Summary
Systemic sclerosis (SSc) is an idiopathic systemic autoimmune disease. It is characterized by a triad of hallmarks: immune dysfunction, fibrosis and vasculopathy. Immune dysfunction in SSc is characterized by the activation and recruitment of immune cells and the production of autoantibodies and cytokines. How immune abnormalities link the fibrosis and vasculopathy in SSc is poorly understood. A plethora of immune cell types are implicated in the immunopathogenesis of SSc, including T cells, B cells, dendritic cells, mast cells and macrophages. How these different cell types interact to contribute to SSc is complicated, and can involve cell‐to‐cell interactions and communication via cytokines, including transforming growth factor (TGF)‐β, interleukin (IL)‐6 and IL‐4. We will attempt to review significant and recent research demonstrating the importance of immune cell regulation in the immunopathogenesis of SSc with a particular focus on fibrosis.
Keywords: arthritis (including rheumatoid arthritis), B cells, T cells, Toll‐like receptors (TLRs)
Introduction
Systemic sclerosis (SSc) is an idiopathic systemic autoimmune disease. The pathogenesis of SSc is mostly unknown. Immunological abnormality, which includes autoimmunity and infiltration and activation of immune cells, is one of three key features of the disease. The other two hallmarks of SSc are fibrosis and vasculopathy. How autoimmunity is linked to fibrosis and vascular damage remains poorly understood, with no single hypothesis or mechanism to explain the links. It is therefore likely that a combination of mechanisms is involved in the pathogenesis of SSc, and picking apart these mechanisms to establish how the triad of fibrosis, vasculopathy and immune cell activation progress in SSc is a mammoth task. Many studies have linked immune abnormality as a cause of or at least a contributor to fibrosis in SSc, while fibrosis may also contribute to immune cell activation. Fibrosis in SSc occurs mainly in the skin but may progress to visceral organs, including heart and lungs. Fibrosis is the result of activation of fibroblasts and excessive extracellular matrix (ECM) deposition, both of which are hallmarks of SSc. As well as fibrosis, immune abnormalities are also linked to vasculopathy in SSc, with most research implicating vascular damage as an activator of immune cells. However, this review will focus mainly on the role of immunological abnormalities in the development of fibrosis in SSc; we recommend the paper by Asano and Sato for a comprehensive review of vasculopathy in SSc 1.
Multiple different cell types of the immune system have been implicated in SSc, including T cells, B cells, dendritic cells, mast cells and macrophages. It appears that both innate and adaptive immunity is critical in the disease. We will attempt to review the old and emerging literature to examine the potential roles of these individual cell types in the immunopathogenesis of SSc. First, we will summarize major cytokines implicated in the immunopathogenesis of SSc, because these major players reappear in many studies, linking immune cell activation with other phenotypes in SSc, especially fibrosis.
Cytokines in SSc
Many cytokines are elevated in SSc, but interleukin (IL)‐4, transforming growth factor (TGF)‐β and IL‐6 are considered to be the major fibrogenic cytokines, or are at least the most studied. Genetic deletion of both IL‐4 and TGF‐β prevents skin fibrosis in a mouse model of SSc 2. Many other in‐vitro studies support the idea that IL‐4 promotes fibrosis through its ability to enhance the production of collagen 3, 4 and other ECM proteins 5, 6 while antibodies against IL‐4 prevent dermal fibrosis in the tight skin (Tsk) mouse model 7 targeted deletion of IL‐4 receptor in the Tsk mouse also reduces fibrosis 2. Skin and lung in SSc have high levels of IL‐4 8 and increased levels of IL‐4 in the blood are a common feature in patients with SSc 9, 10, 11 suggesting systemic release.
TGF‐β is a well‐known potent inducer of fibrosis, with TGF‐β‐stimulated fibroblasts resembling those from SSc patients 12. Activation of the TGF‐β receptor following the binding of TGF‐β results in the phosphorylation and activation of SMAD proteins in the cytoplasm 13. TGF‐β also activates the three mitogen‐activated protein kinase (MAPK) signalling branches, c‐Jun N‐terminal kinase (JNK), p38 and extracellular signal‐regulated kinases 1 and 2 (ERK1 and 2) 12 all of which can promote inflammatory signalling. TGF‐β‐induced collagen production from both healthy and SSc dermal fibroblasts was found to be dependent on p38 14. JNK activation has also been implicated in fibrosis 15. However, in one study ERK activation inhibited skin fibroblast collagens I and III production while, p38 activation up‐regulated collagen I 16.
IL‐6 is a classic proinflammatory cytokine and is also considered to be an important protein in the immunopathogenesis of SSc. For example, IL‐6 levels are increased in SSc patient sera 9 and skin 17. IL‐6 levels also correlate with SSc disease severity 18. A mouse model with development of autoimmune disease with SSc‐like skin thickening and lung fibrosis was found to be mediated by IL‐6 signalling 19. Bleomycin‐induced lung inflammation with collagen deposition was significantly attenuated in IL‐6‐deficient mice 20. IL‐6 signalling through trans‐signalling appears to be important, and we found that IL‐6 and the soluble form of the IL‐6 receptor are necessary for collagen production 21. We further showed in the same study that this was critical, dependent on the downstream signalling molecule signal transducer and activator of transcription (STAT)‐3.
A crucial early step hypothesized to trigger the immune abnormalities and fibrosis in SSc is vasculopathy, including the damage and apoptosis of endothelial cells, resulting in the release of internal damage‐associated molecular patterns (DAMPs), which go on to activate and recruit immune cells 22. IL‐6 was found to mediate endothelial activation and apoptosis caused by the serum of patients with SSc 23, suggesting that it may play a major role in the very early stages of SSc. However, IL‐6 was found to be up‐regulated at the late stage of the disease using immunohistological analysis of skin biopsies from SSc patients 17. In both IL‐6 knock‐out (KO) mice and mice exposed to an IL‐6 blocking antibody, bleomycin‐induced dermal fibrosis was greatly induced by supressing fibroblast activation 24. The anti‐IL‐6 receptor antibody tocilizumab has had promising results with softening of the skin in two patients with SSc in one study 25 while a Phase II trial provided SSc patients with improvement in fibrosis of the skin 26 although statistically this was not significant. Thus, IL‐6 antibody therapy could be the first biological licensed for SSc.
T cells
T cells have been identified early in SSc progression before any evidence of fibrosis 27. SSc skin has a greater propensity to recruit/adhere T cells compared to healthy controls because of a greater expression of intercellular adhesion molecule (ICAM‐1), which is a ligand for the lymphocyte function‐associated antigen 1 (LFA1) receptor found on the surface of lymphocytes such as T cells 28. T cells from SSc skin biopsies have increased expression of the early T cell activation marker CD69 29. TGF‐β, which is elevated in SSc, was also found to be important for the recruitment of T cells to the skin in an SSc mouse model 30. A recent paper demonstrated that abatacept, which is an antibody that interferes with T cell activation, reduced fibrosis in not one, but two animal models of fibrosis 31. This was associated with reduced T cell activation and reduced levels of IL‐6, which may be mediated by blockade of cross‐talk between T cells and antigen‐presenting cells such as monocytes. Abatacept works by blocking the interaction of CD80/86 with cytotoxic T lymphocyte antigen (CTLA)‐4 on T cells, which is required for co‐stimulatory activation of T cells alongside major histocompatibility complex (MHC) and antigen presentation from antigen‐presenting cells such as dendritic cells, macrophages and B cells 32 Abatacept was initiated in three patients with morphea, and all three showed regression of the modified Rodnan skin score 33.
T cells can be further classified into diverse subsets characterized by their distinct function, activators and the cytokines they subsequently release. Some of these T cell types have been found to play a role in SSc, which has been reviewed in more detail 34. However, many studies have identified the T helper type 2 (Th2) subtype as playing the largest role in SSc. Th2 cells are a major IL‐4‐producing cell type, but also secrete IL‐5, IL‐6, IL‐10 and IL‐13. Depletion of CD3+ T cells in a bleomycin mouse model reduced both fibrosis and IL‐4 secretion 35. As described above, IL‐4 can directly induce fibroblasts to promote fibrosis, but in addition IL‐4 can induce TGF‐β production in fibroblasts 36 and macrophages 37, which then leads to further signalling to fibroblasts to produce more collagen. Therefore, it is very likely that T cells have a major role to play in the onset of fibrosis in SSc through activation of macrophages and fibroblasts. An unusual population of CD4+CD8+ double‐positive T cells has been described in the skin that has very high levels of IL‐4 38. Gross et al. showed almost 30 years ago that IL‐4 is required to induce naive T cells to produce further IL‐4 39. If IL‐4 is required for T cells to produce IL‐4, this leads to the question: ‘what is the initial source of IL‐4 in SSc?’. This question remains unanswered, but potentially implicates other immune cells in the onset or progression of SSc.
IL‐13 is produced predominantly by activated Th2 cells 34. IL‐13 was established as a major profibrotic agent in a model of hepatic fibrosis 40. IL‐13 inhibited IL‐1β‐induced matrix metalloproteinase (MMP)‐1 and MMP‐3 production and enhanced tissue inhibitor of metalloproteinase (TIMP)‐1 and collagen generation in fibroblasts 41. In the bleomycin model of SSc pulmonary fibrosis IL‐13 levels increased with pathogenesis, while neutralization of IL‐13 attenuated bleomycin‐induced pulmonary fibrosis 42. The potent fibrosis‐inducer, TGF‐β, may also contribute to an increase in Th2‐originating IL‐13 because TGF‐β up‐regulates GATA binding protein 3 (GATA‐3) expression in the T cells of patients with SSc, resulting in an increase in IL‐13 synthesis 43. As well as CD4+CD8+ double‐positive T cells, CD8+ single‐positive cells have been described that produce exuberant levels of IL‐13 44. These cells appear to be memory CD8+ T cells 45. We have shown that IL‐13 is directly profibrotic and is STAT‐6‐dependent 46. Thus, IL‐13 produced from T cells probably contributes to fibrosis in SSc and a monoclonal IL‐13 clinical‐grade antibody exists. Very recently, IL‐13 was shown to decrease MMP‐1 expression in both healthy and SSc fibroblasts and therefore may have an anti‐fibrotic role 47. However, this was only demonstrated in tumour necrosis factor (TNF)‐α‐induced fibroblasts, thus the effect of IL‐13 may be specific to the mechanism with which TNF‐α induces MMP‐1 expression.
Another cytokine, IL‐17, prevalent in the serum of SSc patients in some studies, also implicates T cells 48. Th17 cells, which are characterized by their production of IL‐17A, IL‐17F, IL‐21 and IL‐22, are elevated in SSc skin compared to healthy controls 49, 50. Thus, Th17 cells and the IL‐17 they produce may play an important role in SSc. However, the roles of Th17 and IL‐17 in SSc are controversial, and have been recently reviewed in detail 51. Importantly, some studies have not detected differences in IL‐17 levels between SSc patients and healthy controls 52, 53 while IL‐17 has been shown to both increase 50 and decrease 54, 55 collagen production. The reasons for the differences in these studies between pro‐ or anti‐fibrotic effects of IL‐17 are not clear, and may reflect the source of the recombinant protein.
A reduction in regulatory T cells has been recorded in the skin lesions of patients with SSc 56 suggesting that there is compromised capacity to regulate immune responses in SSc. A recent study identified an imbalance of regulatory T cells and Th17 cells, with a decrease in the former and an increase in the latter in the peripheral blood of SSc patients compared to healthy controls 57. In addition, a more recent study has confirmed that Th2 and Th17 cells are found in higher frequencies in SSc patients, and this was found to positively correlate with IL‐35 levels, although a causal link has not been established 58. Further evidence of disruption to T cell homeostasis comes from a recent study showing that SSc patients displaying severe peripheral vascular complications have an expansion of the recently discovered angiogenic T cells 59. This expansion in angiogenic T cells may also demonstrate a link between immune cell activation and vasculopathy in SSc.
Macrophages
More than 30 years ago circulating monocytes were found to be strongly activated in patients with SSc 60. Since then, many studies have found evidence of monocyte/macrophage activation in SSc. Higher numbers of macrophages have been observed in skin from SSc patients 27, 61 while cells positive for CD163, a putative marker for M2 macrophages 62, have been found to be increased in the serum of SSc patients 61, 63, 64, 65. M2 macrophages are therefore prominent in SSc, with a very recent study involving more than 200 SSc patients, not only confirming this but also demonstrating that soluble CD163 (sCD163) is significantly elevated in the serum of SSc patients and therefore may be of use as a biomarker 66.
Microarray gene expression data have also supported a role for monocytes/macrophages in the immunopathogenesis of SSc, with studies demonstrating that peripheral blood mononuclear cells (PBMCs) from SSc patients have an increased expression of genes associated with monocyte/macrophages 67, 68, 69. Such large‐scale genomic studies have identified multiple innate immune regulators in SSc. Macrophages are also a major source of TGF‐β 70, which is a potent inducer of fibrosis 71. Many studies have implicated macrophages in the initiation or progression of fibrosis in SSc. Microarray data from SSc lung tissue confirm what has been observed in the blood with markers of macrophage activation 72 and emigration, which correlate with progressive lung fibrosis 73. In another recent study, a novel multi‐network approach to compare gene expression profiles has identified a gene expression signature indicative of profibrotic M2 macrophages in SSc tissues 74. Interestingly, the profibrotic macrophage gene expression profile differed between skin and lung, suggesting that although a role for macrophages in the immunopathogenesis of fibrosis in SSc seems likely in both skin and lung, there may still be subtle differences in what those roles are. Recently nintedanib, an inhibitor of the receptor tyrosine kinase platelet‐derived growth factor receptor, fibroblast growth factor receptor and vascular endothelial growth factor receptor, blocked myofibroblast differentiation and subsequent fibrosis in a mouse model of SSc. Interestingly, these nintedanib‐mediated anti‐fibrotic effects were associated with reduced numbers of M2 macrophages 75.
Many chemokines which both recruit and can be primarily secreted by macrophages are up‐regulated in SSc skin 76, 77, 78, 79 which is not surprising, given that infiltration of macrophages in SSc skin has been known since the early 1990s 80. However, the chemokine (C‐C motif) ligand 19 (CCL19) was up‐regulated in SSc skin and co‐localized with CD163‐positive macrophages, suggesting that it has a role in the recruitment of macrophages 76. The authors of the study also suggest that macrophages are the source of the CCL19, and in‐vivo experiments demonstrate that Toll‐like receptor (TLR)‐3, ‐4 or ‐9 activation is required for CCL19 expression in monocytes. TLR activation is considered a major event in the immunopathogenesis of SSc 81, 82. Cell types which highly express TLRs such as macrophages are thought to play a major role in SSc. However, many different cell types express TLRs, and thus TLR‐mediated signalling in the immunopathogenesis of SSc may not be limited to macrophages. It is possible that TLR activation in any cell type expressing TLRs is also a source of CCL19, and therefore a potential cause of macrophage recruitment in SSc. Indeed, Mathes et al. also observed that CCL19 expression was up‐regulated from TLR activation in T cells and other cell types from PBMCs, but monocytes gave the most robust increase 76. We have found that macrophages that respond to the TLR‐8 stimulus single‐stranded RNA in SSc result in up‐regulation of TIMP‐1 that is functional and leads to increased collagen deposition 83. Interestingly, these monocytes seemed to be perivascular.
IL‐6 appears to play an important part in the role of macrophages in SSc because inhibition of phosphodiesterase‐4, which blocks M2 differentiation, also inhibits IL‐6 production, fibroblast activation and fibrosis in an SSc mouse model 84. IL‐6 may also be an important activator of M2 macrophages, as indicated by IL‐6 receptor blockage by tocilizumab 85 causing down‐regulation of genes associated with M2 macrophages in SSc skin. Oncostatin M is another IL‐6‐like cytokine that appears to be involved in fibrosis, as cells treated with OSM induced ECM accumulation in fibroblasts 86.
SSc patients with higher expression of the M2 macrophage marker, MRC1, were also found to have elevated levels of IL‐13 in their plasma 69 suggesting that macrophage activation in SSc results in production of cytokines from macrophages themselves or indirectly through interaction with other immune cells, because macrophages can both secrete IL‐13 87 and, through being antigen‐presenting cells, can activate T cells which could lead to IL‐13 production.
There have been more than 30 years of research implicating macrophages in the immunopathogenesis of SSc, with various cytokines produced by macrophages and other immune cells, internal molecules from damaged endothelial cells and chemokines all contributing to the recruitment and activation of macrophages, resulting in a profibrotic environment (Fig. 1). However, how macrophages are recruited to and activated in the tissues of SSc patients is not fully understood. What role is played by macrophages in the progression of fibrosis is also not fully known, but activation of TLRs and the subsequent profibrotic signalling remains an obvious choice. Targeting of TLRs may be a therapeutic option.
Figure 1.
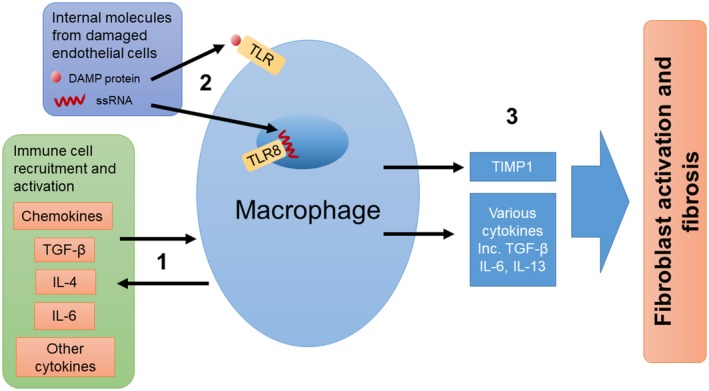
Potential roles for macrophages in the immunopathogenesis of SSc. (1) Macrophages can both be activated by and release cytokines. Cytokines and chemokines released by macrophages may recruit and activate other immune cells to further promote an inflammatory environment. (2) Internal molecules released from damaged endothelial cells can activate macrophages by Toll‐like receptors (TLRs). (3) Activation of macrophages leads to the secretion of profibrotic molecules and the activation of fibroblasts resulting in fibrosis.
B cells
B cells are heavily implicated in the immunopathogenesis of SSc. B cells are known inducers of fibrosis generally and in SSc 88 and mounting evidence suggests that they are modified and activated in patients with SSc. SSc patients have abnormalities of B cell homeostasis in the blood, which includes an expansion of naive B cells and activated but diminished memory B cells 89. The cytokine B cell activating factor (BAFF), which belongs to the TNF ligand family, is a potent activator of B cells 90. Not only are BAFF levels elevated in SSc; levels also correlate with disease severity, while B cells isolated from SSc patients produce more IL‐6 when exposed to BAFF 91. A BAFF antagonist inhibited IL‐6 and IL‐10 expression in the skin of the Tsk mouse model of SSc, while stimulation of B cells with BAFF greatly increased IL‐6 92. IL‐6 can direct the differentiation of T cells into IL‐4‐producing Th2 cells 93, therefore B cell‐secreted IL‐6 could contribute to the Th2 phenotype detected in SSc. As well as being involved in IL‐6 cytokine production, B cells are also suggested to play an important role in fibrosis. Co‐cultures of B cells and fibroblasts from SSc patients induced a fibrotic response, including collagen, TGF‐β1 and IL‐6 secretion. Exposure of co‐cultures to BAFF (and anti‐IgM) further increased secretion of the profibrotic compounds 88.
The majority of B cells are IL‐6‐producing, termed B effector cells (Beffs), and have a proinflammatory and autoimmunity role. However, a small subset of B cells termed regulatory B cells (Bregs) are potent negative regulators of inflammation and autoimmunity, in part through their ability to express IL‐10 94. These Bregs therefore have the potential to inhibit diseases with immune abnormalities such as SSc. Indeed, Bregs were able to inhibit the initiation of a mouse model of multiple sclerosis and depletion of Bregs increased symptom severity 95. Interestingly, the frequency of blood Bregs was found to be significantly lower in SSc patients compared to healthy controls 96 while in a very recent study by Matsushita et al., Bregs were found to have a similar role in a mouse model of SSc. Depletion of IL‐6‐producing Beffs reduced skin and lung fibrosis, while depletion of IL‐10‐producing Bregs caused more severe fibrosis 97 backing up findings from a previous study that Bregs can suppress skin fibrosis in a graft‐versus‐host disease mouse model of SSc 98. A tipping of the balance of Bregs and Beffs in favour of fewer Bregs is therefore a potential important event in the initiation of fibrosis in SSc. However, the cause of this shift in B cell homeostasis remains unknown, and future studies to identify the trigger/s may provide useful early therapeutic targets.
Various response regulators on the surface of B cells exist, with some increasing and others decreasing B cell receptor (BCR) signals. Of these, the positive B cell regulator, CD19, is the most established in SSc disease research. CD19 is an important regulatory molecule expressed by B cells. CD19+ B cells increase in SSc, with expression of CD19 on the surface of B cells significantly higher in SSc patients compared to healthy controls 99. CD19‐deficient mice have decreased sera levels of several autoantibodies 100 suggesting that B cells could be responsible for the autoantibodies detected in SSc patients. In agreement, a transgenic mouse model, with an elevation in CD19 expression similar to SSc human patients, shows an increase in the levels of autoantibodies 101, 102. It is also worth noting that CD19 over‐expression has not been observed in the Tsk mouse model, suggesting that a role for CD19 in B cell activation in SSc is not straightforward or that the animal models can only replicate the human disease phenotypes to a degree. However, the apparent lack of CD19 over‐expression may be explained partly through constitutive activation of CD19 and the downstream signalling pathway via up‐regulated CD19 tyrosine phosphorylation, which could negate the need for over‐expression 102.
Autoantibody production is prevalent, and can be used diagnostically in SSc. Furthermore, autoantibodies against platelet‐derived growth factor receptor (PDGFR) were found in SSc patients, and caused collagen I‐increased expression and transition of normal human primary fibroblasts to a myofibroblast phenotype through activation of an intracellular loop that involves Ha‐Ras, ERK1/2 and reactive oxygen species (ROS) 103. However, it is still possible that B cell activation is the cause of both autoantibody production and fibrosis, as the two may be linked. Interestingly, B cell activation via CD19 specifically may also be a cause of other immune cell type infiltration in SSc. CD19‐deficient mice showed less infiltration of mast cells, macrophages, T cells and B cells after bleomycin treatment compared to wild‐type cells exposed to bleomycin 104. In the same study, it was discovered that B cells, via hyaluronan‐induced TLR‐4 activation, produced various cytokines which were dramatically reduced in CD19‐deficient cells. Therefore, B cells have the potential to contribute to fibrosis directly as well as indirectly through recruitment and activation of other immune cells to promote a cycle of cytokine and fibrogenic compound release.
Mast cells
Mast cell infiltration was detected from lip biopsy in very early stages of SSc and preceded the onset of skin changes 105 and more recently cutaneous mastocytosis was found to precede the onset of SSc in a 36‐year‐old woman 106 suggesting that mast cells may play an early role in the onset of SSc. Mast cell infiltration has also been detected late in the development of SSc 107 and in a subset of SSc patients with localized scleroderma‐like lesions 108. An increase in mast cell numbers has also been detected in SSc skin 109.
Mast cells are able to produce TGF‐β and are found localized to fibroblasts in the skin of SSc patients 110. Interestingly, mast cells are not only a source of TGF‐β; they can also directly transfer TGF‐β to fibroblasts mediated by transgranulation via cell–cell contacts 111, which has also been observed in the dermis of SSc patients 112. The triggers of mast infiltration in SSc or other fibrotic diseases are not known, but a very recent study has identified Snail‐dependent production of plasminogen activator inhibitor‐1 (PAI1) in keratinocytes as a chemoattractant of mast cells, a mediator of mast cell–fibroblast adhesion and a promoter of fibrogenesis 113. Interestingly, PAI1 up‐regulated tenascin‐C (TENC) expression, which is the main activator of TLR‐4 to promote fibrosis and found to be elevated in SSc 114. Therefore, the role of PAI1 in SSc could involve mast cell recruitment and activation of fibroblasts to promote an inflammatory environment.
Dendritic cells
Two major types of dendritic cell are known: conventional (cDC) and plasmacytoid (pDC). pDC are a specialized cell type that when activated produce large amounts of interferon (IFN). A very recent study observed that pDCs infiltrate the skin of SSc patients and are chronically activated, producing IFN‐α and chemokine (C‐X‐C motif) ligand 4 (CXCL4). Fibrosis was reverted in a mouse model of SSc after pDCs depletion 115. In the same study, TLR‐8 signalling was found to be important for CXCL4 production and recruitment of pDCs to fibrotic skin. TLR involvement has also been found to be important for mediating the increased cytokine production in DCs in SSc, which has been comprehensively reviewed 116. pDCs isolated from SSc patients show up‐regulation of CXCL4 115, 117, which can also be detected at elevated levels and correlates with disease severity in SSc patient plasma 118 and has been suggested as a biomarker in SSc.
In another recent study, pDC depletion in a bleomycin mouse model improved the clinical score, lung histopathology score, skin thickness and collagen content. The expression of genes involved in chemotaxis, dendritic cell differentiation, inflammation and fibrosis in the lungs of pDC‐depleted mice was also significantly reduced. Alongside this, B and T cells were also found to be reduced in the lungs, suggesting an important role for ongoing immune abnormalities induced by DCs 119. In another bleomycin‐induced pulmonary fibrosis mouse model, DC accumulation in the lung was reduced through blocking of TGF‐β with the use of inhibitor SB431542 120. TGF‐β was also found to be important for the recruitment of pDCs (and T cells) to the skin in an SSc mouse model 30 suggesting an important role for TGF‐β in DC location and activation. An important role for microRNAs in the pathogenesis SSc is emerging 121. Recently, microRNA expression profiling has identified miRNA‐618 (miR‐618) as being up‐regulated in pDCs from SSc patients, while over‐expression in healthy pDCs resulted in an SSc‐like pDC 122. Thus, microRNAs are disturbed, leading to altered functionality of immune cells. Alterations of microRNAs by restoration using microRNA therapeutics may be one way to reset dendritic cells to a ‘tolerogenic’ phenotype.
Anti‐nuclear antibodies, which are a subset of autoantibodies, are found to be elevated in SSc patients 123. DNA topoisomerase I (TopoI) is an intracellular target of anti‐nuclear antibody anti‐TopoI. The presence of anti‐TopoI antibodies has been clinically associated with a more severe form of SSc 124. Immunization of mice with TopoI peptide‐loaded DCs induces anti‐TopoI autoantibody response and long‐term fibrosis of skin and lung, which is preceded by inflammation with increased IL‐17A and CXCL4 expression 125. TopoI peptide‐loaded DCs also induce proliferation of SSc and healthy‐derived T cells, but activation of T cells requires a fragmented form of TopoI and activation of full‐length TopoI is IL‐2‐dependent 126. Interestingly, antigen‐presenting cells within PBMCs were able to activate T cells more efficiently than dendritic cells, and even processed TopoI peptides differently, suggesting that there is a complex regulation of T cells by dendritic cells and other antigen‐presenting cells with regard to anti‐nuclear antibodies. Overall, experimental manipulation of DCS can mimic some major events in the pathogenesis of SSc, and therefore raises the intriguing prospect that DCs may be capable of initiating SSc. A broken loop that may perpetuate inflammation with cytokine‐mediated up‐regulation of co‐stimulatory molecules may also exist in SSc.
Other immune cells
Dysregulation and activation of immune cells is clearly a major hallmark of SSc involving many cell types. This complex network of signalling between different immune cells is only now beginning to become unravelled, with new evidence emerging regularly to provide new players in SSc immunopathogenesis. For example, only a few studies have highlighted the potential for platelets 127, 128, 129, neutrophils 130, 131, natural killer cells 132, 133 and innate lymphoid cells 134, 135 to be dysregulated and contribute to the pathogenesis of SSc. Type 2 innate lymphoid cells are found in higher numbers in SSc and correlate strongly with the Rodnan skin score and also the presence of lung fibrosis.
Although not considered to be innate immune cells, fibroblasts play a crucial role in the fibrotic phenotype of SSc, but they also express TLRs and are therefore capable of responding to DAMPs. Expression of TLR‐2 was found to be increased in fibroblasts from SSc skin 136. Recently, fibroblasts exposed to TGF‐β and IL‐17A responded with a 100‐fold increase in the production of IL‐6 137, a known profibrotic mediator.
Conclusion
Various cell types of the immune system appear to be involved to some degree in the immunopathogenesis of SSc through promoting other immune cell activation, fibrosis or vascular damage. Very recent studies have highlighted the importance of this plethora of immune cell types in SSc (Table 1). However, research to date provides evidence for macrophages, T cells and B cells having the most important role in SSc. Whether this accurately represents their importance in SSc or is a result of research interests combined with the practicality of investigating specific cell types remains to be seen. Nevertheless, immune cell activation is a recurring observation in SSc research and is leading to the development and testing of therapeutic interventions. Although the over‐production of cytokines is a well‐accepted symptom of SSc, TGF‐β, IL‐6 and IL‐4 specifically seem to be integral to many of the immune abnormalities recorded. Thoroughly understanding how these and other cytokines fully regulate, and are regulated by, all the different immune cells and their subcategories in SSc will be a difficult task, but every study into immune cell activation, regardless of cell type, brings us closer to achieving it and as a result developing therapeutic interventions. Current trials such as the Fassinate trial in SSc blocking IL‐6 seem promising, with the Phase II trial positive, although the primary end‐point was not reached. Phosphodiesterase type 4 (PDE4) inhibitors appear to regulate macrophages leading to reduced fibroblast activation. There is an ongoing trial concerning morphea.
Table 1.
A selection of recent and important studies implicating each of the innate cell types in the immunopathogenesis of systemic sclerosis (SSc)
| Immune cell | Recent findings | Reference |
|---|---|---|
| T cell | Angiogenic T cells were elevated in SSc patients displaying digital ulcers, which is a severe peripheral vascular complication | 59 |
| Macrophage | The soluble form of the M2 macrophage marker CD163 is elevated in the serum of patients with SSc, highlighting it as a potential biomarker | 66 |
| B cell | Used a mouse model of SSc to demonstrate the importance of B cell homeostasis. Depletion of IL‐6‐producing B effector cells reduced fibrosis while depleting of IL‐10‐producing B regulatory cells had the opposite effect | 97 |
| Mast cell | A subset of SSc patients with localized scleroderma‐like lesions were found to have an inflammatory phenotype leading to the activation of mast cells in the dermis of mechanically stressed skin | 108 |
| Dendritic cell | After depletion of pDCs fibrosis was reverted in mice with established SSc‐like disease. pDC depletion prior to induction of disease also prevented fibrosis | 115 |
| Platelets | Activated platelets induced the production of the profibrotic mediator thymic stromal lymphopoietin (TSLP) in human dermal endothelial cells | 128 |
| Neutrophils | Neutrophil activation was induced by SSc microparticles. Microparticles were derived from platelets and expressed the damage‐associated molecular pattern HMGB1. An inhibitor of HMGB1 attenuated neutrophil activation | 131 |
| Natural killer | A peculiar natural killer cell phenotype in SSc patients was identified characterized by decreased chemokine and activation receptors expression. These SSc‐derived natural killer cells were potent inducers of endothelial microparticle release suggesting that there may be a role for natural killer cells in the activation of endothelial cells in SSc | 133 |
| Innate lymphoid | Type 2 innate lymphoid (ILC2) cells are elevated in patients with SSc. ILC2 counts correlated with skin fibrosis. This study highlights that there may be a profibrotic role for ILC2 cells in SSc | 134 |
| Fibroblast | Although not an immune cell type, fibroblasts exposed to TGF‐β and IL‐17a responded with a 100‐fold increase in the production of IL‐6 | 137 |
DCs = dendritic cells; IL = interleukin; TGF = transforming growth factor; HMGB1 = high mobility group box 1.
Disclosures
None.
References
- 1. Asano Y, Sato S. Vasculopathy in scleroderma. Semin Immunopathol 2015; 37:489–500. [DOI] [PubMed] [Google Scholar]
- 2. McGaha T et al Lack of skin fibrosis in tight skin (TSK) mice with targeted mutation in the interleukin‐4R alpha and transforming growth factor‐beta genes. J Invest Dermatol 2001; 116:136–43. [DOI] [PubMed] [Google Scholar]
- 3. Fertin C et al Interleukin‐4 stimulates collagen synthesis by normal and scleroderma fibroblasts in dermal equivalents. Cell Mol Biol 1991; 37:823–9. [PubMed] [Google Scholar]
- 4. Sempowski GD, Derdak S, Phipps RP. Interleukin‐4 and interferon‐gamma discordantly regulate collagen biosynthesis by functionally distinct lung fibroblast subsets. J Cell Physiol 1996; 167:290–6. [DOI] [PubMed] [Google Scholar]
- 5. Postlethwaite AE et al Human fibroblasts synthesize elevated levels of extracellular matrix proteins in response to interleukin 4. J Clin Invest 1992; 90:1479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wegrowski Y, Paltot V, Gillery P, Kalis B, Randoux A, Maquart FX. Stimulation of sulphated glycosaminoglycan and decorin production in adult dermal fibroblasts by recombinant human interleukin‐4. Biochem J 1995; 307:673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ong C, Wong C, Roberts CR, Teh H‐S, Jirik FR. Anti‐IL‐4 treatment prevents dermal collagen deposition in the tight‐skin mouse model of scleroderma. Eur J Immunol 1998; 28:2619–29. [DOI] [PubMed] [Google Scholar]
- 8. Luzina IG, Atamas SP, Wise R et al Occurrence of an activated, profibrotic pattern of gene expression in lung CD8+ T cells from scleroderma patients. Arthritis Rheum 2003; 48:2262–74. [DOI] [PubMed] [Google Scholar]
- 9. Needleman BW, Wigley FM, Stair RW. Interleukin‐1, interleukin‐2, interleukin‐4, interleukin‐6, tumor necrosis factor alpha, and interferon‐gamma levels in sera from patients with scleroderma. Arthritis Rheum 1992; 35:67–72. [DOI] [PubMed] [Google Scholar]
- 10. Hasegawa M et al Elevated serum levels of interleukin 4 (IL‐4), IL‐10, and IL‐13 in patients with systemic sclerosis. J Rheumatol 1997; 24:328–32. [PubMed] [Google Scholar]
- 11. Sakkas LI et al Increased levels of alternatively spliced interleukin 4 (IL‐4delta2) transcripts in peripheral blood mononuclear cells from patients with systemic sclerosis. Clin Diagn Lab Immunol 1999; 6:660–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ihn H. The role of TGF‐beta signaling in the pathogenesis of fibrosis in scleroderma. Arch Immunol Ther Exp (Warsz) 2002; 50:325–31. [PubMed] [Google Scholar]
- 13. Zi Z, Chapnick DA, Liu X. Dynamics of TGF‐beta/Smad signaling. FEBS Lett 2012; 586:1921–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sato M et al Role of p38 MAPK in transforming growth factor beta stimulation of collagen production by scleroderma and healthy dermal fibroblasts. J Invest Dermatol 2002; 118:704–11. [DOI] [PubMed] [Google Scholar]
- 15. Hocevar BA, Brown TL, Howe PH. TGF‐beta induces fibronectin synthesis through a c‐Jun N‐terminal kinase‐dependent, Smad4‐independent pathway. EMBO J 1999; 18:1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reunanen N, Foschi M, Han J, Kähäri V‐M. Activation of extracellular signal‐regulated kinase 1/2 inhibits type I collagen expression by human skin fibroblasts. J Biol Chem 2000; 275:34634–9. [DOI] [PubMed] [Google Scholar]
- 17. Koch AE, Kronfeld‐Harrington LB, Szekanecz Z et al In situ expression of cytokines and cellular adhesion molecules in the skin of patients with systemic sclerosis. Their role in early and late disease. Pathobiology 1993; 61:239–46. [DOI] [PubMed] [Google Scholar]
- 18. Sato S, Hasegawa M, Takehara K. Serum levels of interleukin‐6 and interleukin‐10 correlate with total skin thickness score in patients with systemic sclerosis. J Dermatol Sci 2001; 27:140–6. [DOI] [PubMed] [Google Scholar]
- 19. Yoshizaki K. Pathogenic role of IL‐6 combined with TNF‐alpha or IL‐1 in the induction of acute phase proteins SAA and CRP in chronic inflammatory diseases. Adv Exp Med Biol 2011; 691:141–50. [DOI] [PubMed] [Google Scholar]
- 20. Saito F, Tasaka S, Inoue K‐I et al Role of interleukin‐6 in bleomycin‐induced lung inflammatory changes in mice. Am J Respir Cell Mol Biol 2008; 38:566–71. [DOI] [PubMed] [Google Scholar]
- 21. O’Reilly S et al Interleukin‐6 (IL‐6) trans signaling drives a STAT3‐dependent pathway that leads to hyperactive transforming growth factor‐beta (TGF‐beta) signaling promoting SMAD3 activation and fibrosis via Gremlin protein. J Biol Chem 2014; 289:9952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Henderson J, Bhattacharyya S, Varga J, O’Reilly S. Targeting TLRs and the inflammasome in systemic sclerosis. Pharmacol Ther 2018; 192:163–9. [DOI] [PubMed] [Google Scholar]
- 23. Barnes TC et al Endothelial activation and apoptosis mediated by neutrophil‐dependent interleukin 6 trans‐signalling: a novel target for systemic sclerosis? Ann Rheum Dis 2011; 70:366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kitaba S, Murota H, Terao M et al Blockade of interleukin‐6 receptor alleviates disease in mouse model of scleroderma. Am J Pathol 2012; 180:165–76. [DOI] [PubMed] [Google Scholar]
- 25. Shima Y, Kuwahara Y, Murota H et al The skin of patients with systemic sclerosis softened during the treatment with anti‐IL‐6 receptor antibody tocilizumab. Rheumatology (Oxf) 2010; 49:2408–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khanna D, Denton CP, Jahreis A et al Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet 2016; 387:2630–40. [DOI] [PubMed] [Google Scholar]
- 27. Prescott RJ, Freemont AJ, Jones CJP, Hoyland J, Fielding P. Sequential dermal microvascular and perivascular changes in the development of scleroderma. J Pathol 1992; 166:255–63. [DOI] [PubMed] [Google Scholar]
- 28. Abraham D, Lupoli S, McWhirter A et al Expression and function of surface antigens on scleroderma fibroblasts. Arthritis Rheum 1991; 34:1164–72. [DOI] [PubMed] [Google Scholar]
- 29. Kalogerou A, Gelou E, Mountantonakis S, Settas L, Zafiriou E, Sakkas L. Early T cell activation in the skin from patients with systemic sclerosis. Ann Rheum Dis 2005; 64:1233–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gerber EE, Gallo EM, Fontana SC et al Integrin‐modulating therapy prevents fibrosis and autoimmunity in mouse models of scleroderma. Nature 2013; 503:126–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ponsoye M, Frantz C, Ruzehaji N et al Treatment with abatacept prevents experimental dermal fibrosis and induces regression of established inflammation‐driven fibrosis. Ann Rheum Dis 2016; 75:2142–9. [DOI] [PubMed] [Google Scholar]
- 32. Gaud G, Lesourne R, Love PE. Regulatory mechanisms in T cell receptor signalling. Nat Rev Immunol 2018; 18:485–97. [DOI] [PubMed] [Google Scholar]
- 33. Adeeb F, Anjum S, Hodnett P et al Early‐ and late‐stage morphea subtypes with deep tissue involvement is treatable with Abatacept (Orencia). Semin Arthritis Rheum 2017; 46:775–81. [DOI] [PubMed] [Google Scholar]
- 34. O'Reilly S, Hugle T, van Laar JM. T cells in systemic sclerosis: a reappraisal. Rheumatology (Oxf) 2012; 51:1540–9. [DOI] [PubMed] [Google Scholar]
- 35. Sharma SK, MacLean JA, Pinto C, Kradin RL. The effect of an anti‐CD3 monoclonal antibody on bleomycin‐induced lymphokine production and lung injury. Am J Respir Crit Care Med 1996; 154:193–200. [DOI] [PubMed] [Google Scholar]
- 36. Kodera T et al Disrupting the IL‐4 gene rescues mice homozygous for the tight‐skin mutation from embryonic death and diminishes TGF‐beta production by fibroblasts. Proc Natl Acad Sci USA 2002; 99:3800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seder RA et al Factors involved in the differentiation of TGF‐beta‐producing cells from naive CD4+ T cells: IL‐4 and IFN‐gamma have opposing effects, while TGF‐beta positively regulates its own production. J Immunol 1998; 160:5719–28. [PubMed] [Google Scholar]
- 38. Parel Y, Aurrand‐Lions M, Scheja A, Dayer J‐M, Roosnek E, Chizzolini C. Presence of CD4+CD8+ double‐positive T cells with very high interleukin‐4 production potential in lesional skin of patients with systemic sclerosis. Arthritis Rheum 2007; 56:3459–67. [DOI] [PubMed] [Google Scholar]
- 39. Gross A, Ben‐Sasson SZ, Paul WE. Anti‐IL‐4 diminishes in vivo priming for antigen‐specific IL‐4 production by T cells. J Immunol 1993; 150:2112–20. [PubMed] [Google Scholar]
- 40. Fallon PG, Richardson EJ, McKenzie GJ, McKenzie ANJ. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL‐4 and IL‐13: IL‐13 is a profibrotic agent. J Immunol 2000; 164:2585–91. [DOI] [PubMed] [Google Scholar]
- 41. Oriente A et al Interleukin‐13 modulates collagen homeostasis in human skin and keloid fibroblasts. J Pharmacol Exp Ther 2000; 292:988–94. [PubMed] [Google Scholar]
- 42. Belperio JA, Dy M, Burdick MD et al Interaction of IL‐13 and C10 in the pathogenesis of bleomycin‐induced pulmonary fibrosis. Am J Respir Cell Mol Biol 2002; 27:419–27. [DOI] [PubMed] [Google Scholar]
- 43. Baraut J et al Transforming growth factor‐beta increases interleukin‐13 synthesis via GATA‐3 transcription factor in T‐lymphocytes from patients with systemic sclerosis. Arthritis Res Ther 2015; 17:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fuschiotti P, Medsger TA Jr, Morel PA. Effector CD8+ T cells in systemic sclerosis patients produce abnormally high levels of interleukin‐13 associated with increased skin fibrosis. Arthritis Rheum 2009; 60:1119–28. [DOI] [PubMed] [Google Scholar]
- 45. Li G, Larregina AT, Domsic RT et al Skin‐resident effector memory CD8(+)CD28(–) T cells exhibit a profibrotic phenotype in patients with systemic sclerosis. J Invest Dermatol 2017; 137:1042–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. OReilly S et al IL‐13 mediates collagen deposition via STAT6 and microRNA‐135b: a role for epigenetics. Sci Rep 2016; 6:25066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Brown Lobbins ML et al Chronic exposure of interleukin‐13 suppress the induction of matrix metalloproteinase‐1 by tumour necrosis factor alpha in normal and scleroderma dermal fibroblasts through protein kinase B/Akt. Clin Exp Immunol 2018; 191:84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kurasawa K, Hirose K, Sano H et al Increased interleukin‐17 production in patients with systemic sclerosis. Arthritis Rheum 2000; 43:2455–63. [DOI] [PubMed] [Google Scholar]
- 49. Truchetet M‐E, Brembilla N‐C, Montanari E et al Interleukin‐17A+ cell counts are increased in systemic sclerosis skin and their number is inversely correlated with the extent of skin involvement. Arthritis Rheum 2013; 65:1347–56. [DOI] [PubMed] [Google Scholar]
- 50. Yang X, Yang J, Xing X, Wan L, Li M. Increased frequency of Th17 cells in systemic sclerosis is related to disease activity and collagen overproduction. Arthritis Res Ther 2014; 16:R4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chizzolini C, Dufour AM, Brembilla NC. Is there a role for IL‐17 in the pathogenesis of systemic sclerosis? Immunol Lett 2018; 195:61–7. [DOI] [PubMed] [Google Scholar]
- 52. Gourh P, Arnett FC, Assassi S et al Plasma cytokine profiles in systemic sclerosis: associations with autoantibody subsets and clinical manifestations. Arthritis Res Ther 2009; 11:R147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Olewicz‐Gawlik A, Danczak‐Pazdrowska A, Kuznar‐Kaminska B et al Interleukin‐17 and interleukin‐23: importance in the pathogenesis of lung impairment in patients with systemic sclerosis. Int J Rheum Dis 2014; 17:664–70. [DOI] [PubMed] [Google Scholar]
- 54. Brembilla N, Montanari E, Truchetet M‐E, Raschi E, Meroni P, Chizzolini C. Th17 cells favor inflammatory responses while inhibiting type I collagen deposition by dermal fibroblasts: differential effects in healthy and systemic sclerosis fibroblasts. Arthritis Res Ther 2013; 15:R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nakashima T, Jinnin M, Yamane K et al Impaired IL‐17 signaling pathway contributes to the increased collagen expression in scleroderma fibroblasts. J Immunol 2012;188:3573–83. [DOI] [PubMed] [Google Scholar]
- 56. Klein S, Kretz CC, Ruland V et al Reduction of regulatory T cells in skin lesions but not in peripheral blood of patients with systemic scleroderma. Ann Rheum Dis 2011; 70:1475–81. [DOI] [PubMed] [Google Scholar]
- 57. Krasimirova E, Velikova T, Ivanova‐Todorova E et al Treg/Th17 cell balance and phytohaemagglutinin activation of T lymphocytes in peripheral blood of systemic sclerosis patients. World J Exp Med 2017; 7:84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tang J, Lei L, Pan J, Zhao C, Wen J. Higher levels of serum interleukin‐35 are associated with the severity of pulmonary fibrosis and Th2 responses in patients with systemic sclerosis. Rheumatol Int 2018; 38:1511–9. [DOI] [PubMed] [Google Scholar]
- 59. Manetti M et al Angiogenic T cell expansion correlates with severity of peripheral vascular damage in systemic sclerosis. PLOS ONE 2017; 12:e0183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Andrews BS et al Changes in circulating monocytes in patients with progressive systemic sclerosis. J Rheumatol 1987; 14:930–5. [PubMed] [Google Scholar]
- 61. Higashi‐Kuwata N et al Characterization of monocyte/macrophage subsets in the skin and peripheral blood derived from patients with systemic sclerosis. Arthritis Res Ther 2010; 12:R128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stifano G, Christmann RB. Macrophage involvement in systemic sclerosis: do we need more evidence? Curr Rheumatol Rep 2016; 18:2. [DOI] [PubMed] [Google Scholar]
- 63. Bielecki M, Kowal K, Lapinska A, Chyczewski L, Kowal‐Bielecka O. Increased release of soluble CD163 by the peripheral blood mononuclear cells is associated with worse prognosis in patients with systemic sclerosis. Adv Med Sci 2013; 58:126–33. [DOI] [PubMed] [Google Scholar]
- 64. Nakayama W et al CD163 expression is increased in the involved skin and sera of patients with systemic lupus erythematosus. Eur J Dermatol 2012; 22:512–7. [DOI] [PubMed] [Google Scholar]
- 65. Shimizu K, Ogawa F, Yoshizaki A et al Increased serum levels of soluble CD163 in patients with scleroderma. Clin Rheumatol 2012; 31:1059–64. [DOI] [PubMed] [Google Scholar]
- 66. Frantz C et al Soluble CD163 as a potential biomarker in systemic sclerosis. Dis Markers 2018; 2018:8509583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Grigoryev DN, Mathai SC, Fisher MR et al Identification of candidate genes in scleroderma‐related pulmonary arterial hypertension. Transl Res 2008; 151:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pendergrass SA, Hayes E, Farina G et al Limited systemic sclerosis patients with pulmonary arterial hypertension show biomarkers of inflammation and vascular injury. PLOS ONE 2010; 5:e12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Christmann RB, Hayes E, Pendergrass S et al Interferon and alternative activation of monocyte/macrophages in systemic sclerosis‐associated pulmonary arterial hypertension. Arthritis Rheum 2011; 63:1718–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shapouri‐Moghaddam A, Mohammadian S, Vazini H et al Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 2018; 233:6425–40. [DOI] [PubMed] [Google Scholar]
- 71. Hu HH et al New insights into TGF‐beta/Smad signaling in tissue fibrosis. Chem Biol Interact 2018; 292:76–83. [DOI] [PubMed] [Google Scholar]
- 72. Hsu E, Shi H, Jordan RM, Lyons‐Weiler J, Pilewski JM, Feghali‐Bostwick CA. Lung tissues in patients with systemic sclerosis have gene expression patterns unique to pulmonary fibrosis and pulmonary hypertension. Arthritis Rheum 2011; 63:783–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Christmann RB et al Association of Interferon‐ and transforming growth factor beta‐regulated genes and macrophage activation with systemic sclerosis‐related progressive lung fibrosis. Arthritis Rheumatol 2014; 66:714–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Taroni JN, Greene CS, Martyanov V et al A novel multi‐network approach reveals tissue‐specific cellular modulators of fibrosis in systemic sclerosis. Genome Med 2017; 9:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Huang J, Maier C, Zhang Y et al Nintedanib inhibits macrophage activation and ameliorates vascular and fibrotic manifestations in the Fra2 mouse model of systemic sclerosis. Ann Rheum Dis 2017; 76:1941–8. [DOI] [PubMed] [Google Scholar]
- 76. Mathes AL, Christmann RB, Stifano G et al Global chemokine expression in systemic sclerosis (SSc): CCL19 expression correlates with vascular inflammation in SSc skin. Ann Rheum Dis 2014; 73:1864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Distler O, Pap T, Kowal‐Bielecka O et al Overexpression of monocyte chemoattractant protein 1 in systemic sclerosis: role of platelet‐derived growth factor and effects on monocyte chemotaxis and collagen synthesis. Arthritis Rheum 2001; 44:2665–78. [DOI] [PubMed] [Google Scholar]
- 78. Yamamoto T, Eckes B, Krieg T. High expression and autoinduction of monocyte chemoattractant protein‐1 in scleroderma fibroblasts. Eur J Immunol 2001; 31:2936–41. [DOI] [PubMed] [Google Scholar]
- 79. Hasegawa M, Sato S, Takehara K. Augmented production of chemokines (monocyte chemotactic protein‐1 (MCP‐1), macrophage inflammatory protein‐1alpha (MIP‐1alpha) and MIP‐1beta) in patients with systemic sclerosis: MCP‐1 and MIP‐1alpha may be involved in the development of pulmonary fibrosis. Clin Exp Immunol 1999; 117:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ishikawa O, Ishikawa H. Macrophage infiltration in the skin of patients with systemic sclerosis. J Rheumatol 1992; 19:1202–6. [PubMed] [Google Scholar]
- 81. O’Reilly S. Toll like receptors in systemic sclerosis: an emerging target. Immunol Lett 2018; 195:2–8. [DOI] [PubMed] [Google Scholar]
- 82. Fullard N, O'Reilly S. Role of innate immune system in systemic sclerosis. Semin Immunopathol 2015; 37:511–7. [DOI] [PubMed] [Google Scholar]
- 83. Ciechomska M, Huigens CA, Hügle T et al Toll‐like receptor‐mediated, enhanced production of profibrotic TIMP‐1 in monocytes from patients with systemic sclerosis: role of serum factors. Ann Rheum Dis 2013; 72:1382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Maier C, Ramming A, Bergmann C et al Inhibition of phosphodiesterase 4 (PDE4) reduces dermal fibrosis by interfering with the release of interleukin‐6 from M2 macrophages. Ann Rheum Dis 2017; 76:1133–41. [DOI] [PubMed] [Google Scholar]
- 85. Sebba A. Tocilizumab: the first interleukin‐6‐receptor inhibitor. Am J Health Syst Pharm 2008; 65:1413–8. [DOI] [PubMed] [Google Scholar]
- 86. Mozaffarian A, Brewer AW, Trueblood ES et al Mechanisms of oncostatin M‐induced pulmonary inflammation and fibrosis. J Immunol 2008; 181:7243–53. [DOI] [PubMed] [Google Scholar]
- 87. Hancock A, Armstrong L, Gama R, Millar A. Production of interleukin 13 by alveolar macrophages from normal and fibrotic lung. Am J Respir Cell Mol Biol 1998; 18:60–5. [DOI] [PubMed] [Google Scholar]
- 88. Francois A et al B lymphocytes and B‐cell activating factor promote collagen and profibrotic markers expression by dermal fibroblasts in systemic sclerosis. Arthritis Res Ther 2013; 15:R168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sato S, Fujimoto M, Hasegawa M, Takehara K. Altered blood B lymphocyte homeostasis in systemic sclerosis: expanded naive B cells and diminished but activated memory B cells. Arthritis Rheum 2004; 50:1918–27. [DOI] [PubMed] [Google Scholar]
- 90. Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol 2009; 9:491–502. [DOI] [PubMed] [Google Scholar]
- 91. Matsushita T, Hasegawa M, Yanaba K, Kodera M, Takehara K, Sato S. Elevated serum BAFF levels in patients with systemic sclerosis: enhanced BAFF signaling in systemic sclerosis B lymphocytes. Arthritis Rheum 2006; 54:192–201. [DOI] [PubMed] [Google Scholar]
- 92. Matsushita T, Fujimoto M, Hasegawa M et al BAFF antagonist attenuates the development of skin fibrosis in tight‐skin mice. J Invest Dermatol 2007; 127:2772–80. [DOI] [PubMed] [Google Scholar]
- 93. Rincon M et al Interleukin (IL)‐6 directs the differentiation of IL‐4‐producing CD4+ T cells. J Exp Med 1997; 185:461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Iwata Y, Matsushita T, Horikawa M et al Characterization of a rare IL‐10‐competent B‐cell subset in humans that parallels mouse regulatory B10 cells. Blood 2011;117:530–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Matsushita T, Yanaba K, Bouaziz J‐D, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest 2008; 118:3420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Matsushita T, Hamaguchi Y, Hasegawa M, Takehara K, Fujimoto M. Decreased levels of regulatory B cells in patients with systemic sclerosis: association with autoantibody production and disease activity. Rheumatology (Oxford) 2016; 55:263–7. [DOI] [PubMed] [Google Scholar]
- 97. Matsushita T et al BAFF inhibition attenuates fibrosis in scleroderma by modulating the regulatory and effector B cell balance. Sci Adv 2018; 4:eaas9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Le Huu D, Matsushita T, Jin G et al Donor‐derived regulatory B cells are important for suppression of murine sclerodermatous chronic graft‐versus‐host disease. Blood 2013; 121:3274–83. [DOI] [PubMed] [Google Scholar]
- 99. Sato S, Hasegawa M, Fujimoto M, Tedder TF, Takehara K. Quantitative genetic variation in CD19 expression correlates with autoimmunity. J Immunol 2000; 165:6635–43. [DOI] [PubMed] [Google Scholar]
- 100. Sato S et al CD19 regulates B lymphocyte signaling thresholds critical for the development of B‐1 lineage cells and autoimmunity. J Immunol 1996; 157:4371–8. [PubMed] [Google Scholar]
- 101. Sato S, Fujimoto M, Hasegawa M, Takehara K, Tedder TF. Altered B lymphocyte function induces systemic autoimmunity in systemic sclerosis. Mol Immunol 2004; 41:1123–33. [DOI] [PubMed] [Google Scholar]
- 102. Asano N, Fujimoto M, Yazawa N et al B Lymphocyte signaling established by the CD19/CD22 loop regulates autoimmunity in the tight‐skin mouse. Am J Pathol 2004; 165:641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Baroni SS, Santillo M, Bevilacqua F et al Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med 2006; 354:2667–76. [DOI] [PubMed] [Google Scholar]
- 104. Yoshizaki A, Iwata Y, Komura K et al CD19 regulates skin and lung fibrosis via Toll‐like receptor signaling in a model of bleomycin‐induced scleroderma. Am J Pathol 2008; 172:1650–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hebbar M et al Early expression of E‐selectin, tumor necrosis factor alpha, and mast cell infiltration in the salivary glands of patients with systemic sclerosis. Arthritis Rheum 1996; 39:1161–5. [DOI] [PubMed] [Google Scholar]
- 106. Bagnato G et al Mastocytosis and systemic sclerosis: a clinical association. Clin Mol Allergy 2016; 14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Frigui M, Dupin N, Carlotti A et al Telangiectasic mastocytosis with systemic sclerosis. Presse Med 2013; 42:902–4. [DOI] [PubMed] [Google Scholar]
- 108. Saigusa R et al Systemic sclerosis complicated with localized scleroderma‐like lesions induced by Kobner phenomenon. J Dermatol Sci 2018; 89:282–9. [DOI] [PubMed] [Google Scholar]
- 109. Seibold JR, Giorno RC, Claman HN. Dermal mast cell degranulation in systemic sclerosis. Arthritis Rheum 1990; 33:1702–9. [DOI] [PubMed] [Google Scholar]
- 110. Hugle T et al Mast cells are a source of transforming growth factor beta in systemic sclerosis. Arthritis Rheum 2011; 63:795–9. [DOI] [PubMed] [Google Scholar]
- 111. Trautmann A et al Human mast cells augment fibroblast proliferation by heterotypic cell‐cell adhesion and action of IL‐4. J Immunol 1998; 160:5053–7. [PubMed] [Google Scholar]
- 112. Hugle T, White K, van Laar JM. Cell‐to‐cell contact of activated mast cells with fibroblasts and lymphocytes in systemic sclerosis. Ann Rheum Dis 2012; 71:1582. [DOI] [PubMed] [Google Scholar]
- 113. Pincha N, Hajam EY, Badarinath K et al PAI1 mediates fibroblast‐mast cell interactions in skin fibrosis. J Clin Invest 2018; 128:1807–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bhattacharyya S et al Tenascin‐C drives persistence of organ fibrosis. Nat Commun 2016; 7:11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ah Kioon MD, Tripodo C, Fernandez D et al Plasmacytoid dendritic cells promote systemic sclerosis with a key role for TLR8. Sci Transl Med 2018; 10:8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Affandi AJ, Carvalheiro T, Radstake TRDJ, Marut W. Dendritic cells in systemic sclerosis: Advances from human and mice studies. Immunol Lett 2018; 195:18–29. [DOI] [PubMed] [Google Scholar]
- 117. van Bon L, Affandi AJ, Broen J et al Proteome‐wide analysis and CXCL4 as a biomarker in systemic sclerosis. N Engl J Med 2014; 370:433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Volkmann ER, Tashkin DP, Roth MD et al Changes in plasma CXCL4 levels are associated with improvements in lung function in patients receiving immunosuppressive therapy for systemic sclerosis‐related interstitial lung disease. Arthritis Res Ther 2016; 18:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kafaja S, Valera I, Divekar AA et al pDCs in lung and skin fibrosis in a bleomycin‐induced model and patients with systemic sclerosis. JCI Insight 2018; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Chakraborty K, Chatterjee S, Bhattacharyya A. Modulation of CD11c+ lung dendritic cells in respect to TGF‐beta in experimental pulmonary fibrosis. Cell Biol Int 2017; 41:991–1000. [DOI] [PubMed] [Google Scholar]
- 121. Horsburgh S, Fullard N, Roger M et al MicroRNAs in the skin: role in development, homoeostasis and regeneration. Clin Sci (Lond) 2017; 131:1923–40. [DOI] [PubMed] [Google Scholar]
- 122. Rossato M, Affandi AJ, Thordardottir S et al Association of MicroRNA‐618 Expression With Altered Frequency and Activation of Plasmacytoid Dendritic Cells in Patients With Systemic Sclerosis. Arthritis Rheumatol 2017; 69:1891–902. [DOI] [PubMed] [Google Scholar]
- 123. Okano Y. Antinuclear antibody in systemic sclerosis (scleroderma). Rheum Dis Clin North Am 1996; 22:709–35. [DOI] [PubMed] [Google Scholar]
- 124. Sato S, Hamaguchi Y, Hasegawa M, Takehara K. Clinical significance of anti‐topoisomerase I antibody levels determined by ELISA in systemic sclerosis. Rheumatology (Oxford) 2001; 40:1135–40. [DOI] [PubMed] [Google Scholar]
- 125. Mehta H, Goulet P‐O, Nguyen V et al Topoisomerase I peptide‐loaded dendritic cells induce autoantibody response as well as skin and lung fibrosis. Autoimmunity 2016; 49:503–13. [DOI] [PubMed] [Google Scholar]
- 126. Oriss TB, Hu PQ, Wright TM. Distinct autoreactive T cell responses to native and fragmented DNA topoisomerase I: influence of APC type and IL‐2. J Immunol 2001; 166:5456–63. [DOI] [PubMed] [Google Scholar]
- 127. Dees C, Akhmetshina A, Zerr P et al Platelet‐derived serotonin links vascular disease and tissue fibrosis. J Exp Med 2011; 208:961–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Truchetet M‐E, Demoures B, Eduardo Guimaraes J et al Platelets induce thymic stromal lymphopoietin production by endothelial cells: contribution to fibrosis in human systemic sclerosis. Arthritis Rheumatol 2016; 68:2784–94. [DOI] [PubMed] [Google Scholar]
- 129. Ntelis K, Solomou EE, Sakkas L, Liossis S‐N, Daoussis D. The role of platelets in autoimmunity, vasculopathy, and fibrosis: Implications for systemic sclerosis. Semin Arthritis Rheum 2017; 47:409–17. [DOI] [PubMed] [Google Scholar]
- 130. Barnes TC, Anderson ME, Edwards SW, Moots RJ. Neutrophil‐derived reactive oxygen species in SSc. Rheumatology (Oxf) 2012; 51:1166–9. [DOI] [PubMed] [Google Scholar]
- 131. Maugeri N et al Platelet microparticles sustain autophagy‐associated activation of neutrophils in systemic sclerosis. Sci Transl Med 2018; 10. [DOI] [PubMed] [Google Scholar]
- 132. Almeida I, Silva SV, Fonseca AR, Silva I, Vasconcelos C, Lima M. T and NK cell phenotypic abnormalities in systemic sclerosis: a cohort study and a comprehensive literature review. Clin Rev Allergy Immunol 2015; 49:347–69. [DOI] [PubMed] [Google Scholar]
- 133. Benyamine A et al Natural killer cells exhibit a peculiar phenotypic profile in systemic sclerosis and are potent inducers of endothelial microparticles release. Front Immunol 2018; 9:1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Roan F et al CD4+ Group 1 innate lymphoid cells (ILC) form a functionally distinct ILC subset that is increased in systemic sclerosis. J Immunol 2016; 196:2051–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Wohlfahrt T, Usherenko S, Englbrecht M et al Type 2 innate lymphoid cell counts are increased in patients with systemic sclerosis and correlate with the extent of fibrosis. Ann Rheum Dis 2016; 75:623–6. [DOI] [PubMed] [Google Scholar]
- 136. O'Reilly S et al Serum amyloid A induces interleukin‐6 in dermal fibroblasts via Toll‐like receptor 2, interleukin‐1 receptor‐associated kinase 4 and nuclear factor‐kappaB. Immunology 2014; 143:331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Dufour AM et al Interleukin‐6 and type‐I collagen production by systemic sclerosis fibroblasts are differentially regulated by interleukin‐17A in the presence of transforming growth factor‐beta 1. Front Immunol 2018; 9:1865. [DOI] [PMC free article] [PubMed] [Google Scholar]


