Summary
A balanced microbiota of the gastrointestinal tract (GIT) is a prerequisite for a healthy host. The GIT microbiota in preterm infants is determined by the method of delivery and nutrition. Probiotics can improve the GIT microbiota balance and suitable animal models are required to verify their harmlessness. Preterm gnotobiotic piglets were colonized with Lactobacillus rhamnosus GG (LGG) to evaluate its safety and possible protective action against infection with an enteric pathogen, Salmonella Typhimurium (ST). Clinical signs (anorexia, somnolence, fever and diarrhea), bacterial interference and translocation, intestinal histopathology, transcriptions of claudin‐1, occludin and interferon (IFN)‐γ, intestinal and systemic protein levels of interleukin (IL)‐8, IL‐12/23 p40 and IFN‐γ were compared among (i) germ‐free, (ii) LGG‐colonized, (iii) ST‐infected and (iv) LGG‐colonized and subsequently ST‐infected piglets for 24 h. Both LGG and ST‐colonized the GIT; LGG translocated in some cases into mesenteric lymph nodes and the spleen but did not cause bacteremia and clinical changes. ST caused clinical signs of gastroenteritis, translocated into mesenteric lymph nodes, the spleen, liver and blood, increased claudin‐1 and IFN‐γ transcriptions, but decreased occludin transcription and increased local and systemic levels of IL‐8 and IL‐12/23 p40. Previous colonization with LGG reduced ST colonization in the jejunum and translocation into the liver, spleen and blood. It partially ameliorated histopathological changes in the intestine, reduced IL‐8 levels in the jejunum and plasma and IL‐12/23 p40 in the jejunum. The preterm gnotobiotic piglet model of the vulnerable preterm immunocompromised infant is useful to verify the safety of probiotics and evaluate their protective effect.
Keywords: bacterial interference, gnotobiotic piglets, Lactobacillus rhamnosusGG, preterm, Salmonella Typhimurium
Introduction
Preterm infants are usually defined as infants born before 37 weeks’ completed gestation. They are vulnerable due to underdevelopment of their organs and they show higher morbidity and mortality in comparison to their full‐term counterparts 1. A balanced gastrointestinal tract (GIT) microbiota has normally been established by the first year of life 2. Its formation is highly influenced by the method of delivery. Babies born by vaginal delivery are colonized by maternal vaginal and fecal microbiota immediately during birth 3 while colonization of babies delivered by cesarean section (CS) is impacted by the microbiota of hospital surroundings 4. This hospital microbiota can cause nosocomial infections due to their frequent resistance to a broad spectrum of antibiotics 5. Preterm infants are highly susceptible to dysbiosis and infections that can culminate in early (EOS) or late onset of sepsis (LOS) 6, 7. Probiotics are microbes that are commonly beneficial to their host 8 and their application influences and supports the establishment of a balanced microbiota 2, 9. Lactobacillus rhamnosus GG (LGG) belongs to a group of the most frequently used probiotic bacteria applied as a therapeutic agent 10. It was originally isolated by Goldin and Gorbach from human feces in 1983 and later used successfully for the treatment of relapsing Clostridium difficile colitis 11. Many reports detail the beneficial effects of LGG to host health 10. It was also shown to be effective in preterm infants in improving necrotizing enterocolitis (NEC) 12. Despite the widely accepted beneficial role of probiotics, it is necessary to be careful regarding their application to immunocompromised preterm infants 13. Consequently, suitable in vivo animal models are required to study and evaluate possible probiotic actions of candidate probiotics to verify their safety for the immunocompromised host. The pig shows closer physiological, anatomical and genetic similarities to the human compared to other animal models 14. They have been used as the animal model of human gastrointestinal illnesses 15 and infectious diseases 16. Preterm piglets are used as animal models of the preterm infant gastrointestinal tract 17 and necrotizing enterocolitis (NEC) 18. The preterm germ‐free piglet model has been established recently to study preterm immunocompromised host–bacteria interactions 19.
In contrast to probiotic L. rhamnosus GG, Salmonella Typhimurium is an obligatory human and animal gastrointestinal pathogen. It causes self‐limiting enterocolitis in healthy humans 20, but it can provoke life‐threatening systemic illness in immunocompromised individuals 21, 22.
The aim of this work was to introduce a preterm gnotobiotic piglet model to evaluate the effect of probiotics, including their safety, on the immunocompromised host and their possible efficacy in protecting against infection with enteric pathogens. Possible amelioration of infection with non‐typhoidal S. Typhimurium after previous colonization with L. rhamnosus GG was assessed in preterm gnotobiotic piglets. Clinical signs (anorexia, somnolence and diarrhea), intestinal histopathology, transcriptions of tight junction proteins claudin‐1 and occludin in the ileum and the colon and intestinal and plasma levels of chemokine interleukin (IL)‐8, proinflammatory cytokine IL‐12/23 p40 and interferon (IFN)‐γ were evaluated. Additionally, a transcription of IFN‐γ in the ileum and the colon were detected to elucidate its possible participation and dynamism in Salmonella‐provoked inflammatory response.
Material and methods
Ethical statement
All experiments with animals were approved by the Animal Care and Use Committee of the Institute of Microbiology in accordance with European Community laws.
Bacterial suspensions
Bacterial suspensions comprised L. rhamnosus GG (LGG) ATCC 53103 (via a Czech Collection of Microorganisms, Masaryk University, Brno, Czech Republic – CCM 7091) and S. enterica subsp. enterica serovar Typhimurium strain LT2 (ST) was from a collection of the microorganisms of the Institute of Microbiology, Novy Hradek, Czech Republic.
Fresh cultures of bacteria were prepared for each experiment by cultivation at 37°C overnight. LGG was cultivated in 10 ml MRS broth (Oxoid, Basingstoke, UK). The cells were harvested by centrifugation at 4000 g for 10 min. The pellet was washed twice with 0·05 M phosphate buffer. ST was cultivated on meat‐peptone agar slopes (blood agar base; Oxoid). Both bacteria were resuspended to an approximate density of 5 × 108 colony‐forming units (CFU)/ml. The number of CFU that was estimated by spectrophotometry at 600 nm was verified by a cultivation method.
Gnotobiotic piglets
The preterm gnotobiotic piglets were obtained by hysterectomy of pregnant miniature Minnesota‐derived sows 23 (Animal Research Institute, Kostelec nad Orlici, Czech Republic) on the 104th day of gestation (average full term in these miniature sows is 112 days), under isoflurane anesthesia (Piramal Healthcare UK Ltd, Morpeth, UK) and reared in fiberglass isolators (Supporting information, Fig. S1), as described elsewhere 19. Briefly, the piglets were bottle‐fed using a cow’s milk‐based diet (Mlekarna Hlinsko, Hlinsko, Czech Republic). Initial feeding was performed by dripping the diet onto the tongue to provoke a swallowing reflex. The piglets received 50 mg of the iron–dextran complex (Ferribion; Bioveta, Ivanovice na Hane, Czech Republic) and 5 mg of phytomenadione (vitamin K) (Kanavit; HBM Pharma, Martin, Slovakia) intramuscularly 2 h after hysterectomy. Twenty‐six preterm gnotobiotic piglets were grouped into four groups (Supporting information, Fig. S2): (i) sterile for the whole experimental period (germ‐free; GF, n = 6), (ii) orally colonized with 1 × 108 CFU of L. rhamnosus GG 4 h after hysterectomy (LGG, n = 8), (iii) 1‐week‐old GF piglets orally infected with 1 × 108 CFU of S. enterica serovar Typhimurium for 24 h (ST, n = 6) and (iv) 1‐week‐old LGG‐colonized piglets orally infected with 1 × 108 CFU of S. Typhimurium for 24 h (LGG+ST, n = 6).
Inspection of gnotobiotic state
Amniotic membranes, umbilical cords, meconium, mouth and isolator surface smears after hysterectomy and later, mouth and surface smears and stool, were cultivated twice a week for the presence of aerobic and anaerobic bacteria and mold. Additionally, Gram‐stained rectal swabs were inspected under a light microscope.
Blood plasma and intestinal lavages
At the end of the experiment the piglets were euthanized by cardiac puncture exsanguination under general isoflurane inhalation anesthesia. The citrated blood was spun at 1500 g for 15 min, protease inhibitor cocktail (Roche Diagnostics, Manheim, Germany) was added and the plasma was stored at −45°C until cytokines were measured. Forty cm of the proximal jejunum (marked in the text as jejunum) and the ileum with the distal jejunum (marked in the text as ileum) were filled with 2 ml of Dulbecco’s phosphate‐buffered saline (DPBS; Life Technologies, Carlsbad, CA, USA). They were gently kneaded and rinsed. The colon was cut into small pieces and was rinsed with 4 ml of DPBS. Parts of these intestinal lavages were used immediately for CFU counting. Other parts with added a protease inhibitor cocktail (Roche Diagnostics) were spun at 2500 g, pressed through 0·20 µm acetate cellulose filter (Sartorius AG, Goettingen, Germany), and the aliquots were stored at –45°C until following analyses.
CFU counting
The intestinal lavages before addition of the protease inhibitor cocktail (Roche) were log10 diluted in phosphate‐buffered saline (PBS) and cultivated in 90 mm Petri dishes – LGG on MRS agar (Oxoid) with acetic acid at 37°C for 72 h and ST on McConkey agar (Merck, Darmstadt, Germany) at 37°C for 24 h. The CFU from dishes containing 20–200 colonies were counted. Mesenteric lymph nodes, liver and spleen were homogenized in deionized water in a 2 ml Eppendorf tube by shaking with 3·2 mm stainless steel beads in a TissueLyser LT beadbeater (Qiagen, Hilden, Germany) for 3 min at 50 Hz. The tissue homogenates were cultivated in the same way as the intestinal lavages.
Histopathological evaluation
Terminal ileum samples were fixed in Carnoy’s fluid for 30 min, dehydrated and embedded in paraffin. Five µm tissue sections were cut on a Leica microtome RM2245 (Leica Microsystems, Wetzlar, Germany), stained with hematoxylin and eosin and examined under an Olympus BX 40 microscope with a Olympus Camedia C‐2000 digital camera (Olympus, Tokyo, Japan). Sections were evaluated in a blinded fashion. Averages of 30 evenly spaced radial lamina mucosalis propria widths per each piglet were determined. Ten measurements for each parameter were taken per piglet to assess ileal villus length and crypt depth. A histopathological score to evaluate differences between the piglet groups was suggested: (i) submucosal edema (0–2 score points), (ii) polymorphonuclear neutrophils (PMNs) infiltration into the lamina propria (0–2 score points), (iii) villus atrophy (0–3 score points), (iv) exudate in lumen (0–2 score points), (v) vessels dilatation (0–2 score points), (vi) inflammatory cellularity in lymphatic vessel lumen (0–2 score points), (vii) hyperemia (0–2 score points), (viii) hemorrhage (0–2 score points), (ix) peritonitis (0–1 score points) and (x) erosion of the epithelial layer (0–3 score points). The total score of 0–21 points was obtained.
Purification of total RNA and reverse transcriptions
One to 2 mm‐thin cross‐sections of the terminal ileum and transversal colon were stored in RNAlater at –20°C until the purification of total RNA was performed. A 10 mg sample was moved from RNAlater to a 2 ml Eppendorf tube containing 600 µl RLT buffer of the RNAeasy Mini Plus kit (Qiagen) with addition 1/1000 of anti‐foaming reagent DX (Qiagen) and 2 mm zirconia beads (BioSpec Products, Bartlesville, OK, USA). The tissue was homogenized at 50 Hz for 5 min in TissueLyser LT beadbeater (Qiagen). Other purification steps followed the manufacturer’s instructions. Five hundred ng of total RNA with ratio absorbances A260–A320/A280–A320 ≥ 2·0 measured in DEPC‐treated 10 mM Tris‐HCl buffer pH 7·5 were reverse‐transcribed. The transcriptions with an initial DNA wipe‐out buffer incubation was performed at 42°C for 20 min and finished by heating at 95°C for 3 min using the QuantiTect Reverse Transcription kit (Qiagen). The synthetized cDNA was diluted 1 : 10 by PCR quality water (Life Technologies, Carlsbad, CA, USA) and these prepared templates were stored at –25°C until the following real‐time–polymerase chain reaction (RT–PCR).
RT–PCR
A Universal Probe Library locked nucleic acids (LNA) probe‐based RT–PCR system (www.universalprobelibrary.com) was used. Two µl of cDNA template were added to 18 µl of the FastStart Universal Probe Master (Roche Diagnostics) containing 100 nM LNA probe (Roche Diagnostics) and 500 nM each of the forward and reverse primers (Table 1) to quantify specific sequences in the cDNA templates. The mixtures were incubated and measured on an iQ cycler (Bio‐Rad, Hercules, CA, USA) equipped with the iQ5 Optical System software version 1.0 (Bio‐Rad). Initial heating for 10 min at 95°C followed by 45 cycles at 95°C for 15 s and 60°C for 60 s were used to measure Cq in duplicate. The obtained values for claudin‐1, occludin and IFN‐γ were normalized using β‐actin and cyclophilin A and their relative expressions were calculated by Genex 6 software (Multid AB, Gothenburg, Sweden).
Table 1.
Locked nucleic acids based real‐time polymerase chain reaction systems used in analyses
| Gene | GenBank access | Forward primer | #LNA probe |
|---|---|---|---|
| Reverse primer | |||
| β actin | U07786 | TCCCTGGAGAAGAGCTACGA | 9 |
| AAGAGCGCCTCTGGACAC | |||
| Cyclophilin A | NM214353 | CCTGAAGCATACGGGTCCT | 48 |
| AAAGACCACATGTTTGCCATC | |||
| Claudin‐1 | NM001244539 | CACCACTTTGCAAGCAACC | 3 |
| TGGCCACAAAGATGGCTATT | |||
| Occludin | U79554 | AAAGAGCTCTCTCGACTGGATAAA | 42 |
| AGCAGCAGCCATGTACTCTTC | |||
| Interferon‐γ | NM_213948 | TGGAAAGAGGAGAGTGACAAAAA | 21 |
| GAATGGCCTGGTTATCTTTGA |
Luminex xMAP technology
IL‐8 (attraction and activation of PMNs 24), IL‐12/23 p40 (early sepsis marker in the pig 25) and IFN‐γ (combat intracellular infections 26) in the intestinal lavages and plasma were measured by a paramagnetic sphere‐based Luminex xMAP technology using a Porcine ProcartaPlex kit (Affymetrix, Santa Clara, CA, USA). The spheres were labeled according to the manufacturer’s instructions and were washed on a Hydroflex washer with a magnetic plate (Tecan, Groedung, Austria). The cytokines were measured on Bio‐Plex Array System and evaluated by Bio‐Plex Manager version 4.01 software (Bio‐Rad).
Statistical analysis
Differences between log10 bacterial CFU of LGG (LGG versus LGG+ST) and ST (ST versus LGG+ST) were counted using the unpaired two‐tailed t‐test. Relative transcriptions of claudin‐1, occludin and IFN‐γ were compared among piglet groups using one‐way analysis of variance (anova) with Tukey’s multiple comparisons post‐hoc test and concentrations of IL‐8, IL‐12 p40 and IFN‐γ with Kruskal–Wallis test with Dunn’s multiple comparison post‐hoc test. The statistical analyses and graphs were performed using GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA) and statistical significances were assessed at *P < 0·05, **P < 0·01 and ***P < 0·001 (log10 CFU) or P < 0·05 (multiple comparisons).
Results
The preterm piglets had closed eyes on the day of hysterectomy and usually opened them on the third day of life (Supporting information, Fig. S2). They also showed other signs of immaturity, as described elsewhere 19.
Bacterial colonization, interferences and translocation
L. rhamnosus GG (LGG) and S. Typhimurium (ST) colonization of the intestine and their translocation and interferences were evaluated by the cultivation methods (Fig. 1). Individual piglet CFU in the jejunum, ileum, colon, mesenteric lymph nodes (MLN), liver, spleen and blood are represented by spots, and the group mean is depicted by the horizontal line (Fig. 1a–g). A summary overview of both bacteria CFUs in all organs are shown for each group as columns with mean + standard deviation (s.d.) (Fig. 1h).
Figure 1.
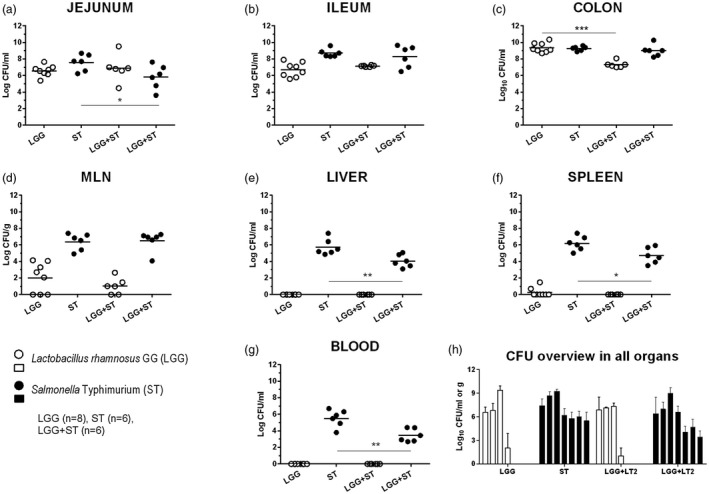
Bacterial counts in the intestine, mesenteric lymph nodes, liver, spleen and blood. Lactobacillus rhamnosus GG (LGG) and Salmonella Typhimurium (ST) colony‐forming units (CFUs) were counted in the jejunum (a, proximal jejunum), the ileum (b, a distal part of the jejunum and the whole ileum), the colon (c), mesenteric lymph nodes (d, MLN), the liver (e), the spleen (f) and blood (g). LGG is depicted as white circles (○, a–g) or columns (□, h) and ST as black circles (●, a–g) or columns (■, h). Differences between LGG CFUs (LGG, n = 8 versus LGG+ST, n = 6) and ST CFUs (ST, n = 6 versus LGG+ST, n = 6) were evaluated by unpaired two‐tailed t‐test and statistical differences were marked with asterisks as statistically significant (*P < 0·01), **P < 0·01 and ***P < 0·01). A summary CFU overview is shown on a column graph (h) as mean + standard deviation (s.d.) (without statistical significance).
Both LGG and ST showed good ability to multiply in the intestine of the gnotobiotic piglets (Fig. 1a–c). LGG decreased ST growth in the LGG+ST piglets in the jejunum (Fig. 1a, *P < 0·05) but not in the ileum and colon (Fig. 1b,c). In contrast, the LGG CFU were diminished in the ST presence in the LGG+ST group in the colon (Fig. 1c, ***P < 0·001). LGG translocated to MLN, but not in all LGG‐colonized piglets (Fig. 1d). LGG did not spread to the liver (Fig. 1e) and blood (Fig. 1g), while it was cultivated from the spleen in two of eight piglets in the LGG group (Fig. 1f). ST translocated to MLN (Fig. 1d), liver (Fig. 1e) and spleen (Fig. 1f) and caused bacteremia (Fig. 1g) in both Salmonella‐infected ST and LGG+ST groups. ST CFU were diminished in the presence of LGG in the liver (Fig 1e, **P < 0·01), spleen (Fig. 1f, *P < 0·05) and blood (Fig. 1g, **P < 0·01). The summary of these results is shown in Fig. 1h.
Histopathological evaluation
Both the enterocytes in the ileum of the GF piglets (n = 6) (Fig. 2a,b) and the LGG piglets (n = 8) (Fig. 2c,d) showed long villi with the abundant presence of vacuolated enterocytes. The villus height was comparable, the crypts were deeper but statistically non‐significant and the muscular layer was thicker (P < 0·01) in LGG than in the GF group (Supporting information, Fig. S3). Villus atrophy in Salmonella‐infected piglets (ST and LGG+ST, both n = 6, Fig. 2e–h) resulted in the markedly lowered presence of the vacuolated enterocytes in the villi. Villi disappeared completely in some parts of the ileum of these piglet groups, but crypts retained their appearance, comparable with the ileum of GF and LGG piglets. The cellularity of the lamina propria and submucosa was increased in the LGG group compared to the GF. No inflammation in the preterm GF piglets and piglets colonized with LGG for 7 days was found on hematoxylin and eosin histological ileum slides. In contrast, the histopathological analysis of the ileal sections in the piglets infected with Salmonella (ST, Fig. 2e,f and LGG+ST, Fig. 2g,h) showed acute inflammation. Using our scoring system, the total histological score was 10·1 for the ST group and 8·2 for the LGG+ST group (Fig. 2i). Both groups showed submucosal edema, PMN infiltration into the lamina propria, villus atrophy, exudate in the lumen, vessel dilatation and inflammatory cellularity in the lymphatic vessel lumen. Additionally, typical features of the ST group were hyperemia, hemorrhage and multiple erosion, but without intensive PMN infiltration (Fig. 2e,f), that was typical for the LGG+ST group (Fig. 2g,h). Erosion of the intestinal epithelium was only sporadic in the LGG+ST group.
Figure 2.
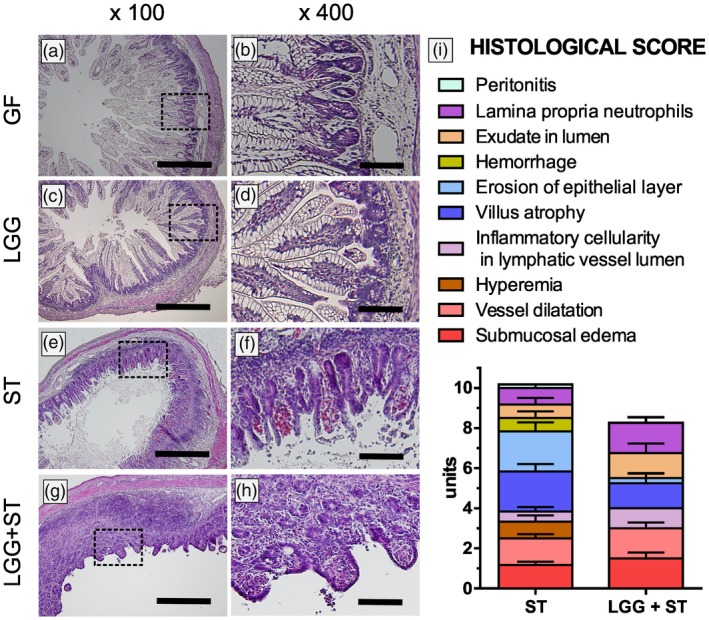
Representative hematoxylin and eosin‐stained cross‐sections of the ileum in the preterm gnotobiotic piglets and a histological score. Representative hematoxylin and eosin‐stained cross‐sections of the ileum in preterm gnotobiotic piglets: 1‐week‐old germ‐free piglets (GF; a,b), piglets colonized with Lactobacillus rhamnosus GG for 1 week (LGG; c,d), 1‐week‐old piglets infected with Salmonella Typhimurium for 24 h (ST; e,f) and piglets colonized with LGG for 1 week and infected with ST for 24 h (LGG+ST; g,h) were compared. Bars represent 500 µm (a,c,e,g) and 100 µm (b,d,f,h). Histological score from the ileum of six piglets per group is depicted (i).
Transcriptions of tight junction proteins claudin‐1 and occludin in the intestine
The transcription of claudin‐1 in the ileum was low and comparable in GF and LGG piglets (Fig 3a,b). Both groups of Salmonella‐infected piglets (ST and LGG+ST) showed statistically significant increased transcriptions (Fig 3a). In the colon, the transcription was higher in the GF than in the LGG group, and the statistically significant differences were between the LGG and ST groups but not between the GF and ST groups. The occludin transcription (Fig 3c,d) showed an opposite trend than claudin‐1 – higher transcription was in the GF and LGG groups and lower in Salmonella‐infected piglets. These changes were without statistical significance among groups in the ileum (Fig. 3c), but statistically significant decreases were found between LGG and both Salmonella‐infected groups in the colon (Fig. 3d).
Figure 3.
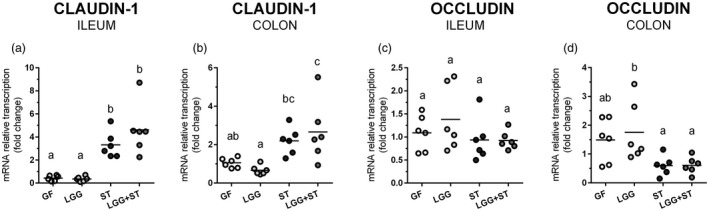
Transcriptions of tight junction proteins claudin‐1 and occludin in the ileum and the colon. Claudin‐1 and occludin mRNAs in the terminal ileum and the transversal colon were normalized to β‐actin and cyclophilin A and their relative transcriptions are depicted. The preterm germ‐free (GF), Lactobacillus rhamnosus GG‐colonized (LGG), Salmonella Typhimurium‐infected (ST) and L. rhamnosus GG‐colonized and S. Typhimurium‐infected (LGG+ST) piglets were compared. One‐way analysis of variance (anova) with Tukey’s multiple comparison post‐hoc test was used to compare the groups and the values are presented as individual dots and mean (horizontal line). Statistical differences P < 0·05 are marked with different letters above the dots and the same letter indicates no significant differences. Six samples in each group were analyzed.
Local and systemic levels of IL‐8, IL‐12/23 p40 and IFN‐γ
Values of IL‐8, IL‐12/23 p40 and IFN‐γ were compared among all groups in the intestinal lavages and plasma. Although we compared differences among all the groups, in the following text we mainly report crucial changes of GF versus ST and ST versus LGG+ST to simplify the description of the results obtained.
IL‐8 medians in the jejunum (Fig. 4a), ileum (Fig. 4b) and colon (Fig. 4c) in GF and LGG groups were lower than 200 pg/ml or were undetectable in plasma (Fig. 4d). Infection with S. Typhimurium in the ST group statistically significantly induced production of IL‐8 in the jejunum, ileum, colon and plasma (Fig. 4a–d). The preliminary colonization of the gastrointestinal tract with LGG in the LGG+ST piglets decreased IL‐8 values in the intestine and plasma compared with the ST group, but these decreases were statistically significant in the jejunum (Fig. 4a) only.
Figure 4.
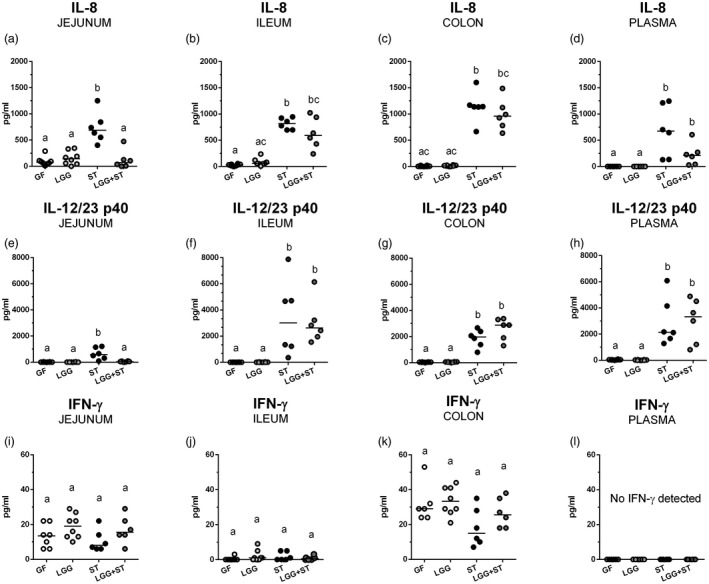
Protein levels of interleukin (IL)‐8, IL‐12/23 p40 and interferon (IFN)‐γ in the jejunum, ileum, colon and plasma. Levels of IL‐8 (a–d), IL‐12/23 p40 (e–h) and IFN‐γ (i–l) in the jejunum (a,e,i), ileum (b,f,j), colon (c,g,k) and plasma (d,h,l) of the preterm germ‐free (GF, n = 6), Lactobacillus rhamnosus GG‐colonized (LGG, n = 8), Salmonella Typhimurium‐infected (ST, n = 6) and L. rhamnosus GG‐colonized and S. Typhimurium‐infected (LGG+ST, n = 6) piglets were compared. Kruskal–Wallis test with Dunn’s multiple comparison post‐hoc test was used to compare the groups. The results are presented as individual dots and median (horizontal line). Statistical differences P < 0·05 are denoted with different letters above the dots, and the same letter indicates no significant differences.
IL‐12/23 p40 levels were minute in the GF and LGG groups (Fig. 4e–h). They were statistically significantly increased in the Salmonella‐infected (ST) jejunum (Fig. 4e), ileum (Fig. 4f), colon and plasma (Fig. 4g,h). Previous colonization with LGG protects piglets infected with Salmonella (LGG+ST) against this increase in the jejunum (Fig. 4e). This IL‐12/23 p40 lowering effect of LGG was absent in other parts of the intestine and plasma, where the levels of this cytokine subunit were significantly higher in the ileum (Fig. 4f), the colon (Fig. 4h) and plasma (Fig. 4g) if compared to the GF group.
IFN‐γ levels reached the highest, but still relatively low values, in the colon (Fig. 4k), lower in the jejunum (Fig. 4i) and lowest in the ileum (Fig. 4j). No statistically significant differences between the groups in any part of the intestine were observed and no IFN‐γ levels were detected in blood plasma (Fig. 4l).
Transcriptions of IFN‐γ in the ileum and the colon
Transcriptions of IFN‐γ were the lowest in the intestine in the GF and LGG piglets (Fig. 5a,b). The presence of ST alone triggered the IFN‐γ transcription in the intestine, and previous colonization with LGG potentiated these transcriptions. Salmonella increased IFN‐γ transcriptions against the GF state in the ileum in ST piglets (Fig. 5a). After previous colonization with LGG (LGG+ST) these differences were even more statistically relevant. This previous colonization with LGG also influenced differences between ST and LGG+ST groups in the ileum. The greatest increase after previous colonization with LGG was found in the colon (Fig. 5b). While the differences between the GF or LGG groups to the ST group were not statistically significant in the colon, the differences in the GF or LGG groups to the LGG+ST group were significant.
Figure 5.
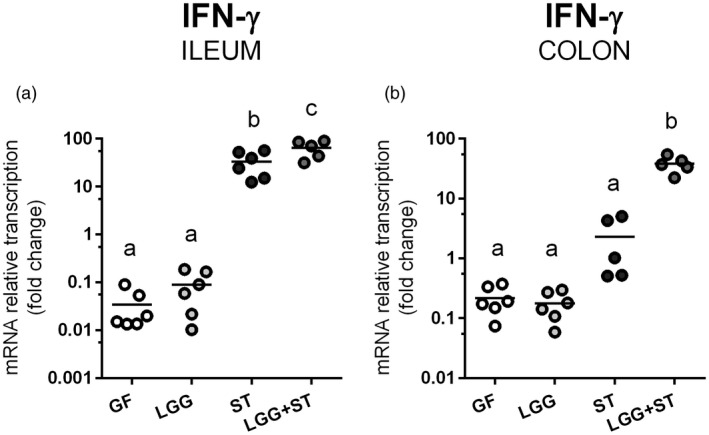
Relative expression of interferon (IFN)‐γ in the ileum and the colon. IFN‐γ mRNA was normalized to β‐actin and cyclophilin A and the relative transcription of the IFN‐γ gene in the ileum (a) and colon (b) in the preterm germ‐free (GF), Lactobacillus rhamnosus GG‐colonized (LGG), Salmonella Typhimurium‐infected (ST) and L. rhamnosus GG‐colonized and S. Typhimurium‐infected (LGG+ST) piglets were compared. One‐way analysis of variance (anova) with Tukey’s multiple range post‐hoc test was used to compare the groups. The results are presented as individual dots and mean (horizontal line). The statistical differences are denoted with different letters above the dots and the same letter indicates no significant differences. Six samples were analyzed in each group.
Discussion
Preterm infants have underdeveloped organ systems, including those of the immune system. They suffer from severe acute and chronic morbidities, e.g. intraventricular hemorrhage, necrotizing enterocolitis, bronchopulmonary dysplasia, chronic lung disease, severe visual impairment, hearing impairment, cerebral palsy and cognitive developmental delay 1. Probiotics are used in preterm infants as prophylaxis of NEC and sepsis 27. L. rhamnosus GG is a useful, time‐proven probiotic bacteria 10 but it is able to endanger immunocompromised patients 28 including newborn infants 29. These events accentuate the need to take into account the increased vulnerability of preterm infants to infections and sepsis.
The epitheliochorial placentation of the pig prevents the prenatal transfer of immunoglobulins, and underdeveloped humoral and cell‐mediated immunity have to be supplied via colostrum intake after birth to thrive in conventional conditions 30, while the colostrum‐deprived conventional piglets succumb to bacterial sepsis 31. The preterm piglet model of immunocompromised infants 18 shows similarities in organ development and clinical features with preterm infants on the 75% of gestation that corresponds to 32 weeks in the human 17, 32. Surgically derived piglets thrive in the sterile conditions of a gnotobiotic isolator without the intake of colostrum 19, 33. Germ‐free animals show lower colonization resistance due to an absence of balanced microbiota. This all assists in the manifestation of the potential opportunistic pathogenicity of probiotics. Additionally, the immaturity of preterm germ‐free piglets 19 contributes to the vulnerability to bacterial attack, as it is known in preterm infants 34. In contrast to the host‐beneficial properties of L. rhamnosus GG, the Salmonella genus comprises food‐borne pathogens 35 that can cause life‐threatening illness in immunocompromised hosts 21, 22.
The hysterectomy‐derived preterm germ‐free piglets in our experiments were colonized with L. rhamnosus GG (LGG) 4 h after hysterectomy. At this time the piglet intestinal barrier is passable for intact colostral immunoglobulins and cells 30. We supposed that possible LGG translocation could be facilitated in this early postnatal period, and the manifestation of opportunistic pathogenicity of LGG occurred as described in immunocompromised hosts 28, 29. While one section of the LGG‐colonized piglets was kept monocolonized for the entire week‐long experiment (LGG), another section was infected with S. Typhimurium strain LT2 for 24 h (LGG+ST) at 1 week of age. Our intention in this section of the experiments was to verify if LGG translocates and if previous colonization with LGG could ameliorate signs of salmonellosis.
Mechanisms of LGG actions against Salmonella are not fully understood, and bacterial interferences use different principles such as inhibition of adhesion and biofilm formation 36, production of lactic acid 37 and others. Positive effects of LGG against enterocolitis in animal models 38, 39, 40 or in infants 9 have been observed. However, other authors did not confirm such beneficial effects. LGG‐caused exacerbation of mouse colitis has also been reported 41.
The balanced microbiota in conventional hosts is essential for their health 2 but dysbiosis, for example, caused by antibiotic therapy, can disturb this balance and commensal bacteria can turn into opportunistic pathogens 42. Antibiotic treatment provoked GIT dysbiosis in C57BL/6 mice, which resulted in the translocation of commensal bacteria into colonic mesenteric lymph nodes 43. The translocation of LGG into mesenteric lymph nodes, spleen and liver but no bacteremia was described in 6‐week‐old gnotobiotic piglets which were colonized at 7 days of age with LGG and were challenged with human rotavirus 2 weeks later 44. In this case, the rotavirus probably disrupted the intestinal barrier which aided the bacterial translocation. Bacterial monocolonization in the gnotobiotic piglets may share some characteristics of the dysbiosis. In our experiments, the LGG successfully colonized the GIT of the preterm monocolonized piglets and its translocation into mesenteric lymph nodes in five of eight piglets, and the spleen in two of eight piglets could suggest the opportunistic pathogenicity of LGG. The lactobacilli were, in the majority of the piglets of the LGG group, successfully trapped in mesenteric lymph nodes which prevented their systemic spread, and no bacteremia was found in any piglet. All these piglets thrived and showed no clinical signs such as anorexia, somnolence, fever and diarrhea.
The ingested Salmonella adhere in the intestine and multiplies itself 45. Its translocation can be proceeded by four different mechanisms: (i) via M cells that overlay Peyer’s patches 46, (ii) by an internalization through enterocytes 47, (iii) by entering between epithelial cells with intact tight junctions engulfed by macrophages 48 or dendritic cells 49 and (iv) by a paracellular way through disrupted tight junctions 50. The virulence of the LT2 strain of S. Typhimurium used for term germ‐free piglets was documented in our previous experiments 51, 52, 53. In the present experiments, S. Typhimurium quickly multiplied in the 1‐week‐old preterm gnotobiotic piglet intestine over 24 h, translocated into all observed organs, caused bacteremia and induced clinical signs of enterocolitis. These Salmonella‐infected piglets (ST) suffered from anorexia, somnolence, fever and diarrhea. In the piglets previously colonized with LGG and later infected with Salmonella (LGG+ST), LGG translocated in the majority of the piglets into mesenteric lymph nodes in a ratio comparable with the LGG group. The translocation of ST in the LGG+ST group was similar in mesenteric lymph nodes to the ST group, but reduced in the spleen, liver and blood. This reduced dissemination of S. Typhimurium may be due to immunomodulation by transforming growth factor (TGF)‐β and IFN‐γ in mesenteric lymph nodes, as was observed in conventional piglets treated by L. reuteri 54.
The GIT microbiota influences the development of the intestine. Conventionalization of germ‐free piglets by pig feces triggered changes leading to intestinal histology that was similar to the histology typical for conventional piglets 55. The lamina propria, submucosa cellularity and muscular layer thickness were increased in the LGG group in comparison with the preterm GF piglets, and histology of their ileum resembled the ileum of the term germ‐free piglets 19, 55. Quite a different picture was seen in the ileum of the Salmonella‐infected preterm piglets (ST and LGG+ST) in which inflammatory cellularity in the lymphatic vessel lumen, villus atrophy, exudate in the lumen and PMN infiltration into the lamina propria shared characteristics with Salmonella‐infected term gnotobiotic piglets 52. Furthermore, the ST group showed hyperemia, the multiple erosion of the intestinal epithelium, hemorrhage and peritonitis that were not obvious in the term Salmonella‐infected piglets in our former experiments 52. The histological score we used indicates that LGG ameliorated the deleterious effect of Salmonella infection on the intestine of the preterm gnotobiotic piglets. Similarly, previous colonization of gnotobiotic piglets with avirulent S. enterica serovar Infantis strain 1326/8 24 or Typhimurium strain SF1591 51 protected the piglets against consecutive infections with virulent S. Typhimurium. The higher abundance of neutrophils in the intestine of gnotobiotic piglets induced by avirulent Salmonella mediated a protective effect.
A thin layer of enterocytes forms the interface between the intestinal lumen and the host body. The tight junction (TJ) proteins join adjacent enterocytes in their apical part. To estimate possible changes in the intestinal barrier we gave our attention to two TJ proteins with different actions. While claudin‐1 seals an epithelial layer for small molecules and protects the host against the loss of electrolytes 56, occludin impedes the paracellular transport of large molecules and cells 57. S. Typhimurium was shown to alter both claudin‐1 and occludin to increase its translocation and transepithelial migration of polymorphonuclear neutrophils 58.
Colonization of the preterm germ‐free piglets with LGG did not influence transcriptions of claudin‐1 and occludin in the intestine compared with the germ‐free piglets. Infection with Salmonella increased the transcription of claudin‐1 but decreased the transcription of occludin. The increased transcription of claudin‐1 was probably an attempt to prevent the loss of electrolytes and dehydration of the organism in both Salmonella‐infected groups (ST and LGG+ST) 56. The decreased transcription of occludin in Salmonella‐infected piglets probably reflected a disrupted intestinal barrier for large molecules that can simultaneously facilitate bacterial translocation 58. To confirm our speculation concerning changes in claudin‐1 and occludin, a suitable test of the functionality and the intestinal barrier function 59 should be performed. Because Salmonella‐infected piglets suffered from anorexia and normal peristalsis was disturbed, their stomachs were full of a precipitated milk diet but the colon was more or less empty. Locally produced biomarkers such as intestinal fatty acid binding protein may provide a suitable leaky gut test in salmonellosis 59, 60.
The immune system is developed during ontogeny but in preterm newborns it has not been fully developed 61. The inflammatory reaction is characterized by movements of leukocytes and fluids from the blood vessels into extravascular space controlled by different mediators. Local and systemic levels of CXCL chemokine interleukin (IL)‐8, proinflammatory cytokine IL‐12/23 p40 subunit and IFN‐γ were measured to reveal whether their levels are related to physiological processes or if they exceed their regulatory function and can cause a self‐damaging over‐exuberant innate immune response called a ‘cytokine storm’ 62. IL‐8 attracts PMNs from intravascular into the interstitial inflammatory site and activates them 63. It has been found that infection with S. Typhimurium increased transepithelial migration of the PMNs 58. We detected relatively high levels of IL‐8 in the jejunum and lower in the ileum and colon in the preterm GF and LGG piglets. Our finding of intestinal IL‐8 occurring in the ileum of preterm gnotobiotic piglets is seemingly in contradiction with the absence of the histopathological changes in the non‐infected piglet groups (GF and LGG). Nevertheless, IL‐8 probably plays a role in the ontogeny of the GIT. IL‐8 is present in the colostrum and mother’s milk and its receptors occur in the human fetal and neonatal intestine 64. Different IL‐8 isoforms were widely expressed in human fetal tissues, although inflammatory changes were not obvious 65. These IL‐8 isoforms during prenatal development minimize IL‐8 inflammatory effects, but their isoform changes make it possible to modify its activity after birth 65. This probably explains the existence of IL‐8 without inflammatory findings in the non‐infected preterm intestine. In concurrence with our findings, IL‐8 was found in the intestinal homogenates from preterm conventional piglets 66. Extremely low birth weight infants that suffer from NEC showed increased levels of IL‐8 67 and significant activation of IL‐8 gene was detected in the preterm piglets suffering from severe NEC 68. It documents the involvement of IL‐8 in pathological processes in the immature intestine. Systemic IL‐8 levels were suggested as a potential biomarker for neonatal sepsis in infants 69.
Salmonella strongly induced IL‐8 levels in the intestine and blood of the ST group. Previous colonization with LGG alleviated these levels only in the jejunum and plasma, and these levels were kept within the range of expected physiological levels comparable with LGG and GF piglets. These findings correspond to the diminished CFUs in the jejunum and blood of LGG+ST piglets. This LGG‐ameliorating effect was not found in the ileum and the colon. In contrast to the preterm GF piglets, no IL‐8 levels were found in the intestine of the term germ‐free piglets and similarly induced IL‐8 values were found in the term gnotobiotic piglets infected with S. Typhimurium 51, 52. Local induction of IL‐8 with S. Typhimurium rough mutants in the intestine of gnotobiotic piglets colonized with avirulent Salmonella serovars and relationships of IL‐8 and PMNs were believed to be responsible for the protective effect against subsequent infection with S. Typhimurium virulent strains 24, 51 as mentioned above. The induction of intestinal and systemic IL‐8 in conventional piglets infected with S. Typhimurium has been described by other authors 70, 71. IL‐8 was also shown 1, 2 and 6 days post‐infection (dpi) in experiments with 1‐month‐old piglets orally infected with Salmonella and the immune response in the jejunum, ileum and colon differed and had various time progressions 70. This may explain the differences in different parts of the intestine.
Another important part of the innate immune response covers the T helper type 1 (Th1) cell‐mediated immune response, that is crucial in host protection against intracellular pathogens. It activates macrophages through IFN‐γ‐controlled mechanisms. IFN‐γ stimulates the anti‐microbial activity of infected macrophages to kill off intracellular pathogens through inducible nitric oxide synthase produced reactive nitrogen intermediates 72. Up‐regulated transcriptions of IFN‐γ, IL‐8 and IL‐12/23 p40 were found in porcine jejunal loops infected with S. Typhimurium 73. IL‐12/23 p40 is an IL‐12 and IL‐23 shared protein subunit that is strongly up‐regulated after microbial stimuli 74. While IL‐12 is a heterodimeric cytokine consisting of p40 and p35 subunits, IL‐23 consists of p40 and p19 subunits 75. Additionally, the IL‐12/23 p40 homodimer can regulate the inflammatory process as an IL‐12 antagonist 76. IL‐12 and IL‐23 regulate the host resistance and intestinal inflammation during infection with Salmonella 77. IL‐12 is primarily produced by antigen‐presenting cells and controls differentiation of naive T‐cells into IFN‐γ‐producing Th1 cells. It is a potent inducer of IFN‐γ production by NK cells and innate lymphoid cells 78. Similarly, IL‐23 is produced by dendritic cells and macrophages 75. Negligible or undetectable levels of IL‐12/23 p40 were present in the intestine and blood of non‐infected preterm gnotobiotic piglets (GF and LGG). Infection with Salmonella strongly induced IL‐12/23 p40, but only in the jejunum was this induction diminished by previous colonization with LGG. This finding agrees with CFUs and IL‐8 in the jejunum.
IFN‐γ shows anti‐bacterial properties against intracellular microbes, including Salmonella. It activates macrophages, promotes phagocytosis and destroys phagocytosed microbes by free radical‐driven toxification of phagosomes and induces the expression of many other genes which participate in anti‐microbial strategies 26. It has been proved that some probiotic bacteria are able to induce IFN‐γ production that ameliorates Salmonella infection 79. Local or systemic levels of IFN‐γ were found in the term gnotobiotic 80, 81 and conventional piglets 82 infected with Salmonella or other enteric bacterial pathogens. We measured local and systemic levels of IFN‐γ to test if LGG shows an IFN‐γ‐stimulatory effect 79, but no levels of IFN‐γ were found in the LGG group. Nevertheless, much more surprising was the absence of the stimulatory effect of Salmonella on IFN‐γ levels in the intestine of both groups of the piglets infected with Salmonella (ST and LGG+ST) and the absence of IFN‐γ levels (or their levels under the lower limit of detection) in blood. Low levels of IFN‐γ or their absence, in contrast to IL‐8 and IL‐12/23, were the reason that we gave our additional attention to IFN‐γ transcription, because IFN‐γ transcription in intestinal tissues in the gnotobiotic piglets after infection with S. Typhimurium has been reported 81. While both Salmonella‐infected groups showed highly activated gene transcription in the ileum, only piglets previously colonized with LGG showed it in the colon. During infection with S. Typhimurium, various transcriptional profiles were found along the porcine intestine and it was determined that the jejunum, ileum and colon respond differently to infection 71. In addition to the variable immune response in different parts of the intestine, it is possible that L. rhamnosus GG modifies the induction of IFN‐γ in the colon. This highly differentiated transcription between the piglet groups suggests that IFN‐γ protein would probably appear later than 24 h post‐infection to support IL‐8‐driven mechanisms in Salmonella infection. The local and systemic absence of IFN‐γ protein contrasted with its highly induced transcription in the ileum and the colon suggests a longer experimental post‐infection period in our future experiments to evaluate the involvement of IFN‐γ in combating Salmonella infection.
Conclusion
We evaluated the effect of the previous colonization of the hysterectomy‐derived preterm gnotobiotic piglets with L. rhamnosus GG against subsequent infection with S. Typhimurium. This colonization with LGG partially ameliorated the deleterious effect of infection with S. Typhimurium, as it was evaluated by CFU enumeration, intestinal histology and the local and systemic values of IL‐8 and IL‐12/23 p40. This is the first use of preterm gnotobiotic piglets as an infectious model of vulnerable immunocompromised preterm infants to verify probiotic safety and its bacterial interference with an enteric pathogen.
Disclosure
The authors have no conflicts of interest.
Author contributions
A. S. planned and participated in experiments, performed microbiological and histological evaluations, arranged figures and wrote the manuscript, V. J. performed experiments and RT‐PCR measurements and partially evaluated results, Z. S. designed histological score criteria and performed histopathological evaluation, I. S. planned experiments, performed xMAP technology measurements, statistical evaluations, graphs, arranged figures and wrote the manuscript. All authors approved the submitted version of the manuscript.
Supporting information
Fig. S1. The preterm gnotobiotic piglet. The hysterectomy‐derived, colostrum‐deprived preterm gnotobiotic piglets were reared in fiberglass isolators for one week and were fed a cow’s milk‐based formula via a nipple. The picture depicts a three‐day‐old preterm gnotobiotic piglet.
Fig. S2. Schema of the experiment. The preterm gnotobiotic piglets (n = 26) were grouped into four groups: i) sterile for the whole experimental period (germ‐free; GF, n = 6), ii) orally colonized with 1 × 108 CFU of Lactobacillus rhamnosus GG 4 hours after hysterectomy (LGG, n = 8), iii) one‐week‐old GF piglets orally infected with 1 × 108 CFU of S. Typhimurium for 24 hours (ST, n = 6), and iv) one‐week‐old LGG‐colonized piglets orally infected with 1 × 108 CFU of S. Typhimurium for 24 hours (LGG+ST, n = 6).
Fig. S3. Intestinal morphometry in the ileum of the preterm gnotobiotic piglets. Villus height, crypt depths and muscular thickness were compared between the GF (n = 6) and LGG (n = 8) groups. Unpaired two‐tailed t‐test was used to compare the groups. The results are presented as individual dots and mean (horizontal line). The statistical significances are denoted with asterisks (*P · 0·05).
Acknowledgements
We thank Jana Machova, Marta Stojkova, Jarmila Jarkovska and Hana Sychrovska for technical assistance. This work was funded by the Czech Science Foundation grants 13‐14736S and 13‐08803S and the Institutional Research Concept of the Institute of Microbiology of the Czech Academy of Sciences RVO 61388971.
References
- 1. Glass HC, Costarino AT, Stayer SA, Brett CM, Cladis F, Davis PJ. Outcomes for extremely premature infants. Anesth Analg 2015; 120:1337–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Backhed F, Roswall J, Peng Y et al Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015; 17:690–703. [DOI] [PubMed] [Google Scholar]
- 3. Marques TM, Wall R, Ross RP, Fitzgerald GF, Ryan CA, Stanton C. Programming infant gut microbiota: influence of dietary and environmental factors. Curr Opin Biotechnol 2010; 21:149–56. [DOI] [PubMed] [Google Scholar]
- 4. Brooks B, Firek BA, Miller CS et al Microbes in the neonatal intensive care unit resemble those found in the gut of premature infants. Microbiome 2014; 2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ramasethu J. Prevention and treatment of neonatal nosocomial infections. Matern Health Neonatol Perinatol 2017; 3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bedford Russell AR. Neonatal sepsis. Paediatr Child Health 2015; 25:271–5. [Google Scholar]
- 7. Ghazal P, Dickinson P, Smith CL. Early life response to infection. Curr Opin Infect Dis 2013; 26:213–8. [DOI] [PubMed] [Google Scholar]
- 8. Hill C, Guarner F, Reid G et al Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014; 11:506–14. [DOI] [PubMed] [Google Scholar]
- 9. Uberos J, Aguilera‐Rodriguez E, Jerez‐Calero A, Molina‐Oya M, Molina‐Carballo A, Narbona‐Lopez E. Probiotics to prevent necrotising enterocolitis and nosocomial infection in very low birth weight preterm infants. Br J Nutr 2017; 117:994–1000. [DOI] [PubMed] [Google Scholar]
- 10. Pace F, Pace M, Quartarone G. Probiotics in digestive diseases: focus on Lactobacillus GG. Minerva Gastroenterol Dietol 2015; 61:273–92. [PubMed] [Google Scholar]
- 11. Gorbach SL, Chang TW, Goldin B. Successful treatment of relapsing Clostridium difficile colitis with Lactobacillus GG. Lancet 1987; 2:1519. [DOI] [PubMed] [Google Scholar]
- 12. Meyer MP, Alexander T. Reduction in necrotizing enterocolitis and improved outcomes in preterm infants following routine supplementation with Lactobacillus GG in combination with bovine lactoferrin. J Neonatal Perinatal Med 2017; 10:249–55. [DOI] [PubMed] [Google Scholar]
- 13. Neu J. Routine probiotics for premature infants: let’s be careful! J Pediatr 2011; 158:672–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lunney JK. Advances in swine biomedical model genomics. Int J Biol Sci 2007; 3:179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Q, Widmer G, Tzipori S. A pig model of the human gastrointestinal tract. Gut Microbes 2013; 4:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V. The pig: a model for human infectious diseases. Trends Microbiol 2012; 20:50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sangild PT, Thymann T, Schmidt M, Stoll B, Burrin DG, Buddington RK. Invited review: the preterm pig as a model in pediatric gastroenterology. J Animal Sci 2013; 91:4713–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nguyen DN, Jiang P, Frokiaer H, Heegaard PM, Thymann T, Sangild PT. Delayed development of systemic immunity in preterm pigs as a model for preterm infants. Sci Rep 2016; 6:36816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Splichalova A, Slavikova V, Splichalova Z, Splichal I. Preterm life in sterile conditions: a study on preterm, germ‐free piglets. Front Immunol 2018; 9:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang S, Kingsley RA, Santos RL et al Molecular pathogenesis of Salmonella enterica serotype Typhimurium‐induced diarrhea. Infect Immun 2003; 71:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keestra‐Gounder AM, Tsolis RM, Baumler AJ. Now you see me, now you don’t: the interaction of Salmonella with innate immune receptors. Nat Rev Microbiol 2015; 13:206–16. [DOI] [PubMed] [Google Scholar]
- 22. Santos RL, Tsolis RM, Baumler AJ, Adams LG. Pathogenesis of Salmonella‐induced enteritis. Braz J Med Biol Res 2003; 36:3–12. [DOI] [PubMed] [Google Scholar]
- 23. Mandel L, Travnicek J. The minipig as a model in gnotobiology. Nahrung 1987; 31:613–8. [DOI] [PubMed] [Google Scholar]
- 24. Foster N, Lovell MA, Marston KL et al Rapid protection of gnotobiotic pigs against experimental salmonellosis following induction of polymorphonuclear leukocytes by avirulent Salmonella enterica . Infect Immun 2003; 71:2182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Castellheim A, Thorgersen EB, Hellerud BC et al New biomarkers in an acute model of live Escherichia coli‐induced sepsis in pigs. Scand J Immunol 2008; 68:75–84. [DOI] [PubMed] [Google Scholar]
- 26. Ingram JP, Brodsky IE, Balachandran S. Interferon‐gamma in Salmonella pathogenesis: new tricks for an old dog. Cytokine 2017; 98:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deshpande G, Jape G, Rao S, Patole S. Benefits of probiotics in preterm neonates in low‐income and medium‐income countries: a systematic review of randomised controlled trials. BMJ Open 2017; 7:e017638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis 2015; 60 (Suppl 2):S129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dani C, Coviello CC, Corsini I, Arena I, Antonelli I, Rossolini GM. Lactobacillus sepsis and probiotic therapy in newborns: two new cases and literature review. AJP Rep 2016; 6:e25–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salmon H, Berri M, Gerdts V, Meurens F. Humoral and cellular factors of maternal immunity in swine. Dev Comp Immunol 2009; 33:384–93. [DOI] [PubMed] [Google Scholar]
- 31. Butler JE, Lager KM, Splichal I et al The piglet as a model for B cell and immune system development. Vet Immunol Immunopathol 2009; 128:147–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sangild PT. Gut responses to enteral nutrition in preterm infants and animals. Exp Biol Med (Maywood) 2006; 231:1695–711. [DOI] [PubMed] [Google Scholar]
- 33. Mandel L, Travnicek J. Haematology of conventional and germfree miniature Minnesota piglets. I. Blood picture. Z Versuchstierkd 1982; 24:299–307. [PubMed] [Google Scholar]
- 34. Melville JM, Moss TJ. The immune consequences of preterm birth. Front Neurosci 2013; 7:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hurley D, McCusker MP, Fanning S, Martins M. Salmonella‐host interactions – modulation of the host innate immune system. Front Immunol 2014; 5:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Petrova MI, Imholz NC, Verhoeven TL et al Lectin‐like molecules of Lactobacillus rhamnosus GG inhibit pathogenic Escherichia coli and Salmonella biofilm formation. PLOS ONE 2016; 11:e0161337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Keersmaecker SC, Verhoeven TL, Desair J, Marchal K, Vanderleyden J, Nagy I. Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella typhimurium is due to accumulation of lactic acid. FEMS Microbiol Lett 2006; 259:89–96. [DOI] [PubMed] [Google Scholar]
- 38. Vlasova AN, Chattha KS, Kandasamy S et al Lactobacilli and bifidobacteria promote immune homeostasis by modulating innate immune responses to human rotavirus in neonatal gnotobiotic pigs. PLOS ONE 2013; 8:e76962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wen K, Liu F, Li G et al Lactobacillus rhamnosus GG dosage affects the adjuvanticity and protection against rotavirus diarrhea in gnotobiotic pigs. J Pediatr Gastroenterol Nutr 2015; 60:834–43. [DOI] [PubMed] [Google Scholar]
- 40. Wu S, Yuan L, Zhang Y et al Probiotic Lactobacillus rhamnosus GG mono‐association suppresses human rotavirus‐induced autophagy in the gnotobiotic piglet intestine. Gut Pathog 2013; 5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mileti E, Matteoli G, Iliev ID, Rescigno M. Comparison of the immunomodulatory properties of three probiotic strains of Lactobacilli using complex culture systems: prediction for in vivo efficacy. PLOS ONE 2009; 4:e7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol 2017; 17:219–32. [DOI] [PubMed] [Google Scholar]
- 43. Knoop KA, McDonald KG, Kulkarni DH, Newberry RD. Antibiotics promote inflammation through the translocation of native commensal colonic bacteria. Gut 2016; 65:1100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kandasamy S, Vlasova AN, Fischer D et al Differential effects of Escherichia coli Nissle and Lactobacillus rhamnosus strain GG on human rotavirus binding, infection, and B cell immunity. J Immunol 2016; 196:1780–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol 2008; 6:53–66. [DOI] [PubMed] [Google Scholar]
- 46. Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J Exp Med 1994; 180:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Francis CL, Starnbach MN, Falkow S. Morphological and cytoskeletal changes in epithelial cells occur immediately upon interaction with Salmonella typhimurium grown under low‐oxygen conditions. Mol Microbiol 1992; 6:3077–87. [DOI] [PubMed] [Google Scholar]
- 48. Vazquez‐Torres A, Jones‐Carson J, Baumler AJ et al Extraintestinal dissemination of Salmonella by CD18‐expressing phagocytes. Nature 1999; 401:804–8. [DOI] [PubMed] [Google Scholar]
- 49. Niess JH, Brand S, Gu X et al CX3CR49‐mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 2005; 307:254–8. [DOI] [PubMed] [Google Scholar]
- 50. Jepson MA, Schlecht HB, Collares‐Buzato CB. Localization of dysfunctional tight junctions in Salmonella enterica serovar Typhimurium‐infected epithelial layers. Infect Immun 2000; 68:7202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Splichal I, Trebichavsky I, Splichalova A, Barrow PA. Protection of gnotobiotic pigs against Salmonella enterica serotype typhimurium by rough mutant of the same serotype is accompanied by the change of local and systemic cytokine response. Vet Immunol Immunopathol 2005; 103:155–61. [DOI] [PubMed] [Google Scholar]
- 52. Splichalova A, Splichal I, Chmelarova P, Trebichavsky I. Alarmin HMGB1 is released in the small intestine of gnotobiotic piglets infected with enteric pathogens and its level in plasma reflects severity of sepsis. J Clin Immunol 2011; 31:488–97. [DOI] [PubMed] [Google Scholar]
- 53. Splichalova A, Trebichavsky I, Rada V, Vlkova E, Sonnenborn U, Splichal I. Interference of Bifidobacterium choerinum or Escherichia coli Nissle 1917 with Salmonella typhimurium in gnotobiotic piglets correlates with cytokine patterns in blood and intestine. Clin Exp Immunol 2011; 163:242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hou C, Liu H, Zhang J et al Intestinal microbiota succession and immunomodulatory consequences after introduction of Lactobacillus reuteri I5007 in neonatal piglets. PLOS ONE 2015; 10:e0119505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shirkey TW, Siggers RH, Goldade BG et al Effects of commensal bacteria on intestinal morphology and expression of proinflammatory cytokines in the gnotobiotic pig. Exp Biol Med (Maywood) 2006; 231:1333–45. [DOI] [PubMed] [Google Scholar]
- 56. Gunzel D. Claudins: vital partners in transcellular and paracellular transport coupling. Pflugers Arch 2017; 469:35–44. [DOI] [PubMed] [Google Scholar]
- 57. Edelblum KL, Shen L, Weber CR et al Dynamic migration of gammadelta intraepithelial lymphocytes requires occludin. Proc Natl Acad Sci U A 2012; 109:7097–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kohler H, Sakaguchi T, Hurley BP, Kase BA, Reinecker HC, McCormick BA. Salmonella enterica serovar Typhimurium regulates intercellular junction proteins and facilitates transepithelial neutrophil and bacterial passage. Am J Physiol Gastrointest Liver Physiol 2007; 293:G178–G187. [DOI] [PubMed] [Google Scholar]
- 59. Galipeau HJ, Verdu EF. The complex task of measuring intestinal permeability in basic and clinical science. Neurogastroenterol Motil 2016; 28:957–65. [DOI] [PubMed] [Google Scholar]
- 60. Zamora IJ, Stoll B, Ethun CG et al Low abdominal NIRS values and elevated plasma intestinal fatty acid‐binding protein in a premature piglet model of necrotizing enterocolitis. PLOS ONE 2015; 10:e0125437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Basha S, Surendran N, Pichichero M. Immune responses in neonates. Expert Rev Clin Immunol 2014; 10:1171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol 2017; 39:517–28. [DOI] [PubMed] [Google Scholar]
- 63. Baggiolini M, Walz A, Kunkel SL. Neutrophil‐activating peptide‐1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest 1989; 84:1045–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Maheshwari A, Lu W, Lacson A et al Effects of interleukin‐8 on the developing human intestine. Cytokine 2002; 20:256–67. [DOI] [PubMed] [Google Scholar]
- 65. Maheshwari A, Voitenok NN, Akalovich S et al Developmental changes in circulating IL‐8/CXCL8 isoforms in neonates. Cytokine 2009; 46:12–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nguyen DN, Sangild PT, Ostergaard MV, Bering SB, Chatterton DE. Transforming growth factor‐beta2 and endotoxin interact to regulate homeostasis via interleukin‐8 levels in the immature intestine. Am J Physiol Gastrointest Liver Physiol 2014; 307:G689–G699. [DOI] [PubMed] [Google Scholar]
- 67. Maheshwari A, Schelonka RL, Dimmitt RA et al Cytokines associated with necrotizing enterocolitis in extremely‐low‐birth‐weight infants. Pediatr Res 2014; 76:100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stoy ACF, Heegaard PMH, Skovgaard K, Bering SB, Bjerre M, Sangild PT. Increased intestinal inflammation and digestive dysfunction in preterm pigs with severe necrotizing enterocolitis. Neonatology 2017; 111:289–96. [DOI] [PubMed] [Google Scholar]
- 69. Chauhan N, Tiwari S, Jain U. Potential biomarkers for effective screening of neonatal sepsis infections: an overview. Microb Pathog 2017; 107:234–42. [DOI] [PubMed] [Google Scholar]
- 70. Collado‐Romero M, Arce C, Ramirez‐Boo M, Carvajal A, Garrido JJ. Quantitative analysis of the immune response upon Salmonella typhimurium infection along the porcine intestinal gut. Vet Res 2010; 41:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Uribe JH, Collado‐Romero M, Zaldivar‐Lopez S et al Transcriptional analysis of porcine intestinal mucosa infected with Salmonella typhimurium revealed a massive inflammatory response and disruption of bile acid absorption in ileum. Vet Res 2016; 47:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Henard CA, Vazquez‐Torres A. Nitric oxide and Salmonella pathogenesis. Front Microbiol 2011; 2:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Meurens F, Berri M, Auray G et al Early immune response following Salmonella enterica subspecies enterica serovar Typhimurium infection in porcine jejunal gut loops. Vet Res 2009; 40:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bette M, Jin SC, Germann T et al Differential expression of mRNA encoding interleukin‐12 p35 and p40 subunits in situ. Eur J Immunol 1994; 24:2435–40. [DOI] [PubMed] [Google Scholar]
- 75. Croxford AL, Kulig P, Becher B. IL‐12‐and IL‐23 in health and disease. Cytokine Growth Factor Rev 2014; 25:415–21. [DOI] [PubMed] [Google Scholar]
- 76. Ling P, Gately MK, Gubler U et al Human IL‐12 p40 homodimer binds to the IL‐12 receptor but does not mediate biologic activity. J Immunol 1995; 154:116–27. [PubMed] [Google Scholar]
- 77. Awoniyi M, Miller SI, Wilson CB, Hajjar AM, Smith KD. Homeostatic regulation of Salmonella‐induced mucosal inflammation and injury by IL‐23. PLOS ONE 2012; 7:e37311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Trinchieri G. Interleukin‐12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen‐specific adaptive immunity. Annu Rev Immunol 1995; 13:251–76. [DOI] [PubMed] [Google Scholar]
- 79. Thiemann S, Smit N, Roy U et al Enhancement of IFN‐gamma production by distinct commensals ameliorates Salmonella‐induced disease. Cell Host Microbe 2017; 21:682–94. [DOI] [PubMed] [Google Scholar]
- 80. Jeong KI, Zhang Q, Nunnari J, Tzipori S. A piglet model of acute gastroenteritis induced by Shigella dysenteriae type 1. J Infect Dis 2010; 201:903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Splichal I, Trebichavsky I, Muneta Y, Mori Y. Early cytokine response of gnotobiotic piglets to Salmonella enterica serotype typhimurium . Vet Res 2002; 33:291–7. [DOI] [PubMed] [Google Scholar]
- 82. Knetter SM, Bearson SM, Huang TH et al Salmonella enterica serovar typhimurium‐infected pigs with different shedding levels exhibit distinct clinical, peripheral cytokine and transcriptomic immune response phenotypes. Innate Immun 2015; 21:227–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. The preterm gnotobiotic piglet. The hysterectomy‐derived, colostrum‐deprived preterm gnotobiotic piglets were reared in fiberglass isolators for one week and were fed a cow’s milk‐based formula via a nipple. The picture depicts a three‐day‐old preterm gnotobiotic piglet.
Fig. S2. Schema of the experiment. The preterm gnotobiotic piglets (n = 26) were grouped into four groups: i) sterile for the whole experimental period (germ‐free; GF, n = 6), ii) orally colonized with 1 × 108 CFU of Lactobacillus rhamnosus GG 4 hours after hysterectomy (LGG, n = 8), iii) one‐week‐old GF piglets orally infected with 1 × 108 CFU of S. Typhimurium for 24 hours (ST, n = 6), and iv) one‐week‐old LGG‐colonized piglets orally infected with 1 × 108 CFU of S. Typhimurium for 24 hours (LGG+ST, n = 6).
Fig. S3. Intestinal morphometry in the ileum of the preterm gnotobiotic piglets. Villus height, crypt depths and muscular thickness were compared between the GF (n = 6) and LGG (n = 8) groups. Unpaired two‐tailed t‐test was used to compare the groups. The results are presented as individual dots and mean (horizontal line). The statistical significances are denoted with asterisks (*P · 0·05).


