Abstract
Background
The World Health Organization (WHO) recommends that women wait at least 24 months after a livebirth before attempting a subsequent pregnancy to reduce the risk of adverse maternal, perinatal, and infant health outcomes. However, the applicability of the WHO recommendations for women in the United States is unclear, as breast feeding, nutrition, maternal age at first birth, and total fertility rate differs substantially between the United States and the low‐ and middle‐resource countries upon which most of the evidence is based.
Methods
To inform guideline development for birth spacing specific to women in the United States, the Office of Population Affairs (OPA) convened an expert work group meeting in Washington, DC, on 14‐15 September 2017 among reproductive, perinatal, paediatric, social, and public health epidemiologists; obstetrician‐gynaecologists; biostatisticians; and experts in evidence synthesis related to women's health.
Results
Presentations and discussion topics included the methodological quality of existing studies, evaluation of the evidence for causal effects of short interpregnancy intervals on adverse perinatal and maternal health outcomes, good practices for future research, and identification of research gaps and priorities for future work.
Conclusions
This report provides an overview of the presentations, discussions, and conclusions from the expert work group meeting.
Keywords: birth spacing, confounding, contraception, interpregnancy interval, maternal health, neonatal health, preterm birth, study design
1. INTRODUCTION
In 2007, the World Health Organization (WHO) issued a recommendation that women wait at least 24 months after a livebirth before attempting the next pregnancy.1 This recommendation was based on a large body of observational studies (published prior to 2006) showing an association between short interpregnancy intervals (see Table 1 for definition) and adverse birth outcomes, particularly preterm birth.1, 2 The applicability of the WHO recommendations for women in the United States is unclear, however, because breast feeding,3, 4 nutrition,5, 6 maternal age at first birth,7, 8 and total fertility rate9, 10 differ between the United States and the low‐ and middle‐income countries upon which most of the evidence reviewed for the WHO recommendation is based. Further, there are concerns that the associations between short interpregnancy intervals and adverse outcomes may not be causal but a result of confounding by maternal characteristics.11, 12, 13, 14 For example, women with short interpregnancy intervals are more likely to be of disadvantaged socio‐economic position and have had an unintended pregnancy,15, 16, 17 both risk factors for adverse pregnancy outcomes such as preterm birth.18, 19
Table 1.
Definition of terms related to timing between pregnancies within a woman
Interbirth interval
|
Interpregnancy interval
|
Post‐abortion interpregnancy interval
|
Post‐pregnancy loss interpregnancy interval
|
On 14‐15 September 2017, the Office of Population Affairs (OPA) convened an expert work group meeting entitled “Birth Spacing and Adverse Pregnancy Outcomes,” in Washington, DC, with the aim of critically evaluating the evidence for the causal effect of short interpregnancy intervals on adverse perinatal and maternal health outcomes in the United States. Participants in the meeting included reproductive, perinatal, paediatric, social, and public health epidemiologists; obstetrician‐gynaecologists; biostatisticians; and experts in evidence synthesis related to women's health. The goals for the meeting were to: (a) obtain expert perspectives on the extent to which current research supports a causal effect of short interpregnancy interval on adverse pregnancy outcomes; (b) reach a consensus on good practices for design, analysis, and interpretation of observational studies of short interpregnancy interval and adverse pregnancy outcomes; and (c) identify knowledge gaps and research priorities for future work. In this report, we summarise the proceedings of the work group meeting.
1.1. Context
The association between short spacing between births and adverse infant outcomes has been recognised in the United States for nearly 100 years. In 1916, a Census Bureau report on births occurring in Gary, Indiana, documented a higher rate of infant mortality among second‐ and higher order births following short interbirth intervals compared with first‐born infants and infants born following longer interbirth intervals (see Table 1 for definition).20 In 1945, an analysis of US national data on infant mortality by birth order among women of similar maternal age suggested that infant mortality increased with shorter interbirth intervals at a national level.21 In 1968, date of last livebirth was added to US birth certificates for the purpose of examining health outcomes associated with birth spacing.22 Consequently, many studies analysing interbirth or interpregnancy intervals on adverse outcomes since then have used state‐ or national‐level data collected on US birth certificates.2, 23, 24, 25, 26, 27
The associations found between short interpregnancy intervals (generally defined as some interval less than 18‐24 months) and adverse outcomes led the American College of Obstetricians and Gynecologists (ACOG) to issue the 2016 committee opinion, Optimizing Postpartum Care, stating that the optimal interval between delivery and subsequent pregnancy is 18 months to 5 years, with the greatest risk of preterm birth and low birthweight for intervals <6 months.28 While this recommendation was primarily based on studies conducted outside the United States,2, 29 reducing the proportion of pregnancies that occur within 18 months of a previous birth has been identified as one of the Healthy People 2020 priorities for the United States;30 and several states monitor progress in reducing short interpregnancy intervals between livebirths as part of their performance measures for improving maternal, infant, and child health.31, 32, 33 Further, the beneficial effect of using contraception to space births is an underlying component of current practice guidelines. These include the 2014 Quality Family Planning Guidelines, published by OPA and the Centers for Disease Control and Prevention (CDC),34 and the 2016 Women's Preventive Services Initiative Report, published by an ACOG‐led collaborative, used to inform women's preventive health care services recommendations.35 Nevertheless, there is no national recommendation for family planning service provision, postpartum or otherwise, included in the US Preventive Services Task Force (USPSTF) recommendations. The USPSTF recommendations play a central role in identifying the preventive services that should be covered, without cost sharing, by health insurance plans in the United States.36
1.2. Importance of understanding the effects of birth spacing
Understanding the causal effect of short birth spacing on adverse pregnancy outcomes is important for two main reasons: (a) providing evidence‐based information to patients to prepare them to make decisions affecting their health and the health of their child and (b) informing allocation of public health resources. During health care visits following a livebirth, a woman may seek information on the optimal time to become pregnant again in terms of her health and the health of her next infant. Sound evidence on the effects of birth spacing will help women (and their partners) make informed decisions on whether or not to use contraception, as well as further consider their plans for subsequent children.34, 37 Although universal access to contraception is a shared value of many providers and patients across the United States,38 access is limited in certain areas and for some women.39, 40, 41, 42, 43 A better understanding of the potential beneficial effects of reducing short birth spacing on health outcomes for women and infants could inform initiatives to improve access to postpartum contraception.
2. EXPERT WORK GROUP MEETING SCOPE
The scope of the meeting focused on evaluating the causal effect of short interpregnancy intervals on adverse pregnancy outcomes in the United States and other high‐resource countries. Although long interpregnancy intervals, such as five or more years between pregnancies, have also been associated with adverse outcomes, in the meeting the expert work group focused on short interpregnancy intervals because they are more amenable to prevention through the provision of family planning services, particularly postpartum contraceptive services.44 For the purposes of this meeting, interpregnancy interval was defined as the time between delivery of a livebirth and either the start of the next pregnancy or the start of the next pregnancy leading to a livebirth, depending on the data source (see Table 1 for definitions of terms related to timing between pregnancies for an individual woman). The meeting did not focus on other types of birth and pregnancy intervals,45, 46 such as interbirth intervals, postpregnancy loss interpregnancy intervals, or postabortion interpregnancy intervals. While these intervals may also be related to adverse pregnancy outcomes, studies using these measures were either methodologically inferior (eg interbirth intervals, which include the gestational length of the subsequent pregnancy) or beyond the scope of the meeting (eg postpregnancy loss or postabortion interpregnancy intervals, which may have unique associations with adverse health outcomes). The expert work group was interested in perinatal and short‐term maternal health outcomes, such as those that can be identified during or after pregnancy. The expert work group did not aim to evaluate longer term health outcomes for the mother, child, or other family members, or nonhealth outcomes such as economic, social, or educational outcomes.
3. EVIDENCE PRESENTED ON SHORT INTERPREGNANCY INTERVAL AND ADVERSE PREGNANCY OUTCOMES
3.1. Systematic reviews
A systematic review and meta‐analysis of interpregnancy interval and adverse perinatal outcomes2 and a systematic review of maternal health outcomes23 have summarised studies published between 1966 and January 2006. Results indicated that interpregnancy intervals <6 months, 6‐11 months, and 12‐17 months compared with 18‐23 months were associated with increased risk of adverse perinatal outcomes, such as preterm birth (pooled adjusted odds ratios [aORs] 1.40, 1.14, and 1.07, respectively), low birthweight (aORs 1.61, 1.14, and 1.05, respectively), and small‐for‐gestational‐age birth (aOR 1.26, 1.11, and 1.06, respectively).2 Interpregnancy intervals of some duration less than 24 months were also associated with increased risk of uterine rupture among women attempting vaginal birth after a caesarean section and uteroplacental bleeding disorders, such a placentae praevia and abruption (individual estimates varied, and data were not pooled).23 However, the applicability of the systematic reviews to US women may be limited because the majority of studies were from lower resource countries. Further, the reviews only covered research published prior to 2006.
In preparation for the expert work group meeting, the existing systematic reviews were updated by selecting studies more applicable to US women and identifying newer studies (ie published between January 2006 and May 2017). The new reviews incorporated more narrow inclusion criteria by restricting included studies to those that defined short birth spacing using the interpregnancy interval (with short interpregnancy interval defined as some duration less than 24 months versus a well‐defined longer duration), controlled for at least maternal age (and socio‐economic position, for perinatal outcomes), and were conducted within countries categorised as “very high” on the United Nations Human Development Index.47 Details on the systematic review methodology, including study quality assessment, and the summary of evidence can be found in other manuscripts in this journal supplement.48, 49 Studies employing a sibling comparison design, which compared differences in a woman's interpregnancy intervals and birth outcomes using a within‐woman analysis, were considered separately from the studies employing a conventional between‐women analysis.
3.2. Short interpregnancy interval and perinatal outcomes
The updated systematic review on short interpregnancy intervals and perinatal outcomes included 21 studies published since 2006 and 11 studies from the previous review that met our revised inclusion criteria.2, 49 Definitions of short interpregnancy interval varied across studies (eg <3 months, <9 months, and 13‐24 months) with most studies specifying more than one mutually exclusive short interpregnancy interval (eg <6, 6‐11, and 12‐17 months). Most studies (31/32) were cohort studies, and the remaining study was a case–control study. Results generally showed modest adverse (aOR <2.00) or null associations between short interpregnancy interval and preterm birth, low birthweight, small‐for‐gestational‐age births, infant mortality, neonatal intensive care unit admission, and specific birth defects. Generally, studies found the shortest interpregnancy interval category showed the strongest association with the study outcome.
Approximately 44% (14 of 32) of studies used state‐level or national US birth certificate data and used a similar set of variables to adjust for maternal demographic characteristics (maternal age, education, maternal race, and marital status). Most of the remaining studies controlled for similar maternal demographic characteristics using other data sources; however, several studies, mostly from outside the United States, controlled for enhanced socio‐economic information. One‐quarter of studies (8 of 32) controlled for history of pregnancy resulting in perinatal death, and two of the 32 studies measured pregnancy intention.50, 51 In general, pregnancy‐related variables included in multivariable modelling were factors measured in the pregnancy following, not preceding, the interpregnancy interval, which could have introduced overadjustment bias.52
Four studies used a sibling comparison design to estimate associations between short interpregnancy interval and adverse perinatal outcomes among women with three or more births (ie two or more interpregnancy intervals). While all four studies reported some degree of increased risk of preterm birth associated with at least one short interpregnancy interval category in the conventional between‐women analysis,53, 54, 55, 56 this association was attenuated or eliminated in all of these studies after controlling for between‐women confounding by performing a sibling comparison analysis.
3.3. Short interpregnancy interval and maternal outcomes
Six new studies and one study from the previous systematic review23 met inclusion criteria for the updated systematic review on short interpregnancy interval and adverse maternal outcomes.49 Our restriction criteria that studies controlled for at least maternal age and that they examined interpregnancy interval (rather than interbirth interval) resulted in the exclusion of at least two large population‐based studies examining interbirth intervals and adverse maternal outcomes.57, 58 All included studies were cohort studies. Two studies reported that short interpregnancy interval was associated with subsequent increased risk of obesity in the mother,54, 59 one found an increased risk of gestational diabetes and decreased risk of preeclampsia,54 two reported increased risk of labour dystocia,60, 61 one found a decreased risk of precipitous labour,62 and one found increased risk of placental abruption.63 A study of women who attempted vaginal birth after caesarean delivery found short interpregnancy interval was associated with increased risk of uterine rupture.64
One study examined the association between short interpregnancy interval and maternal outcomes using a sibling comparison design.54 In contrast to the finding in this study that associations between short interpregnancy interval and perinatal outcomes were attenuated after a sibling analysis, associations between short interpregnancy interval and risk of subsequent gestational diabetes and prepregnancy maternal obesity remained or became more pronounced in the sibling analyses. The protective effect of short interpregnancy interval on risk of preeclampsia also remained.
4. METHODOLOGICAL LIMITATIONS OF STUDIES
The expert work group discussed the methodological limitations of existing studies on short interpregnancy interval and adverse pregnancy outcomes, as well as important considerations for future research. What follows below are summaries of five key issues identified by the expert work group members. More information on good practices when conducting analyses of short interpregnancy interval on adverse pregnancy outcomes using observational study data is detailed elsewhere in this journal supplement.65
4.1. Issue 1: Residual confounding in studies employing conventional between‐women analyses
The expert work group members concluded some of the previously observed associations between short interpregnancy interval and adverse pregnancy outcomes could be attributed to confounding. These confounders include maternal socio‐economic position, perinatal loss (stillbirth or neonatal death) in the previous pregnancy, and pregnancy intention for the subsequent pregnancy. These factors could lead to both short interpregnancy intervals and adverse pregnancy outcomes, as illustrated in our causal diagram (Figure 1).
Figure 1.
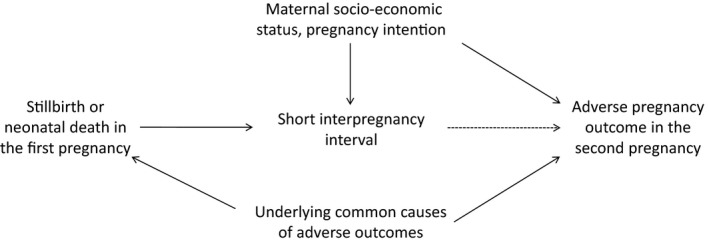
Causal diagram: simplified directed acyclic graph showing key factors to consider for analyses of short interpregnancy interval on adverse pregnancy outcomes
Disadvantaged maternal socio‐economic position is associated with both short interpregnancy intervals and adverse pregnancy outcomes, such as stillbirth, preterm birth, and low birthweight, making it a potential confounder.2, 12, 66, 67 Women's pregnancy intentions prior to conception is also a potential confounder;16, 68 however, measuring intention is complex, as behaviours such as contraception use do not align with intentions, and intentions change over time.69 In addition, women with prior perinatal death (stillbirths or neonatal deaths) are likely to have short interpregnancy interval before becoming pregnant again,66, 67 and prior perinatal death could reflect an underlying condition causing adverse outcomes for multiple pregnancies across a woman's reproductive lifespan.
Studies of short interpregnancy interval on adverse pregnancy outcomes, particularly perinatal outcomes, may be susceptible to positive residual confounding if there is incomplete control for maternal socio‐economic position, pregnancy intention, and prior pregnancy perinatal loss. This conclusion is supported by the attenuated effect on perinatal outcomes after adjusting for maternal demographics and socio‐economic position.2, 49, 70 The expert work group did not reach consensus on how complete control of confounding could be achieved, but did agree that current research could be improved by more diverse study designs, analyses, and sources of data. In addition, researchers should provide a clear explanation for how these factors are incorporated in their analysis and the quality of the variables used.
4.2. Issue 2: Challenges inherent in sibling comparison design studies
The sibling comparison design provides a powerful approach to control for confounding by all factors, both observed and unobserved, that remain constant across a woman's pregnancies. However, the design is intrinsically susceptible to other problems. By definition, these studies are limited to women with three or more pregnancies (to compare at least two interpregnancy intervals). It is unclear to what extent women with at least two interpregnancy intervals are representative of all women with at least one interpregnancy interval. In other words, whether the findings from such sibling designs are generalisable remains in question. Further, estimates from sibling comparison analysis are informed only by women who have had discordant pregnancy outcomes and discordant interpregnancy intervals, not all women with at least two interpregnancy intervals. This limits the study sample to a small subset of the target population, introducing further concerns regarding selection bias and generalisability,71, 72 which are exacerbated when exposures and outcome are categorised and even fewer women provide information for the analysis.73 Furthermore, the reduced sample size owing to this restriction comes at an expense of compromised statistical power to detect associations. Time‐varying confounding factors that vary between a woman's pregnancies (such as prepregnancy body mass index or maternal comorbidities) are not intrinsically controlled for through the sibling comparison design and can introduce bias if not included in multivariable models.74
Despite these concerns, the expert working group felt these studies were valuable in understanding the causal relationship between short interpregnancy interval and adverse pregnancy outcomes because they fully controlled for between‐woman confounding. However, findings from these studies have limited generalisability.
4.3. Issue 3: Discrepancy between evidence on interpregnancy interval and advising patients on when to try for next pregnancy
Although interpregnancy interval can be modified, particularly through the use of effective contraceptive methods, it is not an exposure that can be directly assigned with high treatment adherence. The interpregnancy interval is the result of numerous biological and behavioural factors: postpartum return to ovulation; underlying fecundability and maternal age; sexual activity postpartum; contraception use initiation, effectiveness of method, and consistency of use; and intentions and desires to conceive (Figure 2). As a result, a woman's actual interpregnancy interval may differ from her intended interpregnancy interval due to the interplay of these factors. If recommendations on birth spacing are derived directly from observational data identifying low‐risk interpregnancy intervals, and women follow these recommendations, actual interpregnancy intervals will often be longer than recommended because of the time it normally takes a couple to conceive. This could result in unanticipated adverse effects, particularly because delaying attempting a next pregnancy results in older maternal age at pregnancy, which may increase associated risks and lead to decreased fecundity, particularly for older women.75
Figure 2.
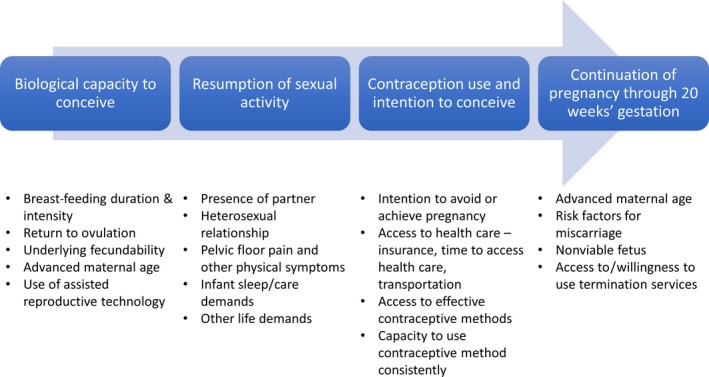
Factors influencing length of interpregnancy interval between livebirth and subsequent pregnancy with perinatal outcomes
Other exposures, such as initiation and duration of postpartum contraception use or timing of unprotected sexual intercourse with a male partner following a birth, may more closely reflect actionable behaviours and be easier for women to modify. For these exposures, interpregnancy interval would function as a mediator variable along the causal pathway. While studies have evaluated pregnancy outcomes as a function of the interval between early pregnancy loss and start of trying for the next pregnancy,76 no studies to our knowledge have evaluated associations as a function of interval between delivery of a livebirth and start of trying for the next pregnancy.
4.4. Issue 4: Poorly defined research questions
The choice of study population, design, data collection, type of analysis, and approach for controlling for confounders all depend on how the research question is formulated. Earlier studies estimated the association between short interpregnancy intervals and adverse events such as preterm birth, while controlling for a limited number of confounding variables. Some studies have interpreted observed associations as causal ones in order to calculate attributable risks and propose ideal interventions, such as the counselling provided to women postpartum.27, 77 This means that a study sometimes addressed three research questions simultaneously including the following: “What is the association?” “What is the causal effect?” and “What is the best intervention?” Studies with multiple, ill‐defined research questions are usually inadequately designed to address them all.
4.5. Issue 5: Consistent exposure and outcome definitions to improve research base
Unlike a dichotomous exposure, interpregnancy interval is a measure of time and can be evaluated in a variety of ways. While researchers should plan their analysis according to their specific research question, consistent definitions of interpregnancy interval are essential to combining study results. Presenting results using standardised cut‐points and reference groups for categorical analyses of interpregnancy intervals and consistent definitions of commonly examined outcomes would improve the research base.65 Further, providing a complete description of the interpregnancy interval, such as whether or not intervening pregnancy losses are included in the interval, would aid in causal interpretation of the study's estimates.
5. PRIORITIES FOR FUTURE RESEARCH
At the conclusion of the expert work group meeting, members discussed priorities for future work in order to understand the potential causal role of short interpregnancy intervals on adverse pregnancy outcomes. Table 2 summarises three areas for future research: (a) understanding whether potential risks associated with short interpregnancy intervals differ among specific subgroups, such as women with intended pregnancies; (b) extending the scope of outcomes to include more maternal outcomes; long‐term maternal, child, and family outcomes, as well as nonhealth outcomes, such as educational or economic attainment; and (c) employing different study designs, with more actionable exposures, such as initiation and duration of postpartum contraception use and timing of unprotected intercourse with male partner.
Table 2.
Future directions for research on short interpregnancy interval and maternal‐child health
Establish whether association between short interpregnancy interval and adverse pregnancy outcome differs according to maternal characteristics
|
Advance understanding of the independent association between short interpregnancy interval and subsequent
|
New study designs of the association between modifiable exposures related to pregnancy spacing and adverse outcomes
|
Finally, new studies examining the associations of short interpregnancy interval on previously studied adverse pregnancy outcomes using only information available from the US birth certificate are unlikely to provide meaningful new insights. A mosaic of new studies is now needed, from more varied populations and using different study designs with rigorous attention to control for confounding (Table 2).
6. CONCLUSIONS
Experts attending the work group meeting Birth Spacing and Adverse Pregnancy Outcomes convened by the Office of Population Affairs on 14‐15 September 2017 identified several key issues for the study of short interpregnancy intervals and adverse pregnancy outcomes. More research is needed on how associations vary by maternal demographics and age and how short interpregnancy interval is associated with maternal and infant health as well as longer term maternal, child, and family outcomes. In addition, the field would benefit from new study designs that can better control for confounding, thereby coming closer to estimating the causal effect of short interpregnancy intervals on adverse pregnancy outcomes and informing the development of US recommendations on birth spacing for optimal maternal and infant health.
CONFLICT OF INTEREST
Peter Briss, Lauren Rossen, and Cynthia Ferré work for the Centers for Disease Control and Prevention, an agency that published the Providing Quality Family Planning Services Recommendations with the Office of Population Affairs in 2014. Mark Klebanoff noted that his participation in the meeting was not intended to disqualify researchers working at The Ohio State University from responding to future requests for proposals from the Office of Population Affairs.
DISCLAIMER
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Office of Population Affairs, Office of the Secretary for Health, Centers for Disease Control and Prevention, National Center for Health Statistics, Health Resources and Services Administration, or Maternal and Child Health Bureau.
ACKNOWLEDGEMENTS
The authors thank Jamie Hart and Julia Rollison, from Atlas Research, for facilitating the expert work group meeting Birth Spacing and Adverse Pregnancy Outcomes, in Washington, DC, 14‐15 September 2017. The authors also acknowledge the critical feedback they received during the meeting from the following participants: Maureen Norton, United States Agency for International Development; Lorrie Gavin, private consultant; Ann Borders, Northwestern University; and Karen Pazol, Centers for Disease Control and Prevention
Ahrens KA, Hutcheon JA, Ananth CV, et al. Report of the Office of Population Affairs’ expert work group meeting on short birth spacing and adverse pregnancy outcomes: Methodological quality of existing studies and future directions for research. Paediatr Perinat Epidemiol. 2019;33:O5–O14. 10.1111/ppe.12504
Funding Information
This product was supported, in part, by a contract between the Office of Population Affairs and Atlas Research, LLC [# HHSP233201450040A]. SLM was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
References
- 1. Report of a WHO Technical Consultation on Birth Spacing: Geneva, Switzerland 13‐15 June 2005. Department of Reproductive Health and Research (RHR). Geneva, Switzerland World Health Organization; 2006. http://apps.who.int/iris/bitstream/10665/69855/1/WHO_RHR_07.1_eng.pdf. Accessed March 15, 2018.
- 2. Conde‐Agudelo A, Rosas‐Bermudez A, Kafury‐Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta‐analysis. J Am Med Assoc. 2006;295:1809‐1823. [DOI] [PubMed] [Google Scholar]
- 3. UNICEF . Breastfeeding: A mother's gift, for every child. Nutrition Section, Programme Division, Data and Analytics Section, Division of Data, Research and Policy, and Division of Communication. 3 United Nations Plaza, New York, NY 10017, USA: Nutrition Section, Programme Division, UNICEF; 2018 https://www.unicef.org/publications/files/UNICEF_Breastfeeding_A_Mothers_Gift_for_Every_Child.pdf. Accessed May 10, 2018.
- 4. World Health Organization, UNICEF . Global Breastfeeding Collective, Global Breastfeeding Scorecard. Geneva, Switzerland: Word Health Organization; 2017. https://www.unicef.org/nutrition/index_100585.html. Accessed March 15, 2018.
- 5. World Health Assembly, 65 . Nutrition of women in the preconception period, during pregnancy and the breastfeeding period. Report by the Secretariat. Geneva, Switzerland: World Health Organization; 2012. http://apps.who.int/gb/ebwha/pdf_files/EB130/B130_11-en.pdf. Accessed March 15, 2018.
- 6. Rosenbloom JI, Kaluski DN, Berry EM. A global nutritional index. Food Nutr Bull. 2008;29:266‐277. [DOI] [PubMed] [Google Scholar]
- 7. Mother's mean age at first birth. The World Factbook. 2018 https://www.cia.gov/library/publications/the-world-factbook/fields/2256.html. Accessed July 13, 2018.
- 8. Matthews T, Hamilton BE. Delayed childbearing: more women are having their first child later in life. NCHS Data Brief. 2009;21:1‐8. [PubMed] [Google Scholar]
- 9. Fertility rate, total (births per woman). The World Bank. High income countries and low & middle income countries. 2016 https://data.worldbank.org/indicator/SP.DYN.TFRT.IN. Accessed July 20, 2018.
- 10. Total Fertility Rate . The World Factbook. Washington, DC: The Central Intelligence Agency, Office of Public Affairs; 2017. https://www.cia.gov/library/publications/the-world-factbook/rankorder/2127rank.html. Accessed March 15, 2018.
- 11. Klebanoff MA. Short interpregnancy interval and the risk of low birthweight. Am J Public Health. 1988;78:667‐670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klebanoff MA. Interpregnancy interval and pregnancy outcomes: causal or not? Obstet Gynecol. 2017;129:405‐407. [DOI] [PubMed] [Google Scholar]
- 13. Klebanoff MA. The interval between pregnancies and the outcome of subsequent births. N Engl J Med. 1999;340:643‐644. [DOI] [PubMed] [Google Scholar]
- 14. Erickson JD, Bjerkedal T. Interpregnancy interval. Association with birth weight, stillbirth, and neonatal death. J Epidemiol Community Health. 1978;32:124‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thoma ME, Copen CE, Kirmeyer SE. Short interpregnancy intervals in 2014: Differences by maternal demographic characteristics. NCHS data brief, no 240. Hyattsville, MD: National Center for Health Statistics, 2016. [PubMed] [Google Scholar]
- 16. White K, Teal SB, Potter JE. Contraception after delivery and short interpregnancy intervals among women in the United States. Obstet Gynecol. 2015;125:1471‐1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahrens KA, Thoma M, Copen C, Frederiksen B, Decker E, Moskosky S. Unintended pregnancy and interpregnancy interval by maternal age. National Survey of Family Growth. Contraception. 2018;98:52‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shah PS, Balkhair T, Ohlsson A, Beyene J, Scott F, Frick C. Intention to become pregnant and low birth weight and preterm birth: a systematic review. Matern Child Health J. 2011;15:205‐216. [DOI] [PubMed] [Google Scholar]
- 19. DeSisto CL, Hirai AH, Collins JW Jr, Rankin KM. Deconstructing a disparity: explaining excess preterm birth among U.S.‐born black women. Ann Epidemiol. 2018;28:225‐230. [DOI] [PubMed] [Google Scholar]
- 20. US Department of Labor . Children’s Bureau. Infant mortality, Results of a field study in Gary, Indiana. Based on Births in One Year, by Elizabeth Hughes. Bureau Publication Number 112. 1923
- 21. Yerushalmy J. On the interval between successive births and its effect on survival of infant. Hum Biol. 1945;17:65‐106. [Google Scholar]
- 22. Lunde AS. Revisions of U.S. standard certificates on vital events. Public Health Rep. 1967;82:913‐916. [PMC free article] [PubMed] [Google Scholar]
- 23. Conde‐Agudelo A, Rosas‐Bermudez A, Kafury‐Goeta AC. Effects of birth spacing on maternal health: a systematic review. Am J Obstet Gynecol. 2007;196:297‐308. [DOI] [PubMed] [Google Scholar]
- 24. Wendt A, Gibbs CM, Peters S, Hogue CJ. Impact of increasing inter‐pregnancy interval on maternal and infant health. Paediatr Perinat Epidemiol. 2012;26:239‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shachar BZ, Lyell DJ. Interpregnancy interval and obstetrical complications. Obstet Gynecol Surv. 2012;67:584‐596. [DOI] [PubMed] [Google Scholar]
- 26. Zhu B‐P. Effect of interpregnancy interval on birth outcomes: findings from three recent US studies. Int J Gynecol Obstet. 2005;89:S25‐S33. [DOI] [PubMed] [Google Scholar]
- 27. Zhu BP, Rolfs RT, Nangle BE, Horan JM. Effect of the interval between pregnancies on perinatal outcomes. N Engl J Med. 1999;340:589‐594. [DOI] [PubMed] [Google Scholar]
- 28. Committee Opinion No. 666 . Optimizing postpartum care. Obstet Gynecol. 2016;127:e187‐e192. [DOI] [PubMed] [Google Scholar]
- 29. Grisaru‐Granovsky S, Gordon ES, Haklai Z, Samueloff A, Schimmel MM. Effect of interpregnancy interval on adverse perinatal outcomes–a national study. Contraception. 2009;80:512‐518. [DOI] [PubMed] [Google Scholar]
- 30. US Department of Health and Human Services . Healthy People 2020 Objectives: Family Planning. Washington, DC http://www.healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=13. Accessed October 17, 2013.
- 31. U.S. Department of Health and Human Services, Administration for Children and Families and Health Resources and Services Administration. Demonstrating Improvement in the Maternal, Infant, and Early Childhood Home Visiting Program. A Report to Congress. 2016 https://mchb.hrsa.gov/sites/default/files/mchb/MaternalChildHealthInitiatives/HomeVisiting/pdf/reportcongress-homevisiting.pdf. Accessed March 23, 2018.
- 32. National Institute for Children's Health Quality Initiatives . Collaborative improvement and innovation network to reduce infant mortality (IM CoIIN). Preconception and interconception care management strategy. http://static.nichq.org/prevention-toolkit/resources/Pre_Interconception_Care_Measurement_Algorithm.pdf. Accessed March 22, 2018.
- 33. Division of Health Start and Perinatal Services . Performance measures. The percent of Healthy Start women participants who conceive within 18 months of a previous birth. https://mchb.hrsa.gov/sites/default/files/mchb/Data/dgis/DGIS_DHSPS_Healthy_Start_Measures.pdf. Accessed March 23, 2018.
- 34. Gavin L, Moskosky S, Carter M, et al. Providing quality family planning services: recommendations of CDC and the U.S. Office of Population Affairs. MMWR Recomm Rep. 2014;63:1‐54. [PubMed] [Google Scholar]
- 35. Women's Preventive Services Initiative . Recommendations for preventive services for women: final report to the U.S. Department of Health and Human Services, Health Resources & Services Administration. Washington, DC: American College of Obstetricians and Gynecologists; 2016 https://www.womenspreventivehealth.org/final-report/. Accessed March 15, 2018.
- 36. Kaiser Family Foundation . Preventive Services Covered by Private Health Plans under the Affordable Care Act. 2015 https://www.kff.org/health-reform/fact-sheet/preventive-services-covered-by-private-health-plans/. Accessed October 15, 2017.
- 37. Files JA, Frey KA, David PS, Hunt KS, Noble BN, Mayer AP. Developing a reproductive life plan. J Midwifery Womens Health. 2011;56:468‐474. [DOI] [PubMed] [Google Scholar]
- 38. Universal Access to Contraception . Policy Statement Number 20153. Washington, DC: American Public Health Association; 2015 https://www.apha.org/policies-and-advocacy/public-health-policy-statements/policy-database/2015/12/17/09/14/universal-access-to-contraception. Accessed March 15, 2018.
- 39. Moniz MH, Chang T, Heisler M, et al. Inpatient postpartum long‐acting reversible contraception and sterilization in the United States, 2008‐2013. Obstet Gynecol. 2017;129:1078‐1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moniz MH, McEvoy AK, Hofmeister M, Plegue M, Chang T. Family physicians and provision of immediate postpartum contraception: a CERA study. Fam Med. 2017;49:600‐606. [PubMed] [Google Scholar]
- 41. Moniz MH, Roosevelt L, Crissman HP, et al. Immediate postpartum contraception: a survey needs assessment of a national sample of midwives. J Midwifery Womens Health. 2017;62:538‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Potter JE, Hopkins K, Aiken AR, et al. Unmet demand for highly effective postpartum contraception in Texas. Contraception. 2014;90:488‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Harney C, Dude A, Haider S. Factors associated with short interpregnancy interval in women who plan postpartum LARC: a retrospective study. Contraception. 2017;95:245‐250. [DOI] [PubMed] [Google Scholar]
- 44. Brunson MR, Klein DA, Olsen CH, Weir L, Roberts TA. Postpartum contraception: initiation and effectiveness in a large universal healthcare system. Am J Obstet Gynecol. 2017;217:55.e1‐55.e9. [DOI] [PubMed] [Google Scholar]
- 45. Conzuelo‐Rodriguez G, Naimi AI. The impact of computing interpregnancy intervals without accounting for intervening pregnancy events. Paediatr Perinat Epidemiol. 2018;32:141‐148. [DOI] [PubMed] [Google Scholar]
- 46. Ahrens KA, Hutcheon JA. Optimal birth spacing: what can we measure and what do we want to know? Paediatr Perinat Epidemiol. 2018;32:149‐151. [DOI] [PubMed] [Google Scholar]
- 47. United Nations Human Development Index, 2015. New York, NY: United Nations; http://hdr.undp.org/en/composite/HDI. Accessed March 21, 2018.
- 48. Hutcheon JA, Nelson HD, Stidd R, Moskosky S, Ahrens KA. Short interpregnancy intervals and adverse maternal outcomes in high resource settings: an updated systematic review. Paediatr Perinat Epidemiol. 2019; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ahrens KA, Nelson HD, Stidd R, Moskosky S, Hutcheon JA. Short interpregnancy intervals and adverse perinatal outcomes in high‐resource settings: an updated systematic review. Paediatr Perinat Epidemiol. 2019; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van Eijsden M, Smits LJ, van der Wal MF, Bonsel GJ. Association between short interpregnancy intervals and term birth weight: the role of folate depletion. Am J Clin Nutr. 2008;88:147‐153. [DOI] [PubMed] [Google Scholar]
- 51. Lang JM, Lieberman E, Ryan KJ, Monson RR. Interpregnancy interval and risk of preterm labor. Am J Epidemiol. 1990;132:304‐309. [DOI] [PubMed] [Google Scholar]
- 52. Ananth CV, Schisterman EF. Confounding, causality, and confusion: the role of intermediate variables in interpreting observational studies in obstetrics. Am J Obstet Gynecol. 2017;217:167‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ball SJ, Pereira G, Jacoby P, de Klerk N, Stanley FJ. Re‐evaluation of link between interpregnancy interval and adverse birth outcomes: retrospective cohort study matching two intervals per mother. BMJ. 2014;349:g4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hanley GE, Hutcheon JA, Kinniburgh BA, Lee L. Interpregnancy interval and adverse pregnancy outcomes: an analysis of successive pregnancies. Obstet Gynecol. 2017;129:408‐415. [DOI] [PubMed] [Google Scholar]
- 55. Shachar B, Mayo J, Lyell D, et al. Interpregnancy interval after live birth or pregnancy termination and estimated risk of preterm birth: a retrospective cohort study. Br J Obstet Gynaecol. 2016;123:2009‐2017. [DOI] [PubMed] [Google Scholar]
- 56. Koullali B, Kamphuis EI, Hof MH, et al. The effect of interpregnancy interval on the recurrence rate of spontaneous preterm birth: a retrospective cohort study. Am J Perinatol. 2017;34:174‐182. [DOI] [PubMed] [Google Scholar]
- 57. Ananth CV, Skjaerven R, Klunssoyr K. Change in paternity, risk of placental abruption and confounding by birth interval: a population‐based prospective cohort study in Norway, 1967‐2009. BMJ Open. 2015;5:e007023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hernández‐Díaz S, Toh S, Cnattingius S. Risk of pre‐eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ. 2009;338:b2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Davis EM, Babineau DC, Wang X, et al. Short inter‐pregnancy intervals, parity, excessive pregnancy weight gain and risk of maternal obesity. Matern Child Health J. 2014;18:554‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhu BP, Grigorescu V, Le T, et al. Labor dystocia and its association with interpregnancy interval. Am J Obstet Gynecol. 2006;195:121‐128. [DOI] [PubMed] [Google Scholar]
- 61. Sandström A, Cnattingius S, Wikström AK, Stephansson O. Labour dystocia–risk of recurrence and instrumental delivery in following labour–a population‐based cohort study. Br J Obstet Gynaecol. 2012;119:1648‐1656. [DOI] [PubMed] [Google Scholar]
- 62. Appareddy S, Pryor J, Bailey B. Inter‐pregnancy interval and adverse outcomes: Evidence for an additional risk in health disparate populations. J Matern Fetal Neonatal Med. 2016;30:1‐5. [DOI] [PubMed] [Google Scholar]
- 63. Blumenfeld YJ, Baer RJ, Druzin ML, et al. Association between maternal characteristics, abnormal serum aneuploidy analytes, and placental abruption. Am J Obstet Gynecol. 2014;211:144. e141‐149. [DOI] [PubMed] [Google Scholar]
- 64. Stamilio DM, DeFranco E, Paré E, et al. Short interpregnancy interval: risk of uterine rupture and complications of vaginal birth after cesarean delivery. Obstet Gynecol. 2007;110:1075‐1082. [DOI] [PubMed] [Google Scholar]
- 65. Hutcheon J, Moskosky S, Ananth C, et al. Good practices for the design, analysis, and interpretation of observational studies on birth spacing and perinatal health outcomes. Paediatr Perinat Epidemiol. 2019; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stephansson O, Dickman PW, Cnattingius S. The influence of interpregnancy interval on the subsequent risk of stillbirth and early neonatal death. Obstet Gynecol. 2003;102:101‐108. [DOI] [PubMed] [Google Scholar]
- 67. Smith GC, Pell JP, Dobbie R. Interpregnancy interval and risk of preterm birth and neonatal death: retrospective cohort study. BMJ. 2003;327:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hall JA, Benton L, Copas A, Stephenson J. Pregnancy intention and pregnancy outcome: systematic review and meta‐analysis. Matern Child Health J. 2017;21:670‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mumford SL, Sapra KJ, King RB, Louis JF, Buck Louis GM. Pregnancy intentions‐a complex construct and call for new measures. Fertil Steril. 2016;106:1453‐1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Howard EJ, Harville E, Kissinger P, Xiong X. The association between short interpregnancy interval and preterm birth in Louisiana: a comparison of methods. Matern Child Health J. 2013;17:933‐939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ahrens KA, Thoma ME, Rossen LM. Interpregnancy interval and adverse pregnancy outcomes: an analysis of successive pregnancies. Obstet Gynecol. 2017;130:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mittleman M. Generalizability of case‐crossover and other case‐only designs in environmental epidemiology. Epidemiology. 2006;17:S224. [Google Scholar]
- 73. Grosz M, Miller D, Shenhav N. Working paper: Long‐term Effects of Head Start: New Evidence from the PSID. 2016; http://www.sole-jole.org/17283.pdf. Accessed September 13, 2018.
- 74. Sjölander A, Frisell T, Kuja‐Halkola R, Öberg S, Zetterqvist J. Carryover effects in sibling comparison designs. Epidemiology. 2016;27:852‐858. [DOI] [PubMed] [Google Scholar]
- 75. Steiner AZ, Jukic AM. Impact of female age and nulligravidity on fecundity in an older reproductive age cohort. Fertil Steril. 2016;105:1584‐1588.e1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Schliep KC, Mitchell EM, Mumford SL, et al. Trying to conceive after an early pregnancy loss: an assessment on how long couples should wait. Obstet Gynecol. 2016;127:204‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. McKinney D, House M, Chen A, Muglia L, DeFranco E. The influence of interpregnancy interval on infant mortality. Am J Obstet Gynecol. 2017;216:316.e311‐316.e319. [DOI] [PMC free article] [PubMed] [Google Scholar]


