0 (0%) | 0 (0%) | 0 (0%) |
Demographic and clinical parameters of the study population are shown. Values are shown as mean ± standard deviation (range) or as number (P %). Demographic parameters between male and female study participants were compared using the unpaired t‐test for parametric data [age, body mass index (BMI)] and Fisher’s exact test for proportions. All reported P‐values are two‐tailed. A P‐value < 0.05 was considered to show a statistically significant difference. K&L score = Kellgren and Lawrence score; DMARD = disease‐modifying anti‐rheumatic drug; NSAID = non‐steroidal anti‐inflammatory drug; s.d.= standard deviation.
Presence of CD4+ T cells in OA joints and peripheral blood
The frequencies of joint‐derived mononuclear cells (SF and SM) were analysed by flow cytometry and compared to corresponding samples from peripheral blood from the same patient. First, the total lymphocyte cell count after MACS CD3+ T cell isolation was quantified and compared between joint‐derived and PB samples. Total lymphocyte cell count was significantly higher in PB compared to SF and SM, with a mean of 118 049 ± 7929 T lymphocytes per sample compared to 17 832 ± 9902; respectively, 30 063 ± 12 018 T lymphocytes per sample.
This was further put in relation to the acquired sample volume/weight (Table 2). Further, CD4+ T lymphocytes were calculated based on the cell surface marker expression and displayed as percentage of the lymphocyte gate and in relation to sample volume/weight. The articular samples showed a substantial infiltration of CD4+ T cells, with 74·7% CD4+ T cells in SM and 49·4% CD4+ T cells in SF. There were no significant differences compared to 78% CD4+ T cells in PB. The percentage of CD4+ T cells in SM was comparable to peripheral blood and considerably higher than SF.
Table 2.
CD4+ T cell infiltration in peripheral blood (PB) and joint‐derived samples (SF, SM)
| PB | SF | SM | P‐values | |||
|---|---|---|---|---|---|---|
| PB:SF | PB:SM | SF:SM | ||||
| Sample volume/weight | 8·14 ± 0·55 | 10·64 ± 2·3 | 2·94 ± 0·14 | n.a. | n.a. | n.a. |
| (PB/SF: ml; SM: g) | (7·5–8·8) | (5·4–15·8) | (2·7–3·2) | |||
| Cell count of CD3+ MACS‐isolated T lymphocytes | 118 049 ± 7929 | 17 832 ± 9902 | 30 063 ± 12 018 | <0·001** | <0·001** | 0·789 |
| (101 717–134 380) | (4230–39 895) | (5310–54 816) | ||||
| CD4+ T cells % of T lymphocytes | 78·0 ± 1·8 | 49·4 ± 3·8 | 74·7 ± 1·3 | 0·116 | 0·842 | 0·260 |
| (74·3–81·6) | (40·9–57·9) | (72·2–77·3) | ||||
| Cell count of T lymphocytes per sample volume/weight (PB/SF: cells/µl; SM: cells/mg) | 14·2 ± 1·2 | 1·4 ± 0·5 | 11·3 ± 4·8 | 0·105 | 1·000 | 0·300 |
| (11·8–16·7) | (0·3–2·5) | (1·3–21·4) | ||||
| Cell count of CD4+ T cells per sample volume/weight (PB/SF: cells/µl; SM: cells/mg) | 11·3 ± 1·1 | 0·7 ± 0·28 | 9·0 ± 4·1 | <0·001** | 0·105 | 0·132 |
| (9·1–13·5) | (0·08–1·3) | (0·6–17·5) | ||||
Mean volume and weight of acquired and analysed samples are given (n = 55). Further, mean cell count of CD3+ MACS‐isolated T lymphocytes and mean percentage rate of CD3+ magnetic‐activated cell sorting (MACS) isolated T lymphocytes stained positive for CD4 (CD4+ T cells) in peripheral blood (PB), synovial fluid (SF) and synovial membrane (SM) of end‐stage osteoarthritis (OA) patients are shown (n = 29). The cell count was taken in relation to the acquired sample volume/weight to illustrate the amount of inflammatory T cells in the different tissues. In brief, mononuclear cells were isolated from PB, SF and SM by density gradient centrifugation. Further, T lymphocytes were isolated by CD3+ magnetic cell separation (MACS), stained with fluorescein isothiocyanate (FITC)‐labelled monoclonal antibody (mAb) against CD4 (clone RPA‐T4, BD Biosciences) and analysed by flow cytometry. Data are shown as mean ± standard error of the mean (s.e.m.) (confidence interval). Significant differences are marked with asterisks: *P < 0·05; **P < 0·01; n.a. = not applicable.
T cell polarization in OA joint‐derived samples
In order to further characterize the T cell population, we utilized flow cytometry analysis of intracellular and cell surface markers to define the following CD4+ T cell subsets: Th1 (IFN‐γ), Th2 (IL‐4), Th17 (IL‐17A) and Treg (CD25+/highCD127low/–) (Fig. 1) and compared the joint‐derived samples to concurrent samples of PB (Table 3, Supporting information, Fig. S1). Whereas in PB only a small proportion of T cells stained positive for the specific markers of the subsets (Th1: 6·1%, Th2: 1·7%, Th17: 0·4%, Treg: 9·6% of total CD4+ T cells), a significant increase of Th1, Th2 and Th17 cells was detected in SF (Th1: 51·8%, Th2: 4·4%, Th17: 1·9%; P < 0·0001). The highest increase was measured for Th1 with 8.49 times, Th2 with 2.59 and Th17 with 4.75 as high as in PB samples (Table 3, Supporting information, Fig. S1). Thus, the Th1/Th2 and the Th17/Treg balance was shifted notably towards the inflammatory CD4+ T cell subsets in SF. No significant differences were detected between SM and PB, although proinflammatory CD4+ T cell subsets were slightly increased compared to PB (Th17, 1·5‐fold; Th1, 1·31‐fold increase compared to PB). The amount of Tregs was comparable between PB, SF and SM. None of the T cell subsets showed a statistically significant correlation with BMI or age (data not shown).
Figure 1.
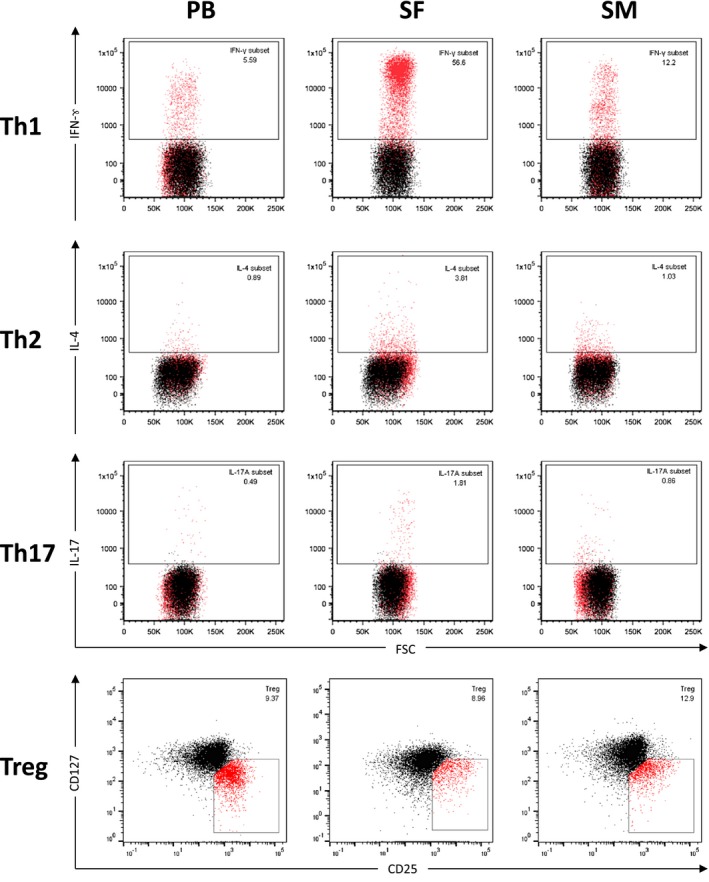
Flow cytometry analysis of CD4+ T cell subsets from samples of peripheral blood, synovial fluid and synovial membrane. Flow cytometry analysis of mononuclear cells derived from synovial membrane (SM), synovial fluid (SF) and peripheral blood (PB) of representative end‐stage OA patients are shown. After isolation and stimulation, T cells were stained with phycoerythrin‐cyanin 7 (PE‐Cy7)‐conjugated monoclonal antibodies (mAb) against CD3 (clone SK7) and VioBlue‐labelled mAb against CD8 (clone BW135/80). Allophycocyanin (APC)‐Cy7‐conjugated mAb against CD4 (clone RPA‐T4) was used to confirm CD4 expression. After permeabilization, cells were stained with APC‐labelled anti‐interferon (IFN)‐γ (clone B27), fluorescein isothiocyanin (FITC)‐labelled anti‐interleukin (IL‐4) (clone MP4‐2502) and PE‐labelled anti‐IL‐17A (clone N49‐653). Mononuclear cells were gated based on their forward‐/side‐scatter (FSC/SSC) profile [numbers in the boxes represent percentage rates (%)] and further defined by cell surface markers as CD3+CD4+CD8– T cells. Th cells were defined by production of their specific cytokines [T helper type 1 (Th1): IFN‐γ, Th2: IL‐4, Th17: IL‐17A) by flow cytometry. Cut‐off was defined by isotype controls (shown as black overlay population). Regulatory T cells (Treg) were identified as CD4+CD25+/highCD127low/– T cells by flow cytometry after staining with FITC‐labelled mAb against CD4 (clone RPA‐T4), PE‐labelled mAb against CD25 (clone MA251) and peridinin chlorophyll (PerCP)‐Cy5.5‐labelled mAb against CD127 (clone RDR5). Cell debris and dead cells were previously excluded [7‐aminoactinomycin D (7‐AAD) staining and FSC profile]. Cut‐off was defined by fluorescence minus one (FMO)/isotype controls, as previously described 19. Representative dot‐plots are shown.
Table 3.
Comparison of T cell polarization in peripheral blood and joint‐derived samples
| PB | SF | SM | P‐values | |||
|---|---|---|---|---|---|---|
| PB:SF | PB:SM | SF:SM | ||||
| Th1 | 6·1 ± 1·4 | 51·8 ± 4·5 | 8·0 ± 2·2 | 0·0001** | 0·925 | 0·0001** |
| (IFN‐γ) | (2·9·–9·2) | (43·4–60·5) | (3·6–17·5) | |||
| Th2 | 1·7 ± 0·2 | 4·4 ± 0·4 | 1·9 ± 0·3 | 0·0001** | 1·000 | 0·0001** |
| (IL‐4) | (1·2–2·1) | (3·7–5·2) | (1·3–2·6) | |||
| Th17 | 0·4 ± 0·1 | 1·9 ± 0·3 | 0·6 ± 0·2 | 0·0001** | 1·000 | 0·001** |
| (IL‐17A) | (0·2–0·6) | (1·4–2·7) | (0·3–1·0) | |||
| Treg | 9·6 ± 1·1 | 7·1 ± 1·2 | 7·8 ± 1·1 | 0·449 | 0·468 | 0·937 |
| (CD25+/highCD127low/–) | (7·3–12·0) | (4·3–9·8) | (5·6–9·9) | |||
| Th1/Th2 ratio | 3·59 | 11·77 | 4·21 | n.a. | n.a. | n.a. |
| Th17/Treg ratio | 0·051 | 0·25 | 0·076 | |||
Percentage rates of CD3+CD4+CD8– T cells stained positive for interferon (IFN)‐γ [T helper type 1 (Th1)], interleukin (IL)‐4 (Th2), IL‐17A (Th17) and CD3+CD4+ T cells stained positive for CD25+/highCD127low/– [regulatory T cells (Treg)] are shown for peripheral blood (PB), synovial fluid (SF) and synovial membrane (SM) of patients with bi‐compartmental end‐stage osteoarthritis (OA) (ICS: n = 17; Treg: n = 24). Intracellular markers were stained with allophycocyanin (APC)‐labelled anti‐IFN‐γ (clone B27), fluorescein isothiocyanate (FITC)‐labelled anti‐IL‐4 (clone MP4‐2502) and phycoerythrin (PE)‐lableled anti‐IL‐17A (clone N49‐653). Surface markers were stained with PE‐labelled anti‐CD25 (clone M‐A251) and peridinin chlorophyll (PerCP)‐cyanin (Cy5.5)‐labelled anti‐CD127 (clone RDR5). Cut‐off was defined by fluorescence minus one (FMO) controls. T helper type 1 (Th1)/Th2 ratios were calculated by mean of Th1 divided by mean of Th2, Th17/Treg ratios were calculated accordingly. Data are shown as mean ± standard error of the mean (s.e.m.) (confidence interval). Significant differences are marked with asterisks: *P < 0·05; ** P < 0·01; n.a. = not applicable.
T cell cytokines in OA synovial fluid and membrane analysed by cytometric bead array
We used CBA to further analyse the secretion of additional T cell cytokines with either proinflammatory (e.g. TNF‐α, IL‐17A, IL‐2) or anti‐inflammatory (IL‐4, IL‐10) effects, as shown in Fig. 2, Supporting information, Table S1. As in the aforementioned analysis, the secreted cytokines of stimulated SF‐derived T cells showed high enrichment, with significantly increased concentrations for proinflammatory IFN‐γ and IL‐2, but also anti‐inflammatory IL‐10 compared to PB and SM. IL‐6 was significantly increased in supernatant of stimulated SM‐derived T cells compared to both PB and SM. IL‐17A and IL‐4 were comparable in PB and synovial tissues. TNF‐α values were notably highest in SF and both SF and SM were higher than PB, albeit not significantly. Overall, supernatant of stimulated PB‐derived T cells showed the lowest secretion of most cytokines, again indicating the local tissues SF and SM to be the source of inflammation in OA.
Figure 2.
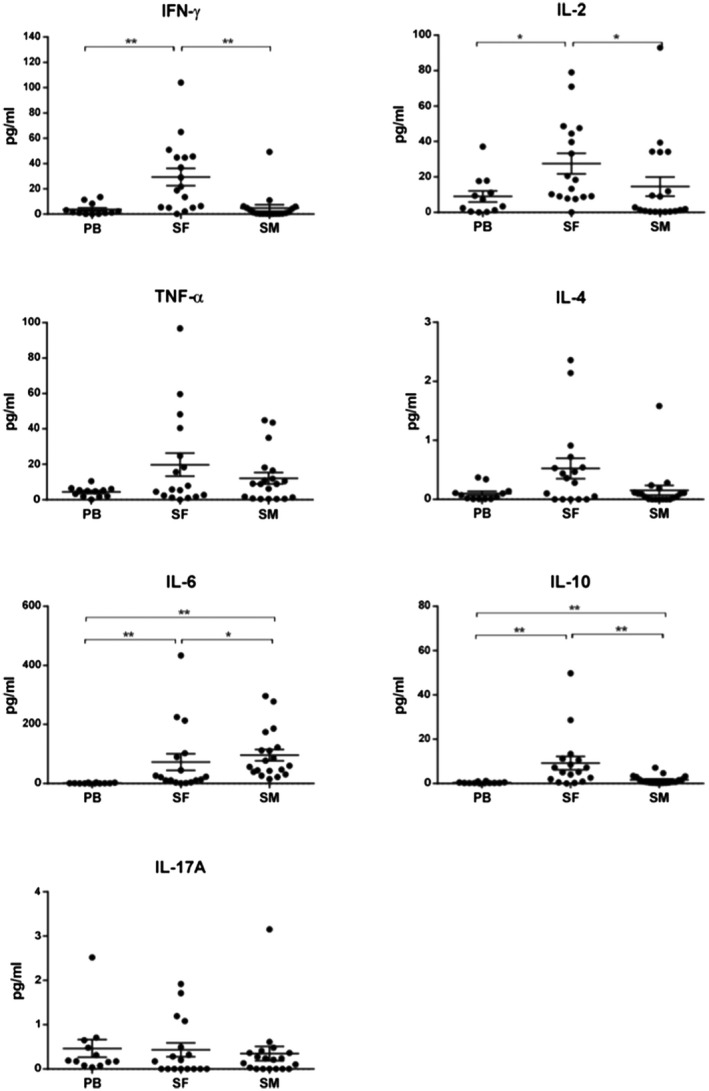
Cytometric bead array (CBA) analysis of secreted cytokines in phorbol–myristate–acetate (PMA)/ionomycin‐stimulated T cell culture supernatants. Amounts of T cell secreted cytokines were analysed using the human T helper type 1 (Th1)/Th2/Th17 CBA kit (BD Biosciences) in PMA/ionomycin‐stimulated CD3+CD8– T cell culture supernatants of peripheral blood (PB), synovial fluid (SF) and synovial membrane (SM) from patients with OA (n = 17). The work steps were performed according to the manufacturer’s instructions. Values in pg/ml are standardized for 1000 cells and shown in scatter dot‐plots as mean ± standard error of the mean (s.e.m.). Significant differences are marked with asterisks: *P < 0·05; **P < 0·01.
Activation status of CD4+ T cells in synovial membrane and peripheral blood
CD4+ T cells from peripheral blood and synovial fluid and synovial membrane were analysed for early, intermediate and late activation markers (Fig. 3, Table 4). Only a small proportion of PB CD4+ T cells expressed CD69 (1·63 ± 0·63%), a common marker for early T cell activation. In SM CD4+ T cells, CD69 expression was significantly increased (76·9 ± 15·3%, P < 0·001), confirming a higher proportion of activated T cells. CD25 as an intermediate activation marker was expressed on 25·0 ± 18·56% in PB and 31·45 ± 9·81% in SM but did not reach statistical significance. A significantly higher amount of joint‐derived CD4+ T cells could be identified as memory cells, as shown by their higher expression of CD45RO (SM, 79·1 ± 5·24; PB, 55·78 ± 16·4%, P < 0·05) and lower expression of CD45RA (SM, 15·82 ± 6·11; PB, 61·9 ± 20·53%, P < 0·0001) in comparison to PB. The significantly higher activation status of SM‐derived T cells was further supported by the expression profile of the cell adhesion molecule L‐selectin (CD62L). CD62L was expressed by 85·9 ± 10·2% of PB CD4+ T cells but decreased significantly in the SM samples to 0·79 ± 0·06%, P < 0·001.
Figure 3.
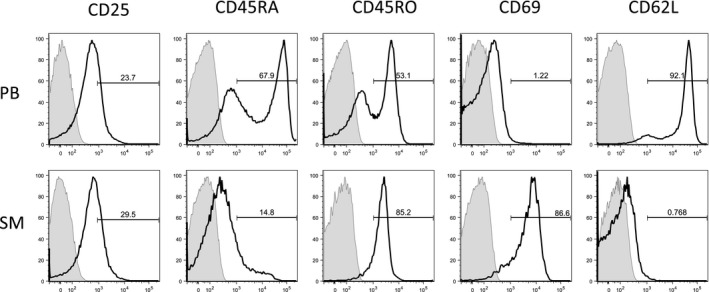
Activation status of CD4+ T cells in in peripheral blood and synovial membrane. Flow cytometry analysis of activation markers from one representative end‐stage bi‐compartmental osteoarthritis (OA) patient is shown for peripheral blood (PB) and synovial membrane (SM). Samples were stained with anti‐CD4‐fluorescein isothiocyanate (clone 2A3) to identify CD4+ cells. The cells were further stained for the following surface markers: CD25 (clone RDR5), CD45RA (clone HI100), CD45RO (clone UCHL1), CD69 (clone FN50) and CD62L (L‐selectin, clone DREG‐56). Cells stained with isotype controls are shadowed in the histograms. The bold‐type line represents the CD4+ T cells.
Table 4.
Activation status of CD4+ T cells in peripheral blood and joint‐derived samples
| PB | SF | SM | P‐values | |||
|---|---|---|---|---|---|---|
| PB:SF | PB:SM | SF:SM | ||||
| CD25 | 25·0 ± 18·56 | 32·28 ± 11·1 | 31·45 ± 9·81 | n.s. | n.s. | n.s. |
| (18·9–31·2) | (27·4–37·2) | (27·2–35·7) | ||||
| CD45RA | 61·9 ± 20·53 | 17·1 ± 6·59 | 15·82 ± 6·11 | <0·0001** | <0·0001** | n.s. |
| (51·93–72·1) | (12·5–29·6) | (11·6–27·4) | ||||
| CD45RO | 55·78 ± 16·4 | 77·9 ± 5·5 | 79·1 ± 5·24 | n.s. | <0·05* | n.s. |
| (47·9–63·6) | (72·3–84·4) | (73·9–83·4) | ||||
| CD69 | 1·63 ± 0·63 | 71·4 ± 21·5 | 76·9 ± 15·3 | <0·001** | <0·001** | n.s. |
| (1·2–1·8) | (63·4–90·3) | (63·4–90·2) | ||||
| CD62L | 85·9 ± 10·2 | 0·79 ± 0·09 | 0·77 ± 0·06 | <0·05* | <0·05* | n.s. |
| (81·11–90·8) | (0·64–0·89) | (0·7–0·9) | ||||
Percentage rates of CD4+ T cells stained positive for activation markers from patients with OA (n=16) are shown. Samples were stained with anti‐CD4‐fluorescein isothiocyanate (clone 2A3) to identify CD4+ cells. The cells were further stained for the following surface markers: CD25 (clone RDR5), CD45RA (clone HI100), CD45RO (clone UCHL1), CD69 (clone FN50), and CD62L (L‐selectin, clone DREG‐56). Values are shown as mean ± standard error of the mean (s.e.m.) (confidence interval). Significant differences are marked with asterisks: *P < 0·05; **P < 0·01; ***P < 0·0001.
Discussion
Even though some studies have described systemic changes in OA patients regarding C‐reactive protein and T cell subsets 20, OA inflammation affects primarily the joint itself. For this reason the analysis of joint‐derived samples has been our focus in order to map the pattern of synovial inflammation 21. The SM is the main site of inflammatory cell interaction, as shown through macroscopic changes (e.g. hypertrophy in end‐stage OA) as well as in MRI studies 10, 13. SF is easily accessible, and its analysis has contributed significantly to the understanding of other inflammatory joint diseases. SF and SM could host different cell populations and play a different role in joint biology and pathology, which is why we utilized matched samples of all three compartments (PB, SF, SM) in this study.
Our data reveal that in end‐stage OA an inflammatory milieu with a significant increase of proinflammatory Th1 cells is present in SF when compared to PB and SM. We did not find any significant increase of Treg cells. Even though Th2 was also significantly increased in SF, the ratios were shifted towards an inflammatory environment with Th1 : Th2 ratios of 3·59 in PB and 11·77 in SF and Th17 : Treg of 0·051 in PB and 0·25 in SF.
The percentage of CD4+ T cells in SM was comparable to PB and higher than SF. This is remarkable, especially in light of the fact that synovial membrane comprises various different cell types, e.g. fibroblasts, synoviocytes and adipocytes. Interestingly, the SM did not display the same pattern as SF and presented a comparable T cell polarization status to PB. This highlights the fact that SF and SM present different compartments, and analysis of both is essential in order to understand inflammatory pathways. It is imaginable that T cells migrate between SF and SM and in end‐stage OA accumulate in the SF 22.
We utilized intracellular staining of cytokines to clearly identify and detect T cell subsets. Even though this is an elegant method to identify cells, it is possible that the cytokine production ability of T cells deteriorates in a chronic inflammatory setting such as the OA joint, which could lead to a detection bias. Our data are in accordance with a recent study by Penatti et al., which showed a Th1 infiltrate and no enrichment of Tregs in SF of OA patients by using cell surface markers 23. Thus, the observed inflammatory milieu in OA joints is not a phenomenon of detection strategy. Further, it should be taken into account that the total CD4+ T cell concentration was higher in SM, which means that the total amount of CD4+ T cell subsets also remain higher in SM. The majority of previous reports either solely analysed SF samples or utilized histology when SM samples were included 24, 25, 26, 27. Although a number of studies demonstrated dominant Th1 polarization and cytokine secretion in OA joints with quantitatively negligible Th2 and Th17 infiltration 14, 25, 28, quantitative analysis of T cell subpopulations comparing joint‐derived and peripheral samples are missing. Our findings complement these immunohistological reports by providing in‐depth analysis of matched SF and SM sample analysis through flow cytometry, which clearly identifies CD4+ T cell subsets and provides quantitative data.
What triggers the infiltration of inflammatory cells into OA joints remains unclear at this stage. A histological study has revealed that infiltrating T cells in the synovium of OA patients were mainly localized in the sublining layer, suggesting that these cells were derived from vessels 25. Degenerative joint pathologies such as meniscopathies or cartilage defects could be a source for the chemotaxis of leucocytes into the SM through increased expression of adhesion molecules and chemokines in the endothelium 22, 29. If and how these cells are released from the SM into the SF remains unknown.
The shift towards proinflammatory subsets in SF could be due to either polarization processes taking place in the joints or to selective recruitment of inflammatory cells. We previously showed the abundant presence of CD14+ macrophages in the SM of patients with uni‐ and bi‐compartmental OA, which have the ability of antigen presentation 15. The study by Nakamura et al. showed oligoclonality of the T cell receptor of joint‐infiltrating T cells through sequencing analysis, suggesting an antigen‐driven immune reaction in the synovium of OA patients. Degradation products of cartilage matrix and secreted proteins from chondrocytes [cartilage intermediate layer protein (CILP) and YKL‐39] could act as autoantigens in OA 30, 31, 32. Conversely, activated T cells in the joints might accumulate in an antigen‐non‐specific manner in response to local inflammation, as shown in rheumatoid arthritis (RA) 33.
The enrichment of T cells in the joints, and the shift towards an inflammatory milieu raises the question of the contribution of these cells to OA onset and progression. Functional studies evaluating the role of T cells on the target tissues such as cartilage, ligaments and bone are rare. Nakamura et al. described the enhanced production of matrix metalloproteinases (MMP)‐1, MMP‐3 and MMP‐13 in direct co‐culture experiments of chondrocytes with autologous T cells 34. An imbalance of Th1/Th2‐type cytokines has been known to play a crucial role in autoimmune diseases such as RA. Our results indicate that T cell recruitment and activation, especially of Th1 cells, is present in end‐stage bi‐compartmental OA. The moderate increase of Th17 cells in OA SF and the fact that CBA analysis did not show a significant increase of IL‐17A in OA joints suggest that these cells play a subordinate role in OA inflammation. It should be taken into account that this study is representative of end‐stage OA patients. IL‐17 has been shown to play a crucial role in the initiation of RA 35. Therefore, it would be of utmost interest to analyse T cell polarization in early OA. It should also be taken into account that the quantity of cells does not necessary translate into quality in terms of their involvement in disease pathogenesis. The exact impact and role of these cells can only be tested in functional studies.
In contrast to these proinflammatory CD4+ T cells, naturally occurring regulatory T cells have been shown to play an essential role in establishing the balance between pro‐ and anti‐inflammatory mechanisms in the periphery and maintaining self‐tolerance 36. The suppressive function of Tregs and their ability to regulate immune reactions ascribes them a key role in the pathophysiology of autoimmune diseases and makes them an interesting target for treatment 37. In this study, there was no enrichment of Tregs in the affected joints. This is contrary to our previous report, where patients with earlier stages of OA were included 24. Thus, the increase of synovial Tregs could be a footprint feature of earlier OA stages.
Some groups have proposed TNF‐α as a key player in OA pathogenesis 38, while others have suggested IL‐1β 39 and IL‐6 39, 40. We could not detect significant differences of TNF‐α in our samples, which is contrary to these reports. TNF‐α could play a more relevant role at earlier stages of OA, and may have contributed to the expression of downstream mediators in late‐stage OA. This hypothesis is strengthened further by a recent study showing that serum levels of IL‐1β and TNF‐α are increased in early compared to advanced OA 29, 41, concluding that they play a more important role in developing OA, while subsiding with disease progression. The synovial analysis performed in our study suggests that the proinflammatory cytokines IFN‐γ and IL‐2 are relevant in maintaining and increasing the proinflammatory milieu in end‐stage OA, thereby attracting the migration of inflammatory cells into the synovial membrane.
We further aimed to analyse the activation status of CD4+ T cells. The majority of the PB CD4+ T cells were naive cells, as shown by their high expression of CD45RA and low expression of CD45RO. CD45RO is associated with proliferative responses to recall antigens, whereas CD45RA is a marker for naive T cells. In contrast, SM CD4+ T cells showed the expression profile for memory cells with a significantly higher expression of CD45RO. Significant differences between the periphery and the SM were also observed when analysed for activation markers CD69 and CD62L. Due to the fact that the aforementioned activation marker status (activated effector memory T cell) of SM‐derived T cells is largely transient, it could be supposed that these cells might be recently activated cells, reacting on antigens in the intra‐articular environment. The presence of activated T cells with a proinflammatory polarization in patients with OA suggests that T cells contribute to chronic inflammation in a large proportion of these patients 24. Consistent with our results, Yamada et al. found that almost all CD4+ T cells in OA joints express activation markers 28.
We did not include healthy joint‐derived samples due to ethical considerations. Histological studies have shown that lymphocyte infiltration is hardly present in healthy joint samples 13. Despite these limitations, to our best knowledge this is one of a very limited number of studies using flow cytometry to analyse the inflammatory pattern in OA. Whereas conventional studies in SM are focused on immunohistochemical analysis, our approach permits a much more detailed study of lymphocyte infiltration. We were able to generate a homogeneous study population in terms of affected joint and disease stage and included a high number of matched peripheral blood and joint‐derived samples, which we consider to be the relevant site of cell interaction. It should be considered that OA remains a heterogeneous disease, and our study population of patients with end‐stage OA might represent only a fraction of OA pathophysiology. A previous report by our group showed significant differences between joint‐derived samples of patients with different OA subtypes 15. In accordance with this, histological examination by Benito et al. has shown that increased mononuclear cell infiltration and over‐expression of mediators of inflammation were seen in early OA, compared with late OA 42. This suggests that mononuclear cell infiltration and polarization shows a temporal pattern and changes with OA stage.
It is important to elucidate the mechanism of chronic inflammation in OA to identify possible new therapeutic approaches. Our study provides insight into mechanisms driving inflammatory infiltration. Even though it remains unclear at this stage whether inflammation predates OA development or is a consequence of it, we consider this not to be the most urgent question. Inflammation clearly contributes to OA pathophysiology and is associated with symptoms and progression of cartilage loss. Further investigation on T cell function to elucidate immunomodulatory treatment options, especially in early OA, are urgently required.
Disclosures
The funding source did not have any involvement in study design, data collection, analysis and interpretation of data, writing of the report and the decision to submit the paper for publication. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript. The authors have no potential or apparent conflicts of interest with regard to this work.
Author contributions
B. M., T. T., L. H.‐M. and N. R. made substantial contributions to study conception and design. N. R. and E. T. carried out the flow cytometry and CBA experiments. N. R., B. M. and E. T. made substantial contributions to data acquisition. N. R., B. M., J. K., F. Z. and S. H. made substantial contribution to data analysis and interpretation. N. R. and B. M. drafted the manuscript and E. T., S. H., J. K., T. G., L. H.‐M., T. T. and F. Z. revised it critically. N. R. and B. M. performed the statistical analysis. All authors read and approved the final manuscript.
Supporting information
Table S1. CBA analysis of secreted cytokines in PMA/Ionomycin‐stimulated T cell culture supernatants.
Fig. S1. Comparison of T cell polarization in peripheral blood, synovial fluid and synovial membrane.
Acknowledgements
The University of Heidelberg funded this study. We are grateful for the technical support of Patrick Göthlich.
References
- 1. Jinks C, Jordan K, Croft P. Measuring the population impact of knee pain and disability with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Pain 2002; 100:55–64. [DOI] [PubMed] [Google Scholar]
- 2. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007; 89:780–5. [DOI] [PubMed] [Google Scholar]
- 3. Carr AJ, Robertsson O, Graves S et al Knee replacement. Lancet 2012; 379:1331–40. [DOI] [PubMed] [Google Scholar]
- 4. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am 2014; 96:624–30. [DOI] [PubMed] [Google Scholar]
- 5. Piscitelli P, Iolascon G, Di Tanna G et al Socioeconomic burden of total joint arthroplasty for symptomatic hip and knee osteoarthritis in the Italian population: a 5‐year analysis based on hospitalization records. Arthritis Care Res (Hoboken) 2012; 64:1320–7. [DOI] [PubMed] [Google Scholar]
- 6. Loza E, Lopez‐Gomez JM, Abasolo L, Maese J, Carmona L, Batlle‐Gualda E. Economic burden of knee and hip osteoarthritis in Spain. Arthritis Rheum 2009; 61:158–65. [DOI] [PubMed] [Google Scholar]
- 7. Hawker GA, Badley EM, Croxford R et al A population‐based nested case–control study of the costs of hip and knee replacement surgery. Med Care 2009; 47:732–41. [DOI] [PubMed] [Google Scholar]
- 8. Goldring MB, Otero M, Tsuchimochi K, Ijiri K, Li Y. Defining the roles of inflammatory and anabolic cytokines in cartilage metabolism. Ann Rheum Dis 2008; 67 (Suppl 3):iii75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis–results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage 2005; 13:361–7. [DOI] [PubMed] [Google Scholar]
- 10. Roemer FW, Guermazi A, Felson DT et al Presence of MRI‐detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30‐month follow‐up: the MOST study. Ann Rheum Dis 2011; 70:1804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oehler S, Neureiter D, Meyer‐Scholten C, Aigner T. Subtyping of osteoarthritic synoviopathy. Clin Exp Rheumatol 2002; 20:633–40. [PubMed] [Google Scholar]
- 12. Smith MD, Triantafillou S, Parker A, Youssef PP, Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol 1997; 24:365–71. [PubMed] [Google Scholar]
- 13. Rollin R, Marco F, Jover JA et al Early lymphocyte activation in the synovial microenvironment in patients with osteoarthritis: comparison with rheumatoid arthritis patients and healthy controls. Rheumatol Int 2008; 28:757–64. [DOI] [PubMed] [Google Scholar]
- 14. Haynes MK, Hume EL, Smith JB. Phenotypic characterization of inflammatory cells from osteoarthritic synovium and synovial fluids. Clin Immunol 2002; 105:315–25. [DOI] [PubMed] [Google Scholar]
- 15. Moradi B, Rosshirt N, Tripel E et al Unicompartmental and bicompartmental knee osteoarthritis show different patterns of mononuclear cell infiltration and cytokine release in the affected joints. Clin Exp Immunol 2015; 180:143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Q, Rozelle AL, Lepus CM et al Identification of a central role for complement in osteoarthritis. Nat Med 2011; 17:1674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kellgren JH, Lawrence JS. Radiological assessment of osteo‐arthrosis. Ann Rheum Dis 1957; 16:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cao D, van Vollenhoven R, Klareskog L, Trollmo C, Malmstrom V. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther 2004; 6:R335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moradi B, Schnatzer P, Hagmann S et al CD4+CD25+/highCD127low/– regulatory T cells are enriched in rheumatoid arthritis and osteoarthritis joints–analysis of frequency and phenotype in synovial membrane, synovial fluid and peripheral blood. Arthritis Res Ther 2014; 16:R97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jin X, Beguerie JR, Zhang W et al Circulating C reactive protein in osteoarthritis: a systematic review and meta‐analysis. Ann Rheum Dis 2015; 74:703–10. [DOI] [PubMed] [Google Scholar]
- 21. Qi C, Shan Y, Wang J et al Circulating T helper 9 cells and increased serum interleukin‐9 levels in patients with knee osteoarthritis. Clin Exp Pharmacol Physiol 2016; 43:528–34. [DOI] [PubMed] [Google Scholar]
- 22. Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity 2014; 41:694–707. [DOI] [PubMed] [Google Scholar]
- 23. Penatti A, Facciotti F, De Matteis R et al Differences in serum and synovial CD4+ T cells and cytokine profiles to stratify patients with inflammatory osteoarthritis and rheumatoid arthritis. Arthritis Res Ther 2017; 19:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sakkas LI, Scanzello C, Johanson N et al T cells and T‐cell cytokine transcripts in the synovial membrane in patients with osteoarthritis. Clin Diagn Lab Immunol 1998; 5:430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ishii H, Tanaka H, Katoh K, Nakamura H, Nagashima M, Yoshino S. Characterization of infiltrating T cells and Th1/Th2‐type cytokines in the synovium of patients with osteoarthritis. Osteoarthritis Cartilage 2002; 10:277–81. [DOI] [PubMed] [Google Scholar]
- 26. Nakamura H, Yoshino S, Kato T, Tsuruha J, Nishioka K. T‐cell mediated inflammatory pathway in osteoarthritis. Osteoarthritis Cartilage 1999; 7:401–2. [DOI] [PubMed] [Google Scholar]
- 27. Sakkas LI, Platsoucas CD. The role of T cells in the pathogenesis of osteoarthritis. Arthritis Rheum 2007; 56:409–24. [DOI] [PubMed] [Google Scholar]
- 28. Yamada H, Nakashima Y, Okazaki K et al Preferential accumulation of activated Th1 cells not only in rheumatoid arthritis but also in osteoarthritis joints. J Rheumatol 2011; 38:1569–75. [DOI] [PubMed] [Google Scholar]
- 29. Marks PH, Donaldson ML. Inflammatory cytokine profiles associated with chondral damage in the anterior cruciate ligament‐deficient knee. Arthroscopy 2005; 21:1342–7. [DOI] [PubMed] [Google Scholar]
- 30. Du H, Masuko‐Hongo K, Nakamura H et al The prevalence of autoantibodies against cartilage intermediate layer protein, YKL‐39, osteopontin, and cyclic citrullinated peptide in patients with early‐stage knee osteoarthritis: evidence of a variety of autoimmune processes. Rheumatol Int 2005; 26:35–41. [DOI] [PubMed] [Google Scholar]
- 31. Tsuruha J, Masuko‐Hongo K, Kato T et al Autoimmunity against YKL‐39, a human cartilage derived protein, in patients with osteoarthritis. J Rheumatol 2002; 29:1459–66. [PubMed] [Google Scholar]
- 32. Tsuruha J, Masuko‐Hongo K, Kato T, Sakata M, Nakamura H, Nishioka K. Implication of cartilage intermediate layer protein in cartilage destruction in subsets of patients with osteoarthritis and rheumatoid arthritis. Arthritis Rheum 2001; 44:838–45. [DOI] [PubMed] [Google Scholar]
- 33. Sattler A, Wagner U, Rossol M et al Cytokine‐induced human IFN‐gamma‐secreting effector‐memory Th cells in chronic autoimmune inflammation. Blood 2009; 113:1948–56. [DOI] [PubMed] [Google Scholar]
- 34. Nakamura H, Tanaka M, Masuko‐Hongo K et al Enhanced production of MMP‐1, MMP‐3, MMP‐13, and RANTES by interaction of chondrocytes with autologous T cells. Rheumatol Int 2006; 26:984–90. [DOI] [PubMed] [Google Scholar]
- 35. Lurati A, Laria A, Gatti A, Brando B, Scarpellini M. Different T cells' distribution and activation degree of Th17 CD4+ cells in peripheral blood in patients with osteoarthritis, rheumatoid arthritis, and healthy donors: preliminary results of the MAGENTA CLICAO study. Open Access Rheumatology: Research and Reviews 2015; 7:63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sakaguchi S, Sakaguchi N, Shimizu J et al Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev 2001; 182:18–32. [DOI] [PubMed] [Google Scholar]
- 37. Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med 2001; 193:1303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Uchida K, Satoh M, Inoue G et al CD11c(+) macrophages and levels of TNF‐alpha and MMP‐3 are increased in synovial and adipose tissues of osteoarthritic mice with hyperlipidaemia. Clin Exp Immunol 2015; 180:551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Goekoop RJ, Kloppenburg M, Kroon HM et al Low innate production of interleukin‐1beta and interleukin‐6 is associated with the absence of osteoarthritis in old age. Osteoarthritis Cartilage 2010; 18:942–7. [DOI] [PubMed] [Google Scholar]
- 40. Riyazi N, Slagboom E, de Craen AJ et al Association of the risk of osteoarthritis with high innate production of interleukin‐1beta and low innate production of interleukin‐10 ex vivo, upon lipopolysaccharide stimulation. Arthritis Rheum 2005; 52:1443–50. [DOI] [PubMed] [Google Scholar]
- 41. Barker T, Rogers VE, Henriksen VT et al Serum cytokines are increased and circulating micronutrients are not altered in subjects with early compared to advanced knee osteoarthritis. Cytokine 2014; 68:133–6. [DOI] [PubMed] [Google Scholar]
- 42. Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis 2005; 64:1263–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. CBA analysis of secreted cytokines in PMA/Ionomycin‐stimulated T cell culture supernatants.
Fig. S1. Comparison of T cell polarization in peripheral blood, synovial fluid and synovial membrane.


