Summary
The identification that T follicular helper (Tfh) cells is critical for the emergence of germinal centre responses prompted the study of CXCR5‐expressing CD4+ T cell subsets in autoimmunity. However, circulating CXCR5‐expressing T cells are heterogeneous by containing Forkhead box protein 3 (FoxP3)+ T follicular regulatory (Tfr) cells in addition to bona fide Tfh cells. Such heterogeneity may hamper the analysis of the contribution of specific follicular T cell subsets for autoimmune pathogenesis. Therefore, separate assessment of Tfh and Tfr populations offer greater opportunities for stratification of autoimmune patients, such as Sjögren’s syndrome patients.
Keywords: Sjögren’s syndrome, T follicular helper cells, T follicular regulatory cells, Foxp3
We read with great interest the work published by Aqrawi et al. 1. In their work, primary Sjögren’s syndrome (SS) patients were shown to have a decreased expression of CXCR5 on distinct peripheral blood B and T cell subsets. As the CXCR5 receptor guides lymphocytes towards B cell zones and germinal centres in lymphoid organs, it has been established as a follicular marker and used to identify follicular T cell subsets. We have demonstrated that besides T follicular helper (Tfh) cells, there is a CXCR5+ forkhead box protein 3 (FoxP3)+ T cell population in human peripheral blood 2. Thus, we have suggested that the study of FoxP3+ and/or CXCR5+ T cells in human blood should consider the distinction of regulatory T cells (Tregs), T follicular regulatory (Tfr) and Tfh cell populations 3.
Aqrawi et al. showed a trend for an increased proportion of blood Tfh cells in SS patients, defined as programmed death‐1 (PD)‐1+CXCR5+CD4+ T cells 1. This population is probably heterogeneous in including CXCR5+FoxP3+ Tfr cells and excluding PD‐1–CXCR5+ Tfh cells 3, 4 (Fig. 1a). Moreover, this heterogeneity may be critical when Tfh1‐, Tfh2‐ and Tfh17‐like cells are studied in autoimmune patients, as blood Tfh and Tfr cells harbour distinct CXCR3 and CCR6 expression profiles (Fig. 1b). We recently studied blood Tfh and Tfr cells as two distinct populations in primary SS patients 5. When we analysed total CXCR5+ T cells, no differences were identified between SS patients and healthy donors. However, distinguishing Tfh and Tfr cells revealed an increased frequency of Tfr cells in SS patients (Fig. 1c). Consistent with the findings of Aqrawi et al., we identified a subgroup of SS patients with increased frequency of PD‐1+ Tfh cells compared to healthy donors but, overall, blood Tfh cells were not increased 1, 5. More unexpected was the observed increased percentage of blood Tfr cells in SS patients 1, 5. By studying Tfh and Tfr cells independently we found that blood PD‐1+ Tfh cells displayed a remarkable correlation with disease activity [assessed by EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI)], while blood Tfr cells could be used as a biomarker for identification of SS patients with lymphocytic infiltration of salivary glands 5. In an independent primary SS cohort, Verstappen et al. validated the correlation between blood Tfh cells and disease activity 6. The low prevalence of extraglandular manifestations on the cohort studied by Aqrawi et al. may also explain differences regarding CXCR5+ T cells, as blood Tfh cells were shown to be selectively increased in SS patients with extraglandular manifestations 7.
Figure 1.
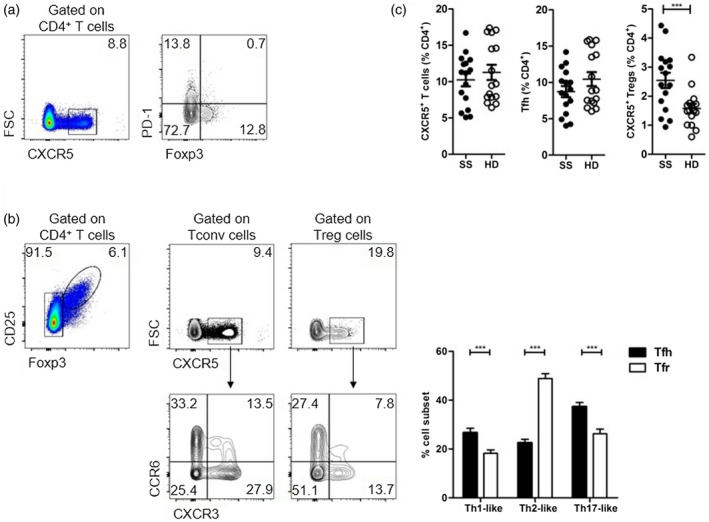
Heterogeneity of human blood CXCR5‐expressing CD4 T cells. (a) Circulating CD4+ T cells that express CXCR5 contain a proportion of cells expressing forhead box protein 3 (FoxP3) (representative plots from healthy donors). (b) When FoxP3+ and FoxP3– CD4+ T cells are analysed separately (i.e. gated as regulatory T cells (Treg) and conventional T (Tconv) cells, respectively), it becomes apparent that among the two populations there is a proportion of cells expressing CXCR5. Follicular subsets defined by expression of CXCR3 and CCR6 are different among T follicular regulatory (Tfr) cells (defined as FoxP3+CXCR5+CD4 T cells) and T follicular helper (Tfh) cells. Representative plots and pooled data from healthy donors (n = 42). (c) Sjögren’s syndrome (SS) patients display an increased frequency of circulating Tfr cells, with no significant changes when Tfh cells or the overall CXCR5‐expressing CD4+ T cells are considered as a whole (n = 16).
The finding of CXCL13‐secreting cells within salivary glands of SS patients by Aqrawi et al. may provide an explanation for salivary gland infiltration by CXCR5‐expressing cells 1. We also observed a substantial number of CXCR5‐expressing cells within salivary glands of SS patients, many of those also expressing FoxP3 5. However, whether increased serum levels of CXCL13 in SS patients are due exclusively to secretion from salivary gland infiltrating cells remains an open question. Novel experimental approaches will be needed for future studies addressing the formation of ectopic lymphoid structures in human tissues 8.
While studying immune reactions in human peripheral tissues remains a challenge for the difficult access to those tissues, compelling evidence suggests that these studies may improve patient stratification and treatment outcomes in disease conditions such as autoantibody‐mediated autoimmune diseases. The analysis of Tfh and Tfr cells as two distinct populations will heighten the potential for patient stratification and, consequently, the clinical relevance of studies addressing follicular T cell biology.
Disclosures
The authors have no competing interests to declare.
This letter is a response to Aqrawi, L. A., Ivanchenko, M., Björk, A., Ramírez Sepúlveda, J. I., Imgenberg‐Kreuz, J., Kvarnström, M., Haselmayer, P., Jensen, J. L., Nordmark, G., Chemin, K., Skarstein, K. and Wahren‐Herlenius, M. Diminished CXCR5 expression in peripheral blood of patients with Sjögren’s syndrome may relate to both genotype and salivary gland homing. Clin Exp Immunol 2018; 192:259–70. doi: 10.1111/cei.13118
Read the response to this letter from Ivanchenko, M., Aqrawi, L., Björk, A., Wahren‐Herlenius, M. and Chemin, K. FoxP3+CXCR5+CD4+ T cell frequencies are increased in peripheral blood of patients with primary Sjögren’s syndrome. Clin Exp Immunol 2019; 195:305–09. doi:10.1111/cei.13244
References
- 1. Aqrawi LA, Ivanchenko M, Björk A et al Diminished CXCR1 expression in peripheral blood of patients with Sjögren’s syndrome may relate to both genotype and salivary gland homing. Clin Exp Immunol 2018;192:259–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fonseca VR, Agua‐Doce A, Maceiras AR et al Human blood Tfr cells are indicators of ongoing humoral activity not fully licensed with suppressive function. Sci Immunol 2017;2:eaan 1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maceiras AR, Fonseca VR, Agua‐Doce A, Graca L. T follicular regulatory cells in mice and men. Immunology 2017;152:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol 2014;35:436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fonseca VR, Romão VC, Agua‐Doce A et al Blood T follicular regulatory cells/T follicular helper cells ratio marks ectopic lymphoid structure formation and PD‐1 + ICOS + T follicular helper cells indicate disease activity in primary Sjögren’s syndrome. Arthritis Rheumatol 2018;70:774–84. [DOI] [PubMed] [Google Scholar]
- 6. Verstappen GM, Nakshbandi U, Mossel E et al Is the T follicular regulatory/T follicular helper cell ratio in blood a biomarker for ectopic lymphoid structure formation in Sjögren’s syndrome? Arthritis Rheumatol 2018;70:1354–5. [DOI] [PubMed] [Google Scholar]
- 7. Szabó K, Papp G, Szántó A, Tarr T, Zeher M. A comprehensive investigation on the distribution of circulating follicular T helper cells and B cell subsets in primary Sjögren’s syndrome and systemic lupus erythematosus. Clin Exp Immunol 2016;183:76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bombardieri M, Lewis M, Pitzalis C. Ectopic lymphoid neogenesis in rheumatic autoimmune diseases. Nat Rev Rheumatol 2017;13:141–54. [DOI] [PubMed] [Google Scholar]


