Summary
Current researches have determined the significance of C‐C chemokine receptor (CCR)6 expression as either a marker of T helper cells (Th) or an effector and regulator of T cell function. However, the roles of CCR6 in the pathogenesis of immune thrombocytopenia (ITP) are unclear. In this study, we aimed to investigate the phenotype and functional characteristics of circulating CCR6+ T cells in blood from chronic ITP patients and healthy controls. We found that the frequency of CCR6+CD4+ cells was higher in ITP patients than in healthy controls. Anti‐CD3/anti‐CD28 stimulation induced rapid expansion of CCR6+CD4+ cells in ITP patients. CCR6+CD4+ cells had a phenotype of activated cells and predominantly expressed CD45RO. Forkhead box protein P3 (FoxP3) and CD25‐positive cells were exclusively detected within the CCR6+CD4+ cells. In ITP patients, CCR6+ regulatory T cells (Treg) were decreased and positively correlated with platelet counts and transforming growth factor (TGF)‐β plasma levels. In contrast to CCR6– counterparts, CCR6+CD4+ cells produced higher levels of interleukin (IL)‐17A. The frequency of CCR6+ Th17 was higher in ITP patients and positively correlated with IL‐17A levels in supernatant. Most importantly, CCR6+CD4+ cell subpopulations, but not CCR6−CD4+, were closely correlated to treatment response of ITP patients. These findings suggest that circulating CCR6+CD4+ cells in ITP patients have characteristics of activated memory Th17 phenotype and could be used to monitor disease activity and treatment response.
Keywords: CCR6, immune thrombocytopenia, memory, regulatory T cell, T helper cell
Introduction
Immune thrombocytopenia (ITP) is currently defined as an acquired autoimmune disorder characterized by a low peripheral blood platelet count 1. The underlying abnormalities involved in the pathogenesis of ITP appear to be complex. In addition to the classical mechanism of glycoprotein (GP)‐specific autoantibody‐mediated platelet destruction, cellular dysfunction has been implicated in ITP pathogenesis, such as antigen‐presenting cell (APC) defects, T cell‐mediated immunity disorders and B cell activation 1, 2, 3.
Abnormal T cells have been described in patients with ITP, including a higher T helper cell (Th) reactivity against platelets 4, 5, 6 and decreased number and function of regulatory T cells (Treg) 7, 8, 9. Th cells are central organizers in immune response 4 and can be distinguished by differential expression of chemokine receptors that facilitate inflammation response 10, 11, 12, 13. For instance, Th1 cells express C‐X‐C chemokine receptor (CXCR)3 and C‐C chemokine receptor (CCR)5 10, 11, whereas Th17 cells express CCR4 and CCR6 12, 13. Chemokine receptors are dynamically modulated during the process of naive CD4+ T cell activation and differentiation 14. Meanwhile, these chemokine receptors are thought to function in positive feedback loops to amplify Th1 and Th17‐like inflammatory responses 15, 16. Human Tregs characterized as CD4+CD25+forkhead box protein 3 (Foxp3+) cells play an important role in maintaining immune tolerance by suppressing both cell‐mediated and antibody‐mediated immune responses 17. Tregs are reported to express chemokine receptors such as chemokine receptor 6 (CCR6), CCR4, C‐X‐C motif chemokine receptor 3 (CXCR3) and CCR10 18. Heterogeneous subsets of FoxP3+ Treg cells identified by chemokine receptors are functionally suppressive and respond differently to Th1 and Th17 responses 18. Constitutive expression of CCR4 and CCR8 on the majority of circulating Treg cells may control the dynamics of other effector T cells for interacting with APCs 19. Because the expression of chemokine receptors is closely linked to the function of the cells, identifying chemokine receptors by immune cells is important in determining favorable immune outcomes in both disease and therapeutic strategies.
CCR6 is a seven transmembrane G‐protein‐coupled receptor (GPCR) that is found predominantly on T cells, B cells and monocyte‐derived dendritic cells (DCs) 20. C‐C motif chemokine ligand 20 (CCL20) is identified to be the sole ligand for CCR6 on peripheral blood T cells 20. CCR6 is present on a substantial fraction of regulatory CD25+CD4+ T cells 21, 22. Furthermore, CCR6+ Treg cells exhibit the phenotype of activated effector‐memory cells and suppress immune responsiveness 21, 22. CCR6+ Treg cells have been identified in some diseases, including recurrent miscarriage and RA, and might play a role in disease development 23, 24. The CCR6+ Th cell population is heterogeneous, and several subpopulations can be distinguished, including interleukin (IL)‐17, IL‐22 and interferon gamma (IFN)‐γ‐secreting cells 25. CCR6+ Th cells and over‐production of their signature cytokines are associated with persistent inflammation and autoimmune diseases 26, 27. Therefore, CCR6 has a role in balancing inflammation versus immune intolerance by regulating the balance between opposing Th cells and Treg cells 15, 18, 25.
Pathogenic roles of such alterations in CCR6 activity and nature of expression by immune cells in ITP have been unexplored to date. In this study, we have characterized the phenotypical differences between CCR6+ and CCR6− subsets to assess its prevalence and activity in peripheral CD4 cells and analyze the correlation with the response of treatment in patients with chronic ITP.
Materials and methods
Patient data and design
Peripheral blood samples from 36 patients with ITP who had not been treated with any therapy for at least 1 week before sampling were enrolled at our hospital between November 2015 and July 2017. All the cases met the diagnostic criteria of chronic ITP, as described previously 28. Among them, 26 patients had evaluation of platelet antibodies. We followed‐up on 31 patients (platelet count < 30 × 109/l) at baseline and at a later time‐point (28 days) following treatment to analyze the dynamic change of CCR6+CD4+ T subsets. The response after each treatment was recorded. A complete response (CR) is defined as any platelet count of at least 100 × 109/l, and a response (R) is defined as a platelet count greater than 30 × 109/l and at least a doubling of the baseline level. No response (NR) is defined as any platelet count lower than 30 × 109/l or less than doubling of the baseline count 28. Age‐ and sex‐matched healthy controls (n = 43) were obtained from the medical examination center. Characteristics of ITP patients and healthy controls can be found in Table 1. The study was approved by the Institutional Review Board and written informed consent was obtained from each study subject.
Table 1.
Main demographic and clinical characteristics of the patients with chronic immune thrombocytopenia and healthy controls
| Characteristics | Patients (n = 36) | Controls (n = 43) |
|---|---|---|
| Female/male, n | 18/18 | 25/18 |
| Age, mean ± s.d. | 46·5 ± 13·9 | 40·0 ± 13·7 |
| Platelet count, × 109/l | 17·9 ± 11·2 | 218·6 ± 40·6 |
| Disease duration, months, mean ± s.d. | 35·1 ± 21·3 | |
| Anti‐platelet autoantibody pattern, n/total number (%) | ||
| GPIIb/IIIa | 4/26 (15·4) | |
| GPIb/IX | 3/26 (11·5) | |
| GPIIb/IIIa+GPIa/IIa | 4/26 (15·4) | |
| GP IIb/IIIa+GPIa/IIa+GPIb/IX | 1/26 (3·8) | |
| None | 14/26 (53·9) | |
| Therapy, n/total number (%) | ||
| GC | 11/36 (30·6) | |
| GC+IVIg | 5/36 (13·8) | |
| GC+DNZ | 4/36 (11·1) | |
| GC+rhTPO | 2/36 (5·6) | |
| GC+IVIg+rhTPO | 8/36 (22·2) | |
| GC+IVIg+VCR | 1/36 (2·8) | |
| None | 5/36 (13·9) |
M = male; F = female; GC = glucocorticoid; IVIg = intravenous immunoglobulin; DNZ = danazol; VCR = vincristine; rhTPO = recombinant human thrombopoietin.
Plasma and cell preparation
Plasma obtained from centrifuged peripheral blood samples anti‐coagulated with ethylenediamine tetraacetic acid (EDTA) was stored at –80°C until assay. Peripheral blood mononuclear cells (PBMCs) were separated by centrifugation over a Ficoll‐Hypaque gradient (Amersham Pharmacia Biotech, Little Chalfont, UK) and resuspended in RPMI‐1640 medium (Invitrogen, Carlsbad, CA, USA) containing 10% fetal calf serum (FCS) for future use.
Cell culture and stimulation
PBMCs were adjusted to 1 × 106/ml in complete RPMI‐1640 medium, and cultured in 24‐well culture plates and incubated in humidified air in 5% CO2 at 37°C. PBMCs were treated with coated 1 μg/ml anti‐CD3 (OKT3; Biolegend, San Diego, CA, USA) and 0·5 μg/ml anti‐CD28 (CD28.2; Biolegend) for up to 12, 24, 48 or 72 h. For Treg cells, 20 U/ml IL‐2 was added to the cultures and cells were cultured overnight.
Flow cytometric analysis
For surface marker detection, PBMCs were labeled by APC anti‐human CD4, APC anti‐human CD8, phycoerythrin‐cyanin 7 (PE‐Cy7) anti‐human CCR6, fluorescein isothiocyanate (FITC) anti‐human CD25, APC anti‐human CD69 or their respective immunoglobulin (IgG) isotype controls (all from Biolegend). For intracellular detection, PBMCs were stimulated with 50 ng/ml phorbol–myristate–acetate (PMA) and 500 ng/ml ionomycin (Sigma‐Aldrich, St Louis, MO, USA) for 5 h in the presence of GolgiStop (BD Biosciences, San Diego, CA, USA). After incubation, the cells were first stained for surface markers, fixed and permeabilized according to the manufacturer’s instructions (BD Biosciences). Cells were then incubated with a selected combination of the following antibodies: FITC anti‐human IFN‐γ, PE anti‐human IL‐17A, PE anti‐human IL‐22, PE anti‐human FoxP3 or their corresponding IgG isotype controls (all from Biolegend). Stained cells were tested on a fluorescence activated cell sorter (FACS) Canto II flow cytometer.
Cytokine assays
PBMCs were stimulated with 50 ng/ml PMA and 500 ng/ml ionomycin at 37°C before supernatants were collected. IFN‐γ, IL‐17A and IL‐22 production in supernatant as well as transforming growth factor (TGF)‐β and IL‐10 plasma levels were measured by enzyme‐linked immunosorbent assay (ELISA) kits, according to the manufacturer’s instructions (Westang Biotech, Shanghai, China and NeoBioscience Technology, Shenzhen, China). The assay was performed in duplicate. The lower limits of detection were 15 pg/ml for TGF‐β, 0·4 pg/ml for IL‐10 and 8 pg/ml for IFN‐γ, IL‐17A and IL‐22.
Statistical analysis
Statistical analysis was performed with GraphPad Prism version 5 (GraphPad Software, San Diego, CA, USA). The results were expressed as mean ± standard deviation (s.d.). Statistical significance was performed by Student’s t‐test, one‐way analysis of variance (anova) or paired t‐test. Correlations were examined using Spearman’s rank correlation analysis. A P‐value < 0·05 was considered statistically significant.
Results
Circulating CCR6+CD4+ T cells are elevated in ITP patients
The frequency of CD4+ T cells expressing CCR6 in the peripheral blood was significantly increased in patients with ITP compared with healthy controls (34·03 ± 7·15% versus·21·97 ± 5·76%, P < 0·001, Fig. 1a). No significant difference was found in the frequency of CCR6+CD8+ T cells between ITP patients and healthy controls (6·15 ± 3·12% versus·6·69 ± 4·24%, P = 0·673, Fig. 1b). In contrast, the frequency of CCR6+CD19+ B cells in ITP patients was lower than that in healthy controls (72·04 ± 16·17% versus· 86·47 ± 8·43%, P = 0·002, Fig. 1c). When ITP patients alone were analyzed, there was no significant association between the frequency of CCR6+CD4+ cells and platelet counts or the presence of autoantibodies (data not shown).
Figure 1.
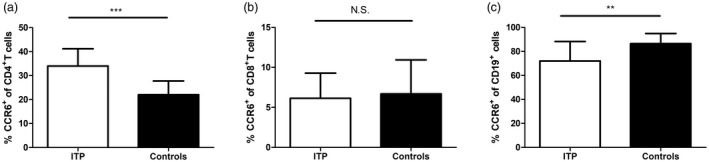
C‐C chemokine receptor (CCR)6 expression in immune thrombocytopenia (ITP) patients. The scatter‐plots showed the frequencies of CCR6 on (a) CD4+ T cells, (b) CD8+ T cells and (c) CD19+ B cells of peripheral blood in ITP patients (n = 16) and healthy controls (n = 18). Bars are shown as means ± standard deviation (s.d.). **P < 0·01, ***P < 0·001; n.s. = not significant.
CCR6+CD4+ T cells in ITP patients exhibit greater expansion and activation
Based on the finding that CCR6+CD4+ cells were significantly increased in ITP, we further sought to determine whether T cell activation can lead to the expansion of CCR6+CD4+ populations. PBMCs were stimulated in vitro with anti‐CD3 and anti‐CD28, then the subsequent frequency of CCR6 was measured (Fig. 2a). CCR6 was induced on CD4+ T cells, starting at 24 h and persisting until at least 72 h in ITP patients (30·22 ± 3·30% at 12 h, 33·18 ± 3·89% at 24 h, 41·84 ± 4·59% at 48 h, 56·29 ± 7·27% at 72 h, Fig. 2b). However, the increase of CCR6 began at 48 h in heathy controls (19·35 ± 2·07% at 12 h, 20·51 ± 2·64% at 24 h, 27·49 ± 4·11% at 48 h, 37·23 ± 5·04% at 72 h, Fig. 2b). The frequency of CCR6+CD4+ T cells in ITP was higher than that in healthy controls after stimulation (P < 0·001, Fig. 2b). Thus, CD4 cells were capable of up‐regulating CCR6 early in response to stimulation via CD3 and CD28 in ITP patients.
Figure 2.
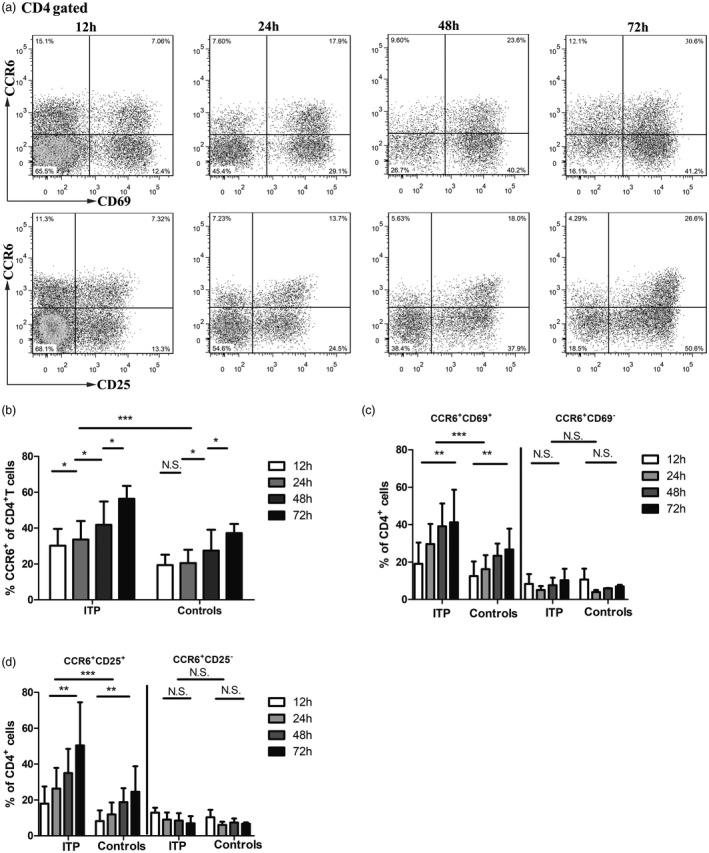
C‐C chemokine receptor (CCR)6 expression following stimulation in vitro. (a) Peripheral blood mononuclear cells (PBMCs) from a healthy control were stimulated using plate‐bound anti‐CD3 (1 μg/ml) and anti‐CD28 (0·5 μg/ml) antibodies. At various times, as shown, the frequency of CCR6 and CD69 or CD25. (b) The frequency of CCR6 among CD4 cells in culture with anti‐CD3 and anti‐CD28 at different times (12, 24, 48 and 72 h) in immune thrombocytopenia (ITP) patients (n = 8) and healthy controls (n = 8). (c) The frequency of CD25 in the CCR6+ versus CCR6− subset in culture with anti‐CD3 and anti‐CD28 at different times (12, 24, 48 and 72 h) in ITP patients (n = 8) and healthy controls (n = 8). (d) The frequency of CD69 in the CCR6+ versus CCR6− subset in culture with anti‐CD3 and anti‐CD28 at different times (12, 24, 48 and 72 h) in ITP patients (n = 8) and healthy controls (n = 8). Bars are shown as means ± standard deviation (s.d.). *P < 0·05, **P < 0·01, ***P < 0·001; n.s. = not significant.
Additionally, we examined the expression of several activation markers on CCR6+CD4+ cells. Although more CCR6+ cells and CCR6– cells expressed the activation markers CD25 and CD69 after stimulation (P < 0·05 in ITP patients and controls, Supporting information, Table S1), the fractions of CD25+/CD25– and CD69+/CD69– were higher for circulating CCR6+ compared with CCR6– cells at every time‐point (P < 0·05 in ITP patients and controls, Supporting information, Table S1). The frequencies of CCR6+CD25+CD4+ and CCR6+CD69+CD4+ cells were increased markedly following increased time of activation (CCR6+CD25+CD4+ cells, P = 0·002 for ITP patients and P = 0·007 for controls; CCR6+CD69+CD4+ cells, P = 0·009 for ITP patients and P = 0·008 for controls, Fig. 2c,d). In contrast, the frequencies of CCR6+CD25–CD4+ and CCR6+CD69–CD4+ cells were slightly decreased or remained unchanged, which did not reach significance (P > 0·05, Fig. 2c,d). Activation markers CD25 and CD69 on CCR6+CD4+ cells were also significantly increased in ITP patients compared with healthy controls (P < 0·001, Fig. 2c,d). Our data suggest that in response to stimuli, increased CCR6+CD4+ cells may represent activated CD4+ T cells in ITP.
Peripheral CCR6+CD4+ T cells in ITP patients exhibit a memory phenotype
We next sought to characterize the phenotype of these CCR6+CD4+ T cells in the peripheral blood of patients with ITP. CD45RO is a well‐established marker of memory T cells 18, 21. CCR6 was expressed on more than 50% of CD45RO+ cells (Fig. 3a). The frequency of CCR6 was greatly increased on CD45RO+ than on CD45RO–CD4+T cells (54·67 ± 7·88% versus 11·49 ± 7·07% for ITP patients, P < 0·001; 46·94 ± 8·62% versus· 6·63 ± 4·83% for controls, P < 0·001, Fig. 3b). The frequency of CCR6+CD4+ cells expressing CD45RO+ was increased in ITP patients compared with healthy controls (P < 0·01, Fig. 3b).
Figure 3.
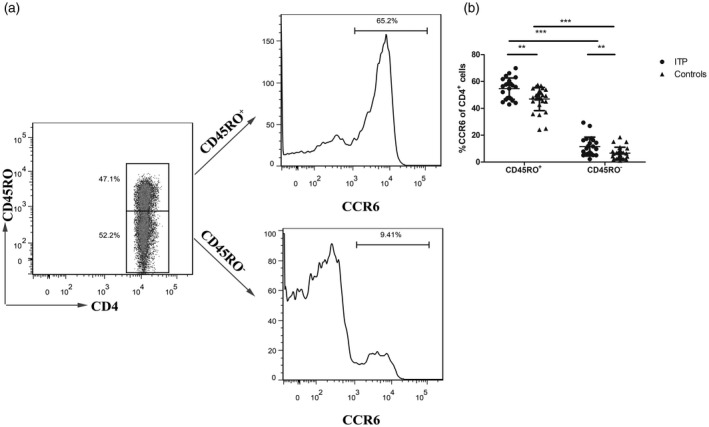
Phenotype of C‐C chemokine receptor (CCR)6+ CD4+ cells in immune thrombocytopenia (ITP) patients. (a) Flow cytometry study of CD45RO status in peripheral blood CD4+ T cells from a healthy control. The frequency of CCR6+ cells among CD45RO+ cells (top) and CD45RO– cells (bottom) was also evaluated. (b) The frequencies of CCR6 on CD45RO+ and CD45RO–CD4+ cells were analyzed in ITP patients (n = 23) and healthy controls (n = 21). Each symbol represents one individual; horizontal bars indicate means ± standard deviation (s.d.). **P < 0·01, ***P < 0·001.
CCR6+ Treg cells are decreased in ITP patients
As our results suggested a memory phenotype for CCR6+CD4+ T cells, we therefore evaluated classic Treg cell markers in CCR6+CD4+ T cells (Fig. 4a). The proportion of CD4+CD25+ cells in ITP patients was similar to that observed in controls (P = 0·9654, Fig. 4b), while the CD4+CD25+ cells expressing FoxP3 were 2·35 times less frequent in ITP patients than in controls (2·79 ± 1·15% versus 6·56 ± 2·94%, P = 0·0012, Fig. 4b). CD4+ cells that expressed both CD25 and FoxP3 had significant enrichment for CCR6 expression (CCR6+ versus CCR6– 2·82 ± 1·15 versus 0·13 ± 0·08% for ITP patients, P < 0·001; 4·75 ± 2·09% versus 0·16 ± 0·12% for controls, P = 0·001, Fig. 4c) and 2·82 ± 1·15% of CCR6+CD25+CD4+ cells in ITP patients were FoxP3+, whereas 4·75 ± 2·09% of CCR6+CD25+CD4+ cells expressed FoxP3 in controls (P < 0·01, Fig. 4c). The frequencies of CD4+CD25+FoxP3+ and CCR6+CD25+CD4+FoxP3+ cells were significantly correlated with platelet counts in ITP patients (r 2 = 0·1734, P = 0·0481 and r 2 = 0·2256, P = 0·0220, respectively, Fig. 4d,e).
Figure 4.
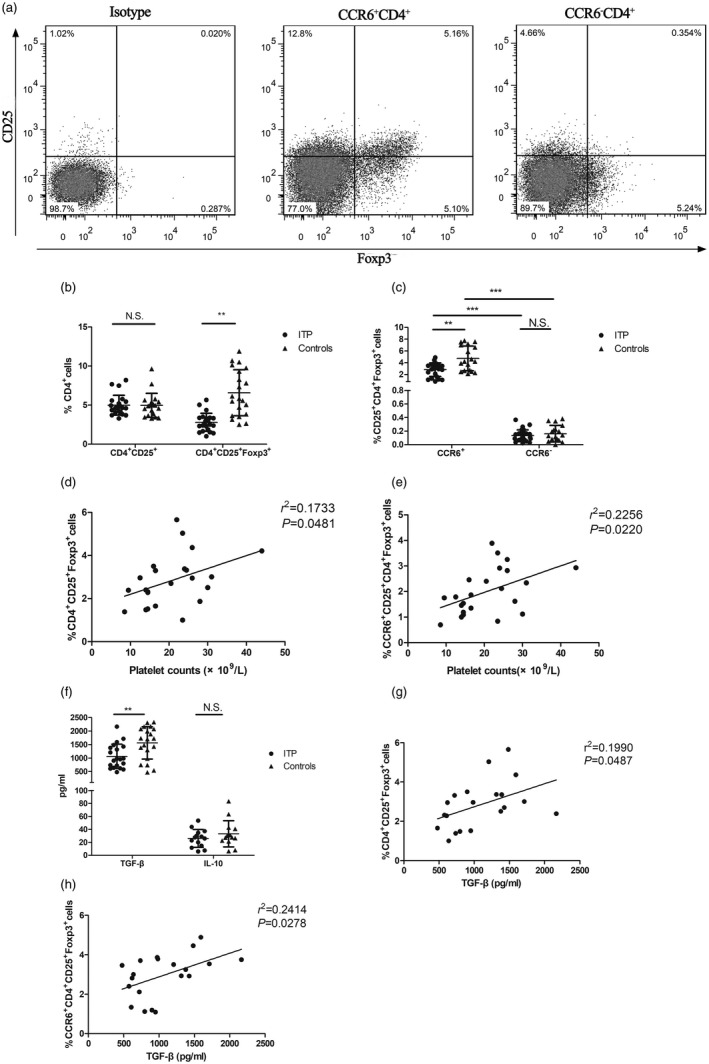
C‐C chemokine receptor (CCR)6+ regulatory T cells (Treg) cells in immune thrombocytopenia (ITP) patients. (a) Representative example of a flow cytometry density plot of CD25 and forkhead box protein 3 (FoxP3) expression in the population of CCR6+CD4+T cells and CCR6–CD4+ T cells from a healthy control. (b) Summary of frequencies of CD4+CD25+ cells and CD4+CD25+FoxP3+ cells in ITP patients (n = 23) and healthy controls (n = 21). (c) Frequencies of CD4+CD25+FoxP3+ cells in the CCR6+ versus CCR6– subset were analyzed in ITP patients (n = 23) and healthy controls (n = 20). Correlation between the frequency of (d) CD4+CD25+FoxP3+ cells or (e) CCR6+CD4+CD25+FoxP3+ cells and platelet counts in ITP patients (n = 23). (f) Transforming growth factor (TGF)‐β and interleukin (IL)‐10 in plasma were measured in ITP patients (n = 20 and n = 16, respectively) and healthy controls (n = 20 and n = 16, respectively). Correlation between the frequency of (g) CD4+CD25+FoxP3+ cells or (H)CCR6+CD4+CD25+FoxP3+ cells and TGF‐β levels in ITP patients (n = 20). Each symbol represents one individual; horizontal bars indicate means ± standard deviation (s.d.). **P < 0·01, ***P < 0·001; n.s. = not significant.
TGF‐β and IL‐10 are primarily considered anti‐inflammatory cytokines 29. We found lower circulating TGF‐β levels in ITP patients compared with controls (1063·67 ± 454·11 pg/ml versus 156 122·57 ± 599·47 pg/ml, P = 0·0053, Fig. 4f). TGF‐β levels were correlated with CD4+CD25+FoxP3+ and CCR6+CD25+CD4+FoxP3+ cells in ITP patients (r 2 = 0·1990, P = 0·0487 and r 2 = 0·2341, P = 0·0263, respectively, Fig. 4g,h). Concurrently, we also tested levels of circulating IL‐10 and found no difference in levels of IL‐10 between ITP patients and healthy controls (25·96 ± 13·67 pg/ml versus 35·96 ± 26·35 pg/ml, P = 0·1775, Fig. 4f). No significant difference was found between platelet counts and TGF‐β or IL‐10 levels in ITP patients (data not shown).
CCR6+CD4+ T cells are a major source of IL‐17A in ITP patients
Next, we detected the secretion of IL‐17A, IL‐22 and IFN‐γ within CCR6+CD4+ T cells (Fig. 5a). As shown in Fig. 5b, increased secretion of IL‐17A, IFN‐γ and IL‐22 by CD4+ T cells was found in ITP patients compared with healthy controls (1·51 ± 1·03% versus·0·97 ± 0·31%, P = 0·016; 15·95 ± 6·03% versus 9·53 ± 3·84%, P = 0·001; 1·91 ± 1·20 versus·1·01 ± 0·72, P = 0·004, Fig. 5b). CCR6–CD4+ cells produced significantly higher levels of IFN‐γ compared to CCR6+ counterparts (11·58 ± 5·44% versus·4·37 ± 2·67% for ITP patients, P = 0·001; 6·74 ± 2·5% versus·2·78 ± 1·64% for controls, P < 0·001, Fig. 5c). In contrast, CCR6+CD4+ cells produced more IL‐17A than their CCR6– counterpart (20·08 ± 0·99% versus 0·41 ± 0·45% for ITP patients, P = 0·001; 0·80 ± 0·35% versus 0·36 ± 0·21% for controls, P = 0·005, Fig. 5d). IL‐22 production was equivalent between CCR6+ and CCR6–CD4+ cells (0·81 ± 0·24% versus 0·91 ± 0·51% for ITP patients, P = 0·468; 0·57 ± 0·33% versus 0·46 ± 0·25% for controls, P = 0·658, Fig. 5e). Our study showed that the frequency of CCR6+CD4+ cells was increased by 1·54‐fold in ITP patients compared with healthy controls (34·03 ± 7·15% versus 21·97 ± 5·76%, P < 0·001).The IL‐17A, IFN‐γ and IL‐22 subsets of CCR6+CD4+ population increased by 2·6‐, 1·57‐ and 1·42‐fold in ITP patients compared with controls (2·08 ± 0·99% versus 0·80 ± 0·35%; 4·37 ± 2·67% versus 2·78 ± 1·64%; 0·81 ± 0·24% versus· 0·57 ± 0·33%, all P < 0·05, Fig. 5c–e). The frequencies of IFN‐γ‐ and IL‐22‐secreting CD4+ T cells in the CCR6– population were 1·78‐ and 1·97‐fold in ITP patients compared with healthy controls (P = 0·025 and P = 0·003, respectively, Fig. 5c,e). Furthermore, the ratio of CCR6+CD4+IL‐17A/CCR6+ Treg in ITP patients was increased significantly when compared with that in controls (1·03 ± 0·63 versus 0·16 ± 0·11, P < 0·001, Fig. 5f).
Figure 5.
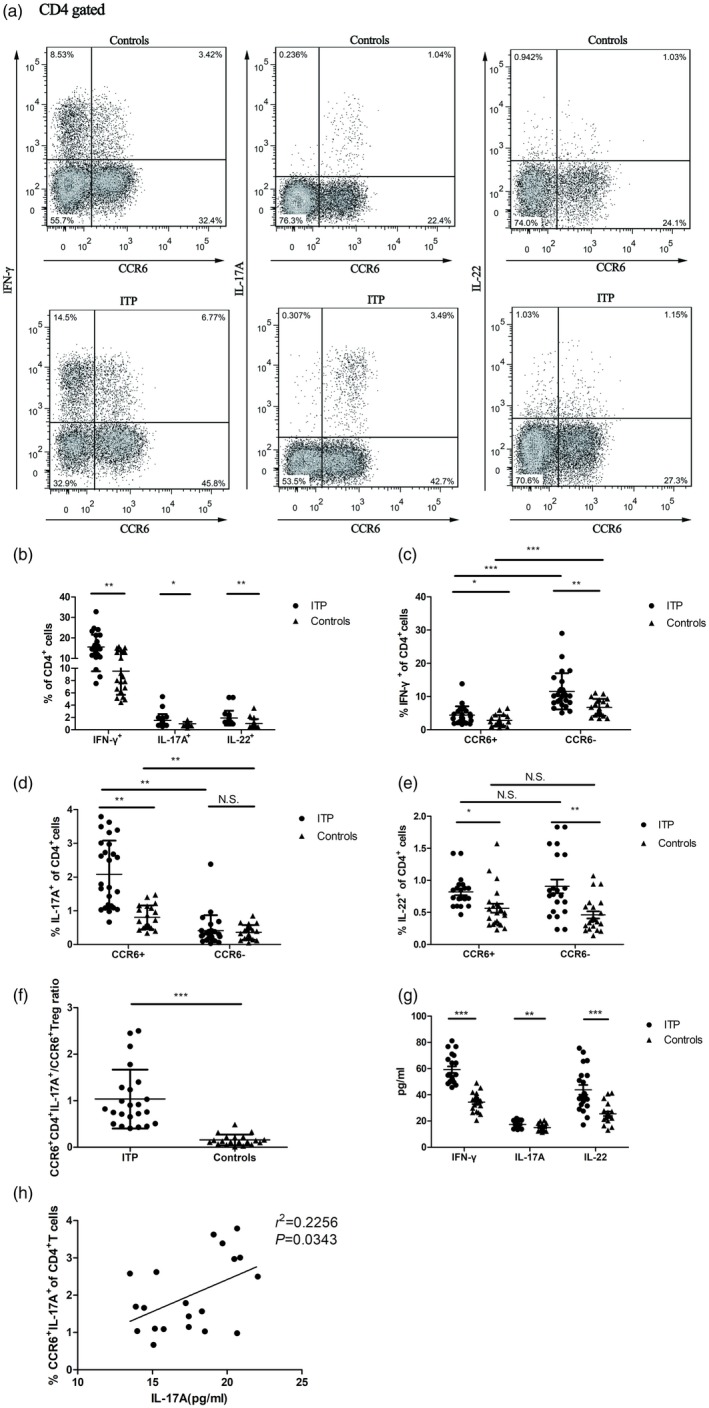
The frequencies of interferon (IFN)‐γ+, interleukin (IL)‐17A+ and IL‐22+‐secreting C‐C chemokine receptor (CCR)6+CD4+ cells in immune thrombocytopenia (ITP) patients. (a) IFN‐γ, IL‐17A and IL‐22 secretion in CD4+ T cells were analyzed based on CCR6 expression. Representative dot‐plots from an ITP patient and a healthy control. (b) Summary of frequencies of CD4+ T cells producing IFN‐γ, IL‐17A and IL‐22 in ITP patients (n = 25) and healthy controls (n = 25). Frequencies of CD4+ T cells producing (c) IFN‐γ, (d) IL‐17A and (e) IL‐22 in the CCR6+ versus CCR6− subset were analyzed in ITP patients (n = 20) and healthy controls (n = 20). (f) The ratio of CCR6+CD4+IL‐17A+/CCR6+ regulatory T cells (Treg) was increased in ITP patients (n = 23) compared to healthy controls (n = 20). (g) IFN‐γ, IL‐17A and IL‐22 in supernatant of ITP patients (n = 20) and healthy controls (n = 20). (h) Correlation between the frequency of CCR6+CD4+IL‐17A+ and IL‐17A in supernatant in ITP patients (n = 20). Each symbol represents one individual; horizontal bars indicate means ± standard deviation (s.d.). *P < 0·05, **P < 0·01, ***P < 0·001; n.s. = not significant.
Similarly in supernatant, the levels of IL‐17A, IFN‐γ and IL‐22 in ITP patients were higher than those in controls (17·47 ± 2·71 pg/ml versus 15·08 ± 2·59 pg/ml, P = 0·0071; 59·16 ± 11·19 pg/ml versus 34·49 ± 7·18 pg/ml, P < 0·001; 43·78 ± 16·69 pg/ml versus 25·59 ± 7·68 pg/ml, P < 0·001, Fig. 5g). Moreover, the frequency of CCR6+CD4+IL‐17A+ cells was significantly correlated with IL‐17A levels in the culture supernatant in ITP patients (r2 = 0·2256, P = 0·0343, Fig. 5h). There was no significant difference between CCR6+CD4+IFN‐γ+ cell frequency and IFN‐γ levels or between CCR6+CD4+IL‐22+ cell frequency and IL‐22 levels (data not shown).
CCR6+CD4+ T cells are associated with treatment response in ITP patients
To further address whether CCR6+CD4+ T cells were associated with treatment response, 31 ITP patients receiving treatment were investigated. CR was observed in eight patients, R in 12 patients and NR in 11 patients. There was no significant difference in the percentages of CCR6+CD4+ T cell subsets between responding and non‐responding patients before treatment (P > 0·05). However, the frequencies of circulating CCR6+CD4+, CCR6+CD4+IFN‐γ+, CCR6+CD4+IL‐17A+ and CCR6+CD4+IL‐22+ cells as well as the ratio of CCR6+CD4+IL‐17A+/CCR6+ Treg were decreased in CR and R patients compared with pretreatment (P < 0·05, Fig. 6a–d,f), while the frequency of circulating CCR6+ Treg cells was increased in CR and R patients (P < 0·05, Fig. 6e). Furthermore, CCR6+ Treg cell frequency after treatment was significantly higher in CR and R patients compared with NR patients (P < 0·01, Fig. 6e). In comparison with pretreatment, the frequencies of CCR6+CD4+, CCR6+CD4+IFN‐γ+ and CCR6+CD4+IL‐17A+ cells in NR patients remained unchanged (P > 0·05, Fig. 6a–c), while the frequencies of CCR6+CD4+IL‐22+ and CCR6+ Treg cells were significantly changed (P < 0·05, Fig. 6d,e). Although the frequencies of CCR6–CD4+IFN‐γ+ and CCR6–CD4+IL‐22+ cells were decreased in CR and R patients compared with pretreatment (P < 0·05, Fig. 6f,h), the frequencies of CCR6–CD4+IL‐17A+ and CCR6– Treg cells had no difference regarding reaction to treatment (P > 0·05, Fig. 6g–i). No significant changes of CCR6–CD4+IFN‐γ+, CCR6–CD4+IL‐17A+, CCR6–CD4+IL‐22+ and CCR6– Treg cells from NR patients were observed in our study (P > 0·05, Fig. 6g–i). Additionally, CCR6+CD4+ subsets in CR and R patients changed in GC alone and combined therapy groups after treatment (Supporting information, Table S2). However, a significant difference was found in patients receiving combined therapy not in patients receiving GC alone, probably because of the small number observations in GC alone group (Supporting information, Table S2).
Figure 6.
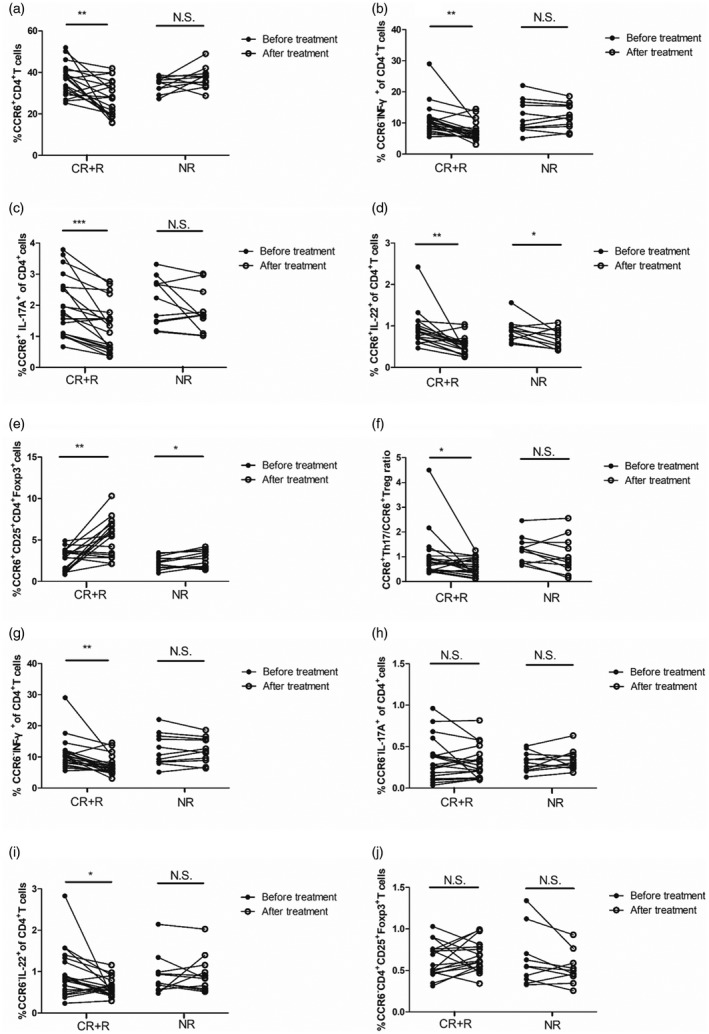
Comparison of C‐C chemokine receptor (CCR)6+CD4+ T cell subsets between responder (n = 20) and non‐responder patients (n = 11). The frequencies of (a) CCR6+CD4+, (b) CCR6+CD4+ interferon (IFN)‐γ+, (c) CCR6+CD4+interleukin (IL)‐17A+, (d) CCR6+CD4+IL‐22+, (e) CCR6+CD4+CD25+forkhead box protein 3 (FoxP3+), (f) CCR6+CD4+IL‐17A+/CCR6+CD4+CD25+FoxP3+ratio, (g) CCR6–CD4+IFN‐γ+, (h) CCR6–CD4+IL‐17A+, (i) CCR6–CD4+IL‐22+ and (j) CCR6–CD4+CD25+FoxP3+ cells were evaluated in responders and non‐responders before and after treatment. Each symbol represents one individual. *P < 0·05, **P < 0·01, ***P < 0·001; n.s. = not significant; CR = complete responders; R = responders; NR = non‐responders.
Discussion
In this study, we provide new information on CCR6 in ITP and report for the first time that the frequency of CCR6+CD4+ cells was increased in ITP patients. We also demonstrate that TCR triggering rapidly induced an increase of CCR6+CD4+ cells, which were enriched of activation markers in ITP patients. CCR6+CD4+ cells exhibiting memory phenotype were particularly susceptible to skew into Th17‐like effector T cells in ITP patients. Finally, we show an association between CCR6+CD4+ cell subsets and the response of treatment in ITP patients.
Among T cell subsets, we found expression of CCR6 on both CD4 and CD8 cells, but a higher frequency of the former stained positive compared with the latter. Stimulation of T cells with anti‐CD3/anti‐CD28, a more physiological stimulus, induced the proportion of CCR6 in CD4 cells, which contained high fractions positive for activation markers (CD69 and CD25). Activated CD4 cells that expressed CCR6 increased following the time of T cell receptor (TCR) stimulation, similar to that reported recently for significant up‐regulation of CCR6 expression on CD4+ cells exposed to proteolipid protein 139‐151 (PLP139–151) 27. This is in contrast to other studies, which have observed no increase in CCR6 surface expression and gene expression by treating cells with IL‐2 for 5 days or with antibody against CD3 for 3 days 20. The differences are probably related to the use of different stimuli, timing of analyses and heterogeneity in the population studied. Although CCR6+ and CCR6–CD4+ subsets contained a greater proportion of activated phenotype cells after stimulation, activated CCR6+CD4+ occurred rapidly in ITP patients. In all eight patients, each of the activated markers tested was over‐represented in the CCR6+ subpopulation of CD4 cells at 24 h, whereas a small fraction of CCR6–CD4+ cells were activated phenotype cells. Thus, CCR6 might be an early marker of CD4+ T cell activation in ITP. Combinatorial analysis of activation markers including CCR6 may improve the assessment of T cell activation in ITP.
CCR6+CD4+ T cells expressed higher levels of conventional memory marker CD45RO and classic Treg cell markers (CD25 and FoxP3). Conversely, these markers were weakly or not expressed in CCR6– counterparts. Our findings might suggest that CCR6+CD4+ cells are characterized by memory‐like phenotype. Furthermore, we observed that the frequency of CCR6+ Treg was lower in ITP patients and positively correlated with platelet counts. Circulating TGF‐β levels were decreased in ITP patients compared with healthy controls, while IL‐10 levels did not differ between two groups. Previous studies have reported that the inhibitory cytokine TGF‐β and IL‐10 produced by Treg cells play a role in immune tolerance 29. Activated CCR6+ Treg cells exhibit stronger suppressive activity compared with CCR6– Treg cells due to enhanced IL‐10 production 21, 30. However, we found that TGF‐β, but not IL‐10, levels were positively correlated with Treg and CCR6+ Treg frequencies in ITP patients. These data indicate that a decrease of CCR6+ Treg and TGF‐β might be involved in the pathogenesis of ITP, and CCR6+ Treg are more capable of exerting TGF‐β‐mediated suppression effects. TGF‐β has also been shown to be released by platelet activation/degranulation and is essential for maintenance of Treg suppressive functions 31. It is possible that, with decreased platelet counts in ITP, there is attenuated release of TGF‐β. Thus, the relatively lower level of TGF‐β fails to effectively promote Treg cell development. In this study, we were unable to find a correlation between platelet counts and TGF‐β levels in ITP patients. Whether the decrease in the frequency of CCR6+ Treg cells is a consequence of TGF‐β released by platelets in ITP requires further investigation.
Of the cytokines examined, circulating CCR6+CD4+ cells were a mixed IL‐17A, IFN‐γ and IL‐22 secretion population. IL‐17A‐producing cells were more frequent in the CCR6+ than in the CCR6– subsets. In contrast to IL‐17A, there was a greater frequency of IFN‐γ‐producing cells among the CCR6– subsets. CCR6+CD4+ cells produced comparable IL‐22 cytokines to CCR6– subsets. In ITP patients, CCR6+CD4+ cells produced more IL‐17A compared with healthy controls. This is consistent with previous reports in which significant increases in CCR6+ Th17 cells were observed in RA and psoriasis patients 25, 32. After short‐term stimulation, IL‐17A, IFN‐γ and IL‐22 levels in supernatant were higher in ITP patients than in controls. However, only up‐regulated IL‐17A was correlated with CCR6+ Th17 cell frequency in ITP patients. These results may indicate that CCR6 expression defines the major source of IL‐17A‐producing T cells that have inflammatory potentials in ITP patients.
Treg cells and Th17 cells have a reciprocal relationship. Conversion of Treg to Th17 cells is regulated partly by epigenetic modifications of transcription factors and signature cytokines 15, 33. The Treg/Th17 ratio may be a useful marker for assessing the severity of diseases in ITP 34. Interestingly, we found that the CCR6+Th17/CCR6+ Treg ratio was higher in ITP patients compared with healthy controls and correlated with the response of treatment, suggesting that the CCR6+Th17/CCR6+ Treg imbalance might be involved in the pathogenesis of ITP. As reported from earlier studies 15, 25, CCR6 is important for Th17 migration to inflammatory tissues and may modulate human Th17 differentiation to sustain inflammatory responses. Similarly, CCR6 on Treg cells is also required for Treg cell localization and migration 15. CCR6+ Treg cells co‐localize with different Th cell subsets and exhibit superior suppressive capacity in vitro 18. These studies suggested that CCR6 might regulate the migration of both proinflammatory and Treg cells in response to inflammatory cues from the immune environment 15, 25. Thus, we postulate that CCR6 may play important roles in dictating the balance between tolerance and immunity. Further studies are needed to delineate precisely how CCR6 control the balance of pro‐ and anti‐inflammatory response in CD4 cells of ITP.
Different strategies have been considered for the treatment of chronic ITP, such as glucocorticoid, intravenous immunoglobulin, danazol, vincristine, thrombopoietin receptor agonists (TPO‐RA) 35 and recombinant human thrombopoietin (rhTPO) 36, 37. As we know, effective treatment might increase bone marrow platelet production, correct the immune dysregulations and lead to a response in patients. In this study, we did not observe any statistical significance in the frequency of circulating CCR6+CD4+ cells between responders and non‐responders before treatment. However, our data showed a significant relationship between CCR6+CD4+ cell subsets and the response to treatment. CCR6+ Treg and CCR6+CD4+IL‐17A+ cells differed significantly in patients with CR/R response between pre‐ and post‐treatment, while CCR6– Treg and CCR6–CD4+IL‐17A+ cells in ITP patients did not alter after treatment. In comparison with pretreatment, the ratio of CCR6+Th17/CCR6+ Treg was decreased in patients with CR/R response. Irrespective of treatments, CCR6+CD4+ cell subsets and the CCR6+Th17/CCR6+ Treg ratio in CR/R patients differed between pre‐ and post‐treatment. On that basis, we postulate that CCR6+CD4+ cells might possibly be involved in the clinical efficacy of ITP treatment.
Our current work reveals that CD4+T cell subsets expressing the chemokine receptor CCR6 are enriched in blood of ITP patients. CCR6+CD4+ cells exhibit characteristics of activated memory effector Th17, rather than regulatory phenotype in ITP patients. Finally, CCR6+CD4+ cell subsets are correlated with the response of treatment. Altogether, our results offer a potential biomarker for a subpopulation of T cells with effector properties, which might contribute to the pathogenesis of ITP.
Disclosure
All authors have no conflicts of interest.
Author contributions
M. L. and R. Y. participated in designing the research, analyzing and interpreting the data and writing the manuscript. M. L., Y. Li and Y. H. contributed to performing the experiments. C. L., Y. H., B. S., H. L., F. X. and X. L. contributed to sample collection and essential reagents or tools.
Supporting information
Table S1. The ratio of positive vs. negative phenotypic markers on CCR6+ and CCR6‐CD4+ T cells in peripheral blood.
Table S2. CCR6+CD4+ T cells within ITP patients stratified by different treatment groups.
Acknowledgements
This work was supported in part by grants of National Natural Science Foundation of China (81500084, 81670118 and 81700111), Tianjin Municipal Science and Technology Commission (14JCZDJC35100, 15ZXLCSY00010).
References
- 1. Audia S, Mahevas M, Samson M, Godeau B, Bonnotte B. Pathogenesis of immune thrombocytopenia. Autoimmun Rev 2017;16:620–32. [DOI] [PubMed] [Google Scholar]
- 2. McKenzie CG, Guo L, Freedman J, Semple JW. Cellular immune dysfunction in immune thrombocytopenia (ITP). Br J Haematol 2013;163:10–23. [DOI] [PubMed] [Google Scholar]
- 3. Semple JW, Provan D. The immunopathogenesis of immune thrombocytopenia: T cells still take center‐stage. Curr Opin Hematol 2012;19:357–62. [DOI] [PubMed] [Google Scholar]
- 4. Semple JW, Freedman J. Increased antiplatelet T helper lymphocyte reactivity in patients with autoimmune thrombocytopenia. Blood 1991;78:2619–25. [PubMed] [Google Scholar]
- 5. Wang T, Zhao H, Ren H et al Type 1 and type 2 T‐cell profiles in idiopathic thrombocytopenic purpura. Haematologica 2005;90:914–23. [PubMed] [Google Scholar]
- 6. Zhang J, Ma D, Zhu X, Qu X, Ji C, Hou M. Elevated profile of Th17, Th1 and Tc1 cells in patients with immune thrombocytopenic purpura. Haematologica 2009;94:1326–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun T, Zhang D, Yang Y et al Interleukin 35 may contribute to the loss of immunological self‐tolerance in patients with primary immune thrombocytopenia. Br J Haematol 2015;169:278–85. [DOI] [PubMed] [Google Scholar]
- 8. Liu B, Zhao H, Poon MC et al Abnormality of CD4(+)CD25(+) regulatory T cells in idiopathic thrombocytopenic purpura. Eur J Haematol 2007;78:139–43. [DOI] [PubMed] [Google Scholar]
- 9. Stasi R, Cooper N, Del Poeta G et al Analysis of regulatory T‐cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell‐depleting therapy with rituximab. Blood 2008;112:1147–50. [DOI] [PubMed] [Google Scholar]
- 10. Kim CH, Rott L, Kunkel EJ et al Rules of chemokine receptor association with T cell polarization in vivo . J Clin Invest 2001;108:1331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qin S, Rottman JB, Myers P et al The chemokine receptors CXCR11 and CCR11 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest 1998;101:746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Acosta‐Rodriguez EV, Rivino L, Geginat J et al Surface phenotype and antigenic specificity of human interleukin 17‐producing T helper memory cells. Nat Immunol 2007;8:639–46. [DOI] [PubMed] [Google Scholar]
- 13. Singh SP, Zhang HH, Foley JF, Hedrick MN, Farber JM. Human T cells that are able to produce IL‐17 express the chemokine receptor CCR13. J Immunol 2008;180:214–21. [DOI] [PubMed] [Google Scholar]
- 14. Colantonio L, Recalde H, Sinigaglia F, D’Ambrosio D. Modulation of chemokine receptor expression and chemotactic responsiveness during differentiation of human naive T cells into Th1 or Th2 cells. Eur J Immunol 2002;32:1264–73. [DOI] [PubMed] [Google Scholar]
- 15. Yamazaki T, Yang XO, Chung Y et al CCR15 regulates the migration of inflammatory and regulatory T cells. J Immunol 2008;181:8391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farber JM. Mig and IP‐10: CXC chemokines that target lymphocytes. J Leukoc Biol 1997;61:246–57. [PubMed] [Google Scholar]
- 17. Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 2010;10:490–500. [DOI] [PubMed] [Google Scholar]
- 18. Duhen T, Duhen R, Lanzavecchia A, Sallusto F, Campbell DJ. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood 2012;119:4430–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iellem A, Mariani M, Lang R et al Unique chemotactic response profile and specific expression of chemokine receptors CCR19 and CCR19 by CD4(+)CD25(+) regulatory T cells. J Exp Med 2001;194:847–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liao F, Rabin RL, Smith CS, Sharma G, Nutman TB, Farber JM. CC‐chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3 alpha. J Immunol 1999;162:186–94. [PubMed] [Google Scholar]
- 21. Kleinewietfeld M. CCR21 expression defines regulatory effector/memory‐like cells within the CD25+CD4+ T‐cell subset. Blood 2005;105:2877–86. [DOI] [PubMed] [Google Scholar]
- 22. Lee JJ, Kao KC, Chiu YL et al Enrichment of human CCR22+ regulatory T cells with superior suppressive activity in oral cancer. J Immunol 2017;199:467–76. [DOI] [PubMed] [Google Scholar]
- 23. Zhang XX, Kang XM, Zhao AM. Regulation of CD4(+)FOXP3(+) T cells by CCL20/CCR23 axis in early unexplained recurrent miscarriage patients. Genet Mol Res 2015;14:9145–54. [DOI] [PubMed] [Google Scholar]
- 24. Li N, Wei W, Yin F et al The abnormal expression of CCR24 and CCR24 on Tregs in rheumatoid arthritis. Int J Clin Exp Med 2015;8:15043–53. [PMC free article] [PubMed] [Google Scholar]
- 25. Paulissen SM, van Hamburg JP, Dankers W, Lubberts E. The role and modulation of CCR25+ Th17 cell populations in rheumatoid arthritis. Cytokine 2015;74:43–53. [DOI] [PubMed] [Google Scholar]
- 26. Paulissen SM, van Hamburg JP, Davelaar N et al CCR26(+) Th cell populations distinguish ACPA positive from ACPA negative rheumatoid arthritis. Arthritis Res Ther 2015;17:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liston A, Kohler RE, Townley S et al Inhibition of CCR27 function reduces the severity of experimental autoimmune encephalomyelitis via effects on the priming phase of the immune response. J Immunol 2009;182:3121–30. [DOI] [PubMed] [Google Scholar]
- 28. Neunert C, Lim W, Crowther M et al The American Society of Hematology 2011 evidence‐based practice guideline for immune thrombocytopenia. Blood 2011;117:4190–207. [DOI] [PubMed] [Google Scholar]
- 29. Palomares O, Martin‐Fontecha M, Lauener R et al Regulatory T cells and immune regulation of allergic diseases: roles of IL‐10 and TGF‐beta. Genes Immun 2014;15:511–20. [DOI] [PubMed] [Google Scholar]
- 30. Kitamura K, Farber JM, Kelsall BL. CCR30 marks regulatory T cells as a colon‐tropic, IL‐10‐producing phenotype. J Immunol 2010;185:3295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bao W, Bussel JB, Heck S et al Improved regulatory T‐cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood 2010;116:4639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol 2010;130:1373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL‐17‐producing cells. Blood 2008;112:2340–52. [DOI] [PubMed] [Google Scholar]
- 34. Yu S, Liu C, Li L et al Inactivation of Notch signaling reverses the Th17/Treg imbalance in cells from patients with immune thrombocytopenia. Lab Invest 2015;95:157–67. [DOI] [PubMed] [Google Scholar]
- 35. Cantoni S, Carpenedo M, Mazzucconi MG et al Alternate use of thrombopoietin receptor agonists in adult primary immune thrombocytopenia patients: a retrospective collaborative survey from Italian hematology centers. Am J Hematol 2018;93:58–64. [DOI] [PubMed] [Google Scholar]
- 36. Zhou H, Xu M, Qin P et al A multicenter randomized open‐label study of rituximab plus rhTPO vs rituximab in corticosteroid‐resistant or relapsed ITP. Blood 2015;125:1541–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kong Z, Qin P, Xiao S et al A novel recombinant human thrombopoietin therapy for the management of immune thrombocytopenia in pregnancy. Blood 2017;130:1097–103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The ratio of positive vs. negative phenotypic markers on CCR6+ and CCR6‐CD4+ T cells in peripheral blood.
Table S2. CCR6+CD4+ T cells within ITP patients stratified by different treatment groups.


